Abstract
Background:
Diabetes is closely linked to obesity, and obesity rates climb during adolescence for reasons that are not clear. Energy efficiency is important to obesity, and we describe a temporary but substantial fall in absolute energy expenditure, compatible with improved energy efficiency, during the rapid growth phase of puberty.
Methods:
In a longitudinal cohort study lasting 10 years, we measured voluntary energy expenditure as physical activity (PA) by accelerometry, involuntary energy expenditure as resting energy expenditure (REE) by oxygen consumption, body mass index (BMI) and body composition by dual energy X-ray absorptiometry annually on 10 occasions from 7 to 16 years in the 347 children of the EarlyBird study. We used mixed effects modelling to analyse the trends in REE and their relationship to BMI, lean mass (LM), fat mass (FM), age, PA and pubertal stage.
Results:
Relative REE and total PA fell during puberty, as previously described, but the longitudinal data and narrow age-range of the cohort (s.d.±4m) revealed for the first time a substantial fall in absolute REE during the period of maximum growth. The fall became clearer still when adjusted for FM and LM. The fall could not be explained by fasting insulin, adiponectin, leptin, luteinising hormone or follicle stimulating hormone.
Conclusions:
There appears to be a temporary but substantial reduction in energy expenditure during puberty, which is unrelated to changes in body composition. If it means higher energy efficiency, the fall in REE could be advantageous in an evolutionary context to delivering the extra energy needed for pubertal growth, but unfavourable to weight gain in a contemporary environment.
Similar content being viewed by others
Introduction
Childhood obesity has become an important issue over recent time because of its association with early metabolic disturbance.1 Metabolic (type 2) diabetes, which in most cases is directly related to obesity, was rarely described in young people a few decades ago, but is now the fastest growing chronic disorder of childhood.2 The factors responsible for childhood obesity are still unclear.
Body mass is the integral of past energy intake and expenditure. Intake is a single variable that can be controlled voluntarily across its range. Energy expenditure, on the other hand, has two components, only one of which (physical activity, PA) is voluntary. PA makes the smaller contribution to energy expenditure,3 and declines progressively during adolescence, the more so in girls.4, 5 The larger part of energy expenditure is involuntary, fuelling the metabolic processes that sustain life, and is commonly reported as resting energy expenditure (REE). REE is accounted for mostly by metabolism within the body’s fat-free tissues (chiefly brain, liver, heart and kidneys), though some is attributable to fat cells. Muscle contributes little at rest.
However, not all energy is used efficiently. Some, such as the thermic response to feeding (dietary-induced thermogenesis), is lost as heat through a process of energy uncoupling in brown adipose tissue,6 and constitutes a further and variable component of involuntary energy expenditure. Conversely, tight energy coupling can conserve energy. The amount of brown adipose tissue wanes with age, but appears to rise temporarily during puberty, though there is wide individual variation.7
REE can be expressed as total (absolute) REE, or as REE relative to size. Absolute REE is deemed to increase during adolescence alongside the increases in lean mass (LM) and fat mass (FM) that characterise the pubertal growth spurt.8 An increase in absolute REE with growth is intuitive, because body mass is demonstrably the single best predictor of REE,9 but the evidence tends be based on cross-sectional studies comparing small numbers of children at different ages,10 or on correlation. Small numbers carry the risk of unintended selection bias which can confound cross-sectional comparisons, and a positive correlation does not necessarily mean that the correlates are moving in the same direction—a correlation can be positive in cross-section, even though the outcome variable (in this case, REE) is falling over time while the explanatory variable (body mass) is rising. Furthermore, unless the age range is tight, a sample incorporating an age-dependent variable will not be fairly represented by the mean age, and ability to detect age-related change over time (resolution) will be compromised.
There have been few cohort studies of REE in childhood, and what truly longitudinal data there is tends to have focused on REE relative to body composition.6, 11 Relative REE appears to fall during puberty, and one theory to explain the fall cites changing body composition, whereby muscle mass increases proportionally during adolescence at the expense of other, metabolically more active, tissues. Thus, the brain, liver, heart and kidneys, all of which have a high metabolic rate, increase in mass by a factor of × 5–12 from birth to maturity, while skeletal muscle, which has a low metabolic rate at rest, increases in mass by a factor of forty.12
Here, we describe the trends in PA and REE over the course of childhood in a single cohort of contemporary children, and use new observations to explore an alternative paradigm—that there are programmed reductions in PA and 'absolute' REE during adolescence, creating energy savings which may have been important over evolutionary time in meeting the additional needs of pubertal growth, but which in a time of plenty now compound the risk of adolescent obesity.
Methods
EarlyBird is a non-intervention longitudinal study of healthy school children reviewed every six months as a single cohort from 5 to 16 years, and has been described in detail elsewhere.13 It was conducted in the city of Plymouth, UK, from 2000–2013, and addressed the question: which children become insulin resistant, and why? All 67 Plymouth primary schools were identified and their head teachers asked for agreement to participate in the study. Fifty-four schools agreed, and were stratified into quartiles according to the proportion of children entitled to free school meals, a socio-economic proxy. A random selection was made from each quartile and registration for the study invited during school induction meetings, where parents expressing interest were given a full explanation. With parents’ written consent and children’s assent, a total of 307 children (137 girls and 170 boys) who started school between January 2000 and January 2001 became the EarlyBird cohort. A further 40 children were added at age 9y to redress a gender imbalance. Importantly for the resolution of age-related change, the variance in age of the cohort did not exceed s.d.±4m throughout, and 80% of the children were retained for the 12 years of the study. Ethical approval was given in the summer of 1999.
Anthropometry
Height was measured every 6 months to the nearest 1mm (Leicester Height Measure, Child Growth Foundation, London), weight to the nearest 200 g in light clothing (Tanita Solar 1632W electronic scales, West Drayton, Middx). A minimum of three 'blind' repeats were made of each anthropometric measure at each visit. BMI was defined as mass(kg)/height2(m).
Physical activity (PA)
PA was measured objectively each year using Actigraph accelerometers (Model: 7164—formerly MTI/CSA—Fort Walton Beach, FL, USA). Actigraph accelerometers are of good technical reproducibility,14 and correlate well with criterion measures of free-living activity-related energy expenditure.15 The accelerometers were worn on an adjustable elastic belt around the child’s waist, and were set to run continuously for 7 days (5 school days and a weekend) at each annual time-point. Only recordings that captured at least 4 days monitoring (each of at least 9 h wear time) were included in the analyses, as this has been shown to be the minimum required to achieve >70% reliability.16 The Actigraph records the intensity of movement every 1/10th of a second, and for this study the counts were collected into epochs of 1 min and stored against clock time. Periods of noncompliance reported by the parents, and periods recording 0 c.p.m. for ⩾17 consecutive minutes (assumed to be unreported noncompliance) were replaced with the mean accelerometer counts recorded at the same clock time on the remaining days of the recording week. The sensitivity of each accelerometer was measured under controlled conditions by a motorised turntable.17
Resting energy expenditure (REE)
REE was measured annually, during the week before PA and on the same day as anthropometry, by indirect calorimetry using a ventilated flow-through hood technique (Gas Exchange Measurement; Nutren Technology Ltd, Manchester, UK). Performance tests report a mean error of 0.3%±2.0% in the measurement of oxygen consumption and 1.8%±1% in that of carbon dioxide production.18 The recording was rejected if the calibration test lay outwith the range 20.90–20.99% O2. The children were fasted overnight, and measured at around 0900 hours in all cases. They were given a ‘settling in’ period of up to 10 min under the hood before data collection over a minimum of 10, usually 15, minutes, once the minute-long readings had stabilised. Any 1-min interval during which the recorded REE lay two s.d. or more above the child's overall mean was ignored. We have referred throughout the text to absolute REE and to relative REE, which is absolute REE adjusted for tissue mass.
Body composition
Whole body dual energy X-ray absorptiometry scans were performed with the Lunar Prodigy Advance fan beam densitometer on the same day as anthropometry and REE, and analysed using EnCore 2004 software version 8.10.027 GE (Lunar Corporation, Madison WI, USA). We were particularly concerned to record FM and fat-free (lean) mass (LM). Coefficients of variation for body composition analysis using this system have been reported to range from 0.18 to 1.97% among paediatric subjects.19
Tanner stage
From 9 years, each child (and their parent in the early years) was shown line drawings representing genital development for boys, breast development for girls and pubic hair development for both, and asked to choose the picture for each that most closely matched their own development. The drawings have been validated,20 and agree, to within one Tanner stage, by 76% with clinical assessment of genital development (k=0.48), and by 88% with pubic hair development (k=0.68). A mean score for both Tanner measures (genital/breast and pubic hair development) was calculated at each age. TS1 represents pre-puberty (no phenotypic change), TS2 early puberty (first phenotypic change), TS3 mid puberty, TS4 late puberty and TS5 the end of puberty (adult phenotype).
Height velocity
Annual height velocity was calculated from serial overlapping 6-month height measurements in order to establish the growth pattern of the cohort, and age at peak height velocity (APHV). Although we incorporated Tanner Stage in the models, APHV is arguably a more objective and metabolic measure of adolescent development, and was included for these reasons.
Statistics
All children age 7–16 years were considered for analysis, and all analyses were carried out in statistical software package Stata version 14.1 (StataCorp. 2015. Stata Statistical Software; StataCorp LP, Release 14. College Station, TX, USA). Three separate linear mixed effect models were developed. The first (M-1) considered PA (c.p.m. per day) as the outcome variable, and BMI, gender and age as explanatory variables. A random coefficient model was developed that permitted each child random intercepts from repeated measurements, and age-related random slopes for PA. A log likelihood ratio test suggested that the random coefficient model was significantly better than a random intercept model (χ2 75.48, P<0.001). The strength of maximum likelihood-based algorithms for mixed effect models lies in their ability to accommodate missing data points, as the best parameter estimate is derived when the likelihood for a probabilistic distribution of the data is at maximum.21 Thus, unlike list-wise deletions applied to conventional regression estimators, maximum likelihood tolerates (within limits) cases where outcomes are missing for some points. Accordingly, 322 out of the 347 children contributed to the analysis of M-1. M-2 (n=323) modelled REE (kcal day−1) as outcome. The number of minute-long measurements used for REE was included as a predictor in the random intercept model, along with age and gender, to adjust for any variance associated with duration of measurement. There was no evidence statistically of random age-related slopes for REE, but a random coefficient model with slopes related to minutes measured fitted the model significantly better than the random intercept model (χ2 18.11, P<0.001). M-3 (n=320) was similar to M-2 except for further adjustments to LM (kg), FM (kg) and APHV, allowing examination of the impact of LM/FM on the relationship between age and REE. The random slope-related variance exhibited in the previous model (M-2) disappeared when the model was adjusted for LM and FM. Accordingly, a random intercept model was fitted which was significantly a better fit than a single level model (χ2 64.11, P<0.001). Interaction effects between gender, age and other variables were included in the model where appropriate. The relationships between age and PA/REE were not linear, so that age was fitted to the second degree higher-order polynomial for PA (M-1), and fourth degree polynomial for REE (M-2 and M-3). To obtain consistent age-related estimates for the linear and higher-order polynomials, age was centred to its overall mean (11.32 years), regardless of gender, to include ‘zero’ in its range. Centring age irrespective of gender did not introduce bias, as the age difference between the genders was negligible (mean 0.12 years, s.d.±0.04 years). M-3 was further adjusted for APHV and Tanner stage (both as a categorical, and as a continuous predictor), but the effect of Tanner stage became insignificant (Tanner stage: P=0.23) once the model was adjusted for LM/FM mass. Outcomes and their residuals at both levels were normally distributed. Results for all three models are presented with their coefficients and 95% confidence intervals (CI). Outcomes are predicted and plotted separately in this report in relation to their explanatory variables.
Results
Population studied
The basic cohort characteristics at ages 7, 11 and 16 years are shown in Table 1, with the proportions deemed obese at each age.
Importantly, the age of the cohort was uniform at each visit, with very little variance, and the boy’s ages closely matched those of the girls. The girls were the same height as the boys at 11 years, but significantly shorter by 16 years. They were fatter than the boys throughout.
Growth velocity
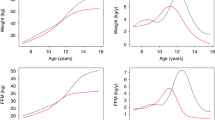
The velocity curves (Figures 1a and b) show the annualised 6-monthly rates of height and weight gain in boys and girls. The period of growth acceleration (growth spurt) spanned the interval 11–15 years. Peak height velocity was achieved by 14 years in both genders, and peak weight velocity by 13 years in the girls and by 16 years in the boys. Weight appeared to accelerate faster in the boys, and height in the girls.
Fat mass and lean mass
FM was systematically higher in the girls, and continued to rise linearly throughout puberty (Figure 1c). In contrast, the early rise of FM in boys tended to level off in puberty. LM followed much the same upward trajectory in boys and girls until early puberty, when it accelerated in the boys but increased little further in the girls (Figure 1d).
Mixed effects models
The analyses here are based on the three models established in Table 2.
Voluntary energy expenditure (PA)
The behaviour of PA predicted by M-1 is plotted in Figure 2. There was a progressive decline in PA from 7 to 16 years in both genders, which accelerated with age. Girls were systematically less active than boys and their activity declined more rapidly with age.
Involuntary energy expenditure (REE)
Figure 3, the key figure in this report, models the age-related trends in REE before (Figure 3a) and after (Figure 3b) adjustment for LM and FM. The unadjusted model shows the rise in REE expected of growing children,22 but only up to the age of 11 years, after which REE fell unexpectedly in both genders to the age of 15 years, when it started to recover. Yet the interval 11–15 years was the period of maximum growth (Figures 1c and d), during which the girls tended to gain fat rather than lean, and boys lean rather than fat. Figure 3b (M-3) models the same age-related REE after removing the variation in REE attributable to metabolically active tissues (LM and FM). Predictably, the rise in REE observed pre-puberty in Figure 3a is lost, as its rise was explained by growth. Paradoxically, however, the decline in REE during puberty is now more, rather than less pronounced, and could not be ascribed to changing body composition. Indeed, none of the variables introduced into the model could explain the fall in REE during the period of rapid adolescent growth.
Interactions
The association between LM and REE weakened with age (Figures 4a and b). Although the correlation between them remained positive throughout at each point in time, REE was falling over time while LM was rising. Thus, an increase in mean LM in boys of 18 kg (girls 10 kg) from 11 to 15 years was accompanied by a fall of REE in the age-related model amounting to 114 kcal day−1 (girls 284), P<0.001. The adolescent decline in REE was greatest among those of highest LM (Figures 4c and d), though the interaction with age had practically disappeared by 16 years. Interaction between age and APHV was significant, suggesting a positive association between APHV and REE over the period of time.
Energy conserved during puberty
Voluntary energy expenditure, expressed as the mean of total PA adjusted for BMI, fell by ~120 000 Actigraph counts/day from age 10–16 years in the boys, and by ~130 000 in the girls. An equation has been proposed in the past to convert Actigraph counts into calories,15 but only in girls of a single age group, so that we have not attempted to use it here.
Involuntary energy expenditure, expressed as the unadjusted mean of REE, fell by ~110 kcal day−1 from age 10–15 years in the boys, and by ~190 kcal day−1 in the girls. The unadjusted figures are drawn from the raw data, so that any fall is offset by the increase in REE associated with the cumulative rise in body mass over the same period and the metabolic energy expended in driving it. Even then, the net result is a decline in REE. Figure 3b, however, models the fall in REE independently of changes in body mass (both lean and fat), and reveals the real reduction in intrinsic REE—up to 450 kcal day−1 over the end of the 5-year period in both genders.
Possible mechanisms
The following hormones were measured alongside REE and body composition, and each was incorporated in turn into the final model shown in Figure 4b: insulin (Diagnostic Products Corporation, Los Angeles, CA, USA), IGF1 (University of Surrey, Guildford, UK), luteinising hormone and follicle stimulating hormone (Bayer Diagnostics, Newbury, Berkshire, UK), leptin and adiponectin (to 14 years, University of Glasgow, UK).
While each showed distinct trends over time, none was able to explain the fall in REE independently of body composition, age, gender or maturity (IGF-1, P=0.28; luteinising hormone, P=0.56; follicle stimulating hormone, P=0.43; adiponectin, P=0.52). Only fasting insulin (P=0.01) and leptin (P=0.02) appeared to have a statistically significant impact on the fall in REE in the final model, but their effect size was small, and not sufficient to explain the fall. Thus, a one-unit increase in fasting insulin was associated with a 3 kcal day−1 increase in REE (15 kcal day−1 for 1 s.d.). Similarly, a one-unit increase in leptin was associated with a 1 kcal decline in REE. Neither altered the age-related decline in REE shown in Figure 3b.
Discussion
The data presented here suggest that both voluntary and involuntary energy expenditure decline systematically during the pubertal years of rapid growth. Where it has been noted before, the decline in REE has been attributed to relative REE (that is, REE adjusted for body mass), and ascribed to the changes in body composition which characterise puberty.8, 11, 23 The decline in absolute REE at a time of rapid growth reported here is counterintuitive, because body mass is deemed the single most important determinant of REE. A decline in absolute REE implies an intrinsic reduction in energy expenditure of metabolically active tissues, one that would conserve energy. Story and Stang estimate that puberty imposes an additional 20–30% in energy needs.24 However speculative, we believe that the decline in intrinsic energy expenditure shown here may represent a period of programmed energy conservation (efficiency savings), which has evolved to assure the extra energy needed for adolescent growth.
Whatever the interpretation given to the behaviour of absolute REE, we believe that the body composition explanation given in the past to the perceived fall in relative REE may not be consistent with observation. First, the fall in REE from 10 to 15 years was not just relative, but absolute. Although there may be changes in body composition associated with pubertal growth, such that the proportion of energy-spending tissues falls, there is no evidence from any source that metabolically active tissue is actually lost, whether lean or fat. Any increase in muscle mass relative to other tissues might attenuate the rise in absolute REE associated with growth, but could arguably not reduce it. Second, whereas the boys gained substantially more LM than the girls, their REE declined less. While the rise in LM from 12 years onwards in the boys was likely to have been attributable in large part to muscle, there is little corresponding change in LM among the girls, whose decline in REE was nevertheless greater than the boys’. The behaviour of REE in boys, compared with that in the girls, appears to be the reverse of what is predicted by the body composition hypothesis. Most importantly, perhaps, the decline in REE over time remains—indeed, becomes clearer—after adjustment for LM and fat mass, and is therefore unlikely to be explained by either. Rather than reflect changing body composition, we believe these data may reveal how the human body adapts to puberty by conserving the energy it needs for rapid growth.
We did not expect the decline in REE, because absolute REE is usually reckoned to increase with body mass—and body mass does not merely increase during puberty, it accelerates. However, as we have shown in Figure 4, there is nothing incompatible with a correlation that remains positive while the mean of the outcome variable (in this case REE) declines. The measures suggest that REE is declining intrinsically, and the small within-cohort variance in REE compared with the large decline over time that the change is systematic. Systematic change in biological systems implies control, and controlled change infers that there is survival advantage to be gained.
We do not know what mechanisms are responsible for reducing voluntary activity, or for switching down the energy expenditure of metabolically active cells, but the falls in PA and REE were profound. We tested a number of candidate hormones that were measured alongside REE and body composition, but none was able to explain the fall in REE. We did not measure oestrogen levels in EarlyBird because of their random fluctuations in adolescent girls, and measurements of testosterone were incomplete. In any event, neither appears to be implicated in the control of REE, at least in adults.25
Growth hormone, and in consequence IGF-1, levels rise from early puberty, but the rise is associated with an increase, rather than a fall, in REE which reportedly occurs before the changes in body composition which might confound it.26 Adiponectin levels are reported to be inversely related to REE in adults,27, 28 though we were not able in an earlier study to show the same relationship in pre-pubertal children.29 Neither of these two studies analysed trends. The study by Ruige et al. was cross-sectional and applied to adults who were either overweight or obese. That of Pannacciulli was also cross-sectional, and confined to Pima Indians. In the present study, adiponectin interacted with age only to the extent that it was inversely related to body fat. When body fat was included in the model, the coefficient for adiponectin fell and lost its statistical significance.
There are strengths and weaknesses to this report. The study was longitudinal and, in view of its unexpected results, every effort was made to account for confounders. The longitudinal design lent itself to mixed effects modelling which can account for missing data and detect interactions over time. The uniform age of the cohort (s.d. ±4m) was crucial to resolving age-related changes, which were central to the analysis. Blind duplicate measurements of height and weight assured optimum precision of the anthropometric measures and AHPV, and dual energy X-ray absorptiometry provided an objective criterion measure of body composition in both absolute and relative terms. However, dual energy X-ray absorptiometry could not resolve the components of LM, so that we cannot be certain how the proportion of each, and of muscle in particular, changed during adolescence. The accelerometers we used provided an objective measure of PA, but recorded only vertical movement, and were unlikely to have recorded fidgeting and movement of the upper body while seated. For this reason, we may have underestimated voluntary energy expenditure, though sedentary PA contributes little to overall volume. Tanner stage was obtained by report, which was inevitable where healthy children are concerned, but we place greater store by APHV as an objective measure of adolescent development, and incorporated it accordingly. The numbers we studied were relatively small by epidemiological standards, but attrition was low (<20% over 10 years), and the multiple time points contributed considerable power to the analysis. The population was 98% white Caucasian, which optimises homogeneity, but arguably limits generalisibility.
Implications
The observations reported here seem reliable, and are novel, but our interpretation of them is inevitably speculative. Sustained growth requires a positive energy balance, and the pubertal dip in REE could represent an evolutionary defence against nutritional pressures during transition from childhood into adulthood—a throwback to an era when nutrition was limited, but maximum fertility at maturity crucial to survival of the species. If so, the same characteristic could have adverse implications for contemporary children, acting unfavourably where calories are widely available. The generalised weight gain which we described earlier in this cohort as they enter adolescence could in part be attributable to this phenomenon.30 Strategies to prevent obesity in children, at its worst among adolescents, might take note of this particularly vulnerable period.
References
Hannon TS . Childhood obesity and type 2 diabetes mellitus. Pediatrics 2005; 116: 473–480.
Pinhas-Hamiel O, Zeitler P . The global spread of type 2 diabetes mellitus in children and adolescents. J Pediatrics 2005; 146: 693–700.
Ball EJ, O'Connor J, Abbott R, Steinbeck KS, Davies PS, Wishart C et al. Total energy expenditure, body fatness, and physical activity in children aged 6-9 y. Am J Clin Nutr 2001; 74: 524–528.
Jago R, Wedderkopp N, Kristensen PL, Møller NC, Andersen LB, Cooper AR et al. Six-year change in youth physical activity and effect on fasting insulin and HOMA-IR. Am J Prev Med 2008; 35: 554–560.
Metcalf BS, Hosking J, Jeffery AN, Henley WE, Wilkin TJ . Exploring the adolescent fall in physical activity. Med Sci Sports Exerc 2015; 47: 2084–2092.
Cannon B . Brown adipose tissue: function and physiological significance. Physiol Rev 2004; 84: 277–359.
Rogers NH . Brown adipose tissue during puberty and with aging. Ann Med 2014; 47: 142–149.
Sun M, Gower BA, Bartolucci AA, Hunter GR, Figueroa-Colon R, Goran MI . A longitudinal study of resting energy expenditure relative to body composition during puberty in African American and white children. Am J Clin Nutr 2001; 73: 308–315.
Wang Z, Heshka S, Zhang K, Boozer CN, Heymsfield SB . Resting energy expenditure: systematic organization and critique of prediction methods*. Obesity 2001; 9: 331–336.
Bitar A, Fellmann N, Vernet J, Coudert J, Vermorel M . Variations and determinants of energy expenditure as measured by whole-body indirect calorimetry during puberty and adolescence. Am J Clin Nutr 1999; 69: 1209–1216.
Wang Z . High ratio of resting energy expenditure to body mass in childhood and adolescence: a mechanistic model. Am J Hum Biol 2012; 24: 460–467.
Sinclaire D . Human Growth After Birth. Oxford Univesity Press: London, 1973.
Voss LD, Kirkby J, Metcalf BS, Jeffery AN, O'Riordan C, Murphy MJ et al. Preventable factors in childhood that lead to insulin resistance, diabetes mellitus and the metabolic syndrome: The EarlyBird Diabetes Study I. J Pediatr Endocrinol Metab 2003; 16: 1211–1224.
Puyau MR, Adolph AL, Vohra FA, Butte NF . Validation and calibration of physical activity monitors in children. Obesity Res 2002; 10: 150–157.
Schmitz KH, Treuth M, Hannan P, McMurray R, Ring KB, Catellier D et al. Predicting energy expenditure from accelerometry counts in adolescent girls. Med Sci Sports Exerc 2005; 37: 155–161.
Basterfield L, Adamson AJ, Frary JK, Parkinson KN, Pearce MS, Reilly JJ . Longitudinal study of physical activity and sedentary behavior in children. Pediatrics 2010; 127: e24–e30.
Metcalf BS, Curnow JSH, Evans C, Voss LD, Wilkin TJ . Technical reliability of the CSA activity monitor: The EarlyBird Study. Med Sci Sports Exerc 2002; 34: 1533–1537.
Nicholson MJ, Holton J, Bradley AP, Beatty PC, Campbell IT . The performance of a variable-flow indirect calorimeter. Physiol Meas 1996; 17: 43–55.
Margulies L, Horlick M, Thornton JC, Wang J, Ioannidou E, Heymsfield SB . Reproducibility of pediatric whole body bone and body composition measures by dual-energy X-Ray absorptiometry using the GE Lunar Prodigy. J Clin Densitom 2005; 8: 298–304.
Taylor SJ, Whincup PH, Hindmarsh PC, Lampe F, Odoki K, Cook DG . Performance of a new pubertal self-assessment questionnaire: a preliminary study. Paediatr Perinat Epidemiol 2001; 15: 88–94.
Krueger C . A comparison of the general linear mixed model and repeated measures ANOVA using a dataset with multiple missing data points. Biol Res Nurs 2004; 6: 151–157.
Hosking J, Henley W, Metcalf BS, Jeffery AN, Voss LD, Wilkin TJ . Changes in resting energy expenditure and their relationship to insulin resistance and weight gain: a longitudinal study in pre-pubertal children (EarlyBird 17). Clin Nutr 2010; 29: 448–452.
Pourhassan M, Humeida H, Braun W, Gluer C, Muller M . Effect of Age on Detailed Body Composition and Resting Energy Expenditure in Normal Weight, Overweight and Obese Children and Adolescents. European Obesity Summit, EOS 2016: Gothenburg, Sweden, 2016.
Story M, Stang J Guidelines for Adolescent Nutrition Services. University of Minnesota: Minneapolis, MN 2005, p 239.
Santosa S, Khosla S, McCready LK, Jensen MD . Effects of estrogen and testosterone on resting energy expenditure in older men. Obesity 2010; 18: 2392–2394.
Burt MG, Gibney J, Hoffman DM, Umpleby AM, Ho KK . Relationship between GH-induced metabolic changes and changes in body composition: a dose and time course study in GH-deficient adults. Growth Hormone IGF Res 2008; 18: 55–64.
Ruige JB, Ballaux DP, Funahashi T, Mertens IL, Matsuzawa Y, Van Gaal LF . Resting metabolic rate is an important predictor of serum adiponectin concentrations: potential implications for obesity-related disorders. Am J Clin Nutr 2005; 82: 21–25.
Pannacciulli N, Bunt JC, Ortega E, Funahashi T, Salbe AD, Bogardus C et al. Lower total fasting plasma adiponectin concentrations are associated with higher metabolic rates. J Clin Endocrinol Metab 2006; 91: 1600–1603.
Hosking J, Metcalf BS, Jeffery AN, Gardner D, Voss LD, Wilkin TJ . Resting energy expenditure, adiponectin and changes in body composition of young children (EarlyBird 34). Int J Pediatr Obes 2008; 3: 46–51.
Mostazir M, Jeffery A, Voss L, Wilkin T . Childhood obesity: evidence for distinct early and late environmental determinants a 12-year longitudinal cohort study (EarlyBird 62). Int J Obes Relat Metab Disord 2015; 39: 1057–1062.
Acknowledgements
We acknowledge the help of Karen Brookes and Val Morgan in maintaining the EarlyBird cohort. We are grateful to the Bright futures trust, Fountain Foundation, BUPA Foundation, EarlyBird Diabetes Trust and countless individual donors who made this study possible.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Mostazir, M., Jeffery, A., Hosking, J. et al. Evidence for energy conservation during pubertal growth. A 10-year longitudinal study (EarlyBird 71). Int J Obes 40, 1619–1626 (2016). https://doi.org/10.1038/ijo.2016.158
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2016.158
- Springer Nature Limited
This article is cited by
-
Short sleep duration is associated with specific food intake increase among school-aged children in China: a national cross-sectional study
BMC Public Health (2019)
-
Energy balance-related factors associating with adolescent weight loss intent: evidence from the 2017 National Youth Risk Behavior Survey
BMC Public Health (2019)
-
Gender-dependent association between sleep duration and overweight incidence in CHINESE school children: a national follow-up study
BMC Public Health (2018)