Abstract
Background/Objectives:
Unhealthy dietary choices are a major contributor to harmful weight gain and obesity. This study interrogated the brain substrates of unhealthy versus healthy food choices in vivo, and evaluated the influence of hunger state and body mass index (BMI) on brain activation and connectivity.
Subjects/Methods:
Thirty adults (BMI: 18–38 kg m−2) performed a food-choice task involving preference-based selection between beverage pairs consisting of high-calorie (unhealthy) or low-calorie (healthy) options, concurrent with functional magnetic resonance imaging (fMRI). Selected food stimuli were delivered to participants using an MRI-compatible gustometer. fMRI scans were performed both after 10-h fasting and when sated. Brain activation and hypothalamic functional connectivity were assessed when selecting between unhealthy–healthy beverage pairings, relative to unhealthy–unhealthy and healthy–healthy options. Results were considered significant at cluster-based family-wise error corrected P<0.05.
Results:
Selecting between unhealthy and healthy foods elicited significant activation in the hypothalamus, the medial and dorsolateral prefrontal cortices, the anterior insula and the posterior cingulate. Hunger was associated with higher activation within the ventromedial and dorsolateral prefrontal cortices, as well as lower connectivity between the hypothalamus and both the ventromedial prefrontal cortex and dorsal striatum. Critically, people with higher BMI showed lower activation of the hypothalamus—regardless of hunger state—and higher activation of the ventromedial prefrontal cortex when hungry.
Conclusions:
People who are overweight and obese have weaker activation of brain regions involved in energy regulation and greater activation of reward valuation regions while making choices between unhealthy and healthy foods. These results provide evidence for a shift towards hedonic-based, and away from energy-based, food selection in obesity.
Similar content being viewed by others
Introduction
The prevalence of obesity has steadily grown in the past 30 years,1 and is now one of the largest causes of preventable disease burden and premature death in Western societies.2 Obesity is a multifactorial condition, linked to both genetic and environmental influences.3 Nonetheless, compelling data show that the overconsumption of high-calorie foods over healthier alternatives is driving the current obesity epidemic.4 Thus, understanding the determinants of unhealthy versus healthy food choices is key to identifying new approaches to tackle this problem.
Food choices are orchestrated by a distributed brain system that includes the hypothalamus, the striatum, and frontal–parietal regions.5 Animal studies show that the hypothalamus has a crucial role in energy balance by sensing changes in metabolic state and then adjusting feeding behaviour to maintain energy homeostasis.6, 7, 8, 9, 10 Human imaging research has shown that choices between foods with different reward values activate the ventromedial prefrontal cortex, the posterior cingulate cortex and the striatum.5, 11, 12 Importantly, the peptides that act on the hypothalamus to regulate feeding (e.g., leptin and ghrelin) also modulate striatal responsivity to food cues,13, 14 linking homeostatic needs with reward value.15 The dorsolateral prefrontal cortex and posterior parietal regions provide additional input about health aspects of food, contributing to cognitive control over eating.16, 17 The ventromedial prefrontal cortex ultimately integrates information about food desirability and food healthiness to determine preferences between unhealthy and healthy food options.12
Functional neuroimaging studies suggest that people with obesity have abnormal activation in brain regions that contribute to food choice. Meta-analytic research on cue reactivity shows that obese versus normal weight participants have higher activation in the medial prefrontal cortex, the striatum and the parahippocampal gyrus when viewing food images.18, 19 Heightened activation in this set of regions reflects overvaluation of food stimuli, which can bias decision-making towards excessive food intake.19, 20 In the only study that has examined the brain signatures of food choice as a function of body mass index (BMI), ventromedial prefrontal cortex activation correlated with subjective food valuation and ‘ad libitum’ consumption of unhealthy foods after scanning.21 However, there were no significant differences between overweight and normal weight individuals.21 An important limitation of the existing neuroimaging studies is that they have measured food motivation and choice using visual cues and hypothetical choices, rather than actual food choice and food delivery. A critical shortcoming of this approach is its lack of sensitivity to measure brain regions involved in homeostatic control of food choice, such as the hypothalamus.22 This is an important research gap, as influential theories posit that the food choices of people with obesity are primarily driven by the reward value of food, bypassing homeostatic regulation mechanisms.23, 24, 25, 26 Moreover, cue-reactivity studies show different patterns of brain activation in pre- versus postmeal states. Hyperactivation of the hippocampus and the amygdala are specifically associated with cue reactivity during hunger, while hyperactivation in the medial prefrontal cortex and the striatum is linked to satiety in obese individuals (see Kennedy and Dimitropoulos19 for a meta-analysis). However, there are no studies about the effect of BMI on food choice as a function of homeostatic status, that is, hunger versus satiety.
In this study, we developed a novel paradigm to interrogate the brain substrates of food choices in vivo, using real food administered during the scan. Brain activations and hypothalamic functional connectivity in response to decisions between unhealthy versus healthy food options were examined as a function of body mass index (BMI) and homeostatic status (hunger versus satiety). Based on combined evidence from animal models and human imaging studies, we hypothesised that: (i) food choices involving unhealthy versus healthy food would elicit significant activation in the hypothalamus, striatum and ventromedial prefrontal and posterior cingulate cortices; (ii) people with higher BMIs would display greater striatal and ventromedial prefrontal cortex activation (reward valuation) and weaker hypothalamic activation (homeostatic regulation), as well as lower connectivity between these regions.
Materials and methods
Participants
Thirty adult participants (16 males; age M=24.17 years, s.d.=5.98 years) were recruited from the general community. The BMI range of the sample was 18.0–37.8 kg m−2, with 16 (53.3%) of the participants being classified as overweight or obese (BMI>25 kg m−2); no participant was considered underweight (BMI<18 kg m−2). Exclusion criteria included (i) history of hypertension or diabetes, (ii) diagnosed psychiatric illness, including depression, substance use or eating disorders, (iii) magnetic resonance imaging (MRI) contraindications and (iv) allergies to ingredients used for the food-choice task (see below), based on self-report. The Monash University Human Research Ethics Committee approved the study, and all participants provided written informed consent.
Experimental design
Participants undertook the Food Choice Task in conjunction with functional MRI (fMRI) on two occasions, 1 week apart: (i) after 10 h of fasting (excluding water), and (ii) 45 min after a standard meal containing 306 kcal (a vegetarian alternative was also offered and contained 280 kcal). Scan order was counterbalanced across participants.
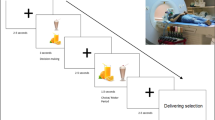
The Food Choice Task was designed to interrogate the neural basis of food choices using actual physiological stimuli. The task presented pseudorandomised images of pairs of healthy (low sugar and fat) and/or unhealthy (high sugar and fat) beverage combinations in the form healthy–healthy (HH), unhealthy–unhealthy (UU) and unhealthy–healthy (UH) options (Figure 1a). The beverage pair combinations were consistent and presented to all participants in the same order. In each trial, participants were asked to select an option based on their usual preferences. Each image pair was displayed for a 3-s viewing-only period followed by a 1.5-s period where participants made their selection using a two-button response box. The chosen beverage was delivered during the 5 s following selection (Figure 1b). The task comprised five runs, each containing 30 choice events (10 from each condition) and lasting 6 min 32 s.
A computer-controlled MRI-compatible food delivery system (gustometer), consisting of six syringe pumps each connected to a different beverage reservoir, was used to deliver the chosen beverages to participants during simultaneous fMRI recording (Supplementary Figure S1 and Supplementary Information). Upon beverage selection, 3 ml of the beverage was delivered via plastic tubing to a mouthpiece mounted on the head coil of the scanner. Task presentation and beverage delivery were controlled using the program ‘Presentation’ (Neurobehavioral Systems Inc., Albany, CA, USA; http://www.neurobs.com).
The beverages were designed by a professional nutritionist and prepared according to a standard operating procedure. ‘Unhealthy’ beverages included chocolate, strawberry and caramel milkshakes. ‘Healthy’ beverages included fruit-blended orange, cranberry/raspberry and veggie juices (including fibre). The unhealthy drinks had significantly more sugar and fat than the healthy drinks: chocolate milkshake (8.93 g of fat and 18.99 g of sugar per 100 ml), strawberry milkshake (8.93 g of fat and 18.93 g of sugar per 100 ml), caramel milkshake (8.93 g of fat and 18.93 g of sugar per 100 ml) versus veggie juice (<1 g of fat and 7.7 g of sugar per 100 ml), cranberry/raspberry juice (<1 g of fat and 9.6 g of sugar per 100 ml) and orange juice (<1 g of fat and 8.4 g of sugar per 100 ml).
MRI data acquisition
Data were acquired using a 3-Tesla Siemens Skyra MRI scanner equipped with a 32-channel head coil at Monash Biomedical Imaging (Melbourne, VIC, Australia). Each of the 10 functional runs (five per session) consisted of 196 gradient-echo echo-planar images comprising 34 interleaved, contiguous axial slices (total acquisition time=6 min 32 s; repetition time=2000 ms; echo time=30 ms; flip angle=90°; 3 mm isotropic voxels; field of view=230 × 230 mm2) covering the cerebrum. A whole-brain T1-weighted magnetisation-prepared rapid gradient-echo structural image was also acquired for each participant (192 sagittal slices; 1.0 mm isotropic voxels; repetition time=2300 ms; echo time=2.07 ms; field of view=256 × 256 mm2).
Functional MRI data analysis: task-related activations
Analyses were undertaken using SPM12 Software (Functional Imaging Laboratory, UCL, UK; http://www.fil.ion.ucl.ac.uk/spm/). Data preprocessing consisted of: (i) slice timing correction, involving temporal registration of all slices to the middle slice of each functional volume; (ii) spatial realignment of all volumes to the first volume of the first run, to account for movement-related displacements and rotations; (iii) spatial unwarping to account for susceptibility-by-movement noise variance; (iv) coregistration to the T1-weighted structural image; (v) skull-stripping, segmentation and nonlinear normalisation of the T1 and coregistered echo-planar images to the standard anatomical space (Montreal Neurological Institute); and (vi) spatial smoothing using a Gaussian kernel of 5 mm full-width at half-maximum.
Task fMRI effects were quantified using hierarchical general linear modelling. For each individual, the 3-s decision-making periods corresponding to each condition-type (UU, HH, UH), convolved with a canonical hemodynamic response function, were coded as predictors of the blood oxygenation level-dependent response. Six additional regressors were included in the general linear modelling to account for residual motion-related variance (three planes of translation; three axes of rotation). All 10 runs were included in the general linear modelling (two runs were excluded in two participants due to excessive movement). A high-pass filter (128 s) was included to account for low-frequency noise in the blood oxygenation level-dependent signal, and temporal autocorrelations were estimated using an AR1 model. Contrasts across runs and conditions were used to derive summary statistic maps for each effect of interest: (i) healthy versus unhealthy food choice, regardless of hunger state [UH−(UU+HH)/2]fasted+sated and (ii) healthy versus unhealthy food choice when fasted versus sated (i.e., choice-by-hunger interactions; [UH−(UU+HH)/2]fasted−[UH−(UU+HH)/2]sated).
fMRI data analysis: task-related connectivity
Task-related hypothalamic connectivity was assessed using a generalised psychophysiological interaction analysis.27 In brief, the individual-level general linear modellings described above were replicated (the psychological and motion-related variables), with the addition of variables coding the time series of activity within the seed region of interest (ROI) (the physiological variable; seed defined below), and the interaction between the physiological variable and each psychological variable. Contrasts were again defined to assess the connectivity change related to: (i) healthy versus unhealthy food choice, regardless of hunger state [UHconnectivity−(UUconnectivity + HHconnectivity)/2]fasted+sated, and (ii) choice-by-hunger interactions.
fMRI data analysis: group-level inference
At the group level, regression models were used to infer the average effects of the above activation and connectivity effects and their linear relations with BMI and Food Preference (proportion of ‘unhealthy’ selections in UH trials), while covarying for average frame-wise displacement (i.e., root mean square of the volume-to-volume movement). Movement-related variance was thus conservatively accounted for at both the individual and group level of assessment. Inference was undertaken across the whole brain and within anatomical masks defined a priori using the Automated Anatomical Labelling (AAL2) cerebral atlas (ventromedial prefrontal cortex and insula, included labels: bilateral Frontal_Sup_Medial, Cingulum_Ant, Frontal_Med_Orb, Insula) (Supplementary Figure S2 and Supplementary Information), the Oxford-Imanova subcortical atlas (caudate, putamen, ventral striatum) and a neuroimaging meta-analytic localisation of the hypothalamus (http://www.neurosynth.org/analyses/terms/hypothalamus).
Statistical thresholds were corrected for multiple comparisons using nonparametric permutation methods (SnPM version 13.1; cluster-based family-wise error (FWE) corrected P<0.05; cluster-forming thresholds set at P<0.001 for whole-brain analyses and P<0.01 within the cerebral anatomical mask). Subcortical ROI analyses were undertaken using the mean signal from within each region averaged across hemispheres (all hemispheric effects, P>0.22). Three participants were excluded from hypothalamic assessments because of incomplete sampling of the structure (>1/3 of mask voxels not captured within the field of view), and one because of the outlier activation (2.96 s.d. above the mean).
Results
Behavioural food choice
The proportion of ‘unhealthy’ food selection in response to UH food pairings was behaviourally matched between the fasted and sated sessions (fasted: 57.2±26.1%; sated: 51.9%±21.8%; paired t29=1.39, P=0.18) and showed relatively high consistency within individuals between sessions (r30=0.64). Similarly, the proportion of healthy versus unhealthy food choices did not differ significantly as a function of BMI in either the fasting (r30=0.14, P=0.48) or sated session (r30=0.18, P=0.35), although we found that BMI was positively correlated with selecting more unhealthy versus healthy choices in the first run of the fasted scan (r30=0.36, P<0.05). This behavioural performance matching ensured that fMRI inferences were not confounded by behavioural effects.
Healthy versus unhealthy food choice: brain activations
Decisions between UH food pairings, relative to HH and UU pairings, were associated with increased functional activation in the left dorsolateral prefrontal cortex (PFC), left anterior insula, bilateral anterior cingulate cortex, bilateral posterior cingulate cortex, bilateral precuneus, bilateral superior and inferior parietal lobules, and right lateral occipital cortex (whole-brain nonparametric cluster-corrected PFWE<0.05; Figure 2a; and Table 1). A significant activation decrease was also evident in the right supramarginal gyrus in the area of the temporoparietal junction (Table 1). No significant correlations between cortical activations and either BMI or food preference were evident.
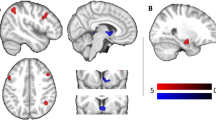
Functional activations related to healthy versus unhealthy food choice. (a) Cortical areas eliciting significantly greater functional activations in response to HU pairings versus HH/UH pairings (whole-brain nonparametric cluster-corrected PFWE<0.05). (b) Functional activations in the subcortical ROI (*P<0.05; #P=0.051); error bars=s.e.m. (c) Significant linear correlation between task-related hypothalamus activation and BMI across participants (r=−0.40, P=0.043). Caud, caudate; HyTh, hypothalamus; Puta, putamen; VStr, ventral striatum.
Subcortical ROI assessments revealed significant activation increases in the hypothalamus (t25=2.37, P=0.028; Figure 2b), which negatively correlated with BMI (r26=−0.40; P=0.043; Figure 2c). Near-significant activation increases were also observed in the caudate (t29=2.04, P=0.051), but not in the putamen (t29=1.49, P=0.15) or ventral striatum (t29=1.27, P=0.22). BMI and food preference did not correlate with striatal activations (all r<0.22, P>0.24).
Healthy versus unhealthy food choice: the influence of hunger
Deciding between a healthy and an unhealthy food when fasted, relative to when sated, was associated with significantly greater activations in the right ventromedial PFC, left dorsolateral PFC, left precuneus and bilateral intraparietal sulcus (Figure 3a and Table 1). No significant functional effects were evident in the opposite contrast, and hunger-related activation differences were not evident in the hypothalamus or striatum (all P>0.10).
Healthy versus unhealthy food choice when fasted versus sated. (a) Cortical areas eliciting significantly greater functional activations during HU food choices when fasted relative to when sated (whole-brain nonparametric cluster-corrected PFWE<0.05). (b) Ventromedial PFC area eliciting an interaction between hunger and BMI during HU food choices (within an a priori anatomical mask of the food-choice network, nonparametric cluster-corrected PFWE<0.05). (c) Scatterplot of the interaction effect between hunger state and BMI in the ventromedial PFC. The colour reproduction of this figure is available on the International Journal of Obesity journal online.
The hunger-related effect on food choice within the right ventromedial PFC was modulated by BMI (Figure 3b and Table 1). As illustrated in Figure 3c, greater BMI was associated with greater task-related ventromedial PFC activation when fasting (r30=0.45), but with lesser activation in this region when sated (r30=−0.35).
Healthy versus unhealthy food choice: functional connectivity
Task-related connectivity between the hypothalamus and both the ventromedial PFC (Table 1) and the caudate (t26=2.08, P=0.047) was significantly modulated by hunger state (Figure 4). Healthy versus unhealthy food choice was associated with less information sharing between the hypothalamus and these regions when fasted, but not when sated. Associations between the strength of hypothalamic connectivity and BMI did not reach corrected-level statistical significance.
Task-dependent functional connectivity. Significant differences in hypothalamic functional connectivity dynamics associated with health-related food choice [UH−(UU+HH)] as a function of hunger state in the ventromedial prefrontal cortex (vmPFC; *PFWE=0.023) and caudate (*P=0.047), displayed in red; blue=seed ROI. HH, healthy–healthy pairings; UH, unhealthy–healthy food pairings; UU, unhealthy–unhealthy pairings. The colour reproduction of this figure is available on the International Journal of Obesity journal online.
Discussion
We found that choices between unhealthy and healthy foods elicit significant activation in brain regions involved in energy regulation, reward valuation and interoception, including the hypothalamus, the medial prefrontal and dorsolateral prefrontal cortices, the anterior insula, and the posterior cingulate. Choosing between healthy and unhealthy options when hungry was associated with higher activation of the ventromedial PFC, the dorsolateral PFC and posterior parietal regions, as well as lower connectivity between the hypothalamus and both the ventromedial PFC and the dorsal striatum. Critically, people with higher body mass index showed lower activation of the hypothalamus—regardless of hunger state—and higher activation of the ventromedial PFC when hungry.
The task-evoked activation in the hypothalamus, the prefrontal cortex, the insula and anterior and posterior cingulate regions show that selecting between unhealthy and healthy foods engage multiple neural drivers of motivation and decision-making, including areas involved in homeostatic regulation, valuation of the reward and health aspects of food and self-control over eating.5, 12, 17 Concurrent activation of dorsomedial and dorsolateral prefrontal along with hypothalamic, insula and posterior cingulate regions, suggest that during unhealthy versus healthy food choices people evaluate the reward and health properties of the different options against their current internal state.12 Our results are generally consistent with previous studies that investigate hypothetical food choice, which report activations in the medial and dorsolateral prefrontal cortices and the posterior cingulate.12, 17, 21 The pattern of activations also partially overlap with that of fMRI studies examining response to individual images of high-calorie foods, which often observe greater activation in regions implicated in cognitive and affective valuation, such as the dorsolateral prefrontal cortex and the orbitofrontal cortex, and attention, such as the anterior cingulate cortex, the superior parietal lobule and the precuneus.18, 28
Hunger-specific modulation of the ventromedial PFC, the dorsolateral PFC and posterior parietal regions (including precuneus) agrees with the assumption that homeostatic inputs modulate the activity of the brain regions involved in food choice.22, 23, 29 Our results align with consistent findings from neuroimaging research showing that hunger upregulates activation of the ventromedial PFC, as well as other reward valuation and attentional regions, during passive observation of highly palatable visual food cues.30, 31 We extend these findings to the context of active food choices, which is more representative of real-life, real-time decisions. One potential mechanism for the observed effect of hunger is the cross-talk between appetite peptides and cortical activity; for example, both fasting and acute administration of ghrelin increase ventromedial PFC activation in response to food cues.32 The ventromedial PFC and the precuneus play an important role in self-reflection and processing affective conflict.33, 34 Together with the dorsolateral PFC, these regions orchestrate value-based action selection.35 Taken together, the observed ventromedial PFC, dorsolateral PFC and precuneus activity suggests that choices between unhealthy and healthy foods become more salient when participants are in a hungry state, potentially because hunger increases the reward value of the high-calorie beverages. Interestingly, this pattern of activation is also associated with less information sharing between the ventromedial PFC (along with the dorsal striatum) and the hypothalamus, suggesting that hunger prioritises the reward valuation system at the expense of energy homeostasis.24, 29
Crucially, we found that people with higher BMIs have lower activation of the hypothalamus, which plays a crucial role in homeostatic regulation,6, 7, 8, 9 and higher activation of the ventromedial PFC during hunger, which mediates the intersection between the reward value of food and subsequent preference-based food choices.21 This finding suggests that overweight and obese people exhibit disjointed activation of brain regions that encode energy states (hypothalamus) and the reward value of food options (ventromedial PFC) during conflicted food choices. This is the first direct experimental evidence of a concept that has been frequently posited in neurobiological theories of obesity.23, 24, 25, 26 In line with these theories, one potential mechanism underlying the observed association between BMI and ventromedial PFC activation during hunger (but not satiety) is that caloric deprivation enhances the response of the brain reward/valuation system to food.31 Nevertheless, it is important to note that meta-analytic research on cue reactivity found greater differences in activation of reward-related regions in obese versus normal weight individuals during sated relative to hunger states,19 and that a previous study testing hypothetical food choices did not find significant effects of BMI on activations related to food choice.21 The observed discrepancies may be explained by the distinctive characteristics of our protocol and task. First, we enforced overnight fasting (10 h), as previous evidence indicates that activation in regions involved in food valuation increases as a function of time since last meal,31 and we conducted the ‘sated scan’ on a different day, with order counterbalanced. This contrasts with previous cue-reactivity studies, which had a shorter fasting period and pre- and postmeal scans on the same day,36, 37 which may result in order effects. Second, the use of real foods (versus hypothetical choices) is known to induce more robust activations within ventromedial PFC and striatal regions,38 thus increasing power to detect group differences.
Since our food choice task contains real food decisions between widely available high-calorie, unhealthy food and healthier alternatives, the observed misalignment between reward-sensitive and energy-sensitive brain systems may have important implications to the unhealthy food choices made by overweight or obese people. However, we have to note that although we expected this relation to be confirmed by reduced functional connectivity between the hypothalamus and the ventromedial PFC among people with higher BMIs, we did not find significant moderating effects of BMI in our connectivity analyses, and future studies with larger samples are needed. In addition, we did not find significant associations between patterns of brain activation/connectivity and behavioural measures of food choice. It is possible that more realistic measures of food intake, such as postscanning buffets, are needed to detect such link.21 Moreover, the relation between ventromedial PFC activation and behavioural measures of food choice is moderated by impulsivity and self-control characteristics.21, 39 Therefore, it is possible that the observed effects of BMI on brain activations are only relevant for everyday food intake among people with heightened impulsivity. Since we did not measure impulsivity in our sample, future studies are needed to test directly this notion. It is also worth noting that although we did not find correlations between BMI and behavioural food choices in the overall percentage of unhealthy choices, we did find a significant correlation in the first run of the fasting scan. This finding suggests that the impact of BMI on behavioural food choices may be particularly significant during the state of maximal caloric deprivation, or as a factor of novelty while initially exploring the range of food options (although we attempted to mitigate this effect by providing samples of the beverage options before commencement of the study).
Strengths of this study include the novel fMRI paradigm of food choice incorporating actual food delivery, which provides high ecological validity and has proven to be sensitive to activity within homeostatic and interoceptive brain regions. An important limitation is the relatively small sample size, especially when considering inferences about obese versus normal weight people. Moreover, our task maximised power to compare food decisions encompassing health-related conflicts (UH) versus unconflicted (UU or HH) evaluations, but we did not have the power to analyse the brain signatures of individual differences in choice patterns (i.e., when specifically selecting the unhealthy option versus the healthy option), or to examine brain activation differences during food consumption independently of food choice. And, although our task used actual physiological stimuli in the context of food choice for the first time, given the movement artefacts induced by chewing solid food, we used caloric beverages. Nevertheless, the beverage pairs differed on caloric and not hydration content, and thus it is unlikely that thirst had an impact on the outcomes. Moreover, previous studies suggest that choices between healthy and unhealthy beverages and healthy and unhealthy solid foods are behaviourally similar.21
In sum, we show that: choices between unhealthy and healthy food activate a distributed brain network that includes the hypothalamus (energy regulation) and the ventromedial PFC, dorsolateral PFC and precuneus (subjective valuation of food); activation in food valuation regions is upregulated by hunger; and people with overweight and obesity show reduced activation of the hypothalamus and increased activation of the ventromedial PFC in response to making choices between high- and low-calorie beverages. This study, including the novel experimental apparatus, motivates innovative avenues of future research, including studies on the neural substrates of subjective food preferences that can inform personalised nutrition approaches and biomedical treatments for obesity.
References
Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014; 384: 766–781.
Hruby A, Manson JE, Qi L, Malik VS, Rimm EB, Sun Q et al. Determinants and consequences of obesity. Am J Public Health 2016; 106: 1656–1662.
Zhou B, Gao W, Lv J, Yu C, Wang S, Liao C et al. Genetic and environmental influences on obesity-related phenotypes in chinese twins reared apart and together. Behav Genet 2015; 45: 427–437.
Crino M, Sacks G, Vandevijvere S, Swinburn B, Neal B . The influence on population weight gain and obesity of the macronutrient composition and energy density of the food supply. Curr Obes Rep 2015; 4: 1–10.
Levy DJ, Glimcher PW . Comparing apples and oranges: using reward-specific and reward-general subjective value representation in the brain. J Neurosci 2011; 31: 14693–14707.
Waterson MJ, Horvath TL . Neuronal regulation of energy homeostasis: beyond the hypothalamus and feeding. Cell Metab 2015; 22: 962–970.
Stuber GD, Wise RA . Lateral hypothalamic circuits for feeding and reward. Nat Neurosci 2016; 19: 198–205.
Dietrich MO, Horvath TL . Hypothalamic control of energy balance: insights into the role of synaptic plasticity. Trends Neurosci 2013; 36: 65–73.
Sternson SM, Atasoy D, Betley JN, Henry FE, Xu S . An emerging technology framework for the neurobiology of appetite. Cell Metab 2016; 23: 234–253.
Krashes MJ, Lowell BB, Garfield AS . Melanocortin-4 receptor-regulated energy homeostasis. Nat Neurosci 2016; 19: 206–219.
Arana FS, Parkinson JA, Hinton E, Holland AJ, Owen AM, Roberts AC . Dissociable contributions of the human amygdala and orbitofrontal cortex to incentive motivation and goal selection. J Neurosci 2003; 23: 9632–9638.
Hare TA, Malmaud J, Rangel A . Focusing attention on the health aspects of foods changes value signals in vmPFC and improves dietary choice. J Neurosci 2011; 31: 11077–11087.
Malik S, McGlone F, Bedrossian D, Dagher A . Ghrelin modulates brain activity in areas that control appetitive behavior. Cell Metab 2008; 7: 400–409.
Farooqi IS, Bullmore E, Keogh J, Gillard J, O'Rahilly S, Fletcher PC . Leptin regulates striatal regions and human eating behavior. Science 2007; 317: 1355.
Zheng H, Corkern M, Stoyanova I, Patterson LM, Tian R, Berthoud HR . Peptides that regulate food intake: appetite-inducing accumbens manipulation activates hypothalamic orexin neurons and inhibits POMC neurons. Am J Physiol Regul Integr Comp Physiol 2003; 284: R1436–R1444.
Polania R, Moisa M, Opitz A, Grueschow M, Ruff CC . The precision of value-based choices depends causally on fronto-parietal phase coupling. Nature Commun 2015; 6: 8090.
Hare TA, Camerer CF, Rangel A . Self-control in decision-making involves modulation of the vmPFC valuation system. Science 2009; 324: 646–648.
Brooks SJ, Cedernaes J, Schiöth HB . Increased prefrontal and parahippocampal activation with reduced dorsolateral prefrontal and insular cortex activation to food images in obesity: a meta-analysis of fMRI studies. PLoS One 2013; 8: e60393.
Kennedy J, Dimitropoulos A . Influence of feeding state on neurofunctionaldifferences between individuals who are obese and normal weight: a meta-analysis of neuroimaging studies. Appetite 2014; 75: 103–109.
Demos KE, Heatherton TF, Kelley WM . Individual differences in nucleus accumbens activity to food and sexual images predict weight gain and sexualbehavior. J Neurosci 2012; 32: 5549–5552.
Medic N, Ziauddeen H, Forwood SE, Davies KM, Ahern AL, Jebb SA et al. The presence of real food usurps hypothetical health value judgment in overweight people. eNeuro 2016; 3: (pii: ENEURO.0025-16.2016) doi:10.1523/ENEURO.0025-16.2016.
Val-Laillet D, Aarts E, Weber B, Ferrari M, Quaresima V, Stoeckel LE et al. Neuroimaging and neuromodulation approaches to study eating behavior and prevent and treat eating disorders and obesity. Neuroimage Clin 2015; 8: 1–31.
Clemmensen C, Muller TD, Woods SC, Berthoud HR, Seeley RJ, Tschop MH . Gut–brain cross-talk in metabolic control. Cell 2017; 168: 758–774.
Morrison CD, Berthoud HR . Neurobiology of nutrition and obesity. Nutr Rev 2007; 65 (Part 1): 517–534.
Volkow ND, Wang GJ, Baler RD . Reward, dopamine and the control of food intake: implications for obesity. Trends Cogn Sci 2011; 15: 37–46.
Ziauddeen H, Alonso-Alonso M, Hill JO, Kelley M, Khan NA . Obesity and the neurocognitive basis of food reward and the control of intake. Adv Nutr 2015; 6: 474–486.
McLaren DG, Ries ML, Xu G, Johnson SC . A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage 2012; 61: 1277–1286.
Stice E, Yokum S . Gain in body fat is associated with increased striatal response to palatable food cues, whereas body fat stability is associated with decreased striatal response. J Neurosci 2016; 36: 6949–6956.
Berthoud HR . The neurobiology of food intake in an obesogenic environment. Proc Nutr Soc 2012; 71: 478–487.
van der Laan LN, de Ridder DT, Viergever MA, Smeets PA . The first taste is always with the eyes: a meta-analysis on the neural correlates of processing visual food cues. Neuroimage 2011; 55: 296–303.
Stice E, Burger K, Yokum S . Caloric deprivation increases responsivity of attention and reward brain regions to intake, anticipated intake, and images of palatable foods. Neuroimage 2013; 67: 322–330.
Goldstone AP, Prechtl CG, Scholtz S, Miras AD, Chhina N, Durighel G et al. Ghrelin mimics fasting to enhance human hedonic, orbitofrontal cortex, and hippocampal responses to food. Am J Clin Nutr 2014; 99: 1319–1330.
Verdejo-Garcia A, Verdejo-Roman J, Rio-Valle JS, Lacomba JA, Lagos FM, Soriano-Mas C . Dysfunctional involvement of emotion and reward brain regions on social decision making in excess weight adolescents. Hum Brain Mapp 2015; 36: 226–237.
Murray RJ, Debbane M, Fox PT, Bzdok D, Eickhoff SB . Functional connectivity mapping of regions associated with self- and other-processing. Hum Brain Mapp 2015; 36: 1304–1324.
O'Doherty JP . Contributions of the ventromedial prefrontal cortex to goal-directed action selection. Ann NY Acad Sci 2011; 1239: 118–129.
Dimitropoulos A, Tkach J, Ho A, Kennedy J . Greater corticolimbic activation to high-calorie food cues after eating in obese vs normal-weight adults. Appetite 2012; 58: 303–312.
Martin LE, Holsen LM, Chambers RJ, Bruce AS, Brooks WM, Zarcone JR et al. Neural mechanisms associated with food motivation in obese and healthyweight adults. Obesity 2010; 18: 254–260.
Blechert J, Klackl J, Miedl SF, Wilhelm FH . To eat or not to eat: effects of food availability on reward system activity during food picture viewing. Appetite 2016; 99: 254–261.
Lawrence NS, Hinton EC, Parkinson JA, Lawrence AD . Nucleus accumbens response to food cues predicts subsequent snack consumption in women and increased body mass index in those with reduced self-control. Neuroimage 2012; 63: 415–422.
Acknowledgements
This study was funded by a Medical Research Grant from the Ian Potter Foundation (2015) and a Strategic Grant from the Faculty of Medicine, Monash University (Strategic Grant Scheme, 2015) (to AVG). IHH supported by Australian National Health and Medical Research Council Fellowship (1106533). ZBA supported by Australian National Health and Medical Research Council Fellowship (1084344). CSM supported by a Miguel Servet contract from the Carlos III Health Institute (CPII16/00048). We would like to acknowledge Helen Truby and Alastair Kwok for developing the beverages used in the task, the Monash Instrumentation Office for developing the gustometer, and Erynn Christensen and Sarah Giles for invaluable help with data collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Rights and permissions
About this article
Cite this article
Harding, I., Andrews, Z., Mata, F. et al. Brain substrates of unhealthy versus healthy food choices: influence of homeostatic status and body mass index. Int J Obes 42, 448–454 (2018). https://doi.org/10.1038/ijo.2017.237
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2017.237
- Springer Nature Limited
This article is cited by
-
Neural underpinnings of food choice and consumption in obesity
International Journal of Obesity (2022)
-
Human Taste-Perception: Brain Computer Interface (BCI) and Its Application as an Engineering Tool for Taste-Driven Sensory Studies
Food Engineering Reviews (2022)
-
A systematic review of resting-state functional connectivity in obesity: Refining current neurobiological frameworks and methodological considerations moving forward
Reviews in Endocrine and Metabolic Disorders (2022)
-
Neural network modelling reveals changes in directional connectivity between cortical and hypothalamic regions with increased BMI
International Journal of Obesity (2021)
-
Reliability of neural food cue-reactivity in participants with obesity undergoing bariatric surgery: a 26-week longitudinal fMRI study
European Archives of Psychiatry and Clinical Neuroscience (2021)