Abstract
Background:
If an infant fails to initiate spontaneous breathing after birth, international guidelines recommend a positive pressure ventilation (PPV). However, PPV by face mask is frequently inadequate because of leak between the face and mask. Despite a variety of available face masks, none have been prospectively compared in a randomized fashion. We aimed to evaluate and compare leak between two commercially available round face masks (Fisher & Paykel (F&P) and Laerdal) in preterm infants <33 weeks gestational age in the delivery room.
Methods:
Infants born at the Royal Alexandra Hospital from April to September 2013 at <33 weeks gestational age who received mask PPV in the delivery room routinely had a flow sensor placed between the mask and T-piece resuscitator. Infants were randomly assigned to receive PPV with either a F&P or Laerdal face mask. All resuscitators were trained in the use of both face masks. We compared mask leak, airway pressures, tidal volume and ventilation rate between the two groups.
Results:
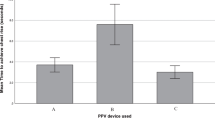
Fifty-six preterm infants (n=28 in each group) were enrolled; mean±s.d. gestational age 28±3 weeks; birth weight 1210±448 g; and 30 (52%) were male. Apgar scores at 1 and 5 min were 5±3 and 7±2, respectively. Infants randomized to the F&P face mask and Laerdal face mask had similar mask leak (30 (25–38) versus 35 (24–46)%, median (interquartile range), respectively, P=0.40) and tidal volume (7.1 (4.9–8.9) versus 6.6 (5.2–8.9) ml kg−1, P=0.69) during PPV. There were no significant differences in ventilation rate, inflation time or airway pressures between groups.
Conclusion:
The use of either face mask during PPV in the delivery room yields similar mask leak in preterm infants <33 weeks gestational age.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Respiratory support is the cornerstone of neonatal resuscitation in the delivery room (DR).1, 2 Although recent randomized controlled trials reported similar outcomes by using either a face mask or nasal prong during the positive pressure ventilation (PPV), face mask ventilation remains the primary mode of resuscitation. At this time, there are a variety of face masks available to provide PPV.3, 4 However, one issue is that face mask ventilation is often complicated by mask leak.5, 6 Effective and consistent face mask ventilation is important to facilitate gas exchange and should occur in a predictable manner so that the clinician can avoid under- or over-inflating the lungs.7
The Fisher & Paykel (F&P) (Fisher & Paykel Healthcare, Auckland, New Zealand) and Laerdal round masks are commonly used commercially available products.8 To our knowledge, no study has compared the efficacy of the F&P and Laerdal mask during PPV in the DR. We therefore aimed to compare the performance of both masks in a randomized controlled trial. Based on manufacturer’s information, we hypothesized that the F&P mask would result in less mask leak during PPV compared with the Laerdal mask in preterm infants <33 weeks gestational age.
Methods
This study was carried out at the Royal Alexandra Hospital in Edmonton, Canada, a tertiary perinatal center admitting approximately 350 infants with a birth weight of <1500 g annually. The trial was conducted between April 2013 and September 2013. Infants <33 weeks post menstrual age and who were judged clinically to have inadequate breathing in the first minutes after birth were eligible for the trial. Potentially eligible infants were randomized immediately prior to delivery. Infants were excluded if there was uncertainty about their gestational age or if they had a congenital abnormality that might adversely affect their breathing. The research team attended deliveries in addition to the resuscitation-stabilization-triage team (usually a neonatal nurse, neonatal respiratory therapist and neonatal nurse practitioner and/or neonatal fellow). The research team was not involved in the clinical care of the infants. The Royal Alexandra Hospital Research Committee and Health Ethics Research Board, University of Alberta approved the study and was registered with clinicaltrials.gov: NCT01685697.
Randomization
Infants were randomly allocated in 1:1 ratios to either have a F&P or Laerdal mask during PPV. Infants randomized to the F&P group were given either the F&P mask size of ∅ 35 or ∅ 42 mm. Allocation was block randomized with variable sized blocks (4–8). A sequentially numbered, sealed, opaque envelope containing the allocation was opened by a researcher before the birth of a potentially eligible infant. Twins and triplets were randomized as individuals.
Blinding
There was no blinding of the resuscitation-stabilization-triage team, as it would not be feasible to conduct a study with mask ventilation with two different face masks without the resuscitation-stabilization-triage team knowing the actual intervention that the subject will receive. However, after admission the clinical team was not aware of the treatment allocation. In addition, the data collector and outcome assessor were both unaware of the group allocation.
Consent
The Royal Alexandra Hospital Research Committee and Health Ethics Research Board, University of Alberta granted deferred consent as both face masks as well as respiratory function monitoring were already routinely used in the DR at the Royal Alexandra Hospital. The study was therefore classified as 'minimal risk'. Written consent was sought from the parents of these infants as soon as possible after the birth so acquired data could be utilized for research.
Face masks and ventilation devices
Potentially eligible infants were randomized immediately prior to delivery and then received PPV as clinically necessary with either the F&P ∅35 or ∅42 mm F&P mask or Laerdal size 0 (Laerdal, Stavanger, Norway) round silicone face masks. Respiratory support was provided with a T-piece device (Giraffe Warmer, GE Health Care, Burnaby, BC, Canada), which is a continuous flow, pressure-limited device with a built-in manometer and a positive end expiratory pressure (PEEP) valve. The default settings used were a gas flow of 8 l min−1, peak inflation pressure of 24 cm H2O and PEEP of 6 cm H2O. Staff members attending deliveries were trained to use the ventilation devices and both face masks. In addition, all staff members were trained in the mask holds recommended by Wood et al.8, 9
Monitoring systems
A Respironics NM3 respiratory profile monitor (Philips Healthcare, Philips Electronics, Markham, ON, Canada) was used to continuously measure tidal volume (VT), airway pressures, gas flow and exhaled CO2 (ECO2). The combined flow and ECO2 sensor was placed between the T-Piece and the face mask. Airway pressure and gas flow were measured by using a fixed orifice differential pressure pneumotachometer. VT was calculated by integrating the flow signal. ECO2 was measured by using nondispersive infrared absorption technique. According to the manufacturer the accuracy for gas flow is ±0.125 l min ml kg−1 and for ECO2 is ±2 mm Hg, with a dead space of <1 ml. In the DR, neither the respiratory profile monitor nor the computer screen was visible to the resuscitation-stabilization-triage team and the monitor’s alarm was disabled.
An IntelliVue MP50 patient monitor (Philips Healthcare, Philips Electronics, Markham, ON, Canada) was used to continuously measure the heart rate, arterial oxygen saturation and intermittent blood pressure. A Masimo pulse oximeter (Masimo Corporation, Irvine CA, USA) probe set at maximum sensitivity and two second averaging was placed around the infant’s right wrist to measure oxygen saturation.10 Heart rate was measured using three Micro-Premie leads (Vermed, Bellows Falls, VT, USA) and blood pressure by using a noninvasive blood pressure cuff of appropriate size on the left upper arm. The left upper arm was chosen to avoid interference with the pulse oximetry measurements.
An Invos Cerebral/Somatic Oximeter Monitor (Invos 5100, Somanetics, Troy, MI, USA) with the neonatal sensor was applied to the infant’s forehead to measure cerebral regional tissue oxygen saturation (crSO2). The sensor contains a light emitting diode and two detectors at different distances from the light source. The Invos Cerebral/Somatic Oximeter Monitor calculates the rSO2, which is expressed as the percentage of oxygenated hemoglobin. The transducer was positioned on the left frontoparietal forehead in each infant regardless of mode of delivery. The sensor on the forehead was secured with a wrap.
Resuscitation
Resuscitation was started with air for infants ⩾29 weeks and with 30% oxygen for infants <29 weeks postmenstrual age. Intubation criteria were defined a priori as (i) a heart rate <100 beats per minute despite adequate PPV for 60 s; (ii) a heart rate <60 beats per minute despite adequate PPV for 30 s (in which case chest compressions and 100% oxygen would be indicated); (iii) persistent or significant apnea requiring ongoing PPV for more then 10 min; and (iv) a sustained fraction of inspired oxygen >40% despite PEEP of 6 cm H2O or more after 10 min of birth. All resuscitative measures (for example use of supplemental oxygen, intubation, cardiac massage or drugs) were at the discretion of the clinical team, following the 2010 guidelines for neonatal resuscitation.11
Sample size and power estimates
Our primary outcome measure was face mask leak during PPV. Our previous observational data showed a mean (±s.d.) mask leak of 55 (20)%. We hypothesized that the mask leak would be less in the F&P mask group. A sample size of 56 (28 in each group) was sufficient to detect a clinically important (15%) reduction in mask leak, that is, 55 versus 40%, with 80% power and a two-tailed alpha error of 0.05.
Data collection and analysis
Demographics and clinical characteristics of study infants were recorded. All variables were stored continuously in a multichannel system ‘alpha-trace digital MM’ (B.E.S.T. Medical Systems, Vienna, Austria) for subsequent analysis. Values of gas flow, VT, airway pressure, and ECO2 were recorded at 200 Hz, arterial oxygen saturation, and heart rate were stored every second, and the sampling time of rSO2 was every 8 s (0.13 Hz). Blood pressure was measured every minute for the first 15 min, then at 20, 25 and 30 min. A breath-by-breath analysis (total of 8940 inflations) of airway pressure, gas flow, VT and ECO2 waves were performed and peak inflation pressure, PEEP, VT and ECO2, inflation time, ventilation rate and minute ventilation were measured. We calculated the leak from the mask by expressing the volume of gas that did not return through the flow sensor during expiration as a percentage of the volume that passed through the flow sensor during inflation (Leak (%)=[(inspiratory tidal volume – expiratory tidal volume)÷inspiratory tidal volume] × 100).12 In each infant, the duration of mask ventilation was included in the analysis. In addition, the secondary outcomes (including intraventricular hemorrhage, necrotizing enterocolitis, patent ductus arteriosus, retinopathy of prematurity, bronchopulmonary dysplasia, length of hospital stay and mortality) were collected during the hospital stay until discharge. The data are presented as mean (s.d. for normally distributed continuous variables and median (interquartile range (IQR)) when the distribution was skewed. All infants were analyzed according to their group at randomization (that is, analysis was by intention-to-treat). The clinical characteristics and outcome parameters were compared by using Student’s t-test for parametric and Mann–Whitney U-test for nonparametric comparisons of continuous variables, and χ2 for categorical variables. For all respiratory parameters, the median value for each infant was calculated first, and then either the mean or median of the median values were analyzed and presented for normal or skewed distribution, respectively. Mean (s.d.) measurements of every minute of arterial and regional oxygen saturation, heart rate, and fraction of inspired oxygen were compared using Student’s t-tests with correction for multiple comparisons using Holm–Sidak method. P-values are two sided and P<0.05 was considered statistically significant. Statistical analyses were performed with Stata (Intercooled 10, Statacorp, College Station, TX, USA).
Results
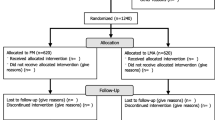
Between April 2013 and September 2013, there were 99 eligible infants <33 weeks gestational age (Figure 1). Sixty-two preterm infants (n=31 in each group) were enrolled and randomized; within the F&P group, 10 infants were stratified to the ∅35 mm F&P mask group and 18 infants to the ∅42 mm F&P mask group. During the study period, 37 infants were not enrolled (born before arrival of neonatal team (n=9), no research team available (n=20) or trial coordinator not contacted (n=8)). After randomization, a further three infants in each group were excluded because (i) no or incomplete data recorded (n=2), or (ii) did not require mask PPV (n=4) (Figure 1). A total of 28 infants in each group were included in the final analysis. The median (IQR) level of experience of the primary resuscitator was similar in both groups; 12 (5–36) months in infants resuscitated with F&P mask and 10 (6–14) months with the Laerdal mask. The groups did not differ in demographic characteristics (Table 1). The demographic characteristics of babies enrolled in the study were similar to those not included (Table 1). One infant in each group received chest compression, however, none required epinephrine. A total of 4 sets of twins and no triplets were included in the study.
Primary outcome measures
The primary outcome measure was a reduction of mask leak with the F&P mask compared with the Laerdal mask. However, median (IQR) mask leak did not differ between the two groups 30 (25–36)% in the F&P mask and 35 (24–46)% in the Laerdal mask (P=0.40) (Table 2 and Figure 2). In the F&P group, infants randomized to the ∅35 m F&P mask had 31 (22–36)% leak and ∅42 mm F&P mask had 30 (24–36)% leak (P=0.90).
Tidal volume, airway pressures, ventilation rate
All respiratory parameters are presented in Table 2. Tidal volume did not differ between groups with VT 7.1 (4.9–8.9) ml kg−1 in the F&P group versus 6.6 (5.2–8.9) ml kg−1 in the Laerdal group. Peak inflation pressure and PEEP were, respectively, 25 (2) and 6 (1) cmH2O in the F&P group and 25 (2) and 6 (1) cmH2O in the Laerdal group.
Heart rate, arterial oxygen saturation, cerebral regional tissue oxygen saturation, fraction of inspired oxygen, and blood pressure
We found no significant differences in heart rate, arterial and crSO2 or fraction of inspired oxygen in either the F&P or Laerdal group. In addition, the systolic and diastolic blood pressure did not differ between the groups over the first 30 min. However, mean (s.d.) arterial blood pressure was significantly higher in the F&P group at 25 and 30 min compared with the Laerdal group (at 25 min 40 (9) versus 33 (9) mm Hg (P<0.0001) and at 30 min: 38 (5) versus 32 (5) (P<0.0001)), respectively.
Secondary outcomes
Major neonatal morbidities (including intraventricular hemorrhage, necrotizing enterocolitis, patent ductus arteriosus, retinopathy of prematurity, bronchopulmonary dysplasia, length of hospital stay and mortality) did not differ between groups (Table 3).
Discussion
International guidelines recommend that an appropriate sized face mask must seal around the mouth and nose, but not cover the eyes or overlap the chin.11 In addition, the shape of the face mask can be either round or triangular: ‘anatomically’ shaped with a cushioned rim.13, 14 Unfortunately, mask leak remains a common problem during PPV, which can potentially lead to underventilation.15, 16 Currently, there are several different face masks available; however, the majority of them are too large for very-small preterm infants and little data exist to demonstrate the superiority of one face mask versus another.13, 17 In our institution, we recently introduced the F&P mask in addition to the Laerdal mask. We therefore aimed to compare both masks in a randomized controlled trial to assess mask leak during PPV. Our study demonstrated that the mask leak did not differ between both the F&P and Laerdal masks. In addition, DR room outcomes and secondary outcomes, although our trial was not powered to find significant differences, did not appear to differ between groups.
When preterm infants fail to breathe adequately immediately after birth, it is important to apply PPV to create a functional residual capacity, facilitate gas exchange and initiate spontaneous breathing. Although some mask leak is likely unavoidable, the delivery of adequate PPV in the DR is dependent on good face mask technique. Good face mask techniques include maintenance of upper airway patency with various opening maneuvers,11, 18 correct positioning and holding of the mask8, 19 and an appropriate face mask.13, 17 Several factors can reduce the effectiveness of PPV including poor face mask technique resulting in leak or airway obstruction, spontaneous movements of the baby, movements by or distraction of the resuscitator and procedures such as changing the wraps or fitting a hat.15, 20 In addition, resuscitator fatigue and stress factors have been reported to exaggerate the mask leak during PPV in neonatal resuscitation.15
A manikin study by Wood et al.8 reported similar mask leaks between the F&P and Laerdal mask with 57% and 55%, respectively. In addition, when participants were instructed in optimal mask positioning, mask leak was reduced to 33% and 32%, respectively.9 In the current study, all providers were instructed on the correct mask position described by Wood et al,9 which resulted in similar mask leaks as described in the manikin study. In addition, several manikin and DR studies have reported similar percentages of mask leak during PPV.6, 14, 16, 21 At this point, none of the face masks have been shown to be superior to the others. Face masks should be chosen based on familiarity, preference, and prior experience. It appears that skilled operators, not mask selection, remains the most significant factor for effective ventilation.9, 14, 22
Limitations
One limitation of the study was that we only examined mask leak within the first five minutes after birth. However, a recent study by Schmölzer et al.15 suggested that mask leak more commonly appears at the start of PPV. We aimed to observe a 15% reduction in mask leak, which we regarded as clinically important; however, mask leak improved in both groups compared with our pretrial observation period. We can only speculate why mask leak was much improved compared with the pretrial period. A greater awareness of the resuscitation team that mask leak was being measured or the pretrial education of correct mask placement might have contributed to the improved mask ventilation technique. An additional area of future research would be to compare an anatomically shaped mask to a round mask given the lack of significant difference between two round face masks. Our current study was not powered to examine long-term outcomes; however, our early evidence suggests that face mask selection will not affect important long-term outcomes such as bronchopulmonary dysplasia. Finally, it should be noted that the neonatal resuscitation team could not be blinded to the mask allocation; however, subsequent data analysis was completed in a blinded fashion.
Conclusions
F&P and Laerdal face masks did not differ in mask leak during PPV in the DR. Given the limited availability of adequate face masks currently, both masks are comparable options for respiratory support of preterm infants <33 weeks gestational age.
References
Perlman JM, Wyllie J, Kattwinkel J, Atkins DL, Chameides L, Goldsmith JP et al. Neonatal resuscitation: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Pediatrics 2010; 126 (5): e1319–e1344.
Kattwinkel J, Niermeyer S, Nadkarni V, Tibballs J, Phillips B, Zideman D et al. An advisory statement from the Pediatric Working Group of the International Liaison Committee on Resuscitation. Pediatrics 1999; 103 (4): e56.
Palme C, Nystrom B, Tunell R . An evaluation of the efficiency of face masks in the resuscitation of newborn infants. Lancet 1985; 1 (8422): 207–210.
McCarthy LK, Twomey AR, Molloy EJ, Murphy JF, O'Donnell CP . A randomized trial of nasal prong or face mask for respiratory support for preterm newborns. Pediatrics 2013; 132 (2): e389–e395.
O'Donnell CP, Davis PG, Lau R, Dargaville PA, Doyle LW, Morley CJ . Neonatal resuscitation 2: an evaluation of manual ventilation devices and face masks. Arch Dis Child Fetal Neonatal Ed 2005; 90 (5): F392–F396.
Schilleman K, Witlox RS, Lopriore E, Morley CJ, Walther FJ, te Pas AB . Leak and obstruction with mask ventilation during simulated neonatal resuscitation. Arch Dis Child Fetal Neonatal Ed 2010; 95 (6): F398–F402.
Tracy M, Downe L, Holberton J . How safe is intermittent positive pressure ventilation in preterm babies ventilated from delivery to newborn intensive care unit? Arch Dis Child Fetal Neonatal Ed 2004; 89 (1): F84–F87.
Wood FE, Morley CJ, Dawson JA, Kamlin CO, Owen LS, Donath S et al. Assessing the effectiveness of two round neonatal resuscitation masks: study 1. Arch Dis Child Fetal Neonatal Ed 2008; 93 (3): F235–F237.
Wood FE, Morley CJ, Dawson JA, Kamlin CO, Owen LS, Donath S et al. Improved techniques reduce face mask leak during simulated neonatal resuscitation: study 2. Arch Dis Child Fetal Neonatal Ed 2008; 93 (3): F230–F234.
Dawson JA, Kamlin CO, Vento M, Wong C, Cole TJ, Donath SM et al. Defining the reference range for oxygen saturation for infants after birth. Pediatrics 2010; 125 (6): e1340–e1347.
Kattwinkel J, Perlman JM, Aziz K, Colby C, Fairchild K, Gallagher J et al. Part 15: neonatal resuscitation: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2010; 122 (18 Suppl 3): S909–S919.
O'Donnell CP, Kamlin CO, Davis PG, Morley CJ . Neonatal resuscitation 1: a model to measure inspired and expired tidal volumes and assess leakage at the face mask. Arch Dis Child Fetal Neonatal Ed 2005; 90 (5): F388–F391.
Wood FE, Morley CJ . Face mask ventilation-the dos and don'ts. Semin Fetal Neonatal Med 2013; 18 (6): 344–345.
Deindl P, O'Reilly M, Zoller K, Berger A, Pollak A, Schwindt J et al. Influence of mask type and mask position on the effectiveness of bag-mask ventilation in a neonatal manikin. Eur J Pediatr 2014; 173 (1): 75–79.
Schmölzer GM, Dawson JA, Kamlin CO, O'Donnell CP, Morley CJ, Davis PG . Airway obstruction and gas leak during mask ventilation of preterm infants in the delivery room. Arch Dis Child Fetal Neonatal Ed 2011; 96 (4): F254–F257.
Schmölzer GM, Kamlin OC, O'Donnell CP, Dawson JA, Morley CJ, Davis PG . Assessment of tidal volume and gas leak during mask ventilation of preterm infants in the delivery room. Arch Dis Child Fetal Neonatal Ed 2010; 95 (6) p F393–F397.Abstract No. 2938.531..
O'Shea JE, Thio M, Owen LS, Dawson JA, Davis PG . Facial Measurements in Preterm Infants: Appropriate Mask Fit for Respiratory Support. Annual Meeting of Pediatric Academic Societies 2014 Abstract No. 2938.531.
Chua C, Schmölzer GM, Davis PG . Airway manoeuvres to achieve upper airway patency during mask ventilation in newborn infants - an historical perspective. Resuscitation 2012; 83 (4): 411–416.
Wilson EV, O'Shea JE, Thio M, Dawson JA, Boland R, Davis PG . A comparison of different mask holds for positive pressure ventilation in a neonatal manikin. Arch Dis Child Fetal Neonatal Ed 2014; 99 (2): F169–F171.
Finer NN, Rich W, Wang C, Leone T . Airway obstruction during mask ventilation of very low birth weight infants during neonatal resuscitation. Pediatrics 2009; 123 (3): 865–869.
Binder C, Schmölzer GM, O'Reilly M, Schwaberger B, Urlesberger B, Pichler G . Human or monitor feedback to improve mask ventilation during simulated neonatal cardiopulmonary resuscitation. Arch Dis Child Fetal Neonatal Ed 2014; 99 (2): F120–F123.
Schilleman K, van der Pot CJ, Hooper SB, Lopriore E, Walther FJ, te Pas AB . Evaluating manual inflations and breathing during mask ventilation in preterm infants at birth. J Pediatr 2013; 162 (3): 457–463.
Acknowledgements
We thank the parents and infants agreeing to be part of the study and the Resuscitation-Stabilization-Triage team at the Royal Alexandra Hospital for their assistance and support of the study. GMS is a recipient of the Heart and Stroke Foundation/University of Alberta Professorship of Neonatal Resuscitation. F&P (Fisher & Paykel Healthcare, Auckland, New Zealand) provided the Fisher & Paykel face masks for the study. No study sponsor or company that manufactures markets or sells any equipment used in the study had involvement in study design, data collection or interpretation or the decision to present or publish the results.
Author Contributions
Conception and design: GMS, KA, PYC. Collection and assembly of data: DC, QM, GMS, KA, GP, MOR, PYC. Analysis and interpretation of the data: DC, QM, GMS, KA, GP, MOR, PYC. Drafting of the article: DC, QM, GMS, KA, GP, MOR, PYC. Critical revision of the article for important intellectual content: DC, QM, GMS, KA, GP, MOR, PYC. Final approval of the article: DC, QM, GMS, KA, GP, MOR, PYC.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Cheung, D., Mian, Q., Cheung, PY. et al. Mask ventilation with two different face masks in the delivery room for preterm infants: a randomized controlled trial. J Perinatol 35, 464–468 (2015). https://doi.org/10.1038/jp.2015.8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2015.8
- Springer Nature America, Inc.