Abstract
We aimed to establish reference ranges for USCOM parameters in preterm infants, determine factors that affect cardiac output, and evaluate the measurement repeatability. This retro-prospective study was performed at Fondazione IRCCS San Gerardo dei Tintori, Monza, Italy. We included infants below 32 weeks of gestational age (GA) and/or 1500 g of birth weight (BW). We excluded infants with congenital heart diseases or hemodynamic instability. Measurements were performed at 3 ± 1, 7 ± 2, and 14 ± 2 postnatal days. We analyzed 204 measurements from 92 patients (median GA = 30.57 weeks, BW = 1360 g). The mean (SD) cardiac output (CO) was 278 (55) ml/min/kg, cardiac index (CI) was 3.1 (0.5) L/min/m2, and systemic vascular resistance (SVRI) was 1292 (294) d*s*cm−5/m2. CO presented a negative correlation with postmenstrual age (PMA), while SVRI presented a positive correlation with PMA. The repeatability coefficient was 31 ml/kg/min (12%).
Conclusion: This is the first study describing reference values for USCOM parameters in hemodynamically stable preterm infants and factors affecting their variability. Further studies to investigate the usefulness of USCOM for the longitudinal assessment of patients at risk for cardiovascular instability or monitoring the response to therapies are warranted.
What is Known: • The ultrasonic cardiac output monitoring (USCOM) has been widely used on adult and pediatric patients and reference ranges for cardiac output (CO) by USCOM have been established in term infants. | |
What is New: • We established reference values for USCOM parameters in very preterm and very-low-birth-weight infants; the reference ranges for CO by USCOM in the study population were 198-405 ml/kg/min. • CO normalized by body weight presented a significant negative correlation with postmenstrual age (PMA); systemic vascular resistance index presented a significant positive correlation with PMA. |
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Preterm infants are at high risk of hemodynamic instability and inadequate systemic perfusion due to several factors, including immature myocardium with poor ventricular function, the sudden shift from low to high vascular resistance occurring at birth, intravascular volume depletion, and the impact of positive pressure ventilation on venous return and cardiac output [1]. In addition, very preterm and very-low-birth-weight infants often present a patent ductus arteriosus (PDA) [2], which might result in either pulmonary overflow or systemic steal.
It is, therefore, important for physicians to have rapid and direct measures of cardiovascular parameters to diagnose and monitor hemodynamic and myocardial dysfunction in this critical population. Several methods are available for cardiac output (CO) monitoring in critically ill patients, but few of them are feasible and clinically applicable in preterm newborns [3, 4]. Invasive methods requiring pulmonary artery catheterization are considered the gold standard to measure CO in terms of accuracy but are of limited applicability in newborns. Blood pressure, heart rate, urine output, capillary refill, and lactate are indirect clinical indicators of perfusion but are poorly correlated with CO [5,6,7]. Non-invasive techniques for CO estimation that are feasible and clinically applicable in critically ill newborn infants are transthoracic echocardiography, electrical biosensing technologies, and transcutaneous Doppler. Echocardiography is the most widely used method; however, it requires well-trained personnel and an expensive instrument and is time-consuming. Electrical biosensing technologies are based on the assumption that electrical impedance changes in the thorax are correlated to blood volume changes. In particular, electrical cardiometry has been recently validated in preterm neonates [8]. The advantage of this approach is that it provides continuous, operator-independent hemodynamic monitoring; however, it tends to overestimate CO, as assessed by echocardiography, and has relatively high variability [8].
The ultrasonic cardiac output monitor (USCOM; USCOM Ltd., Sydney, Australia) provides rapid trans-thoracic estimates of CO. The device performs continuous wave Doppler blood flow measurements in large vessels and estimates the vessel cross-sectional area based on validated nomograms derived from height and weight. The flow in the ascending aorta, related to blood pressure and aortic valve area, provides information about cardiovascular function, such as CO, cardiac index (CI), and systemic vascular resistance index (SVRI). The advantage of USCOM is that it is compact and cheaper than a conventional ultrasound machine; moreover, it is designed for users without prior echocardiography experience, and competence can be achieved in a few days of training: 20 to 30 measurements are usually considered sufficient for learning the technique [9, 10].
The USCOM has been widely used on adult and pediatric patients [11], but data about its applicability to neonates are scant. Previous studies performed in hemodynamically stable term infants suggested that the agreement between CO measured by USCOM and conventional echocardiography is broad, and so are the reference ranges [9, 12,13,14]. However, the reproducibility and the intra-rater reliability of the measurement are high, suggesting that USCOM may allow the longitudinal monitoring of cardiovascular parameters in infants. For instance, Liu et al. have used USCOM to evaluate hemodynamic changes after PDA ligation in very- and extremely-low-birth-weight infants [15]. Furtherly, among cardiovascular parameters obtained by USCOM measurements, SVRI is a useful indicator of vascular status and can help the clinician understand whether the patient is vaso-constricted or vaso-dilated [16].
The feasibility of CO, CI, and SVRI measurements by the USCOM system in a population of very preterm and/or very-low-birth-weight infants has never been systematically evaluated. To fill this gap, this study aimed at (1) establishing reference ranges for USCOM parameters in this specific population, (2) assessing the effect of patients’ characteristics and other possible confounders on USCOM parameters, and (3) evaluating the measurement short-term repeatability.
Materials and methods
Study design and setting
This retro-prospectively study was performed in the Neonatal Intensive Care Unit of Fondazione IRCCS San Gerardo dei Tintori, Monza, Italy. Data were collected between November 2020 and June 2023. The protocol was approved by the local ethical committee (nr. 4309).
Participants
In our unit, serial USCOM assessments are routinely performed on infants with gestational age (GA) at birth below 32 weeks and/or birth weight (BW) below 1500 g. We included in the analysis measurements performed at 3 ± 1, 7 ± 2, and 14 ± 2 postnatal days on infants with GA < 32 weeks and/or BW < 1500 g. Exclusion criteria were congenital abnormalities; heart diseases, as assessed by echocardiography; hemodynamic instability, defined as the presence of a hemodynamically significant PDA or treatment with inotropes at any time; and sepsis, defined as positive blood cultures at the time of evaluation.
USCOM measurements
USCOM records blood flow through the aortic valve using a continuous Doppler wave (2.2 MHz) and obtains the velocity time integral (VTI). An anthropometric algorithm, based on length and weight, calculates the aortic cross-sectional area (CSA). The product of CSA times VTI is stroke volume (SV). The USCOM software obtains CO as SV times heart rate (HR), estimated from the distance between flow systolic peaks.
Infants were studied in the supine position during sleep or quiet wake. One neonatologist (DD) who completed the recommended USCOM training by an experienced company representative and had considerable experience with hemodynamic measurements and USCOM examination supervised the study and trained more naif operators. Blood flow in the ascending aorta was assessed using a compact ultrasound probe (diameter of 12 mm) placed at the suprasternal notch. The angle of insonation was adjusted until an optimal velocity-time profile was obtained, as previously described [9], and then five to ten reproducible cycles were selected to calculate the average CO.
Cardiac index (CI) was determined as CO normalized for the body surface, which was calculated using the formula of Dubois et al. [17]. At the time of USCOM assessment, we also measured systolic, diastolic, and mean arterial pressure non-invasively (DINAMAP Procare 100, GE Medical Systems) to calculate afterload parameters, namely systemic vascular resistance (SVR = mean blood pressure/CO) and systemic vascular resistance indexed (SVRI, SVR normalized for body surface). For quality control, about one-half of the tracings stored on the device were carefully reviewed by the same practitioner (EZ).
Intra-observer short-term repeatability of CO measures was assessed in a subset of patients by performing two consecutive measurements, 2 min apart, taking the probe off the skin between measurements, and applying it again.
Clinical data
Clinical data were extracted from the electronic clinical record. For each USCOM evaluation, we recorded the presence of a PDA, as assessed by an echocardiography performed on the same day. We considered PDA as hemodynamically significant when the internal diameter was > 1.5 mm, the flow pattern was pulsatile [18], and one of the following conditions was present: (i) end-diastolic blood flow velocity in the left pulmonary artery > 20 cm/s or (ii) diastolic flow reversal in the descending aorta.
Statistical analysis
The primary endpoint was to determine reference ranges of CO. Secondary endpoints were the reference ranges of CI and SVRI, the short-term intra-observer repeatability of CO measurements, and the relationship between USCOM parameters and relevant clinical data.
Aiming for 80% power at the 5% significance level, we calculated a minimum required sample size of 73 patients for a linear regression model with one independent continuous predictor of small effect size (f2 = 0.1) [19] (Gpower 3.1).
Aiming for 90% power at a significance level of 0.05, hypothesizing a mean and a standard deviation of differences between repeated measurements of 0 and 16 ml/kg/min, respectively [12], and allowing for a maximum difference of 50 ml/kg/min [20], we calculated a minimum of 34 measurements to determine the intra-observer short-term repeatability of CO measurements using Bland-Altman analysis (MedCalc).
CO was corrected for body weight and expressed as ml/kg/min. All parameters were reported as median (2.5–97.5 percentile). We calculated the coefficient of variation (CV) to assess the consistency of measurements. The relationships between USCOM parameters and potential factors were examined using mixed-effect models. Tested factors included GA, postmenstrual age (PMA), gender, small for gestational age (SGA), postnatal age (PNA), PDA, respiratory support level (0, no respiratory support; 1, high flow nasal cannula; 2, non-invasive respiratory support; 3, invasive respiratory support). We first performed univariable regressions and then adjusted for GA. The repeatability of CO measurements was assessed using the two-way mixed effects intra-class correlation coefficient (ICC) with a focus on consistency to account for intra-observer reliability [21]. The repeatability coefficient was determined as twice the SD of the differences between measurements, computed both in absolute and percentage values. P-values < 0.05 were considered statistically significant. We analyzed the data using Matlab R2019b (MathWorks, Natick, USA).
Results
One hundred and twenty-eight infants with a BW < 1500 g and/or GA < 32 weeks were admitted to our unit during the study period; 19 were missed, 19 met the exclusion criteria, and 92 were included in the analysis, for a total of 204 measurements (see Fig. 1). The characteristics of the study participants are reported in Table 1. Infants had a median (Q1, Q3) GA of 30.57 (29.43, 31.36) weeks and BW of 1360 (1135, 1521) g. The GA, BW, proportion of males, and SGA did not differ significantly between infants included and infants who satisfied inclusion criteria but were missed.
Reference ranges of USCOM parameters
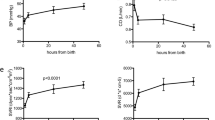
Table 2 reports the USCOM and other hemodynamic parameters at different postnatal ages. Pooling all data points together, the median (2.5, 97.5 percentile) CO was 268 (198, 405) ml/min/kg, CI was 3.0 (2.3, 4.2) L/min/m2, and SVRI was 1261 (796, 1862) d*s*cm−5/m2. CV was 20%, 17%, and 23% for CO, CI and SVRI, respectively. Figure 2 shows the trajectory of USCOM parameters over the first 2 weeks of life.
Factors affecting USCOM parameters
Figure 3 shows CO, CI, and SVRI versus PMA. Table 3 shows the correlation between USCOM parameters and relevant clinical factors. CO presented a negative correlation, and SVRI a positive correlation with GA and PMA. CI presented a negative correlation with GA. CO and CI were significantly higher in infants being born small for GA. In univariable analysis, CO was significantly correlated with the respiratory support level, but the correlation did not remain significant after adjustment for GA. None of the other factors significantly correlated with USCOM parameters, including a non-significant PDA.
USCOM parameters against postmenstrual age. PMA, postmenstrual age; CO, cardiac output; CI, cardiac index; SVRI, systemic vascular resistance indexed. Individual empirical values are reported as closed circles. Solid lines, 0 SD; long-dashed line, ± 1 SD; short-dashed lines, ± 2 SD. SD lines are based on the univariable regression models. CO = 645 – 12*PMA, SEE = 43 ml/kg/min; CI = 3.98 – 0.03*PMA, SEE = 0.52 L/min/m2; SVRI = 378 + 29*PMA, SEE = 270 d*s*cm−5/m2
Repeatability of USCOM measurements
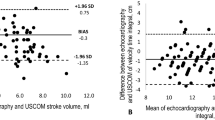
The short-term repeatability was assessed in 45 infants with a median (Q1, Q3) GA of 31.00 (29.97, 31.86) weeks and BW of 1408 (1168, 1487) g. Measurements were performed at a mean PNA of 7 (3, 9) days. The intra-rater reliability of CO measurements by USCOM was high, as determined by the ICC (95% CI) = 0.98 (0.95–0.9). Figure 4 shows the absolute and percentage differences in CO between repeated measurements. The mean (SD) difference between repeated measures was −2 (15) ml/kg/min, corresponding to 0 (6)%. The Bland–Altman plots showed no trend between the measurement error and the mean CO value. The repeatability coefficient was 31 ml/kg/min, corresponding to 12%.
Discussion
We assessed the feasibility of USCOM measurements in preterm infants born before 32 weeks of GA and/or below 1500 g of BW. We established reference ranges in hemodynamically stable preterm infants without cardiovascular abnormalities, which is crucial for applying the technique in clinical practice. We also evaluated the correlation between USCOM parameters and relevant clinical factors and assessed the measurement repeatability.
The median (2.5, 97.5 percentile) for CO by USCOM in the study population was 268 (198, 405) ml/kg/min. CO values considered normal in preterm and term infants range between 150 and 300 ml/kg/min[22]. The established reference ranges for CO, CI, and SVRI are comparable to those reported in previous studies using USCOM in term or near-term healthy neonates [12, 14]. CO and CI were slightly higher, and SVRI values were slightly lower than in term infants, as expected from the correlation we observed between these parameters and PMA.
The observed variability is likely the combination of physiological (related to clinical factors that might affect hemodynamic parameters in preterm infants) and measurement variability. PMA significantly correlated with CO and SVRI. Moreover, CO and CI were significantly higher in infants being born small for GA. We did not observe a statistically significant correlation of USCOM parameters with gender, PNA, the presence of a non-significant PDA, and the respiratory support level. However, we cannot exclude that such factors might play a role in the variability of CO measures. We speculate that PNA was not significantly associated with USCOM parameters because measurements were performed starting from the 3rd day of life when the adaptation to the extrauterine life is likely completed and up to 2 postnatal weeks. The finding that CO was not significantly associated with a non-significant PDA is consistent with the fact that left ventricular CO, as assessed at the suprasternal notch, is measured upstream of the PDA.
Hudson et al. observed that about half the variability in the measurement of CO in term infants measured by independent observers was the result of the measurement variability [23]. The repeatability of CO measurements by USCOM observed in the present study is comparable with previous reports in infants and children, ranging from 2.5 to 22% [12, 23], and accounted for 28% of the variability of the reference ranges. We assessed the short-term intra-observer variability, which reflects the variability associated with the measurement technique. We found a repeatability coefficient of 31 ml/kg/min, corresponding to 12%, indicating that for the USCOM device to track a change in CO with a 95% CI, such change should be at least 61 ml/kg/min or 24%.
Accuracy of CO by USCOM
Several factors might affect the accuracy and repeatability of USCOM measurements. Prior validation studies comparing CO measured by USCOM versus the gold-standard CO measurements by pulmonary artery thermodilution found variable agreement between the two techniques in adults [24] and children [25].
CO measurement errors by USCOM could arise from variations in aortic cross-sectional area between infants of similar body weight not accounted for by the formula. A validation of the absolute accuracy of CO measurements by USCOM in preterm infants has not been performed due to the lack of a gold standard for CO measurements suitable for preterm infants. USCOM validation in infants was performed versus echocardiography [9, 12]. Echocardiography is the most accepted and widely used CO measurement technique in neonatal intensive care units, but it is not a gold standard. In the present study, we decided not to compare CO measures by USCOM with those obtained by echocardiography because such a comparison has already been done in term [9] and preterm infants [12]. Fraga et al. found that USCOM overestimated CO compared to echocardiography [9]. Patel et al. reported wide limits of agreement between the USCOM and standard echocardiography [12]. Measuring CO with Doppler ultrasound requires measuring the aortic cross-sectional area and blood flow velocity. Hudson et al. reported that the main difficulty of measuring CO in infants is the accurate measurement of aortic diameter; indeed, small errors in aortic diameter may produce large errors in cardiac output, especially in preterm infants with small aortic dimensions [23]. Possible errors in estimating the aortic valve diameter based on anthropometric algorithms are more reproducible and operator-independent than measurement errors.
Moreover, the accurate assessment of CO might be affected by measurement errors of blood flow velocity inherent in the Doppler technique. Indeed, the Doppler beam must be aligned with the long axis of the aorta, and any deviation will result in an underestimation [9]. Such a problem affects the accuracy of CO measurement both by USCOM and echocardiography. While USCOM uses continuous wave Doppler (CWD) measurements for VTI, echocardiography uses pulsed wave Doppler (PWD). While CWD might overestimate VTI as compared with PWD, CWD was found to be more reproducible [23].
Limitations
Limitations include the retrospective study design, which precluded the possibility of assessing inter-rater agreement. Another limitation is the single-center study design, which may limit the generalizability of results due to differences in population and hemodynamic management in different units. Blood pressure was measured non-invasively, limiting the accuracy of the afterload measurements.
One of the challenges of studies aimed at defining reference ranges for preterm infants is defining what is normal and avoiding the selection of a cohort that is not representative of the most at-risk population. We used a pragmatic approach, including all infants considered hemodynamically stable by the attending physician who did not have a hemodynamically significant PDA. Such criteria led to the inclusion of a limited number of extremely preterm infants (10%); therefore, caution must be used to extrapolate the reference ranges to infants with GA below 28 weeks. Similarly, the first measurement was taken at 3 days of life because hemodynamics might be unstable during the transition to extrauterine life; caution must be used to extrapolate reference values obtained on day 3 to the first 2 days of life. Reference values obtained between 3 and 14 days are relevant, for example, for the management of late-onset sepsis. We acknowledge a limited sample size for establishing normative values or target ranges to be achieved. Ours is a pragmatic study that provides expected values of USCOM parameters in preterm infants unaffected by hemodynamic problems and describes their variability and dependence on relevant clinical factors. Reference values are reported as a function of postmenstrual age to allow comparing values collected in different patients or interpreting changes within the same patient over time. We believe that our data can help clinicians in interpreting data coming from the USCOM and might serve as pilot data to design future studies addressed at defining normative data on larger cohorts of patients, evaluating the effect of interventions on hemodynamics, or clinical trials evaluating the effect of decision-making strategies based on USCOM parameters.
Conclusion
This is the first study establishing reference values for USCOM parameters in hemodynamically stable preterm infants. Such ranges are too broad to be meaningful in clinical practice to diagnose alterations of cardiovascular function. However, the repeatability of the measurement is acceptable, suggesting that serial measurements within the same infant might reduce the measurement variability and represent a meaningful use of the technique. Indeed, since USCOM is easy to learn and use, it can have a role as a bedside tool for the longitudinal assessment of patients at risk for cardiovascular instability or in monitoring the response to therapies. Future studies addressing the clinical utility of USCOM in guiding treatment (e.g., inotropes) are warranted.
Data availability
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
References
Evans JR, Lou Short B, Van Meurs K, Cheryl Sachs H (2006) Cardiovascular support in preterm infants. Clin Ther 28:1366–1384. https://doi.org/10.1016/j.clinthera.2006.09.006
Koch J, Hensley G, Roy L et al (2006) Prevalence of spontaneous closure of the ductus arteriosus in neonates at a birth weight of 1000 grams or less. Pediatrics 117:1113–1121. https://doi.org/10.1542/peds.2005-1528
Vrancken SL, van Heijst AF, de Boode WP (2018) Neonatal hemodynamics: from developmental physiology to comprehensive monitoring. Front Pediatr 6:1–15. https://doi.org/10.3389/fped.2018.00087
McGovern M, Miletin J (2018) Cardiac output monitoring in preterm infants. Front Pediatr 6:1–10. https://doi.org/10.3389/fped.2018.00084
Groves A, Kuschel CA, Knight DB, Skinner JR (2008) Relationship between blood pressure and blood flow in newborn preterm infants. Arch Dis Child Fetal Neonatal Ed 93:29–33. https://doi.org/10.1136/adc.2006.109520
Kharrat A, Rios DI, Weisz DE et al (2019) The relationship between blood pressure parameters and left ventricular output in neonates. J Perinatol 39:619–625. https://doi.org/10.1038/s41372-019-0337-6
Tibby SM, Hatherill M, Marsh MJ, Murdoch IA (1997) Clinicians’ abilities to estimate cardiac index in ventilated children and infants. Arch Dis Child 77:516–518. https://doi.org/10.1136/adc.77.6.516
Boet A, Jourdain G, Demontoux S (2016) De Luca D (2016) Stroke volume and cardiac output evaluation by electrical cardiometry: accuracy and reference nomograms in hemodynamically stable preterm neonates. J Perinatol 369(36):748–752. https://doi.org/10.1038/jp.2016.65
Fraga MV, Dysart KC, Rintoul N et al (2019) Cardiac Output measurement using the ultrasonic cardiac output monitor: a validation study in newborn infants. Neonatology 116:260–268. https://doi.org/10.1159/000501005
Meyer S, Todd D (2009) Assessment of portable continuous wave Doppler ultrasound (ultrasonic cardiac output monitor) for cardiac output. J Paediatr Child Health 45:464–468. https://doi.org/10.1111/j.1440-1754.2009.01535.x
Cattermole GN, Leung PYM, Ho GYL et al (2017) The normal ranges of cardiovascular parameters measured using the ultrasonic cardiac output monitor. Physiol Rep 5:1–9. https://doi.org/10.14814/phy2.13195
Patel N, Dodsworth M, Mills JF (2011) Cardiac output measurement in newborn infants using the ultrasonic cardiac output monitor: an assessment of agreement with conventional echocardiography, repeatability and new user experience. Arch Dis Child Fetal Neonatal Ed 96. https://doi.org/10.1136/adc.2009.170704
Doni D, Nucera S, Rigotti C et al (2020) Evaluation of hemodynamics in healthy term neonates using ultrasonic cardiac output monitor. Ital J Pediatr 46:1–9. https://doi.org/10.1186/s13052-020-00872-x
He SR, Zhang C, Liu YM et al (2011) Accuracy of the ultrasonic cardiac output monitor in healthy term neonates during postnatal circulatory adaptation. Chin Med J (Engl) 124:2284–2289. https://doi.org/10.3760/cma.j.issn.0366-6999.2011.15.008
Liu YM, Zheng ML, Sun X et al (2022) The clinical value of ultrasonic cardiac output monitor in very-low-birth-weight and extremely-low-birth-weight infants undergoing PDA ligation. Early Hum Dev 165:105522. https://doi.org/10.1016/j.earlhumdev.2021.105522
Quezado ZMN, Natanson C (1992) Systemic hemodynamic abnormalities and vasopressor therapy in sepsis and septic shock. Am J Kidney Dis 20:214–222. https://doi.org/10.1016/S0272-6386(12)80693-7
Du Bois D, Du Bois EF (1916) Clinical calorimetry: tenth paper a formula to estimate the approximate surface area if height and weight be known. Arch Intern Med XVII:863–871. https://doi.org/10.1001/ARCHINTE.1916.00080130010002
Su BH, Watanabe T, Shimizu M, Yanagisawa M (1997) Echocardiographic assessment of patent ductus arteriosus shunt flow pattern in premature infants. Arch Dis Child Fetal Neonatal Ed 77:F36-40. https://doi.org/10.1136/fn.77.1.f36
Cohen J, Cohen P, West SG, Aiken LS (2002) Applied multiple regression/correlation analysis for the behavioral sciences, 3rd edn. Lawrence Erlbaum Associates, New Jersey
Evans N, Kluckow M (1996) Early determinants of right and left ventricular output in ventilated preterm infants. Arch Dis Child 74:88–94. https://doi.org/10.1136/fn.74.2.f88
Koo TK, Li MY (2016) A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 15:155. https://doi.org/10.1016/J.JCM.2016.02.012
De BW (2020) Advanced hemodynamic monitoring in the neonatal intensive care unit shock hemodynamic monitoring cardiac output. Clin Perinatol 47:423–434. https://doi.org/10.1016/j.clp.2020.05.001
Hudson I, Houston A, Aitchison T et al (1990) Reproducibility of measurements of cardiac output in newborn infants by Doppler ultrasound. Arch Dis Child 65(1 Spec No):15–19. https://doi.org/10.1136/adc.65.1_spec_no.15
Thom O, Taylor DM, Wolfe RE et al (2009) Comparison of a supra-sternal cardiac output monitor (USCOM) with the pulmonary artery catheter. Br J Anaesth 103:800–804. https://doi.org/10.1093/bja/aep296
Knirsch W, Kretschmar O, Tomaske M et al (2008) Cardiac output measurement in children: comparison of the ultrasound cardiac output monitor with thermodilution cardiac output measurement. Intensive Care Med 34:1060–1064. https://doi.org/10.1007/s00134-008-1030-y
Acknowledgements
The authors thank Dr. Giulia Dognini, Dr. Valentina Privitera, Dr. Marianna Zicoia, and Dr. Ester De Luca for their help in data collection.
Author information
Authors and Affiliations
Contributions
DD was the principal investigator, interpreted the data, critically revised the manuscript, and approved the final manuscript as submitted. MF collected the data, drafted the initial manuscript, performed the literature search, and approved the final manuscript as submitted. EZ performed statistical analysis, drafted the figures, reviewed and revised the manuscript, and approved the final manuscript as submitted. AR, CC, and LI contributed to data collection and approved the final manuscript as submitted. VC and MTS contributed to data interpretation, critically revised the manuscript, and approved the final manuscript as submitted. CR contributed to data collection, critically revised the manuscript, and approved the final version as submitted. TF and MLV obtained the resources to conduct the study, contributed to conceiving the study, and approved the final version of the manuscript as submitted.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study has been conducted in compliance with the principles of the Helsinki Declaration and was approved by the ethical review board of the Fondazione IRCCS San Gerardo dei Tintori, Monza, Italy (protocol nr. 4309/23). The study has been registered, NCT05961657. Informed consent was obtained from the parents/legal guardians of the participants included in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by Daniele De Luca
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Doni, D., Faraguna, M.C., Zannin, E. et al. Hemodynamic evaluation in preterm infants using ultrasonic cardiac output monitor (USCOM). Eur J Pediatr 183, 2183–2192 (2024). https://doi.org/10.1007/s00431-024-05465-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-024-05465-y