Abstract
Background:
Cross-sectional studies show that white adipose tissue hypertrophy (few, large adipocytes), in contrast to hyperplasia (many, small adipocytes), associates with insulin resistance and increased risk of developing type 2 diabetes. We investigated if baseline adipose cellularity could predict improvements in insulin sensitivity following weight loss.
Methods:
Plasma samples and subcutaneous abdominal adipose biopsies were examined in 100 overweight or obese individuals before and 10 weeks after a hypocaloric diet (7±3% weight loss) and in 61 obese subjects before and 2 years after gastric by-pass surgery (33±9% weight loss). The degree of adipose tissue hypertrophy or hyperplasia (termed the morphology value) in each individual was calculated on the basis of the relationship between fat cell volume and total fat mass. Insulin sensitivity was determined by homeostasis model assessment-estimated insulin resistance (HOMAIR).
Results:
In both cohorts at baseline, subjects with hypertrophy displayed significantly higher fasting plasma insulin and HOMAIR values than subjects with hyperplasia (P<0.0001), despite similar total fat mass. Plasma insulin and HOMAIR were normalized in both cohorts following weight loss. The improvement (delta insulin or delta HOMAIR) was more pronounced in individuals with hypertrophy, irrespective of whether adipose morphology was used as a continuous (P=0.0002–0.027) or nominal variable (P=0.002–0.047). Absolute adipocyte size associated (although weaker than morphology) with HOMAIR improvement only in the surgery cohort. Anthropometric measures at baseline (fat mass, body mass index, waist-to-hip ratio or waist circumference) showed no significant association with delta insulin or delta HOMAIR.
Conclusions:
In contrast to anthropometric variables or fat cell size, subcutaneous adipose morphology predicts improvement in insulin sensitivity following both moderate and pronounced weight loss in overweight/obese subjects.
Similar content being viewed by others
Introduction
White adipose tissue (WAT) expands by increasing the number and/or size of the fat cells.1 This leads to the development of basically two forms of cellularity phenotypes in WAT, hyperplasia characterized by many small cells or hypertrophy characterized by few but large cells. It is well-established that adipocyte hypertrophy is associated with insulin resistance.2, 3, 4 Recent studies, comparing different adipose regions, have shown that this relationship is particularly evident for subcutaneous adipose tissue, which is by far the largest adipose depot in humans.5, 6, 7 Conversely, hypertrophy in visceral adipose tissue is more closely linked to dyslipidemia.6, 7 Determination of adipose cellularity may be of clinical value. Thus, long-term prospective studies have shown that subjects with subcutaneous adipose hypertrophy, irrespective of body weight status, have increased the risk of developing type 2 diabetes.8, 9 Furthermore, improvement in insulin sensitivity following pronounced weight loss by bariatric surgery is more strongly correlated to the decrease in subcutaneous fat cell size than to the reduction in fat mass per se.10
As described previously,11 it is possible to categorize and quantitatively determine the degree of hyperplasia/hypertrophy (termed the morphology value) by investigating the curve-linear relationship between adipose mass and fat cell size. Subjects with an adipocyte volume above the correlation curve have hypertrophy, whereas those below have hyperplasia. Importantly, the two forms of morphology are only marginally influenced by gender and, unlike fat cell size per se, independent of body weight status.11
As far as we know there are no reports on the predictive value of adipose morphology measures for improvements in insulin sensitivity following weight loss. This notion was presently examined in two separate cohorts. Cohort 1 included 100 overweight or obese subjects before and after a 10-week low-calorie diet, resulting in moderate weight loss. Cohort 2 included 61 morbidly obese subjects before and 2 years after marked weight reduction induced by bariatric surgery. Subjects were categorized according to subcutaneous adipose morphology at baseline, which was compared with the improvement in insulin sensitivity after weight loss.
Subjects and methods
Subjects
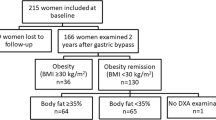
Cohort 1 consisted of 100 overweight or obese subjects (79 women and 21 men) who were otherwise healthy and free of any continuous medication. Body mass index (BMI) ranged from 29 to 49 kg m−2 (36.5±4.5, mean±s.d.). They participated in a large pan-European randomized study (Nutrient-gene interactions in human obesity: implications for dietary guidelines) assessing the effect on metabolic parameters and weight loss following a 10-week treatment with either of two hypocaloric diets.12 The study showed that the diets, composed of equal total amounts of calories but different fat content, resulted in similar improvements in insulin, glucose and body weight parameters, that is, the measures used in the present study. We confirmed that there was no difference between the two diets in weight loss or metabolic improvement in our Swedish subcohort (values not shown). We have therefore combined baseline and outcome data obtained from the two diet arms. Cohort 2, consisted of 61 morbidly obese women (BMI 35–55 kg m−2) investigated before and 2 years after Roux-en-Y gastric by-pass surgery when body weight had been stabilized at a new level for at least 3 months. Some of the clinical data in cohort 2, not included here, have recently been presented.10 Five subjects had type 2 diabetes and were treated with either lifestyle intervention alone (n=1) or with the addition of metformin (n=4). Twenty-one subjects were treated with antihypertensive agents. Further clinical details are given in the published paper.10 Cohort 3, only used for calculations of adipose tissue morphology (see below), consisted of 52 obese or non-obese women described in detail previously.10 All subjects in Cohort 3 were otherwise healthy and free of continuous medication and none had undergone any important weight reduction before measurements of total fat mass and fat cell volume. The study was approved by the regional committee of ethics in Stockholm, Sweden and conducted in full accordance with the principles of the Declaration of Helsinki. It was explained in detail to each subject and informed written consent was obtained.
Examinations
The subjects in cohort 1 and 2 came to the laboratory between 07.30 and 08.00 in the morning after an overnight fast. Height and weight were determined and a venous blood sample was obtained for measurements of insulin and glucose as described.13 These values were used to calculate homeostasis model assessment-estimated insulin resistance (HOMAIR) index using the formula (fasting plasma insulin (mU l−1) x fasting plasma glucose (mmol l−1) divided by 22.5).14 This index shows a satisfactory correlation with values for insulin sensitivity measured using the 'gold standard' method (hyperinsulinemic euglycemic clamp) in both cross-sectional and longitudinal studies.14, 15 In cohort 1, body fat mass was determined by bio-impedance (Body Stat, QuadScan 4000, Isle of Man, British Isles). In cohort 2, lean body mass and total body fat were measured by dual-energy X-ray absorptiometry using a GE Lunar iDXA with the software EnCore (Version 14.10.022). Automatic calibration checks of the dual-energy X-ray absorptiometry were performed daily throughout the study, and calibrations were performed three times weekly using a spine phantom (for bone mineral density, provided by the manufacturer). The coefficient of variation for the spine phantom testing was 1.5 %. No hardware or software changes were made during the course of the trial. The subjects were scanned using standard imaging and positioning protocols and the same scan mode (set for obese subjects) was used throughout the study. Subcutaneous abdominal fat biopsies (1–5 g) were obtained by needle aspiration using a knee puncture needle (2.1-mm diameter) under local anesthesia. Fat cell size was determined using a common and validated method as described.16, 17 This provides similar measures of fat cell diameters as direct assessments in histological sections.18 In brief, fat cells were isolated and the diameter of 100 cells was measured by light microscopy. The diameters were distributed in a unimodal way, and it has been shown in several independent studies that counting 100 or 300 cells by microscopy gives a reliable estimate of fat cell size, which is comparable with results obtained using either manual or automated image analysis software.19 Fat cell volume (expressed in pl) was calculated as ((π × d3)/6) where d is the cell diameter in μm. As previously discussed,17, 20 the diameter (d) is a normally distributed variable but its cube (d3) is skewed, and the arithmetic mean of d3 can therefore not be used to calculate mean fat cell volume. The average fat cell volume is instead better approximated by using the formula  (where
(where  is the mean diameter and σ is the s.d. of the diameter). The curve-linear relationship between mean fat cell volume and total fat mass was determined as described and discussed in detail elsewhere.6, 11 The adipose morphology value (expressed in pl) was obtained by plotting the subject’s body fat mass and measured fat cell volume and subtracting the latter with the expected cell volume on the curve-linear fit. A positive value indicates hypertrophy and a negative hyperplasia. All subjects in cohort 1 had been included in a previous study to establish their morphology value.11 In cohort 2, the curve-linear relationship between total adipose mass and fat cell volume was determined by combining the data with that from Cohort 3 as described in detail previously.10 The addition of Cohort 3 was necessary because calculations of adipose cellularity require the inclusion of subjects within a broad range of fat mass to obtain a valid curve-linear relationship.11 This relationship was estimated through least squares non-linear fitting to be V=(31.6 × m)/(1+0.0161 × m), where V is mean fat cell volume in picoliter and m is the amount of fat in kilogram as measured by dual-energy X-ray absorptiometry. The data are presented in Supplementary Figure S1.
is the mean diameter and σ is the s.d. of the diameter). The curve-linear relationship between mean fat cell volume and total fat mass was determined as described and discussed in detail elsewhere.6, 11 The adipose morphology value (expressed in pl) was obtained by plotting the subject’s body fat mass and measured fat cell volume and subtracting the latter with the expected cell volume on the curve-linear fit. A positive value indicates hypertrophy and a negative hyperplasia. All subjects in cohort 1 had been included in a previous study to establish their morphology value.11 In cohort 2, the curve-linear relationship between total adipose mass and fat cell volume was determined by combining the data with that from Cohort 3 as described in detail previously.10 The addition of Cohort 3 was necessary because calculations of adipose cellularity require the inclusion of subjects within a broad range of fat mass to obtain a valid curve-linear relationship.11 This relationship was estimated through least squares non-linear fitting to be V=(31.6 × m)/(1+0.0161 × m), where V is mean fat cell volume in picoliter and m is the amount of fat in kilogram as measured by dual-energy X-ray absorptiometry. The data are presented in Supplementary Figure S1.
Statistics
Values are presented as mean±s.d or box plots. Absolute values were compared by paired or unpaired t-test. Values representing net changes (before minus after weight reduction=delta values) for fasting plasma insulin and HOMAIR were not normally distributed and were therefore analyzed using non-parametric tests; that is, Mann–Whitney U-test and Spearman correlation. For the latter test, power calculations showed that in cohort 1, a sample size of 100 with Rho=0.23 would yield 80% power at P<0.05, whereas in cohort 2, a sample size of 61 and Rho=0.50 would yield 99.5% power at P<0.05.
Results
At baseline, subjects with hypertrophy or hyperplasia in cohort 1 displayed similar BMI, plasma glucose and body fat levels although there was a trend toward larger waist circumference in WAT hypertrophy (P=0.08, Table 1). As expected, fat cell size was smaller in the hyperplastic group. Individuals with hypertrophy displayed significantly higher waist-to-hip (WHR) ratio, plasma insulin and HOMAIR. Although there was a small difference in age between the groups, age did not correlate with any of the measured parameters (values not shown). Results in cohort 2 (Table 2) were similar as in cohort 1. Thus, whereas BMI and fat mass were similar between the groups, hypertrophic patients had larger fat cells and higher WHR, plasma levels of insulin, glucose and HOMAIR. There was no difference in age or waist circumference between the two groups in cohort 2.
At follow-up, subjects in cohort 1 and 2 displayed significant weight loss (7±3 % and 33±9 %, respectively). As expected, BMI, waist circumference, WHR, fat mass, plasma insulin, glucose and HOMAIR decreased significantly (P<0.001 by paired t-test) in all subgroups in cohort 1 and 2 (Tables 1 and 2). In the weight-reduced state, there were no major differences in the measured variables between subjects with hyperplasia and those with hypertrophy except for fasting plasma insulin which was slightly, but significantly, higher in hypertrophic compared with hyperplastic patients in cohort 2. A similar trend (P=0.052) was observed in cohort 1. Moreover, in cohort 1, waist circumference and WHR were higher in the hypertrophic subjects.
Although adipose morphology had no impact on weight loss in either cohort (values not shown), the improvements (delta) in insulin (Figures 1a and b for cohort 1 and Figures 2a and b for cohort 2, respectively) and HOMAIR (Figures 1c and d for cohort 1 and Figures 2c and d for cohort 2, respectively) were significantly more prominent in the hypertrophic compared with the hyperplastic group. The degree of adipose tissue morphology correlated significantly with the decrease in insulin and HOMAIR, that is, those with more pronounced hypertrophy had a larger improvement in circulating insulin levels and insulin sensitivity. In cohort 1, three subjects displayed outlying delta HOMAIR values (two >8 and one <−4). Similarly, in cohort 2, two subjects displayed pronounced improvements in HOMAIR (>8). However, the results of the statistical analyses were not different when these individuals were omitted.
The relationship between adipose morphology and changes in fasting plasma insulin and HOMAIR following short-term/moderate weight reduction. Delta values from Cohort 1 for (a–b) fasting plasma insulin and (c–d) HOMAIR in relation to adipose morphology shown as box plots or graphs for individual values, respectively. Mann–Whitney U-test and Spearman correlation were used as statistical tests.
The relationship between adipose morphology and changes in fasting plasma insulin and HOMAIR following long-term/pronounced weight reduction. Delta values from Cohort 2 for (a–b) fasting plasma insulin and (c–d) HOMAIR in relation to adipose morphology shown as box plots or graphs for individual values, respectively. Mann–Whitney U-test and Spearman correlation were used as statistical tests.
Because adipose morphology is a measure adjusted for total fat mass, we also investigated if absolute fat cell size at baseline was associated with the reduction in HOMAIR following weight loss (graphs not shown). In cohort 1, there was no significant relationship (Rho=0.17; P=0.10), whereas in cohort 2 initial fat cell size and HOMAIR reduction correlated significantly (Rho=0.38; P=0.005).
The relationship between insulin and HOMAIR improvement and different anthropometric variables at baseline was also determined. However, neither BMI, total fat mass, WHR nor waist circumference showed any significant association with delta insulin or delta HOMAIR in either cohort (Supplementary Table S1).
Finally, we assessed whether baseline adipose morphology was associated with improvements in other cardiometabolic risk factors including circulating levels of total or HDL-cholesterol, triglycerides as well as systolic and diastolic blood pressure. Although plasma lipids and blood pressure were improved in both cohorts following weight reduction (all P<0.001 by paired t-test, data not shown), the only statistically significant difference between subjects discordant in adipose morphology was a more pronounced improvement in diastolic blood pressure among the hypertrophic individuals in Cohort 2 (P=0.04, graph not shown).
Discussion
From both an individual and societal point of view, it is important to identify predictors of improved metabolic outcome following different forms of weight-reduction therapies. Markers of insulin resistance may be of particular importance given the impact this parameter has on the development of type 2 diabetes. As reviewed,21 several factors have been suggested to aid in the selection of patients who might benefit the most from therapies resulting in substantial weight loss (that is, by bariatric surgery). For remission of type 2 diabetes after weight loss, markers of insulin production/sensitivity, glucose control, BMI, diabetes duration and hypertension have been reported to be useful.22, 23, 24 However, bariatric surgery can only be used in selected cases with the most pronounced forms of obesity and metabolic complications. In this study we report for the first time on the predictive value of subcutaneous adipose tissue morphology for improvements in insulin sensitivity following short-term/moderate or long-term/pronounced weight loss in obese patients. We could confirm that individuals with WAT hypertrophy, compared with subjects with hyperplasia, had higher fasting plasma insulin levels and HOMAIR, despite similar BMI and fat mass.11 More importantly, although WAT morphology did not associate with weight loss per se, it correlated with improvements in circulating fasting insulin levels and insulin sensitivity. Thus, in both cohorts, subjects with hypertrophy had more to gain in terms of insulin sensitivity improvements than those with hyperplasia. These results extend recently published data, demonstrating that subjects displaying the largest reduction in fat cell size following bariatric surgery are the ones with the most marked improvement in insulin sensitivity.10
As mentioned above, several investigators have shown that large fat cells are linked to increased risk of developing type 2 diabetes.8, 9 However, the prognostic value of fat cell size following weight loss is less clear. Although older studies have demonstrated that small fat cells at baseline associate with increased risk of weight regain following diet-induced weight loss,25 changes in insulin sensitivity were not determined in that work. A recent study in morbidly obese women scheduled for bariatric surgery suggested that there is a threshold for baseline adipocyte volume (~800–1000 pl) above which insulin resistance/type 2 diabetes is significantly more prevalent.26 Moreover, 6 months after surgery, subjects with larger fat cells at baseline displayed a significantly lower resolution of diabetes or risk of diabetes (defined by HOMAIR> the 75th percentile). In our present study, fat cell size associated with improvements in insulin sensitivity only in cohort 2. However, in contrast with the data from Cotillard et al.,26 we observed a positive relationship between baseline fat cell size and HOMAIR reduction. These differences may depend on several factors, for example, that cohort 2 was re-investigated significantly later after surgery, when body weight had attained a new steady state. It is well-established that most individuals are still in a catabolic weight-losing state 6 months after bariatric surgery. Furthermore, almost one third of the subjects in the study by Cotillard et al.26 had established type 2 diabetes. In any case, the present observation that adipocyte size corrected for total fat mass (that is, morphology value) had a prognostic value in both cohort 1 and 2 suggests that this estimate provides more information than fat cell size per se. In fact, the influence of fat cell size alone is difficult to determine because of its strong dependency on body fat mass and by itself, adipocyte size can therefore not distinguish between different WAT morphologies.
The results reported from cohort 1 were obtained within Nutrient-gene interactions in human obesity: implications for dietary guidelines, a pan-European diet intervention study where the primary aim was to determine the effects on metabolic parameters and weight loss following two hypocaloric diets. The prognostic values on weight loss of other parameters have been published previously.27, 28, 29 These have shown that early weight reduction28 and to a lesser extent global gene expression27 can predict final weight loss, whereas allelic variants in the melanocortin 3 receptor gene do not.29 However, only one report has evaluated factors possibly related to changes in insulin sensitivity, demonstrating that plasma adiponectin levels (irrespective of isoform) showed no association with improved HOMAIR.30 Our present data can therefore for the first time identify an adipose-related parameter, predicting improved insulin sensitivity. Admittedly, in both the present and previous studies, the r- and Rho-values suggest that although significant, the prognostic value of the different markers is modest. Nevertheless, by combining adipose morphology with other clinical variables it may be possible to develop algorithms that are stronger predictors for beneficial outcomes of weight-reducing therapies.
Excess body fat status is usually classified on the basis of BMI and sometimes according to the fat distribution (that is, waist and/or WHR). Waist circumference correlates with insulin resistance13 and the degree of reduction in this measure associates with improvement in insulin sensitivity following weight loss by gastric by-pass surgery.31 The hypertrophic subjects displayed significantly higher WHR in both cohorts at baseline. Despite this, neither WHR nor other anthropometric variables predicted improvements in insulin sensitivity. This observation supports recent suggestions that better classification tools are needed to identify overweight/obese subjects where therapeutic interventions should be prioritized.32, 33, 34 The present study suggests that subcutaneous adipose morphology may add a further dimension to obesity phenotypes and that determination of this parameter could be useful in selecting patients for weight reduction therapy. In a clinical setting, fat cell size can be measured histologically in small pieces of adipose tissue obtained by needle aspiration,17 a method which yields similar results as the more tedious protocol for fat cell isolation (by collagenase treatment) used in the present study.18 Total body fat can easily be estimated using a formula based on age, sex and BMI.35 For the purpose of measuring adipose morphology, this indirect method gives essentially the same results as direct assessments using bio-impedance.11
What are the mechanisms behind the predictive role of adipose morphology for improvements in insulin sensitivity? Although this study was not designed to answer this question, some speculations can be offered. Adipose tissue cellularity is related to the inflammatory and metabolic status of adipose tissue (reviewed in36). Subjects with adipose tissue hypertrophy have increased secretion of inflammatory proteins, enhanced lipolytic activity and decreased insulin-induced glucose metabolism in fat cells,37 factors that are well-established to favor the development insulin resistance. Furthermore, it has been suggested that the capacity to store lipids in adipose hypertrophy is less efficient than in hyperplasia.38 This may result in increased ectopic lipid deposition, which in turn promotes insulin resistance. The well-recognized observation, that these disturbances are normalized even upon modest weight loss, could explain why individuals with adipose hypertrophy display larger improvements in insulin sensitivity than weight-matched subjects with hyperplasia.
Although subcutaneous adipose morphology may predict improvements in insulin sensitivity, it appears to be of less value as a marker for beneficial effects on plasma lipid or blood pressure. Thus, whereas all these variables were normalized upon weight loss, adipose morphology had only a modest impact on change in diastolic blood pressure in Cohort 2. These results are in line with the previously mentioned observation that subcutaneous adipocyte size associates more closely with insulin sensitivity, whereas visceral fat cell volume is linked to dyslipidemia.6
There are some limitations with the present study. Results are solely based on analyses of abdominal subcutaneous adipose tissue. Whether other depots display similar associations remains to be defined. Also, because the majority of the subjects were women, we cannot exclude that there may be gender differences. However, similar correlations between morphology and delta HOMAIR were observed when analyzing the 21 men in cohort 1 separately (data not shown). All our subjects were white Caucasians. Adipose morphology may possibly have a different predictive value in individuals of other ethnicities. Finally, insulin sensitivity was determined by HOMAIR. Nevertheless, this indirect estimate shows a strong relationship with direct measures of insulin sensitivity as discussed in detail previously.14
In conclusion, subcutaneous adipose tissue morphology predicts improvements in insulin sensitivity after both short-term/moderate and long-term/pronounced weight reduction in obese subjects. Adipose morphology could therefore serve as a useful tool when selecting obese subjects for various weight loss interventions. Although adipose tissue morphology is a significantly better prognostic marker than fat cell size or commonly used anthropometric variables, its clinical value needs to be validated in larger cohorts including a higher proportion of men as well as subjects of different ethnicities.
References
Hirsch J, Batchelor B . Adipose tissue cellularity in human obesity. Clin Endocrinol Metab 1976; 5: 299–311.
Salans LB, Knittle JL, Hirsch J . The role of adipose cell size and adipose tissue insulin sensitivity in the carbohydrate intolerance of human obesity. J Clin Invest 1968; 47: 153–165.
Stern JS, Batchelor BR, Hollander N, Cohn CK, Hirsch J . Adipose-cell size and immunoreactive insulin levels in obese and normal-weight adults. Lancet 1972; 2: 948–951.
Krotkiewski M, Sjostrom L, Bjorntorp P, Smith U . Regional adipose tissue cellularity in relation to metabolism in young and middle-aged women. Metabolism 1975; 24: 703–710.
Lundgren M, Svensson M, Lindmark S, Renstrom F, Ruge T, Eriksson JW . Fat cell enlargement is an independent marker of insulin resistance and 'hyperleptinaemia'. Diabetologia 2007; 50: 625–633.
Hoffstedt J, Arner E, Wahrenberg H, Andersson DP, Qvisth V, Lofgren P et al. Regional impact of adipose tissue morphology on the metabolic profile in morbid obesity. Diabetologia 2010; 53: 2496–2503.
Veilleux A, Caron-Jobin M, Noel S, Laberge PY, Tchernof A . Visceral adipocyte hypertrophy is associated with dyslipidemia independent of body composition and fat distribution in women. Diabetes 2011; 60: 1504–1511.
Weyer C, Foley JE, Bogardus C, Tataranni PA, Pratley RE . Enlarged subcutaneous abdominal adipocyte size, but not obesity itself, predicts type II diabetes independent of insulin resistance. Diabetologia 2000; 43: 1498–1506.
Lonn M, Mehlig K, Bengtsson C, Lissner L . Adipocyte size predicts incidence of type 2 diabetes in women. Faseb J 2010; 24: 326–331.
Andersson DP, Eriksson Hogling D, Thorell A, Toft E, Qvisth V, Naslund E et al. Changes in subcutaneous fat cell volume and insulin sensitivity after weight loss. Diabetes Care 2014; 37: 1831–1836.
Arner E, Westermark PO, Spalding KL, Britton T, Ryden M, Frisen J et al. Adipocyte turnover: relevance to human adipose tissue morphology. Diabetes 2010; 59: 105–109.
Petersen M, Taylor MA, Saris WH, Verdich C, Toubro S, Macdonald I et al. Randomized, multi-center trial of two hypo-energetic diets in obese subjects: high- versus low-fat content. Int J Obes (Lond) 2006; 30: 552–560.
Wahrenberg H, Hertel K, Leijonhufvud BM, Persson LG, Toft E, Arner P . Use of waist circumference to predict insulin resistance: retrospective study. BMJ 2005; 330: 1363–1364.
Wallace TM, Levy JC, Matthews DR . Use and abuse of HOMA modeling. Diabetes Care 2004; 27: 1487–1495.
Katsuki A, Sumida Y, Gabazza EC, Murashima S, Furuta M, Araki-Sasaki R et al. Homeostasis model assessment is a reliable indicator of insulin resistance during follow-up of patients with type 2 diabetes. Diabetes Care 2001; 24: 362–365.
Arner P, Andersson DP, Thorne A, Wiren M, Hoffstedt J, Naslund E et al. Variations in the size of the major omentum are primarily determined by fat cell number. J Clin Endocrinol Metab 2013; 98: E897–E901.
Hirsch J, Gallian E . Methods for the determination of adipose cell size in man and animals. J Lipid Res 1968; 9: 110–119.
Smith U, Sjostrom L, Bjornstorp P . Comparison of two methods for determining human adipose cell size. J Lipid Res 1972; 13: 822–824.
Tchoukalova YD, Harteneck DA, Karwoski RA, Tarara J, Jensen MD . A quick, reliable, and automated method for fat cell sizing. J Lipid Res 2003; 44: 1795–1801.
Goldrick RB . Morphological changes in the adipocyte during fat deposition and mobilization. Am J Physiol 1967; 212: 777–782.
Neff KJ, Olbers T, le Roux CW . Bariatric surgery: the challenges with candidate selection, individualizing treatment and clinical outcomes. BMC Med 2013; 11: 8.
Hayes MT, Hunt LA, Foo J, Tychinskaya Y, Stubbs RS . A model for predicting the resolution of type 2 diabetes in severely obese subjects following Roux-en Y gastric bypass surgery. Obes Surg 2011; 21: 910–916.
Aarts EO, Janssen J, Janssen IM, Berends FJ, Telting D, de Boer H . Preoperative fasting plasma C-peptide level may help to predict diabetes outcome after gastric bypass surgery. Obes Surg 2013; 23: 867–873.
Dixon JB, Chuang LM, Chong K, Chen SC, Lambert GW, Straznicky NE et al. Predicting the glycemic response to gastric bypass surgery in patients with type 2 diabetes. Diabetes Care 2013; 36: 20–26.
Krotkiewski M, Sjostrom L, Bjorntorp P, Carlgren G, Garellick G, Smith U . Adipose tissue cellularity in relation to prognosis for weight reduction. Int J Obes 1977; 1: 395–416.
Cotillard A, Poitou C, Torcivia A, Bouillot JL, Dietrich A, Kloting N et al. Adipocyte size threshold matters: link with risk of type 2 diabetes and improved insulin-resistance after gastric bypass. J Clin Endocrinol Metab 2014; 99: E1466–E1470.
Mutch DM, Temanni MR, Henegar C, Combes F, Pelloux V, Holst C et al. Adipose gene expression prior to weight loss can differentiate and weakly predict dietary responders. PLoS One 2007; 2: e1344.
Handjieva-Darlenska T, Holst C, Grau K, Blaak E, Martinez JA, Oppert JM et al. Clinical correlates of weight loss and attrition during a 10-week dietary intervention study: results from the NUGENOB project. Obes Facts 2012; 5: 928–936.
Santos JL, De la Cruz R, Holst C, Grau K, Naranjo C, Maiz A et al. Allelic variants of melanocortin 3 receptor gene (MC3R) and weight loss in obesity: a randomised trial of hypo-energetic high- versus low-fat diets. PLoS One 2011; 6: e19934.
Polak J, Kovacova Z, Holst C, Verdich C, Astrup A, Blaak E et al. Total adiponectin and adiponectin multimeric complexes in relation to weight loss-induced improvements in insulin sensitivity in obese women: the NUGENOB study. Eur J Endocrinol 2008; 158: 533–541.
Andersson DP, Wahrenberg H, Toft E, Qvisth V, Lofgren P, Hertel K et al. Waist circumference to assess reversal of insulin resistance following weight reduction after bariatric surgery: cohort and cross-sectional studies. Int J Obes (Lond) 2014; 38: 438–443.
Ahima RS, Lazar MA . Physiology. The health risk of obesity—better metrics imperative. Science 2013; 341: 856–858.
Sharma AM, Kushner RF . A proposed clinical staging system for obesity. Int J Obes (Lond) 2009; 33: 289–295.
Arner E, Arner P . Health and obesity: not just skin deep. Science 2013; 342: 558–559.
Gallagher D, Visser M, Sepulveda D, Pierson RN, Harris T, Heymsfield SB . How useful is body mass index for comparison of body fatness across age, sex, and ethnic groups? Am J Epidemiol 1996; 143: 228–239.
Sun K, Kusminski CM, Scherer PE . Adipose tissue remodeling and obesity. J Clin Invest 2011; 121: 2094–2101.
Gao H, Mejhert N, Fretz JA, Arner E, Lorente-Cebrian S, Ehrlund A et al. Early B Cell Factor 1 regulates adipocyte morphology and lipolysis in white adipose tissue. Cell Metab 2014; 19: 981–992.
Virtue S, Vidal-Puig A . Adipose tissue expandability, lipotoxicity and the Metabolic Syndrome—an allostatic perspective. Biochim Biophys Acta 2010; 1801: 338–349.
Acknowledgements
We are grateful for the excellent technical assistance of research nurses Britt-Marie Leijonhufvud, Katarina Hertel and Yvonne Widlund as well as laboratory technicians Eva Sjölin, Gaby Åström, Elisabeth Dungner and Kerstin Wåhlén. MR and PA conceived the study and wrote the first version of the manuscript, which was then read and approved by all co-authors. All authors contributed to subject recruitment and analyses. Mikael Rydén and Peter Arner are the guarantors of this work. This study was supported by grants from Swedish Research Council, Swedish Diabetes Association, Novo Nordisk Foundation, EASD/Eli-Lilly, The Erling-Persson Family Foundation, the EU/EFPIA Innovative Medicines Initiative Joint Undertaking (EMIF grant n° 115372) and the Diabetes Program at Karolinska Institutet.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Supplementary information
Rights and permissions
About this article
Cite this article
Eriksson-Hogling, D., Andersson, D., Bäckdahl, J. et al. Adipose tissue morphology predicts improved insulin sensitivity following moderate or pronounced weight loss. Int J Obes 39, 893–898 (2015). https://doi.org/10.1038/ijo.2015.18
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2015.18
- Springer Nature Limited
This article is cited by
-
Associations between subcutaneous adipocyte hypertrophy and nonalcoholic fatty liver disease
Scientific Reports (2022)
-
Using metabolic markers to identify insulin resistance in premenopausal women with and without polycystic ovary syndrome
Journal of Endocrinological Investigation (2021)
-
Elucidating nanoscale mechanical properties of diabetic human adipose tissue using atomic force microscopy
Scientific Reports (2020)
-
Consumption of out-of-season orange modulates fat accumulation, morphology and gene expression in the adipose tissue of Fischer 344 rats
European Journal of Nutrition (2020)
-
Metabolic Impact of Body Fat Percentage Independent of Body Mass Index in Women with Obesity Remission After Gastric Bypass
Obesity Surgery (2020)