Abstract
The present study assessed the ability of optogenetics techniques to provide a better understanding of the control of insulin secretion, particularly regarding pancreatic β-cell function in homeostasis and pathological conditions such as diabetes mellitus (DM). We used optogenetics to investigate whether insulin secretion and blood glucose homeostasis could be controlled by regulating intracellular calcium ion concentrations ([Ca2+]i) in a mouse pancreatic β-cell line (MIN6) transfected with the optogenetic protein channelrhodopsin-2 (ChR2). The ChR2-transfected MIN6 (ChR2-MIN6) cells secreted insulin following irradiation with a laser (470 nm). The increase in [Ca2+]i was accompanied by elevated levels of messenger RNAs that encode calcium/calmodulin-dependent protein kinase II delta and adenylate cyclase 1. ChR2-MIN6 cells suspended in matrigel were inoculated into streptozotocin-induced diabetic mice that were then subjected to a glucose tolerance test. Laser irradiation of these mice caused a significant decrease in blood glucose, and the irradiated implanted cells expressed insulin. These findings demonstrate the power of optogenetics to precisely and efficiently controlled insulin secretion by pancreatic β-cells ‘on demand', in contrast to techniques using growth factors or chemical inducers. Optogenetic technology shows great promise for understanding the mechanisms of glucose homeostasis and for developing treatments for metabolic diseases such as DM.
Similar content being viewed by others
Introduction
Over 300 million people worldwide suffer from diabetes mellitus (DM) that is caused by either insulin insufficiency (type 1 diabetes, T1D) or insulin resistance (T2D).1, 2 T1D and T2D, which account for 5–10% and 90–95% of patients with diabetes, respectively, are caused by defects in the secretion (T1D) or response to insulin (T2D) or both (T2D).2 Insulin is produced by pancreatic β-cells, and the pathogenesis of T1D involves their destruction or dysfunction.3 Although insulin sensitivity is impaired in T2D, the disease progresses to insulin insufficiency as pancreatic β-cells fail to produce insulin at levels required to regulate glucose metabolism.3, 4 Because of the critical role of pancreatic β-cells in DM, they are the focus of intensive research.4, 5, 6
Insulin injection, which is the predominant treatment for diabetes, is incapable of replenishing the lost insulin-producing β-cell in patients. Restoring β-cell mass through replacement therapy such as islet transplantation or β-cell regeneration has attracted particular attention because of its potential to cure diabetes. Gene therapy and stem cell therapy have been tested as alternatives to islet transplantation in various preclinical animal models to improve the longevity and function of human islets against posttransplantation challenges. However, they are limited by a shortage of pancreas donors and the requirement for lifelong immunosuppression, which carries adverse side effects and can compromise the survival of transplanted tissue.7 The goal of gene therapy of DM is to produce a cell that processes, stores and secretes insulin and maintains normal glucose tolerance in response to fluctuating blood glucose concentrations. Thus, over the past decade, there have been several attempts to generate ‘artificial’ β-cells that produce insulin in response to glucose in a regulated manner.8 The production of a functional ‘artificial’ β-cell via genetic manipulation requires a comprehensive understanding pancreatic developmental.
Optogenetics techniques employ light-sensitive proteins that are introduced into cells using molecular genetic manipulations that, for example, target specific cell types.9, 10 These genetically modified cells are stimulated by light to influence cellular behaviors. Membrane depolarization of neurons induces transient electrical signals (spikes or action potentials) that ultimately convey information between neurons within the same circuit. For neurons to be artificially depolarized by light, they are engineered to express photoreceptor proteins that alter the ion permeability of membranes in response to light. The first photoreceptor used as a ‘switch’ to control neuronal activity was the Chlamydomonas protein channelrhodopsin-2 (ChR2), a cation-selective channel that permits entry of Na+ and Ca2+ ions in response to blue light (470 nm).11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 ChR2, an algal phototaxis receptor that depolarizes the plasma membrane when exposed to light,22 acts as a light-gated cation channel when expressed in animal cells.23 Among studies, Nagel et al.23 demonstrated that various cell types that express ChR2 are depolarized when they are simply irradiated with the appropriate wavelength of laser light. For example, when ChR2 is expressed in a neuron exposed to blue light (470 nm), the channel immediately depolarizes the neuron, triggering a spike.
In pioneering studies, Boyden et al.24 used ChR2 to reliably control neuronal spiking on millisecond timescales to control excitatory and inhibitory synaptic transmissions even at the level of single spikes and synaptic events. Li et al. developed systems for millisecond- to second-scale control of light activation of neurons using vertebrate rat rhodopsin 4 and ChR2 that permits precise and reversible antagonistic control of neuronal function in cultured neurons and intact spinal cords.25 Antagonistic control of neural activation is achieved using alternative light-activated proteins with different wavelength sensitivities and ion permeabilities. For example, the chloride pump halorhodopsin hyperpolarizes neurons in response to yellow light (590 nm), in contrast to ChR, which depolarizes cells irradiated with blue light (470 nm).10
Irradiation methods (such as those using optical fibers) optimized for in vivo contexts combined with transgenic technologies have enabled ChR2-mediated optogenetics to leap out of the culture dish into live organisms. For example, Arenkiel et al.26 expressed a transgene encoding a ChR2-YFP fusion protein in the central nervous system of mice and exploited the in vivo activation of neurons to map neural circuits involved in olfaction. Their data suggest that olfactory processing in mice depends on the convergence of mitral cells with the olfactory cortex and subsequent integration by cortical cells.
The utility of optogenetics is not limited to experimental manipulations, but can be applied to potentially significant therapeutic applications. Promising opportunities for the clinical use of optogenetic methods are available, perhaps unsurprisingly in the visual system, where ChR2’s original function as a photosensitive pigment is most relevant. When ChR2 is introduced into second-order neurons (ON bipolar cells) in the rd1 mouse model of retinal degeneration, the resultant photosensitive cells stimulate light-evoked responses in the retinal ganglion and visual cortex.27 Likewise, the introduction of ChR2 into retinal ganglion cells restores vision to genetically blind rats.28 The results of these studies raise the possibility of using ChR2 for gene therapy of certain forms of congenital or acquired blindness in humans.
Two very recent studies contribute further compelling evidence of the potential value of optogenetic therapeutics.29, 30 Cheng et al.29 selectively stimulated neurons expressing ChR2 in stroke mice and found that mechanisms associated with plasticity are enhanced and are associated with improved recovery. Moreover, their studies represent the first demonstration that recovery from stroke is enhanced using optogenetic techniques. Bryson et al.30 used motor neurons derived from mouse embryonic stem cells that express ChR2 to engraft branches of the sciatic nerve of adult mice that were partially denervated. Optogenetic stimulation not only controlled restoration of nerve function but also enabled the engrafted motor neurons to reinnervate lower hind-limb muscles.
The goal of the present study was to assess the ability of optogenetics techniques to provide a better understanding of the control of insulin secretion, particularly regarding pancreatic β-cell function in homeostasis and pathological conditions such as DM. Glucose-stimulated insulin secretion by pancreatic β-cells consists of a transient phase followed by a sustained phase. These two phases correspond to ‘triggering' and ‘amplifying’ pathways, respectively.31, 32 In the former, the increase in cytosolic ATP concentration via mitochondrial glucose metabolism leads to plasma membrane depolarization mediated by closure of the ATP-sensitive potassium channel. Depolarization induces the opening of L-type voltage-dependent Ca2+ channels that mediate Ca2+ entry and subsequent insulin secretion by fusion of secretory vesicles with the plasma membrane. Glucose activates the metabolic amplifying pathway that is independent of ATP-sensitive potassium channel channels or further increases intracellular Ca2+ concentrations ([Ca2+]i), which augment insulin secretion in response to the Ca2+ signal.33
Ca2+ acts as a second messenger that mediates diverse cellular functions such as exocytosis, cell migration, cardiac contractility, platelet aggregation and the formation of memory.34 Elevated levels of [Ca2+]i induce Ca2+ influx through voltage-dependent Ca2+ channels, Ca2+ mobilization from internal Ca2+ stores and activation of Ca2+-activated non-selective cation channels facilitates exocytosis. In contrast, stimuli that elevate Ca2+, such as high levels of KCl or glucose, increase [Ca2+]i as well as [cAMP]i in pancreatic β-cells.35
To determine whether insulin secretion and blood glucose homeostasis can be controlled by regulating [Ca2+]i, we took advantage of optogenetics techniques to study these processes in vitro in a cell line (MIN6) derived from a mouse insulinoma that faithfully reflects the phenotype of pancreatic β-cells, including insulin secretion in response to glucose and other secretagogues. We further evaluated optogenetic regulation of glucose homeostasis using a mouse model of diabetes. We show here that ‘on demand’ laser irradiation of ChR2 ectopically expressed in MIN6 cells induced insulin expression through elevation of [Ca2+]i and that laser irradiation of diabetic mice implanted with these cells ameliorated hypoglycemic.
Results
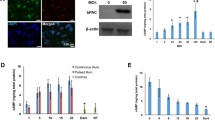
To assess the value of optogenetic techniques in understanding the mechanisms of pathogenesis of diseases with a major impact on society, such as DM, we transfected MIN6 cells, which display the phenotype of insulin-secreting pancreatic β-cells, with an expression vector encoding ChR2 (Figure 1a). ChR2-transfected MIN6 (ChR2-MIN6) cells were irradiated using a nanosecond-pulsed laser (470 nm) with 50 μJ intensity for each of the time intervals in the no-glucose culture medium, and samples of the medium harvested at each time were assayed for insulin. Insulin secretion was highest when the cells were irradiated for 20 s (Figure 1b). Lengthier irradiation decreased insulin concentrations, likely because the cells were damaged by the laser irradiation. The same result was obtained when the glucose concentration was 1000 mg l−1 in the culture medium (Supplementary Figure 1a). However, the effect of laser irradiation was not observed and the basal insulin secretion was increased when the glucose concentration was 4500 mg l−1 in the culture medium because glucose metabolic turnover and insulin secretion responded to glucose concentration in the culture medium could be enhanced before (Supplementary Figure 1b). In addition, insulin secretion was not enhanced by the laser irradiation in the presence of Ca2+ cannel blocker, mibefradil dihydrochloride (Supplementary Figure 1c). This result indicates that the insulin secretion by the laser irradiation to ChR2-MIN6 cells are really mediated by Ca2+ influx.
(a) The EYFP—ChR2 fusion protein was stably expressed in the MIN6 cell membrane. The nucleus was stained with DAPI (blue). (bar=50 μm) (b) Analysis of insulin secretion in cultures of irradiated ChR2-MIN6 cells. ChR2-MIN6 cells were cultured overnight in DMEM containing 1000 mg l−1 of glucose and incubated for 1 h without glucose. Cells were exposed to laser irradiation for the indicated times and incubated further for 1 h. The concentration of insulin was then measured using an ELISA kit described in methods. *P<0.005 compared with unirradiated cells (n=12). (c) Analysis of mitochondrial activity in cultures of (i) irradiated and (ii) unirradiated ChR2-MIN6 cells. *P<0.005 compared with unirradiated cells (n=4). (d) Analysis of ATP levels in cultures of (i) irradiated and (ii) unirradiated ChR2-MIN6 cells. *P<0.005 compared with unirradiated cells (n=4). (e) Whole-cell currents (pA) were measured during the indicated irradiation times. Irradiation times are indicated by the blue lines. (f) Effect of laser irradiation on the [Ca2+]i of ChR2-MIN6 cells. ChR2-MIN6 cells were washed once with PBS and loaded with the calcium indicator Fluo-4-AM in the dark for 15 min in glucose-free medium. The fluorescence intensities of cells irradiated for 10 s (upper line) and unirradiated cells (lower dotted line) were measured at the indicated times. The data are expressed as the fluorescence intensity greater than baseline divided by the baseline intensity (F/F0). (g) Effect of laser irradiation on the levels of (i) Camk2d and (ii) Adcy1 mRNAs. The levels of Camk2d and Adcy1 mRNAs at the indicated times were determined using real-time RT-PCR. *P<0.05 compared with unirradiated cells (n=6).
To determine the mechanism of the enhancement of insulin secretion by irradiated ChR2-MIN6 cells, we used an MTT assay to assess the level of mitochondrial activity as a function of exposure time (Figure 1c). Mitochondrial activity was unaffected when cells were irradiated for as long as 30 s and decreased by a factor of ~0.25 after they were irradiated for 60 s. These data indicate that the optogenetic control of insulin secretion was independent of the activation of mitochondrial activity and therefore independent of the glycolytic pathway. Because irradiation times longer than 60 s damaged the cells, optogenetic manipulations must be carefully optimized. There were no significant differences in ATP level as a function of exposure time (Figure 1d), indicating that the pathways that control insulin secretion were independent of the glycolytic pathway. The level of ATP decreased significantly after irradiation of the ChR2-MIN6 cells for 60 s, consistent with irradiation-induced damage to the cells. ATP levels did not change during the times when insulin secretion by ChR2-MIN6 cells increased, which supports the conclusion that insulin secretion was not mediated by the glycolytic pathway. These data are consistent with those of the mitochondrial activity assays described above.
Taken together, the analyses of mitochondrial activity and ATP levels during the first 30 s of irradiation of ChR2-MIN6 cells described above suggest that insulin secretion was induced directly by ChR2-mediated Ca2+ rather than through the glucose transporter and that ChR2-mediated Ca2+ entry into the cells during irradiation. To support this conclusion, we performed patch clamp analysis of irradiated ChR2-MIN6 cells (Figure 1e). The cells were depolarized while irradiated, indicating uptake of Ca+2. We next analyzed Ca+2 influx using Fluo-4-AM and found that a 10 s pulse of laser light increased [Ca2+]i by a factor of ~2 compared with unirradiated cells (Figure 1f). This increase in [Ca2+]i persisted for the duration of the experiment (140 s). Similar results were obtained when the cells were irradiated with the laser for 20 or 30 s.
We next analyzed the levels of messenger RNAs (mRNAs) encoding calcium/calmodulin-dependent protein kinase II delta (Camk2δ)36 and adenylate cyclase 137 to determine whether the light-induced increase in [Ca2+]i influenced calcium signalling in ChR2-MIN6 cells. Laser irradiation of ChR2-MIN6 cells increased the levels of CamK2δ and adenylate cyclase 1 (Figure 1g) mRNAs by factors of ~1.5 and 2, respectively, compared with unirradiated cells.
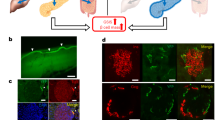
The results of the preceding experiments indicate the potential of optogenetics to treat type I diabetes (T1D) by potently regulating glucose homeostasis. Therefore, we used streptozotocin to induce T1D in mice, engrafted the diabetic mice with optogenetic ChR2-MIN6 cells, and assessed the status of glucose homeostasis after the implanted cells were irradiated with laser light delivered using a fiber-optic cable. We then subjected the mice to glucose tolerance tests. The blood glucose levels of unirradiated mice increased to a peak level of 26.8 mM after 30 min (Figure 2a). The most marked reductions in glucose concentrations induced by laser irradiation were detected in mice implanted with ChR2-MIN6 cells 30 min after the mice were irradiated for 10 and 20 s (18.6 and 19.1 mM, respectively).
(a) Effect of laser irradiation on glucose levels of normal or diabetic mice implanted with ChR2-MIN6 cells. ChR2-MIN6 cells suspended in matrigel were injected subcutaneously into the backs of mice with normal or STZ-induced diabetes mice, which were fasted for 16 h and then subjected to a glucose tolerance test. Immediately thereafter, the mice were irradiated for 0 s (closed circles), 5 s (open circles), 10 s (closed triangles), 20 s (open triangles) and 30 s (closed squares), and blood glucose levels were then determined at the indicated intervals for 120 min. *P<0.05, **P<0.01, ***P<0.001 compared with unirradiated mice (n=5–6). (b) Analysis of insulin expression and apoptosis in mice implanted with ChR2-MIN6 cells. Implanted ChR2-MIN6 cells were excised from STZ-induced diabetes mice irradiated for the indicated times and subjected to H&E staining, immunohistochemical analysis of insulin expression, and TUNEL analysis to assess apoptosis. Insulin-positive cells stained brown, and TUNEL staining was undetectable. As a positive control for TUNEL staining, ChR2-MIN6 cells were exposed to UV light for 1 h in PBS (pH 7.4) before implantation (bar=100 μm).
To determine whether the decrease in blood glucose levels of the implanted, irradiated mice was associated with insulin secretion, the ChR2-MIN6 cells were excised from the implants and subjected to immunohistochemical staining to detect insulin (Figure 2b). Unirradiated cells did not express detectable levels of insulin. A small number of insulin-positive cells were detected in mice irradiated for 5 s, and most cells from mice irradiated from 10–30 s expressed insulin. To determine the contribution, if any, of apoptosis to insulin release by ChR2-MIN6 cells, we performed TUNEL assays of laser-irradiated cells. Apoptotic cells were not detected in control or irradiated cells (Figure 2b). These findings show that optogenetics techniques provide a better understanding of pancreatic β-cell function regulated by [Ca2+]i in homeostasis and pathological conditions, and taken together with the results of glucose tolerance tests, support the conclusion that optogenetic techniques maintain glucose homeostasis in vivo and show promise for translation to the clinic.
Discussion
The present study demonstrates the value of optogenetic techniques that employ laser irradiation to understand the biochemical mechanisms underlying the pathogenesis of complex metabolic diseases such as DM. We were specifically interested in whether insulin secretion and blood glucose homeostasis can be controlled by ‘on demand’ regulation of [Ca2+]i and took advantage of in vitro and in vivo models to analyze insulin secretion by pancreatic β-cells. We stably expressed ChR2 in MIN6 cells that exhibit the phenotype of pancreatic β-cells and demonstrated that the transfected cells secreted insulin when irradiated with 470 nm laser light. The results of assays for mitochondrial activity and ATP levels indicate a direct role for ChR2 in the release of insulin which is analogous to that of P/Q- and L-type Ca2+ channels, in contrast to a mechanism involving glucose metabolism that raises the ATP/ADP ratio (Figure 3).38, 39 Moreover, using optimized conditions, we found that laser irradiation depolarized ChR2-MIN6 cells, allowing entry of Ca2+. The increase in [Ca2+]i was supported by findings of elevated levels of mRNAs that encode proteins involved in Ca2+ metabolism, such as CamK2δ and adenylate cyclase 1.
Illustration of possible optogenetic mechanism of insulin secretion from ChR2-MIN6 cells. (a) The increase in cytosolic ATP concentration via mitochondrial glucose metabolism leads to plasma membrane depolarization mediated by closure of the ATP-sensitive potassium channel (K(ATP)) channel. Depolarization induces the opening of L-type voltage-dependent Ca2+ channels (VDCCs) that mediate Ca2+ entry and subsequent insulin secretion by fusion of secretory vesicles with the plasma membrane. (b) Mitochondrial activity and ATP levels did not change when insulin secretion by ChR2-MIN6 cells increased, which supports the conclusion that insulin secretion was not mediated by the glycolytic pathway. These results indicate a direct role for ChR2 in the release of insulin, in contrast to a mechanism involving glucose metabolism that raises the ATP/ADP ratio.
We were able to show here that it is possible to regulate glucose metabolism in vivo using optogenetic techniques. For this purpose, we suspended ChR2-MIN6 cells in matrigel and inoculated them into mice with STZ-induced diabetic mice that were then irradiated and subjected to a glucose tolerance test. Irradiation induced a significant and sustained decrease in the concentration of blood glucose. Moreover, the implanted cells that expressed insulin in response to laser irradiation did not undergo apoptosis, which eliminated the possibility of artifactual insulin release. The present study complements a recent study on transgenic mice that express ChR2 controlled by the insulin promoter, and our present data are consistent with its findings that increased Ca2+-influx induced by light-stimulation triggers insulin secretion independent of glucose metabolism.40 This new experimental model makes it possible to perform highly sensitive analyses of β-cells that are precisely stimulated at intervals of milliseconds. Such measurements are not technically possible using intact islets. Thus, Reinbothe et al. observed rapid increases in [Ca2+]i when the islets of ChR2+/−Rip-Cre+/− mice were irradiated using blue light generated by a light-emitting diode device.40 Further, low and intermediate, but not high, glucose concentrations increase insulin secretion by these islets but do not affect the release of glucagon. When diabetes is induced by feeding mice a high-fat diet, Ca2+ influx increases significantly (3.5-fold) in β-cells. The increased release of insulin by these mice in the presence of high glucose levels indicates that there is compensatory potentiation of the Ca2+ response in β-cells.
In addition to those described above, other studies have demonstrated the enormous potential of optogenetics to enhance our understanding of homeostasis and pathogenesis, and show promise for translation to the clinic.41 In particular, an optogenetic transcription device has been shown to enhance blood glucose homeostasis in mice.42 Moreover, optogenetic techniques induce insulin secretion by pancreatic β-cells only when cells are irradiated ‘on demand’, and their ability to precisely control glucose metabolism may make them more advantageous for treating DM than growth factors or chemicals. Taken together with these studies, our present results further demonstrate the potential of optogenetic technology in understanding the mechanisms of insulin secretion, as well as in developing treatments for complex metabolic diseases such as DM.
Materials and methods
Cells
The MIN6 cell line (kindly provided by Professor Miyazaki, Osaka University, Japan) was derived from a mouse insulinoma43 and is one of a few cell lines that display the phenotypes of pancreatic β-cells, such as insulin secretion induced by glucose and other secretagogues.44 MIN6 cells were grown in Dulbecco’s modified eagle’s medium (DMEM) with 4500 mg l−1 glucose (Sigma-Aldrich, St Louis, MO, USA) containing 10% fetal calf serum (FCS).
Generation of cells stably transfected with ChR2
MIN6 cells were transfected with pcDNA3.1 ChR2-EYFP20 (Addgene Inc., Cambridge, MA, USA) using a Nucleofector II System (Lonza Japan, Tokyo, Japan), and transfected cells were selected by culturing them in DMEM containing 10% FCS and 500 μg ml−1 G418 (Sigma-Aldrich). The stably transfected cell line was designated ChR2-MIN6.
Laser irradiation
Cells plated at a density of 1 × 105 cells per well in 96-well Black with Clear Bottom 96-well Microtest Optilux Plates (BD Bioscience, Inc., San Jose, CA, USA) to protect them from light were cultured for 24 h in DMEM containing 1000 mg l−1 glucose and 10% FCS. The medium was changed to DMEM with 10% FCS but without glucose 1 h before laser irradiation. Cells were irradiated using an optical fiber attached to the bottom of the culture plate. The position of culture plate was controlled with an automated positioning stage (Sigma Koki, Co., Ltd, Tokyo, Japan), and the cells were irradiated for 0, 5, 10, 20, 30 and 60 s using an integrated single compact housing a diode pumped Q-switched laser and a no-gap wavelength tuning OPO system (NT242, EKSPLA, Vilnius, Lithuania) as follows: 470 nm, 50 μJ, 1000 Hz pulse repetition rate, 3–6 ns pulse duration. The cells attached to the bottom of culture plate were uniformly irradiated with the laser. After irradiation, the cells were incubated at 37 °C in an atmosphere containing 5% CO2.
Analysis of insulin secretion
One hour after laser irradiation, culture supernatants were collected and centrifuged for 5 min. The insulin concentrations of the supernatants were measured using an ELISA Kit (Shibayagi, Co., Ltd, Gunma, Japan) for mouse insulin.
Assay of mitochondrial activity and ATP
Independent cell preparations were analyzed using MTT assays before and after laser irradiation using a Cell Counting Reagent SF kit (Nacalai Tesque, Inc., Kyoto, Japan), and ATP levels were determined using a luciferase assay kit (Wako Pure Chemical Industries, Ltd, Osaka, Japan) according to the manufacturer’s instructions.
Patch clamp analysis
Measurements of whole-cell currents in ChR2-MIN6 cells were performed using an automated patch clamp platform with a chip size of 5 MV and an ECP10 amplifier (HEKA, Lambrecht, Germany). Cells were irradiated using an optical fiber attached to the cell stage. The laser intensity was adjusted as described above.
Measurements of intracellular calcium
ChR2-MIN6 cells were washed once with phosphate-buffered saline (PBS) pH 7.4, resuspended in DMEM without FCS or glucose and loaded with the calcium indicator Fluo-4-AM (Sigma-Aldrich) (10 μM final concentration) at 37 °C for 15 min in the dark. The treated cells were recorded using a BZ-9000 fluorescence microscope (Keyence corporation, Osaka, Japan) equipped with a 10 × objective. Immediately after laser irradiation, changes in fluorescence intensity indicating intracellular Ca2+ levels were recorded, analyzed using ImageJ software (National Institutes of Health, Bethesda, MD, USA), and quantified as the change in fluorescence intensity before irradiation above baseline divided by the baseline fluorescence intensity (F/F0).
Real-time PCR
Total cellular RNA was extracted from ChR2-MIN6 cells (0, 5, 10, 20, 30 and 60 s after laser irradiation) using an RNeasy MINI kit (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions. All samples were treated with DNase, followed by reverse transcription using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Ltd, Tokyo, Japan). The levels of the mRNAs encoding indicators of intracellular calcium metabolism Camk2δ and adenylate cyclase 1 were quantified using real-time reverse transcription-PCR. Reactions were carried out using a 7500 Real-Time PCR System (Applied Biosystems Ltd) with a TaqMan gene expression assay (Applied Biosystems Ltd) according to the manufacturer’s instructions. Purified complementary was used to generate standard curves. The efficiency of the primer sets was determined to confirm that the dilution of the samples did not affect the reactions. The level of each mRNA was normalized to the amount of mouse β-actin mRNA in each sample.
In vivo experiments
Female nude mice (BALB/c-nu/nu) 7 weeks of age were obtained from Nihon SLC Co. Ltd (Shizuoka, Japan). Diabetes was induced in mice using streptozotocin, a compound that is preferentially toxic to pancreatic β-cells. The STZ solution was administered intraperitoneally (200 mg kg−1). After 5 days, mice were fasted for 16 h and analyzed for blood glucose levels. In the present study, we used mice with a fasting blood glucose concentration >16 mM. All animal procedures were performed according to protocols approved by the National Defense Medical College Committee for Animal Use (approval number 12037).
ChR2-MIN6 cells were cultured for 24 h in DMEM containing 1000 mg l−1 glucose and 10% FCS. Cells were collected using accutase and suspended in matrigel (growth-factor reduced, phenol red-free; Corning Incorporated, Tewksbury, MA, USA) on ice. The cells (1 × 105) were suspended in 300 μl of matrigel and injected subcutaneously into the back of each normal and diabetic mouse, and the mice were then fasted for 16 h.
To test glucose tolerance, glucose solution (1.5 g kg−1) was injected intraperitoneally immediately before the mice were irradiated with the laser. The mice were lightly anaesthetized using diethyl ether and irradiated using the optical fiber inserted into the implanted site. Long-term diethyl ether exposure elevates blood glucose levels; however, no changes were observed in control experiments (no-glucose solution injection, no laser irradiation). The mice were irradiated for 0, 10, 20 and 30 s. Blood glucose levels of blood taken from the tail vein 0, 30, 60, 90 and 120 min after laser irradiation were determined using a glucometer (EIDIA Co., Ltd, Tokyo, Japan). Implanted matrigel implants were extirpated after experiments, fixed in 5% formalin solution for pathological analysis.
Statistical analysis
Data are presented as the mean±s.d. of the mean. Statistical significance (defined as P-values of <0.01 or 0.05 vs non-laser-irradiated group) was evaluated using the unpaired Student's t-test (two-tailed).
References
Guo JH, Chen H, Ruan YC, Zhang XL, Zhang XH, Fok KL et al. Glucose-induced electrical activities and insulin secretion in pancreatic islet beta-cells are modulated by CFTR. Nat Commun 2014; 5: 4420.
American Diabetes A. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014; 37 (Suppl 1): S81–S90.
National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. Centres for Disease Control and Prevention, US Department of Health and Human Services, 2014.
Ashcroft FM, Rorsman P . Diabetes mellitus and the beta cell: the last ten years. Cell 2012; 148: 1160–1171.
Vetere A, Choudhary A, Burns SM, Wagner BK . Targeting the pancreatic beta-cell to treat diabetes. Nat Rev Drug Discov 2014; 13: 278–289.
Newby BN, Terada N, Mathews CE . In search of a surrogate: engineering human beta cell lines for therapy. Trends Endocrinol Metab 2014; 25: 378–380.
Marzorati S, Melzi R, Citro A, Cantarelli E, Mercalli A, Scavini M et al. Engraftment versus immunosuppression: cost-benefit analysis of immunosuppression after intrahepatic murine islet transplantation. Transplantation 2014; 97: 1019–1026.
Gerace D, Martiniello-Wilks R, O'Brien BA, Simpson AM . The use of beta-cell transcription factors in engineering artificial beta cells from non-pancreatic tissue. Gene Ther 2014; 22: 1–8.
Deisseroth K . Optogenetics. Nat Methods 2011; 8: 26–29.
Pastrana E . Optogenetics: controlling cell function with light. Nat Methods 2010; 8: 24–25.
Aravanis AM . An optical neural inteface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J Neural Eng 2007; 4: S143–S156.
Arenkiel BR . In vivo light-induced activation of neural circuitry in transgenic mice expressing channelrhodopsin-2. Neuron 2007; 54: 205–218.
Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K . Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci 2005; 8: 1263–1268.
Han X, Boyden ES . Multiple-color optical activation, silencing and desynchronization of neural activity with single-spike temporal resolution. PloS One 2007; 2: e299.
Ishizuka T, Kakuda M, Araki R, Yawo H . Kinetic evaluation of photosensitivity in genetically engineered neurons expressing green algae light-gated channels. Neurosci Res 2006; 54: 85–94.
Nagel G . Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses. Curr Biol 2005; 15: 2279–2284.
Petreanu L, Huber D, Sobczyk A, Svoboda K . Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections. Nat Neurosci 2007; 10: 663–668.
Schroll C . Light-induced activation of distinct modulatory neurons triggers appetitive or aversive learning in Drosophila larvae. Curr Biol 2006; 16: 1741–1747.
Wang H . High-speed mapping of synaptic connectivity using photostimulation in Channelrhodopsin-2 transgenic mice. Proc Natl Acad Sci USA 2007; 104: 8143–8148.
Zhang F . Multimodal fast optical interrogation of neural circuitry. Nature 2007; 446: 633–639.
Zhang F, Wang LP, Boyden ES, Deisseroth K . Channelrhodopsin-2 and optical control of excitable cells. Nat Methods 2006; 3: 785–792.
Sineshchekov OA, Jung KH, Spudich JL . Two rhodopsins mediate phototaxis to low- and high-intensity light in Chlamydomonas reinhardtii. Proc Natl Acad Sci US A 2002; 99: 8689–8694.
Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P et al. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc Natl Acad Sci USA 2003; 100: 13940–13945.
Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K . Millisecond-timescale genetically targeted optical control of neural activity. Nat Neurosci 2005; 8: 1263–1268.
Li X, Gutierrez DV, Hanson MG, Han J, Mark MD, Chiel H et al. Fast noninvasive activation and inhibition of neural and network activity by vertebrate rhodopsin and green algae channelrhodopsin. Proc Natl Acad Sci USA 2005; 102: 17816–17821.
Arenkiel BR, Peca J, Davison IG, Feliciano C, Deisseroth K, Augustine GJ et al. In vivo light-induced activation of neural circuitry in transgenic mice expressing channelrhodopsin-2. Neuron 2007; 54: 205–218.
Lagali PS, Balya D, Awatramani GB, Munch TA, Kim DS, Busskamp V et al. Light-activated channels targeted to ON bipolar cells restore visual function in retinal degeneration. Nat Neurosci 2008; 11: 667–675.
Tomita H, Sugano E, Isago H, Tamai M . Channelrhodopsins provide a breakthrough insight into strategies for curing blindness. J Genet 2009; 88: 409–415.
Cheng MY, Wang EH, Woodson WJ, Wang S, Sun G, Lee AG et al. Optogenetic neuronal stimulation promotes functional recovery after stroke. Proc Natl Acad Sci USA 2014; 111: 12913–12918.
Bryson JB, Machado CB, Crossley M, Stevenson D, Bros-Facer V, Burrone J et al. Optical control of muscle function by transplantation of stem cell-derived motor neurons in mice. Science 2014; 344: 94–97.
Henquin JC . Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes 2000; 49: 1751–1760.
Rorsman P, Eliasson L, Renstrom E, Gromada J, Barg S, Gopel S . The Cell Physiology of Biphasic Insulin Secretion. News Physiol Sci 2000; 15: 72–77.
Gembal M, Gilon P, Henquin JC . Evidence that glucose can control insulin release independently from its action on ATP-sensitive K+ channels in mouse B cells. J Clin Invest 1992; 89: 1288–1295.
Borodinsky LN, Spitzer NC . Second messenger pas de deux: the coordinated dance between calcium and cAMP. Sci STKE 2006; 2006: pe22.
Seino S, Shibasaki T . PKA-dependent and PKA-independent pathways for cAMP-regulated exocytosis. Physiol Rev 2005; 85: 1303–1342.
Mohlig M, Wolter S, Mayer P, Lang J, Osterhoff M, Horn PA et al. Insulinoma cells contain an isoform of Ca2+/calmodulin-dependent protein kinase II delta associated with insulin secretion vesicles. Endocrinology 1997; 138: 2577–2584.
Kitaguchi T, Oya M, Wada Y, Tsuboi T, Miyawaki A . Extracellular calcium influx activates adenylate cyclase 1 and potentiates insulin secretion in MIN6 cells. Biochem J 2013; 450: 365–373.
Rorsman P, Braun M, Zhang Q . Regulation of calcium in pancreatic alpha- and beta-cells in health and disease. Cell Calcium 2012; 51: 300–308.
Braun M, Ramracheya R, Bengtsson M, Zhang Q, Karanauskaite J, Partridge C et al. Voltage-gated ion channels in human pancreatic beta-cells: electrophysiological characterization and role in insulin secretion. Diabetes 2008; 57: 1618–1628.
Reinbothe TM, Safi F, Axelsson AS, Mollet IG, Rosengren AH . Optogenetic control of insulin secretion in intact pancreatic islets with beta-cell-specific expression of Channelrhodopsin-2. Islets 2014; 6: e28095.
Kushibiki T, Okawa S, Hirasawa T, Ishihara M . Optogenetics: novel tools for controlling mammalian cell functions with light. Int J Photoenergy 2014; 2014: 1–10.
Ye H, Daoud-El Baba M, Peng RW, Fussenegger M . A synthetic optogenetic transcription device enhances blood-glucose homeostasis in mice. Science 2011; 332: 1565–1568.
Miyazaki J, Araki K, Yamato E, Ikegami H, Asano T, Shibasaki Y et al. Establishment of a pancreatic beta cell line that retains glucose-inducible insulin secretion: special reference to expression of glucose transporter isoforms. Endocrinology 1990; 127: 126–132.
Ishihara H, Asano T, Tsukuda K, Katagiri H, Inukai K, Anai M et al. Pancreatic beta cell line MIN6 exhibits characteristics of glucose metabolism and glucose-stimulated insulin secretion similar to those of normal islets. Diabetologia 1993; 36: 1139–1145.
Acknowledgements
This work was supported by The Ministry of Education, Culture, Sports, Science and Technology (MEXT) Grant-in-Aid Project for Scientific Research on Innovative Areas 'Crosstalk between transcriptional control and energy pathways, mediated by hub metabolites' (26116728) and Japan Society for the Promotion of Science (JSPS) Grant-in-Aid for Young Scientists (A) (25713009).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Gene Therapy website
Supplementary information
Rights and permissions
About this article
Cite this article
Kushibiki, T., Okawa, S., Hirasawa, T. et al. Optogenetic control of insulin secretion by pancreatic β-cells in vitro and in vivo. Gene Ther 22, 553–559 (2015). https://doi.org/10.1038/gt.2015.23
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2015.23
- Springer Nature Limited
This article is cited by
-
Optogenetic Approach in Trigeminal Neuralgia and Potential Concerns: Preclinical Insights
Molecular Neurobiology (2023)
-
Optogenetic regulation of insulin secretion in pancreatic β-cells
Scientific Reports (2017)
-
Shining Light on the Sprout of Life: Optogenetics Applications in Stem Cell Research and Therapy
The Journal of Membrane Biology (2016)