Abstract
Background/Objectives:
We assessed the effect of weight loss-associated changes in detailed body composition on plasma insulin levels and homeostatic model assessment (HOMA) index to calculate the magnitude of reduction in different adipose tissue depots required to improve insulin sensitivity.
SubjectS/Methods:
A total of 50 subjects aged 20–69 years were studied. The participants were compiled from low-calorie diet interventions and bariatric surgery and differed in their baseline body mass index (BMI; range 21.6–54.4 kg/m2) and degree of weight losses (range −3.3 to −56.9 kg). Detailed body composition and liver fat were measured using whole-body magnetic resonance imaging (MRI). Insulin resistance was assessed by HOMA.
Results:
Mean body weight decreased by −16.0±13.6 kg. Significant changes were observed in total adipose tissue (TATMRI, range −0.5 to −36.0 kg), total subcutaneous adipose tissue (SATMRI), visceral adipose tissue (VATMRI), skeletal muscle, liver fat, plasma insulin levels and HOMA. Decreases in insulin and HOMA were correlated with reductions in TATMRI, SATMRI, VATMRI (just with HOMA) and liver fat. Losses of 2.9 and 6.5 kg body weight, 2.0 and 5.0 kg TATMRI as well as 1.6 and 6% liver fat were required to decrease plasma insulin levels by 1 μU/ml and HOMAadjusted for baseline HOMA by 1 point. Multiple regression analysis showed that baseline liver fat and changes in liver fat explained 49.7% and 55.1% of the variance in weight loss-associated changes in plasma insulin and HOMA, respectively.
Conclusions:
Decreases of adipose tissues and liver fat are the major determinants of reduction in plasma insulin levels and improvement in HOMA index.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Insulin resistance is frequently associated with obesity and is considered an important link between adiposity and the related risk for cardiovascular disease and type 2 diabetes.1, 2 The association of obesity with insulin resistance does not depend on the degree of obesity alone. Fat mass, fat distribution and hepatic fat add to the variance in insulin sensitivity.3, 4 Visceral adipose tissue (VAT) is considered a major determinant of metabolic disorders,5 but a causal relationship between VAT and metabolic abnormalities remains unclear. Loss of VAT has a therapeutic role in obesity management.6 In addition, most studies have shown that VAT is associated directly with liver fat content,7, 8 and higher liver fat content is correlated with VAT.7, 9, 10 Therefore, it is possible that liver fat loss, not VAT loss, is a better predictor of metabolic risk factor for obesity-related metabolic disorders.
Weight loss-associated changes in individual fat depots indicated that the reductions in subcutaneous adipose tissue (SAT11) and liver fat12 were correlated with improvement in insulin sensitivity in obese individuals following a low-calorie diet and a weight loss of −11.2 and −8.5 kg, respectively. Overweight patients are encouraged to lose 5–10% of their body weight to improve insulin sensitivity,1, 13 but quantitative data supporting this recommendation are limited. One study showed improvements in insulin sensitivity and cardiovascular risks with 10–15% decreases in body weight.14 In addition, the study by Hamman et al.15 revealed that there was a 16% reduction in risk for progression to diabetes with 1 kg of weight loss and that 5% weight loss would produce about 50% reduction in the incidence of type 2 diabetes. The results of the Look AHEAD trial13 demonstrated a strong relationship between glycemic measures and weight loss, with improvement beginning at 2.5–5% weight loss. These studies indicated an average reduction in body weight and risk factors, but little research has been conducted to identify the associations between magnitude of weight loss and individual body components—that is, different adipose tissue depots, such as SAT, VAT or liver fat—along with subsequent improvements in insulin sensitivity.
The choice of method used to measure insulin sensitivity is important. Although changes in insulin sensitivity—that is, the responsiveness of target tissue (mainly skeletal muscle (SM), liver and adipose tissue) to metabolic actions of insulin, such as insulin-mediated glucose disposal and inhibition of hepatic glucose production16, 17—often develop simultaneously, the degree of the effect may be different among the various tissues.18 The hyperinsulinemic–euglycemic clamp is considered the gold standard for measuring whole-body insulin sensitivity.19 In addition, surrogate measures of insulin sensitivity that have been developed from measurements of glucose and insulin in fasting state20, 21 are primarily related to hepatic insulin action and not to insulin sensitivity in SM. In our previous study,22 homeostatic model assessment of insulin resistance (HOMA-IR) has been validated against clamp data, and M-value was significantly correlated with HOMA-IR (r=−0.69, P<0.05). In this study, an estimate of fasting insulin sensitivity was obtained by HOMA-IR.
The aim of our study was to assess the relationship between weight loss-associated changes in detailed body composition and plasma insulin levels and HOMA to calculate the magnitude of reduction in different adipose tissue depots required to improve insulin sensitivity. Whole-body magnetic resonance imaging (MRI) was used to assess body composition.
Materials and methods
This was a post-hoc analysis of previously obtained data of 50 subjects (22 women and 28 men), aged 20–69 years with body mass index (BMI) ranging from 21.6 to 54.4 kg/m2, who had been assessed at two different occasions at the Institute for Human Nutrition at Christian-Albrechts-University, Kiel, Germany. Subjects were compiled from different studies in the investigators’ archived databases (see below). The sample size was chosen by power analysis using the software G*Power (version 3.1.9.2, Düsseldorf, Germany). The participants were compiled from low-calorie diet interventions as well as bariatric surgery and differed in their initial body weight and weight losses. Net weight changes were calculated, and subjects were grouped into three categories as follows:
Group 1
The original study population comprised 32 subjects who were recruited between February 2010 and September 2012. The study protocol and experimental data of the participants have been described previously.22, 23 Briefly, subjects had participated in a controlled nutritional intervention study and consumed a low-calorie diet, by which their daily energy intake was reduced by 50% of energy requirements with a follow-up period of 3 weeks. Out of the 32 subjects, baseline and follow-up whole-body MRI scans were measured in 17 subjects (aged 20–29 years, mean BMI 24.6 kg/m2) who were selected for the analysis. This trial was registered at clinicaltrials.gov as NCT01737034.
Group 2
The original study population comprised 96 overweight subjects who were recruited between 2006 and 2009. Subjects participated in a 6-month intervention study. Details of the study design and recruitment procedure have been described elsewhere.24, 25 Out of 96, 30 subjects with weight loss of >3% of the initial body weight were considered as weight losers.26 Out of 30, baseline and follow-up of whole-body MRI results of 17 subjects (mean follow-up period of 3.0±2.6 years) were selected for the analysis.
Group 3
Thirty-two extremely obese subjects (aged 24–68 years, mean BMI 46.7 kg/m2) were studied between 2009 and 2010. Subjects underwent bariatric surgery with a follow-up period of 6 months. Baseline and follow-up whole-body MRI scans were measured in 17 subjects.
In all groups, exclusion criteria were smoking, pregnancy, acute or chronic disease and use of any medication that could influence energy metabolism or body composition. The study protocol had been approved by the local ethical committee of the Christian-Albrechts-University zu Kiel, Germany, and each subject provided informed written consent before participation.
Anthropometric measurements and detailed body composition analysis
Body weight was measured to the nearest 0.1 kg on an electronic Tanita scale. Height was assessed using a stadiometer (Seca, Hamburg, Germany) to the nearest 0.5 cm.
Volumes of four internal organs (brain, heart, liver and kidneys), SAT, VAT and SM were measured by using transversal MRI images as described previously.24, 27 Briefly, scans were obtained using a 1.5T Magnetom Vision scanner (Siemens, Erlangen, Germany). Brain and abdominal organs were examined with a T1-weighted sequence (FLASH) (time to repeat (TR): 177.8 ms for abdominal organs; TR: 170.0 ms for the brain; time of echo (TE): 4.1 ms/echo). Electrocardiogram-triggered, T2-weighted, turbo spin-echo ultrashot scans (HASTE) (TR: 800.0 ms; TE: 43 ms/echo) were used to examine the heart. The slice thickness ranged from 6 mm for the brain to 7 mm for the heart to 8 mm for internal organs without interslice gaps. Cross-sectional organ areas were determined manually using segmentation software (SliceOmatic, version 4.3; Tomo-Vision Inc., Montreal, Canada). Volumes of SAT, VAT and SM were examined with T1-weighted gradient echo sequences (TR: 575 ms; TE: 15 ms) as described previously.28 Continuous transversal images with an 8-mm slice thickness and 2-mm interslice gaps were obtained and analyzed from the wrist to the ankle using SliceOmatic software. Images in abdominal and thoracic regions were measured with subjects holding their breath. Volume data were transformed into organ and tissue weights using the following densities: 1.036 g/cm3 for the brain, 1.06 g/cm3 for the heart and liver, 1.05 g/cm3 for kidneys, 0.92 kg/l for VAT and 1.04 for SAT.29, 30
Liver fat was determined in a subgroup of 28 subjects by MRI (Magnetom Avanto 1.5-T Siemens) along with the two-point Dixon method with a volume interpolated breath-hold examination as previously described.22, 31 Data of two and four subjects in groups 1 and 3, respectively, were disregarded for analysis of liver fat because of non-attendance at one time point (for example, at follow-up) or low quality of images. There are no liver fat data available in group 2.
Briefly, a T1-weighted gradient echo sequence with in-phase and out-of-phase imaging was performed using the following variables: repetition time, 10.4 ms; echo time, 4.76 (in-phase) and 7.14 (opposed-phase) ms; flip angle, 10°; matrix, 80 3128; and field of view, 440 mm. Fat-only and water-only images were calculated from in-phase and opposed-phase images as follows: water only = 1/2 × (in phase +opposed phase); fat only = 1/2 × (in phase-opposed phase). Forty adjacent slices were acquired within a 19-s breath-hold to cover the liver with a slice thickness of 5-mm and 1-mm interslice gap. Images were analyzed and processed using ImageJ software (US NIH)32 to calculate hepatic fat fraction images from fat-only and water-only images. A single continuous region of interest was defined (20.62 × 20.62) in each of five adjacent hepatic fat fraction images and was placed in the liver parenchyma, avoiding major blood vessels. The region of interest was placed in the same area for all repeated measurements. The quantity of liver fat was determined as percentage of the total liver core and was averaged for the five hepatic fat fraction images. The intra-organ fat percentage was evaluated from two liver regions of interest, defined and averaged by one observer.
Clinical and metabolic variables
Blood samples were taken after an 8-h overnight fast and analyzed according to standard procedures. Plasma insulin and plasma glucose levels were measured using a radioimmunoassay (Adaltis, Rome, Italy) and hexokinase enzymatic method respectively. An estimate of fasting insulin sensitivity obtained by HOMA-IR was calculated as fasting insulin (μU/ml) x fasting glucose (mmol/l)/22.5.20
Statistical analysis
The statistical analysis was performed using SPSS statistical software (SPSS Statistics for Windows, IBM Corp, Version 22.0, Armonk, NY, USA). Differences between variables and between baseline and follow-up were analyzed using paired samples t-test for normally distributed variables. Differences in variables between three groups at baseline were analyzed by one-way analysis of variance with post-hoc t-test (with Bonferroni correction) in normally distributed variables. In addition, differences in liver fat between group 1 and group 3 were analyzed using an unpaired t-test in normally distributed variables. HOMA was adjusted for their baseline values by using a regression analysis. A stepwise regression analysis was performed to explain the effect of body composition (as an independent variable) on the variance in changes in plasma insulin levels and changes in HOMA. Pearson’s correlation coefficient was calculated for relations between variables. All tests were two-tailed, and P<0.05 was accepted as the limit of significance.
Results
An overall 44% of subjects were women, and 56% of subjects were men. The age range was between 20 and 69 years. Of 50 participants, 68% of study participants were obese (Table 1). Mean body weight decreased in the total population as well as in each group. Weight loss was associated with significant decreases in total adipose tissue (TATMRI), SAT of the trunk (SATtrunk MRI), SAT of the extremities (SATextremities MRI) and VATMRI as well as sizes of total and regional SMMRI, total organ masses, plasma insulin, glucose and HOMA in the whole population (Table 1).
At baseline, 86% of the total population was hyperinsulinemic (plasma insulin levels >8.4 μU/ml). With weight loss, the percentage of subjects with insulin >8.4 μU/ml significantly decreased to 38%.
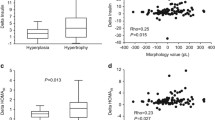
Mean plasma insulin, HOMA and liver fat declined by −10.9±8.6 (μU/ml, P=0.000), −3.5±2.4 (P=0.000) and −6.7% (P=0.005, Table 1), respectively, whereas magnitude of the reductions was significantly higher in bariatric surgery subjects (group 3) compared with that in others (insulin, −17.4 vs 7.7 μU/ml; HOMA, −6.6 vs −1.8; liver fat, −15.7% vs 1.1%, respectively). This effect depended on baseline plasma insulin, HOMA and liver fat. The results clearly indicate that subjects with high plasma insulin, HOMA and liver fat at baseline tended to greater decreases in plasma insulin, HOMA (Figure 1d) and liver fat (Figure 1f) with weight loss (both P=0.000). In addition, despite the significant differences in mean weight changes between groups 1 and 2 (P=0.007), no differences were observed in terms of changes in glucose levels, insulin and HOMA (P=0.551, P=0.896 and P=0.916, respectively; Table 1).
Relationship between changes in HOMAadjusted for baseline HOMA vs (a) changes in body weight, (b) changes in total adipose tissue (TATMRI) measured by MRI, (c) changes in visceral adipose tissue (VATMRI) measured by MRI and (d) association between changes in HOMA vs baseline HOMA in the total study population of 50 subjects differing with respect to weight changes. Relationship between changes in liver fat percentage measured by MRI vs (e) changes in HOMAadjusted for baseline HOMA and (f) baseline liver fat in a subgroup of 28 subjects. Solid lines are regression lines.
Reductions in plasma insulin levels were correlated with decreases in body weight (r=0.54, P=0.000), TATMRI (r=0.48, P=0.000) and liver fat (r=0.70, P=0.000), whereas no association was observed between reduction in insulin and decrease in VATMRI (r=0.24, P=0.092). In addition, decreases in HOMAadjusted for baseline HOMA were associated with reductions in body weight (r=0.85, P=0.000, Figure 1a), TATMRI (r=0.68, P=0.000, Figure 1b), VATMRI (r=0.39, P=0.005; Figure 1c) and liver fat (r=0.71, P=0.000, Figure 1e). Furthermore, significant correlations were observed between changes in liver fat and decreases in TATMRI (r=0.67) and VATMRI (r=0.73, both P=0.000).
The amount of body weight, TAT and liver fat percentage required to decrease plasma insulin levels by 1 μU/ml is 2.9 kg, 2.0 kg and 1.6%, respectively. Moreover, loss of −6.5 kg weight (5–10%), −5.0 kg TATMRI, −1.2 VATMRI kg and −6 % liver fat was required to decrease HOMAadjusted for baseline HOMA by 1 point.
To test the effect of changes in body composition in response to weight loss on the variance in changes in plasma insulin (dependent variable), we performed a series of stepwise multiple regression analyses. In a first analysis, we included baseline insulin, weight, TATMRI, total SMMRI, age as well as changes in TATMRI and changes in total SMMRI. Baseline insulin explained 82.5% of the variance in changes in insulin. In a second analysis, we added baseline and changes in SATextremities MRI, SATtrunk MRI, VATMRI, SMextremities MRI and SMtrunk MRI and baseline insulin. Baseline insulin and baseline SATextremities MRI explained 82.5% and 2.1% of the variance in changes in insulin, respectively, and changes in SMtrunk MRI explained an additional 1.7%. When baseline and changes in liver fat percentage were included in the second model (except baseline insulin), only baseline liver fat percentage explained 49.7% of the variance in changes in insulin.
We also performed a similar series of stepwise multiple regression analyses to test the effect of changes in body composition in response to weight loss on the variance in changes in HOMA (dependent variable). In a first analysis, we included baseline HOMA, weight, TATMRI, total SMMRI, age as well as changes in TATMRI and changes in total SMMRI. Baseline HOMA and baseline TATMRI explained 93.5% and 1.4% of the variance in changes in HOMA, respectively. In a second analysis, we added baseline and changes in SATextremities MRI, VATMRI, SATtrunk MRI, SMextremities MRI and SMtrunk MRI and baseline HOMA. Baseline HOMA and baseline SATextremities MRI explained 93.5% and 2.1 % of the variance in changes in HOMA, respectively, and changes in SATextremities MRI explained an additional 1.0%. When baseline and changes in liver fat were included in the second model (except baseline HOMA), only changes in liver fat percentage explained 55.1% of the variance in changes in HOMA.
In order to determine the independent association of either VAT loss or liver fat loss with metabolic function, we evaluated groups of participants who differed in liver fat loss ( Figure 2a; n=14, P=0.007) but matched on VAT loss (
Figure 2a; n=14, P=0.007) but matched on VAT loss ( Figure 2a; n=14; P=0.157), or differed in VAT reduction (
Figure 2a; n=14; P=0.157), or differed in VAT reduction ( Figure 2b; n=14; P=0.024) but matched on liver fat loss (
Figure 2b; n=14; P=0.024) but matched on liver fat loss ( Figure 2b; n=14; P=0.794). The results showed that reduction in liver fat rather than decrease in VAT was associated with significant decreases in plasma insulin (
Figure 2b; n=14; P=0.794). The results showed that reduction in liver fat rather than decrease in VAT was associated with significant decreases in plasma insulin ( ) and HOMA (• Figure 2a). In contrast, no differences in plasma insulin loss and HOMA loss were observed between subjects with different VAT loss, matched on liver fat loss (Figure 2b).
) and HOMA (• Figure 2a). In contrast, no differences in plasma insulin loss and HOMA loss were observed between subjects with different VAT loss, matched on liver fat loss (Figure 2b).
(a) Comparison between changes in plasma insulin levels and HOMA between patients with similar amount of visceral adipose tissue reductions (ΔVATMRI) but differing in liver fat changes (Δ liver fat %) and (b) between subjects matched for liver fat reduction (%) but differing in VATMRI changes in a subgroup of 28 subjects.
Changes in SMMRI relative to changes in TATMRI were larger in group 1 (1.2±0.9) with low TAT MRI at baseline compared with those in the others (group 2: 0.1± 0.3; group 3: 0.3±0.2; all P=0.000). In the whole population, changes in SMMRI/TATMRI ratio (ΔSMMRI/ΔTATMRI) were significantly associated with baseline TATMRI. Subjects with low TATMRI at baseline tended to have greater increases in SMMRI/TATMRI ratio.
Discussion
Treating obese patients typically aims to reduce body weight and improve metabolic risks. The associations between loss in individual adipose tissue depots and liver fat and metabolic risk are unclear. Although a 5–10% weight reduction appears to have a beneficial effect on metabolic risk factors and improves the metabolic parameters, there are no cutoff values of different adipose tissue depots reduction (for example, TAT, VAT) or ectopic fat reduction for improving the metabolic disorders. To address this question, we investigated a group of weight loss subjects with a BMI range from 21.6 to 54.4 kg/m2 to determine a target value of weight loss and reduction of individual adipose tissue depots for improving plasma insulin levels and HOMA.
Our results show that the amount of body weight, TATMRI and liver fat percentage required to decrease plasma insulin levels by 1 μU/ml are 2.9 kg, 2.0 kg and 1.6%, respectively, whereas reductions of −6.5 kg (5–10%) body weight, −5.0 kg TATMRI, −1.2 kg VATMRI and −6% liver fat are required to reduce HOMA by 1 point (Figure 1).
Despite the fact that baseline BMI and the magnitude of weight losses differed between groups, significant improvements in risk factors were observed in normal weight, obese and severely obese subjects. Larger weight losses were accompanied with more advanced improvements in insulin sensitivity. Our finding is in line with one study that reported that the odds of clinically significant improvements in most risk factors were even greater in those who lost 10–15% of their body weight.13 A study by Wing et al.34 indicating a weight loss of 4.5 kg at 2 years reduced the risk of developing type 2 diabetes by 30% in diabetic overweight and obese individuals. Su et al.35 have demonstrated that weight loss of 8 kg in moderately obese individuals (mean BMI 30.2±1.0) is accompanied by significant decreases in plasma glucose and insulin concentrations in response to an oral glucose challenge and degree of insulin resistance. Furthermore, Wing et al.36 have shown that modest weight loss of 10 kg in 1 year due to a behavioral weight loss program in patients with type II diabetes leads to reduce fasting blood glucose by 1.6 mmol/l. In this study, loss of 2.9 kg body weight and 2.0 kg TATMRI was required to decrease plasma insulin levels by 1 μU/ml.
In addition, the decreases in individual fat depots and liver fat were associated with significant reduction in plasma insulin and HOMA index. However, the results of correlation analyses revealed that the relationships between liver fat loss and improving metabolic risks were more significant than relationships with adipose tissue depots loss (that is, Pearson’s correlation coefficient reached 0.71 in terms of liver fat loss and HOMA improvement compared with 0.39 for VATMRI). Individual contribution to insulin sensitivity has to be demonstrated owing to the interrelation of fat depots. Therefore, we assessed groups of patients who differed in liver fat loss (%, high or low reduction) but matched on VAT loss (kg) compared with subjects who differed in VAT reduction (kg, high or low reduction) but matched on liver fat loss content. In subjects matched for liver fat loss, a twofold difference in VAT volume between low and high VAT loss groups was not associated with a detectable improvement in insulin sensitivity. In contrast, subjects with high loss of liver fat were accompanied by significant reduction in plasma insulin and HOMA. These results are in agreement with other studies on the role of liver fat rather than VAT in improving hepatic insulin sensitivity.9, 37, 38 Our data demonstrated that liver fat, not VAT, is a better predictor of obesity-related metabolic dysfunction and suggested that association between VAT and metabolic disorders is due to a direct relationship between VAT and liver fat. However, the mechanism responsible for ectopic triglyceride accumulation is still unknown but one hypothesis is that fat accumulation in non-adipose tissues is due to insufficient adipose tissue capacity to store triglycerides.
Study strengths and limitations
Our findings are based on indirect indices of insulin resistance and therefore the results need to be interpreted cautiously. It is unclear whether the underlying assumptions of the HOMA index are fulfilled during caloric restriction. Hence, we have performed the analysis using measured insulin to prevent any assumption regarding the HOMA index. In addition, HOMA index does not provide information about the relative contributions of the liver vs SM to the observed reduction in whole-body insulin sensitivity. However, HOMA index has been shown to be significantly related to whole-body insulin sensitivity measured with the insulin clamp.22, 39 Moreover, these analyses examined the effect of weight loss; but physical activity and fitness have not been addressed, which may be the important independent determinants of weight loss/or changes in risk factor.40 Study strengths included the use of whole-body MRI technology to assess regional changes in individual fat mass and fat-free mass components with weight changes.
Altogether, the present evidence suggests that losses of 6.5 kg (5–10%) body weight, 5.0 kg TATMRI and 6.0% liver fat are considered meaningful markers of weight loss success for medical treatment, which have a positive impact on metabolic risk factors. However, increased amounts of weight loss (>15%) provided even greater benefits. Detailed changes in tissue masses and liver fat add to the explanation for improvement in insulin sensitivity.
References
Douketis JD, Macie C, Thabane L, Williamson DF . Systematic review of long-term weight loss studies in obese adults: clinical significance and applicability to clinical practice. Int J Obes (2005) 2005; 29: 1153–1167.
Diabetes Prevention Program Research G Diabetes Prevention Program Research G, Knowler WC Diabetes Prevention Program Research G, Fowler SE Diabetes Prevention Program Research G, Hamman RF Diabetes Prevention Program Research G, Christophi CA Diabetes Prevention Program Research G, Hoffman HJ et al. 10-Year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009; 374: 1677–1686.
Reaven G . All obese individuals are not created equal: insulin resistance is the major determinant of cardiovascular disease in overweight/obese individuals. Diabetes Vasc Dis Res 2005; 2: 105–112.
Stefan N, Haring HU . The metabolically benign and malignant fatty liver. Diabetes 2011; 60: 2011–2017.
Vega GL, Adams-Huet B, Peshock R, Willett D, Shah B, Grundy SM . Influence of body fat content and distribution on variation in metabolic risk. J Clin Endocrinol Metab 2006; 91: 4459–4466.
Despres JP, Lemieux I . Abdominal obesity and metabolic syndrome. Nature 2006; 444: 881–887.
Korenblat KM, Fabbrini E, Mohammed BS, Klein S . Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology 2008; 134: 1369–1375.
Jakobsen MU, Berentzen T, Sorensen TI, Overvad K . Abdominal obesity and fatty liver. Epidemiol Rev 2007; 29: 77–87.
Fabbrini E, Magkos F, Mohammed BS, Pietka T, Abumrad NA, Patterson BW et al. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci USA 2009; 106: 15430–15435.
Hwang JH, Stein DT, Barzilai N, Cui MH, Tonelli J, Kishore P et al. Increased intrahepatic triglyceride is associated with peripheral insulin resistance: in vivo MR imaging and spectroscopy studies. Am J Physiol Endocrinol Metab 2007; 293: E1663–E1669.
Pourhassan M, Bosy-Westphal A, Schautz B, Braun W, Gluer CC, Muller MJ . Impact of body composition during weight change on resting energy expenditure and homeostasis model assessment index in overweight nonsmoking adults. Am J Clin Nutr 2014; 99: 779–791.
Bosy-Westphal A, Kossel E, Goele K, Blocker T, Lagerpusch M, Later W et al. Association of pericardial fat with liver fat and insulin sensitivity after diet-induced weight loss in overweight women. Obesity (Silver Spring, MD) 2010; 18: 2111–2117.
Wing RR, Lang W, Wadden TA, Safford M, Knowler WC, Bertoni AG et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care 2011; 34: 1481–1486.
Wing RR, Jeffery RW . Effect of modest weight loss on changes in cardiovascular risk factors: are there differences between men and women or between weight loss and maintenance? Int J Obes Related Metab Disorders 1995; 19: 67–73.
Hamman RF, Wing RR, Edelstein SL, Lachin JM, Bray GA, Delahanty L et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care 2006; 29: 2102–2107.
Erdmann J, Kallabis B, Oppel U, Sypchenko O, Wagenpfeil S, Schusdziarra V . Development of hyperinsulinemia and insulin resistance during the early stage of weight gain. Am J Physiol Endocrinol Metab 2008; 294: E568–E575.
Matthaei S, Stumvoll M, Kellerer M, Haring HU . Pathophysiology and pharmacological treatment of insulin resistance. Endocr Rev 2000; 21: 585–618.
Abdul-Ghani MA, Matsuda M, Balas B, DeFronzo RA . Muscle and liver insulin resistance indexes derived from the oral glucose tolerance test. Diabetes Care 2007; 30: 89–94.
DeFronzo RA, Tobin JD, Andres R . Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979; 237: E214–E223.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC . Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–419.
Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 2000; 85: 2402–2410.
Lagerpusch M, Bosy-Westphal A, Kehden B, Peters A, Muller MJ . Effects of brief perturbations in energy balance on indices of glucose homeostasis in healthy lean men. Int J Obes (2005) 2012; 36: 1094–1101.
Lagerpusch M, Enderle J, Later W, Eggeling B, Pape D, Muller MJ et al. Impact of glycaemic index and dietary fibre on insulin sensitivity during the refeeding phase of a weight cycle in young healthy men. Br J Nutr 2013; 109: 1606–1616.
Bosy-Westphal A, Kossel E, Goele K, Later W, Hitze B, Settler U et al. Contribution of individual organ mass loss to weight loss-associated decline in resting energy expenditure. Am J Clin Nutr 2009; 90: 993–1001.
Bosy-Westphal A, Schautz B, Lagerpusch M, Pourhassan M, Braun W, Goele K et al. Effect of weight loss and regain on adipose tissue distribution, composition of lean mass and resting energy expenditure in young overweight and obese adults. Int J Obes (2005) 2013; 37: 1371–1377.
Stevens J, Truesdale KP, McClain JE, Cai J . The definition of weight maintenance. Int J Obes (2005) 2006; 30: 391–399.
Pourhassan M, Schautz B, Braun W, Gluer CC, Bosy-Westphal A, Muller MJ . Impact of body-composition methodology on the composition of weight loss and weight gain. Eur J Clin Nutr 2013; 67: 446–454.
Pourhassan M, Eggeling B, Schautz B, Johannsen M, Kiosz D, Gluer CC et al. Relationship between submaximal oxygen uptake, detailed body composition, and resting energy expenditure in overweight subjects. Am J Hum Biol 2014; 27: 397–406.
FA D . Physical Properties of Tissue. Academic: New York, NY, USA, 1990.
Snyder WS CM, Nasset ES, Karhausen LR, Howells GP, Tipton IH . Report of the Task Group on Reference Man. Pergamon Press: Oxford, UK, 1975.
Lagerpusch M, Enderle J, Eggeling B, Braun W, Johannsen M, Pape D et al. Carbohydrate quality and quantity affect glucose and lipid metabolism during weight regain in healthy men. J Nutr 2013; 143: 1593–1601.
Schneider CA, Rasband WS, Eliceiri KW . NIH Image to ImageJ: 25 years of image analysis. Nat Methods 2012; 9: 671–675.
Wing RR, Venditti E, Jakicic JM, Polley BA, Lang W . Lifestyle intervention in overweight individuals with a family history of diabetes. Diabetes Care 1998; 21: 350–359.
Su HY, Sheu WH, Chin HM, Jeng CY, Chen YD, Reaven GM . Effect of weight loss on blood pressure and insulin resistance in normotensive and hypertensive obese individuals. Am J Hypertension 1995; 8: 1067–1071.
Wing RR, Koeske R, Epstein LH, Nowalk MP, Gooding W, Becker D . Long-term effects of modest weight loss in type II diabetic patients. Arch Int Med 1987; 147: 1749–1753.
Kantartzis K, Machann J, Schick F, Fritsche A, Haring HU, Stefan N . The impact of liver fat vs visceral fat in determining categories of prediabetes. Diabetologia 2010; 53: 882–889.
Muller MJ, Lagerpusch M, Enderle J, Schautz B, Heller M, Bosy-Westphal A . Beyond the body mass index: tracking body composition in the pathogenesis of obesity and the metabolic syndrome. Obes Rev 2012; 13 (Suppl 2), 6–13.
Retnakaran R, Shen S, Hanley AJ, Vuksan V, Hamilton JK, Zinman B . Hyperbolic relationship between insulin secretion and sensitivity on oral glucose tolerance test. Obesity (Silver Spring, MD) 2008; 16: 1901–1907.
Kyle UG, Zhang FF, Morabia A, Pichard C . Longitudinal study of body composition changes associated with weight change and physical activity. Nutrition 2006; 22: 1103–1111.
Acknowledgements
We thank Britta Jux, Klinik fur Radiologische Diagnostik, University Hospital Schleswig Holstein Kiel, for help with MRI scanning. The study was funded by Deutsche Forschungsgemeinschaft (DFG Mü 714/ 8-3) BMBF Kompetenznetz Adipositas, Core domain 'Body composition' (Kö rperzusammensetzung; FKZ 01GI1125).
Author contributions
MP and MJM designed the study; MP did the segmentations and analyzed the data; C-CG was responsible for MRI examinations; PP and WT are surgeons and are responsible for recruitments and treatments of severely obese patients undergoing bariatric surgery; MP and MJM wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Pourhassan, M., Glüer, CC., Pick, P. et al. Impact of weight loss-associated changes in detailed body composition as assessed by whole-body MRI on plasma insulin levels and homeostatis model assessment index. Eur J Clin Nutr 71, 212–218 (2017). https://doi.org/10.1038/ejcn.2016.189
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2016.189
- Springer Nature Limited
This article is cited by
-
How Does Fat Mass Change in the First Year After Bariatric Surgery? A Systemic Review and Meta-Analysis
Obesity Surgery (2021)
-
Differential unfolded protein response in skeletal muscle from non-diabetic glucose tolerant or intolerant patients with obesity before and after bariatric surgery
Acta Diabetologica (2020)
-
Changes in Bile Acid Metabolism, Transport, and Signaling as Central Drivers for Metabolic Improvements After Bariatric Surgery
Current Obesity Reports (2019)
-
Weight Loss and Changes in Adipose Tissue and Skeletal Muscle Volume after Laparoscopic Sleeve Gastrectomy and Roux-en-Y Gastric Bypass: a Prospective Study with 12-Month Follow-Up
Obesity Surgery (2019)