Abstract
Background
Pubertal changes in body composition significantly influence future health, with links to various diseases. This study aimed to evaluate the weight changes, fat-free mass (FFM), and body fat mass (BFM) during pubertal growth in Korean children and adolescents.
Methods
We utilized mixed longitudinal data, employing bioelectrical impedance analysis for 4641 height measurements (2204 boys, 2437 girls) from 361 individuals (170 boys, 191 girls) aged 7–18 years. Utilizing the Superimposition by Translation and Rotation (SITAR) model, a shape-invariant growth curve model, reference curves for height, weight, and body composition change velocities were estimated.
Results
Korean boys experience peak weight velocity (PWV) at an average age of 12.41 years, with a rate of 8.19 kg/year, peak fat-free mass velocity (PFFV) at 12.70 years (7.60 kg/year), and peak body fat mass velocity (PBFV) at 9.69 years (2.67 kg/year). Korean girls show PWV at 11.28 years (6.33 kg/year), PFFV at 11.13 years (4.86 kg/year), and PBFV at 12.33 years (2.72 kg/year). Positive correlations exist among the ages of peak height velocity, PWV, PFFV, and PBFV.
Conclusions
This research represents the groundbreaking application of the SITAR model in analyzing changes in body composition during pubertal growth in Korean children and adolescents.
Impact
-
This study utilized the SITAR model to analyze longitudinal changes in the body composition of the general pediatric population in Korea across pre- and post-pubertal stages, addressing overlooked aspects in cross-sectional studies.
-
Examining growth parameters, including size (mean mass), tempo (timing), and velocity (compression and expansion) for each body component, revealed positive correlations among ages at peak velocities for various body composition parameters.
-
This study can be employed for further investigations that compare the tempo, size, and velocity of various body composition parameters in pediatric disease cohorts and the general population.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Childhood obesity is a widespread epidemic that severely impacts global public health. There has been a marked increase in the prevalence of childhood obesity in recent years. This rise is linked to a growing incidence of chronic diseases, such as type 2 diabetes mellitus, metabolic syndrome, cardiovascular diseases, and non-alcoholic fatty liver disease.1 Additionally, the scope of research has expanded beyond traditional body mass index (BMI)-focused studies. There is now a greater emphasis on sarcopenic obesity, with active exploration into body fat mass (BFM) and fat-free mass (FFM).2,3 In pediatric populations, there is evidence of heightened vulnerability to metabolic disorders among individuals with high BFM, even those with a normal BMI.4,5
The life course perspective reveals a surge in obesity rates during adolescence and young adulthood, both critical stages for obesity development. This increase is attributed to physiological changes that favor central fat accumulation and lifestyle changes typical of adolescence.6 These findings indicate that weight gain during childhood and adolescence may significantly affect adult BFM more than during infancy, despite infancy being a crucial period for cardio-metabolic disease risk.7,8
Bioelectrical impedance analysis (BIA) is recognized as a practical and non-invasive technique among various body composition analysis methods.9 Studies have shown a strong correlation between BIA and dual-energy X-ray absorptiometry (DEXA) measurements of BFM and FFM. DEXA is noted for its precision in body composition measurement.9,10 While numerous studies have linked body composition, especially BFM measured via BIA, to various diseases in cross-sectional analyses, longitudinal studies focusing on body composition changes during adolescence are scarce. Body composition growth is suggested to decrease after reaching peak velocities (PVs). However, few studies have utilized age at PVs (APVs) to examine the adolescent period when adipose tissue accrual or weight gain may significantly affect adult health outcomes.
The primary objectives of this study are to evaluate the size, timing, and velocity of weight, FFM, and BFM gains during childhood and adolescence in a longitudinal cohort of Korean children and adolescents.
Methods
Study sample
The GP Cohort Study, a mixed longitudinal investigation, is conducted by Global Prediction Co., Ltd.(GP) in Gwangmyeong City, Republic of Korea. GP is a company specializing in growth research utilizing children’s biometric data. It has undertaken numerous national projects focused on child and adolescent growth and has been awarded clinical Good Manufacturing Practices accreditation from the Ministry of Food and Drug Safety for its growth testing software. GP Cohort Study encompasses elementary, middle, and high school students (aged 7–18 years) in Korea, specifically in Gyeonggi Province. Each year, on average, 35 schools participate in the study.
Data collection involves biannual school visits, during which trained examiners measure all students’ height, weight, and body composition, following a standardized protocol. To be eligible for the study, participants must receive consent from their guardians and agree to participate themselves. Subjects unable to adhere to the standard measurement methods were excluded, as well as those whose individuals or guardians did not provide consent for the examination. Since its inception on January 1, 2013, the study has gathered 588,546 data points from 96,485 children and adolescents (50,480 boys and 46,005 girls) born between 1998 and 2016. It received approval from the Institutional Review Board (IRB) of Yonsei Medical University. Due to the study’s retrospective nature and the use of de-identified data, the IRB waived the requirement for informed consent (IRB No. 4-2023-1312).
Anthropometric and body composition parameters
Height (in centimeters), weight (in kilograms), FFM (in kilograms), and BFM (in kilograms) were assessed using an octapolar multifrequency bioelectrical impedance analyzer (Inbody models J10 and J30, Inbody, Seoul, Korea). Highly trained personnel conducted all measurements in accordance with established standard operating procedures. The utilization of the stadiometer for height measurement adhered to CDC (Centers for Disease Control and Prevention) guidelines, and body composition examinations were carried out following the manual provided by InBody Co., Ltd.
Data management
Cole et al. devised a growth curve estimation model based on known stylized facts about growth in children and adolescents.11 The SITAR methodology is a shape-invariant growth model employed to estimate a reference growth velocity curve for the entire dataset, with individual curves being estimated through three random parameters. These parameters encompass the size parameter, which adjusts the curve’s height; the tempo parameter, responsible for changing the left and right timing; and the velocity parameter, which scales the curve accordingly.
Using the SITAR method, growth parameters such as age and magnitude of peak velocity can be estimated. To enhance estimation performance, this study utilizes a subset of the GP Cohort data as follows. The dataset included boys and girls who had at least one height measurement falling within the ranges of 7 to <8.8 years, 8.8 to <10.6 years, 10.6 to <12.4 years, 12.4 to <14.2 years, and 14.2 to <16 years. Participants lacking confirmed measurements at least once within the five designated age groups were excluded from the analysis. Exclusions encompassed instances where measurement data were lost, the measurement period needed to comprehensively cover all age groups, or there were refusals to participate in certain measurement intervals. In a cohort of 96,485 children and adolescents, we identified 22,277 individuals aged 7–16 years for our data analysis, excluding those with fewer than 5 measurements in the designated age range. Among these, 9368 individuals without measurements in the 7 to <8.8-year-old range were excluded, as were 5014 individuals without measurements in the 8.8 to <10.6-year-old range, 3586 individuals without measurements in the 10.6 to <12.4-year-old range, 3310 individuals without measurements in the 12.4 to <14.2-year-old range, and 1138 individuals without measurements in the 14.2 to <16-year-old range. Ultimately, the total dataset comprises 4641 observations (2204 for boys and 2437 for girls) across 361 individuals (170 boys and 191 girls), which were included in the final analysis. (Supplementary Fig. 1) On average, there were 12.9 height measurements available for each individual, which served as the basis for the subsequent SITAR analysis. We employed the SITAR package in R version 4.2.2 to fit the SITAR model.
Data analysis
We estimated three parameters for each individual that correspond to different aspects of growth variation: size (whether an individual is larger or smaller than average), tempo (whether the timing of pubertal growth spurt is earlier or later than average), and velocity (whether the duration of the pubertal growth spurt is faster or slower than average). These parameters were used to identify the pubertal growth spurt, a rapid increase and decrease in growth velocity. Based on the results, we estimated each participant’s age and magnitude of peak velocity for height, weight, FFM, and BFM. We also determined the timing and magnitude of height, weight, FFM, and BFM gain using the age at peak height velocity (APHV), peak height velocity (PHV), age at peak weight velocity (APWV), peak weight velocity (PWV), age at peak FFM velocity (APFFV), peak FFM velocity (PFFV), and age at peak BFM velocity (APBFV), peak BFM velocity (PBFV).
Statistical analysis
We analyzed differences between boys and girls for each parameter and within each sex group. We also calculated Pearson correlation coefficients and determined significance levels to explore the interrelationships among APVs, including APHV, APWV, APFFV, and APBFV. Furthermore, we divided the dataset into the highest 20% and lowest 20% based on each APV. Independent two-sample t-tests were then utilized to investigate potential differences in mean pubertal growth parameters between these two groups. APVs were quantified in years, while PVs such as PHV, PWV, PFFV, and PBFV were measured in cm/year for PHV and kg/year for the remaining PVs.
All statistical analyses were conducted using Python version 3.9.7 and R version 4.2.2. A p value of 0.05 or lower was deemed to indicate statistical significance.
Results
SITAR trajectories
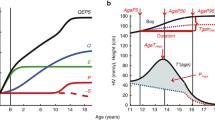
Figure 1 illustrates the mean SITAR trajectory curves, with (a) the growth curve and (b) the velocity curve, differentiated by sex. These SITAR trajectories for height, weight, FFM, and BFM revealed significant variability in both the timing and magnitude of growth across the parameters.
When comparing the growth curves of boys and girls, it is observed that the height and weight trajectories are similar in shape up to approximately 11.8 years of age, with no notable differences between the sexes. However, after 11.8 years, boys demonstrate a marked increase in height and weight, while the growth rates for girls in these parameters plateau.
Analyzing weight in terms of FFM and BFM uncovers distinct sex-specific patterns. Until around age 12, boys and girls exhibit similar FFM growth curves. After this age, boys experience a notable increase in FFM, while the rate of FFM growth in girls slows down. In the case of BFM, younger boys initially have larger measurements, but these begin to decrease around age 11.5. Conversely, girls consistently rise in BFM, with a sharp increase noted around age 10.5, reflecting typical pubertal body changes in females.
Overall, during pubertal growth, boys show a sustained increase in FFM, a pattern of initial rise, and then a decrease in BFM. In contrast, girls display a continual increase in BFM and a slowing growth rate in FFM.
Estimates of the timing and magnitude of peak velocities
Table 1 highlights a statistically significant difference in the timing and magnitude of PVs between boys and girls. Analyzing APHV and PHV, boys average 12.52 years and 9.96 cm/year, respectively, compared to girls at 10.88 years and 8.38 cm/year. In terms of APWV and PWV, boys have values of 12.41 years and 8.19 kg/year, respectively, whereas girls record 11.28 years and 6.33 kg/year, showing earlier APWV and smaller PWV in girls.
Figure 1b depicts distinct sex-specific characteristics in FFM and BFM gains. For boys, APFFV and PFFV are 12.70 years and 7.60 kg/year, respectively, while APBFV and PBFV are 9.69 years and 2.67 kg/year. Here, FFM in boys grows significantly during pubertal growth, whereas BFM velocity peaks before age 10 and then declines. Conversely, in girls, APFFV and PFFV are noted at 11.13 years and 4.86 kg/year, and APBFV and PBFV at 12.33 years and 2.72 kg/year, indicating steady BFM increases into later stages and modest FFM gains.
While APHV, APWV, and APFFV show minor differences between sexes, APBFV demonstrates a notable discrepancy, with boys experiencing earlier APBFV and girls showing later APBFV. The boxplots in Fig. 2 further validate these observations, revealing that girls have earlier APHV, APWV, and APFFV, along with higher PHV, PWV, and PFFV, yet exhibit significantly later APBFV and relatively higher PBFV compared to boys.
APVs age of peak velocity, APHV age of peak height velocity, APWV age of peak weight velocity, APFFV age of peak fat-free mass velocity, APBFV age of peak body fat mass velocity, PVs peak velocity, PHV peak height velocity, PWV peak weight velocity, PFFV peak fat-free mass velocity, PBFV peak body fat mass velocity. Panel a shows the results for boys, while Panel b shows the results for girls.
Table 2’s correlation matrix elucidates the relationships between the APVs for various parameters using the three random parameters of the SITAR model. The column labeled “PVs” indicates the correlations between APVs and PVs for each parameter.
Notably, in boys, there’s a significant negative correlation between APHV and PHV (ρ = −0.50), contrasted with positive correlations between APWV and PWV (ρ = 0.24), and between APBFV and PBFV (ρ = 0.61). For girls, however, negative correlations exist between APHV and PHV (ρ = −0.43), APBFV and PBFV (ρ = −0.20), and APFFV and PFFV (ρ = −0.23). This indicates that a later body mass gain correlates with a greater peak velocity in boys, whereas the trend is the opposite in girls.
Further examining the inter-parameter relationships, there is a strong positive correlation between APHV and APFFV (ρ = 0.91 in boys and ρ = 0.90 in girls) and a moderate positive correlation between APFFV and APBFV (ρ = 0.21 in boys and ρ = 0.42 in girls). These findings suggest that overall body growth tends to occur at similar times for individuals of both sexes, with a particularly notable synchrony in girls.
The data were divided into the top and bottom 20th percentiles based on each APV to examine the relationship between pubertal growth parameters. Table 3 categorizes the samples into these top and bottom 20% groups based on specific APVs and calculates the mean differences in other APVs. Notably, the diagonal elements indicate that the differences in APBFV between the late and early 20% groups are substantially larger (6.76 years for boys and 5.93 years for girls) than those for other APVs. This suggests a higher individual variation in BFM gain relative to different parameters.
Moreover, a positive correlation is observed between APVs at the individual level. In boys, those in the faster APHV group exhibit APWs 1.38 years earlier and APFFVs 1.96 years earlier compared to those in the slower APHV group. Additionally, the group with a faster APFFV shows an APBFV that is 1.70 years earlier, and the group with a faster APBFV has an APFFV that is 0.46 years earlier. In girls, the faster APHV group displays APWs that are 2.07 years earlier, APFFVs that are 2.14 years earlier, and APBFVs that are 2.41 years earlier than their slower counterparts. Those in the faster APFFV group have an APBFV 2.32 years earlier, and the group with a faster APBFV shows an APFFV 0.87 years earlier. Figure 3 visually represents these mean differences and confidence intervals.
Discussion
This study utilized the SITAR method to conduct a mixed longitudinal analysis of the body composition of Korean children and adolescents. This approach enabled us to examine the timing, magnitude, and intensity of weight, FFM, and BFM gain during childhood and adolescence. Our findings showed marked differences in growth trajectories between boys and girls. Boys demonstrated a sustained increase in FFM and a rise followed by a decrease in BFM, whereas girls exhibited an increase in BFM and a slowing of FFM growth. These differences are highlighted by earlier APFFV in girls (11.13 years) versus boys (12.70 years) and later APBFV in girls (12.33 years) compared to boys (9.69 years). We also observed positive correlations among APHV, APWV, APFFV, and APBFV.
The SITAR model comprehensively analyzed body composition changes over pre- and post-pubertal periods, exploring aspects not covered in prior cross-sectional studies. Our study also included correlation analyzes between these growth parameters, offering insights into the dynamic changes in body composition during puberty.
Given the established link between rapid weight gain in mid-childhood and a heightened risk of obesity later in life12,13,14, the puberty period, characterized by significant body composition changes, is increasingly recognized as critical for understanding obesity risks. However, only some studies have explored this by evaluating APVs and PVs for different body compositions during puberty. For instance, Zheng et al. analyzed pubertal timing and body composition in China, reporting positive association of BFM with early puberty for girls while negative for boys.15 Using longitudinal data, the Taiwan Children’s Health Study investigated the relationship between body composition and early puberty, identifying higher adiposity as a risk factor for early puberty in both sexes.16 However, our study uniquely showed significant correlations between APHV and other APVs in both sexes, diverging from previous research on absolute z-scores of FFM and BFM relative to Tanner stages.
Our approach differed by using APHV as a marker of pubertal growth and employing the SITAR model for comparing APVs, making direct comparisons with our results more nuanced. Our study aligns with previous research highlighting sex-specific associations between FFM and BFM during puberty. Although limited, these studies underscore the importance of understanding the relationship between APVs, PVs, and later-life body composition measures like BFM and FFM, which could be instrumental in advancing public health initiatives.
The differences in the timing and velocity of body composition changes observed in our study, which sampled the general population, can be insightfully compared with data from various disease groups. Such comparisons may offer valuable insights into predicting risks of obesity, metabolic disorders, growth-related issues, and pubertal-related conditions during different stages of childhood and adolescence. Furthermore, they can serve as a reference in determining whether individuals fall into risk groups for chronic adult obesity and related metabolic diseases.
Nyati et al. studied South African children and adolescents, finding a correlation between higher pre-pubertal weight gain and early APWV, as well as high PWV during puberty.17 They also noted that early puberty onset was linked to increased BMI and fat mass index in early adulthood, especially in females. Similarly, research on the Chinese population using the SITAR model showed that PWV and PBFV during puberty correlated with increased adult fat mass index.7 Cheng et al., who estimated BFM through skinfold thicknesses and applied the SITAR model, suggested a relationship between earlier APWV in mid-childhood and higher adult fat mass.18 A British study using the SITAR model found that earlier puberty, greater weight, and slower PWV were associated with better bone health outcomes in individuals aged 60–64.19 While these studies mainly focused on obesity and related diseases in adulthood, further research is needed to explore associations with diverse disease groups during childhood and adolescence.
Tanner and Whitehouse, pioneers in longitudinal growth studies of children and adolescents, served as the inspiration for our comparative analysis between their previous research in the UK and our current study.20 Utilizing the SITAR model, we conducted a thorough comparison and reflection on the disparities, with a particular focus on APHV, a widely recognized parameter of pubertal growth, and APBFV, one of the main body composition parameters in our paper. Cole, renowned for his application of the SITAR method, examined APHV using longitudinal height measurements from the Harpenden Growth Study, which is a legacy of Tanner and Whitehouse.11,21 In our investigation, APHV, derived from data collected from Korean children and adolescents born between 1998 and 2020, diverged from Cole’s findings, indicating an earlier onset by approximately 1.5 years for boys and 1.1 years for girls. This discrepancy could be attributed to temporal differences in data collection, with our study spanning a 60-year period after the Harpenden Growth Study. On this basis, we observed global trends toward earlier puberty onset and APHV.22,23,24,25,26,27,28 Moreover, racial disparities between East Asian and Western populations contribute to variations in pubertal growth patterns, with longitudinal studies on Western populations generally indicating a later APHV compared to studies involving Asian populations.29,30,31 Additionally, Tanner and Whitehouse’s study on BFM, utilizing triceps skinfold measurements, provided valuable insights.20 Although direct comparison was challenging due to methodological differences, our findings suggest an earlier APBFV in Korean children compared to the British cohort studied by Tanner and Whitehouse. This discrepancy may also be influenced by racial and pubertal onset differences, as demonstrated in the analysis of the difference in APHV above. Furthermore, challenges arise in comparing triceps skinfold measurements with the BIA method in our study. In the same cohort of British children and adolescents in 2012, correlations with DEXA and BIA were examined.32 The correlation coefficient between triceps skinfold and DEXA fat mass, as well as BIA fat mass, was 0.83 (p < 0.0001), lower than the correlation coefficient of 0.98 (p < 0.0001) between DEXA fat mass and BIA fat mass. Also, there have been reports of an average underestimation of 8.1% in BFM calculated through triceps skinfold thickness, and triceps skinfold thickness is also associated with high inter-measurer bias depending on the measurement method.33 Hence, direct comparison with our study utilizing BIA may be challenging. In conclusion, while racial differences exist between UK and Korean children and adolescents, contributing to variations in pubertal growth, global trends indicate an earlier onset of puberty. Therefore, applying the SITAR method to longitudinal growth data across different races during similar time periods could facilitate comparative analysis and elucidate racial differences in pubertal growth characteristics.
Our study has limitations. Firstly, the age range was confined to 7–18 years. Previous research has shown increased BFM in early adulthood among individuals with high weight gain velocity during infancy and early childhood.12,13,34 Our study’s focus on ages 7 and above meant we could not examine the effects of infant weight gain on pre-pubertal body composition.
Secondly, we employed BIA for assessing body composition, which derives fat percentage from body fluid measurements. BIA has limitations wherein measurements may be influenced by factors such as body posture, obesity, malnutrition, body temperature, fluid and food intake, changes in hydration and electrolyte balance, physical activity, prolonged supine position, examination procedure, and electrode placement method.35 To address these limitations, the measurement of body composition via BIA in this study was conducted by trained examiners following the standard guidelines provided by InBody Co., Ltd. This included excluding individuals unable to assume the standard posture, ensuring a minimum of 5 min in the orthostatic position, adhering to a fasting period of at least 2 h postprandially, and conducting measurements in a post-voiding state, as well as minimizing interference during electrode attachment by restricting bodily contact or foreign materials. Although there were limitations such as the inability to confirm uniform hydration status or electrolyte balance within all subjects and the lack of temperature measurements, it is considered that the measurement data adhered to a standard protocol applicable in actual clinical practice. Despite these shortcomings, in adopting BIA as the method for measuring body composition in this study, several strengths are also present. Various methodologies are accessible to evaluate body composition, including techniques like magnetic resonance imaging, computed tomography, DEXA, and BIA. Among them, BIA is a simple, non-invasive, and low-cost method for estimating body composition, which consequently serves as a practical avenue for assessing body fat and lean body mass, making it suitable for large-scale investigations.36 While some reports suggest that the BIA method may underestimate BFM and overestimate FFM in morbid obesity states, it is not presumed that these limitations would significantly affect our analysis of velocity in longitudinal data in the present study, given the high reproducibility of the test.37 Recent investigations suggest that multi-frequency BIA devices may have a lower susceptibility to errors and offer highly reproducible tests, ensuring reliability in longitudinal observations, with reports indicating almost perfect reproducibility of body composition estimation using BIA in both sexes among pediatric and adolescent populations.10,38,39,40 Studies reporting the utility and accuracy of BIA, particularly in the pediatric and adolescent domain, have indicated excellent test-retest reliability and moderate to strong correlations compared to DEXA.41 Therefore, BIA emerges as an affordable, convenient, non-invasive, and highly reproducible approach for assessing body composition, underscoring its applicability for monitoring changes in individual body composition within clinical settings among pediatric and adolescent cohorts.
Thirdly, it should be noted that this cohort study, primarily carried out in Gwangmyeong City, Gyeonggi Province, may not precisely reflect the characteristics of the entire Korean general population. Nevertheless, as of 2023, the population aged 0 to 19 in South Korea comprises 1,246,773 individuals in Seoul and 2,310,181 individuals in Gyeonggi Province, constituting 44.5% of this demographic in the Seoul-Gyeonggi region. Gwangmyeong City, situated in Gyeonggi Province, is closely linked to Seoul and is recognized as a representative area of the Seoul-Gyeonggi region. Thus, despite this limitation, we assert that it offers a valid foundation for reflecting the broader Korean population. Moreover, to enhance the representativeness of our study, we are gradually expanding data collection to include children from other regions beyond Gwangmyeong City. However, it is widely acknowledged that the onset of puberty and body composition exhibit significant differences across various racial groups.42,43 Consequently, the applicability of our findings is constrained to Korean children and adolescents, given their minimal racial diversity, and may not be generalized to the global population.
A significant strength of our study is its pioneering use of the SITAR model to analyze pubertal body composition changes in Korean children and adolescents. Our team plans to conduct further studies comparing various body composition parameters’ tempo, size, and velocity in diverse groups, including pediatric disease cohorts and the general population, to analyze their associations.
Conclusions
This study is a groundbreaking application of the SITAR model to examine body composition development in Korean children and adolescents, utilizing mixed longitudinal data. The results underscore pronounced differences in growth trajectories between boys and girls. In this study, boys consistently showed increased FFM and a rising and declining BFM pattern. In contrast, girls exhibited an earlier and sustained increase in BFM, alongside a slowdown in FFM growth. There was also a notable positive correlation among the APVs. Future studies should investigate the relationships between body composition changes during childhood and adolescence and their links to various disease groups.
Data availability
The data that support the findings of this study are available from The Global Prediction Co., Ltd. but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of The Global Prediction Co., Ltd.
References
Lister, N. B. et al. Child and adolescent obesity. Nat. Rev. Dis. Prim. 9, 24 (2023).
Choi, K. M. Sarcopenia and sarcopenic obesity. Korean J. Intern. Med. 31, 1054–1060 (2016).
Kyle, U. G., Schutz, Y., Dupertuis, Y. M. & Pichard, C. Body composition interpretation. Contributions of the fat-free mass index and the body fat mass index. Nutrition 19, 597–604 (2003).
Córdoba-Rodríguez, D. P. et al. Fat-free/lean body mass in children with insulin resistance or metabolic syndrome: a systematic review and meta-analysis. BMC Pediatr. 22, 58 (2022).
Xu, R., Zhang, X., Zhou, Y., Wan, Y. & Gao, X. Percentage of free fat mass is associated with elevated blood pressure in healthy Chinese children. Hypertens. Res. 42, 95–104 (2019).
Alberga, A. S., Sigal, R. J., Goldfield, G., Prud’ homme, D. & Kenny, G. P. Overweight and obese teenagers: why is adolescence a critical period? Pediatr. Obes. 7, 261–273 (2012).
Cheng, G. et al. Velocities of weight, height and fat mass gain during potentially critical periods of growth are decisive for adult body composition. Eur. J. Clin. Nutr. 69, 262–268 (2015).
Uauy, R., Kain, J. & Corvalan, C. How can the developmental origins of health and disease (DOHaD) hypothesis contribute to improving health in developing countries? Am. J. Clin. Nutr. 94, 1759S–1764S (2011).
Kyle, U. G., Earthman, C. P., Pichard, C. & Coss-Bu, J. A. Body composition during growth in children: limitations and perspectives of bioelectrical impedance analysis. Eur. J. Clin. Nutr. 69, 1298–1305 (2015).
Chula de Castro, J. A., Lima, T. R. & Silva, D. A. S. Body composition estimation in children and adolescents by bioelectrical impedance analysis: a systematic review. J. Bodyw. Mov. Ther. 22, 134–146 (2018).
Cole, T. J., Donaldson, M. D. & Ben-Shlomo, Y. SITAR—a useful instrument for growth curve analysis. Int. J. Epidemiol. 39, 1558–1566 (2010).
McCarthy, A. et al. Birth weight; postnatal, infant, and childhood growth; and obesity in young adulthood: evidence from the Barry Caerphilly growth study. Am. J. Clin. Nutr. 86, 907–913 (2007).
Kuzawa, C. W. et al. Birth weight, postnatal weight gain, and adult body composition in five low and middle income countries. Am. J. Hum. Biol. 24, 5–13 (2012).
Dietz, W. H. Critical periods in childhood for the development of obesity. Am. J. Clin. Nutr. 59, 955–959 (1994).
Zheng, Y. et al. Association of body composition with pubertal timing in children and adolescents from Guangzhou, China. Front. Public Health 10, 943886 (2022).
Chen, Y. C. et al. Assessing causality between childhood adiposity and early puberty: a bidirectional Mendelian randomization and longitudinal study. Metabolism 100, 153961 (2019).
Nyati, L. H., Pettifor, J. M., Ong, K. K. & Norris, S. A. The association between the timing, intensity and magnitude of adolescent growth and body composition in early adulthood. Eur. J. Clin. Nutr. https://doi.org/10.1038/s41430-023-01293-9 (2023).
Cheng, H. L. et al. The tempo and timing of puberty: associations with early adolescent weight gain and body composition over three years. Child Adolesc. Obes. 5, 16–27 (2022).
Cole, T. J. et al. Using super-imposition by translation and rotation (SITAR) to relate pubertal growth to bone health in later life: the Medical Research Council (MRC) National Survey of Health and Development. Int. J. Epidemiol. 45, 1125–1134 (2016).
Tanner, J. M. & Whitehouse, R.H. Atlas of Children’s Growth (Academic Press, 1982).
Cole, T. J. Tanner’s tempo of growth in adolescence: recent SITAR insights with the Harpenden Growth Study and ALSPAC. Ann. Hum. Biol. 47, 181–198 (2020).
Eckert-Lind, C. et al. Worldwide secular trends in age at pubertal onset assessed by breast development among girls: a systematic review and meta-analyzis. JAMA Pediatr. 174, e195881 (2020).
Pierce, M. & Hardy, R. Commentary: the decreasing age of puberty—as much a psychosocial as biological problem? Int. J. Epidemiol. 41, 300–302 (2012).
Gluckman, P. D. & Hanson, M. A. Changing times: the evolution of puberty. Mol. Cell. Endocrinol. 254, 26–31 (2006).
Cole, T. J. Secular trends in growth. Proc. Nutr. Soc. 59, 317–324 (2000).
Kaplowitz, P. B. Link between body fat and the timing of puberty. Pediatrics 121, S208–216, (2008).
Aksglaede, L., Olsen, L. W., Sørensen, T. I. & Juul, A. Forty years trends in timing of pubertal growth spurt in 157,000 Danish school children. PLoS ONE 3, e2728 (2008).
Ohlsson, C. et al. Secular trends in pubertal growth acceleration in Swedish boys born from 1947 to 1996. JAMA Pediatr. 173, 860–865, (2019).
Tanner, J. M. & Davies, P. S. Clinical longitudinal standards for height and height velocity for North American children. J. Pediatr. 107, 317–329 (1985).
Gerver, W. J. M. & De Bruin, R. Growth velocity: a presentation of reference values in Dutch children. Horm. Res. 60, 181–184, (2003).
De Onis, M. et al. Comparison of the World Health Organization growth velocity standards with existing US reference data. Pediatrics 128, e18–26, (2011).
Wells, J. C. et al. Body-composition reference data for simple and reference techniques and a 4-component model: a new UK reference child. Am. J. Clin. Nutr. 96, 1316–1326 (2012).
Forte, G. C. et al. Can skinfold thickness equations be substituted for bioimpedance analysis in children? J. Pediatr. 97, 75–79 (2021).
Ekelund, U. et al. Upward weight percentile crossing in infancy and early childhood independently predicts fat mass in young adults: the Stockholm weight development study (SWEDES). Am. J. Clin. Nutr. 83, 324–330 (2006).
Wasyluk, W., Wasyluk, M., Zwolak, A. & Łuczyk, R. J. Limits of body composition assessment by bioelectrical impedance analysis (BIA). J. Educ. Health Sport 9, 35–44 (2019).
Khalil, S. F., Mohktar, M. S. & Ibrahim, F. The theory and fundamentals of bioimpedance analysis in clinical status monitoring and diagnosis of diseases. Sensors 14, 10895–10928 (2014).
Coppini, L. Z., Waitzberg, D. L. & Campos, A. C. Limitations and validation of bioelectrical impedance analysis in morbidly obese patients. Curr. Opin. Clin. Nutr. Metab. Care 8, 329–332 (2005).
Faria, S. L., Faria, O. P., Cardeal, M. D. & Ito, M. K. Validation study of multi-frequency bioelectrical impedance with dual-energy X-ray absorptiometry among obese patients. Obes. Surg. 24, 1476–1480 (2014).
Antonio, J. et al. Comparison of dual-energy X-ray absorptiometry (DXA) versus a multi-frequency bioelectrical impedance (InBody 770) device for body composition assessment after a 4-week hypoenergetic diet. J. Funct. Morphol. Kinesiol. 4, 23 (2019).
Potter, A. W. et al. High precision but systematic offset in a standing bioelectrical impedance analysis (BIA) compared with dual-energy X-ray absorptiometry (DXA). BMJ Nutr. Prev. Health 5, 254–262 (2022).
Kabiri, L. S., Hernandez, D. C. & Mitchell, K. Reliability, validity, and diagnostic value of a pediatric bioelectrical impedance analysis scale. Child. Obes. 11, 650–655 (2015).
Ramnitz, M. S. & Lodish, M. B. Racial disparities in pubertal development. Semin. Rneprod. Med. 31, 333–339 (2013).
Meyer, K. A. et al. Ethnic variation in body composition assessment in a sample of adolescent girls. Int. J. Pediatr. Obes. 6, 481–490 (2011).
Acknowledgements
We would like to thank Enago for their English editing services.
Funding
This research was supported by “Regional Innovation Strategy (RIS)” through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (MOE) in 2024 (2022RIS-005).
Author information
Authors and Affiliations
Contributions
Dohyun Chun: acquisition of data, analysis, drafting the article, final approval of article. Seo Jung Kim: drafting the article, revising it critically for important intellectual content, final approval of article. Junghwan Suh: conception, design, interpretation of data, final approval of article. Jihun Kim: conception, design, interpretation of data, final approval of article. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
D.C. and J.K. are employees and holding stocks of Global Prediction Co., Ltd. S.J.K. and J.S. declare that they have no competing interests.
Ethics approval and consent to participate
This study was approved by the Institutional Review Board (IRB) of Yonsei Medical University, and the need for informed consent was waived because this was a de-identified retrospective study (IRB No. 4-2023-1312).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chun, D., Kim, S.J., Suh, J. et al. Timing, velocity, and magnitude of pubertal changes in body composition: a longitudinal study. Pediatr Res (2024). https://doi.org/10.1038/s41390-024-03299-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41390-024-03299-w
- Springer Nature America, Inc.