Abstract
In light of the emerging diabetes epidemic, new experimental approaches in islet research are needed to elucidate the mechanisms behind pancreatic islet dysfunction and to facilitate the development of more effective therapies. Optogenetics has created numerous new experimental tools enabling us to gain insights into processes little was known about before. The spatial and temporal precision that it can achieve is also attractive for studying the cells of the pancreatic islet and we set out to explore the possibilities of this technology for our purposes. We here describe how to use the islets of an “optogenetic beta-cell” mouse line in islet batch incubations and Ca2+ imaging experiments. This protocol enables light-induced insulin release and provides an all-optical solution to control and measure intracellular Ca2+ levels in pancreatic beta-cells. The technique is easy to set up and provides a useful tool for controlling the activity of distinct islet cell populations.
Access provided by CONRICYT – Journals CONACYT. Download protocol PDF
Similar content being viewed by others
Key words
1 Introduction
Optogenetics has lead to groundbreaking discoveries in medical science [1] and the number of related articles being published per year has grown exponentially over the last 5 years. However, its main advantage, the cell-specific and spatiotemporally precise control of distinct cell subpopulations, has to date been mainly exploited in the field of neuroscience [2]. Although the brain is central to the regulation of energy homeostasis and metabolism [3], and optogenetics has also contributed to advances in this area of research [4], the sole tissue that controls the release of the key blood glucose-regulating hormones are the islets of Langerhans . Their two main cell-types , the beta- and alpha-cells are responsible for maintaining glucose homeostasis by releasing insulin and glucagon, respectively. Under normal circumstances, insulin is released from beta-cells upon increased blood glucose levels while glucagon is secreted when the glucose concentration falls below a critical point during fasting. In patients suffering from type-2 diabetes , the counter-regulatory pattern of the two hormones is greatly perturbed, with a lack of insulin and an excess of glucagon both leading to hyperglycemia, the main hallmark of the disease [5]. The pathways leading to the opposing effects of these two hormones, under the same pathophysiological stimuli , are incompletely understood [6]. To experimentally isolate externally induced glucose-dependent effects from their intrinsic (basal) activity, islets are often routinely isolated from rodents and tested for their in vitro secretory capacity in batch incubations. This approach however does neither allow for the precise stimulation of one distinct cell population within the islet, let alone one cell, nor to swiftly manipulate the stimulus, i.e. both the spatial and temporal precision is greatly limited. To overcome this, electrophysiological (“patch-clamping”) measurements are traditionally performed. However, this requires the islets to be dissociated into single cells with the consequent loss of physiologically important interactions inherent to their architecture. Alternatively, the (electrical) activity of individual cells can also be assessed in intact islets [7], but this allows measurement of only one (peripheral) cell at the time and is both a very difficult and time-consuming undertaking.
The prospect to control both the activity of a complete islet cell subpopulation and to adjust the stimulatory pattern on a millisecond time scale encouraged us to explore the suitability of optogenetics for islet studies, that we recently reported [8]. In the following, we describe this approach in detail to allow other researchers in the field to take advantage of this possibility.
2 Materials
2.1 Buffers and Solutions
Buffers should be prepared with double-deionized water (ddH2O) and can be stored at room temperature unless otherwise specified.
-
1.
1 M Tris-HCl: Weigh 60.6 g Tris Base into ddH2O, adjust pH to 8 with HCl and the volume to 500 ml, autoclave.
-
2.
1 M d-Glucose: Add 9 g of d-glucose to 25 ml ddH2O and adjust to a final volume of 50 ml while stirring. Sterile filter by passing the solution through a 0.2 μm filter. Store at 4°C.
-
3.
1 M NaOH: Add 2 g of NaOH to 30 ml ddH2O, adjust to 50 ml and sterile filter.
Caution: Corrosive—may damage skin and eyes. Wear safety equipment!
-
4.
50 mM NaOH: Prepare 100 ml by dilution with ddH2O from 1 M NaOH (see Subheading 3).
Caution: Corrosive—may damage skin and eyes. Wear safety equipment!
-
5.
Hank’s buffer salt solution (HBSS): Dissolve a vial of Hank’s buffer salt in ddH2O, add 0.35 g NaHCO3, adjust to 1 l, if necessary adjust pH to 7.4 with 1 M NaOH, keep at 4 °C.
-
6.
13.3 U/ml collagenase type V stock (Sigma): Add 10.5 ml HBSS to 100 mg collagenase. Mix well. Store at −20 °C as 1 ml aliquots.
-
7.
0.1 M NaHCO3: Prepare a solution of 0.1 M NaHCO3 by adding 0.42 g of NaHCO3 to a final volume of 50 ml ddH2O and adjust pH to 8.
-
8.
Islet medium: RPMI 1640 supplemented with 4 mM l-Glutamine, 10 mM glucose, 5 % FBS, 100 μg/ml streptomycin and 100 units/ml penicillin.
-
9.
Krebs-Ringer’s bicarbonate buffer (KRBB): 120 mM NaCl (7.01 g), 4.7 mM KCl (0.35 g), 1.2 mM KH2PO4 (0.16 g), 1.2 mM MgSO4 (0.14 g), 25 mM NaHCO3 (2.1 g), 2.5 mM CaCl2 (0.28 g), 10 mM HEPES (2.38 g) in ddH2O, adjust pH to 7.4 with NaOH and volume to 1 l. Store at 4 °C for a maximum of 3 days. Add 0.1 % [w/v] BSA (1 g) and 1 M d-glucose to the desired concentration on the day of use. Waterbath and carbogen gas (95 % O2 and 5 % CO2) are needed for final preparation. See Note 1 .
-
10.
Calcium imaging buffer 1: 140 mM NaCl (8.18 g), 3.6 mM KCl (0.35 g), 0.5 mM NaH2PO4 (0.06 g), 0.5 mM MgSO4 (0.06 g), 2 mM NaHCO3 (0.17 g), 2.5 mM CaCl2 (0.28 g), 5 mM HEPES (1.19 g), adjust pH to 7.4 with NaOH, adjust volume to 1 l, and add d-glucose to desired concentration freshly.
-
11.
Calcium imaging buffer 2 (High potassium buffer): Same as above but with 50 mM KCl (3.73 g for 1 l) and 93.6 mM NaCl (5.47 g) instead of 140 mM NaCl.
2.2 Animals and Genotyping
Important: For animal breeding and experiments, national animal welfare regulations and guidelines must be followed and ethical permission be granted prior to commencement.
-
1.
Mouse strain #012569 (The Jackson Laboratory, Bar Harbor, ME, USA).
-
2.
Mouse strain #003573 (The Jackson Laboratory).
-
3.
Mouse ear puncher.
-
4.
Table-top centrifuge.
-
5.
RipCre PCR: Forward primer 5′TGC CAC GAC CAA GTG3′ and reverse primer 5′CAA GGT TAC GGA TAT3′ in 10 mM mix (each).
-
6.
ChR2-YFP PCR: Wild-type allele primer forward 5′AAG GGA GCT GCA GTG GAG TA3′ and reverse 5′CCG AAA ATC TGT GGG AAG TC3′; for the transgenic allele forward/reverse primers 5′ACA TGG TCC TGC TGG AGT TC3′ and 5′GGC ATT AAA GCA GCG TAT CC3′ in 10 mM mix.
-
7.
Standard Taq polymerase.
-
8.
Hot Start polymerase.
2.3 Islet Preparation and Culture
-
1.
Noncoated plastic petri dishes 35 × 10 mm.
-
2.
Autoclavable glass or nonstick plastic petri dishes 60 × 10 mm.
-
3.
Centrifugation tubes PS 11 ml for washing islets.
-
4.
Waterbath, instruments for mouse surgery, dissection microscope.
-
5.
Picking microscope and dimmable light source.
-
6.
Petri dishes 35 mm with No. 1 thickness (0.13–16 mm) glass bottom.
-
7.
Cell-Tak (BD Biosciences, Franklin Lakes, NJ, USA).
-
8.
Bovine serum albumin (BSA).
-
9.
Humidified incubator 37 °C, 5 % CO2.
2.4 Batch Incubation LED Illuminations
-
1.
Ellerman plastic tubes 55 × 11 mm and 11 mm plug caps.
-
2.
Pointy forceps e.g. Dumont #3C or needle.
-
3.
Light-emitting diodes (LED) 470.
-
4.
Laboratory power supply such as Easy Power PS2000B (Elektro-Automatik GmbH, Germany).
-
5.
Plastic housing or an empty pipette tip box.
-
6.
Resistors 160 Ω.
-
7.
Highly flexible twin cable 0.75 mm2.
-
8.
Banana plugs 4 mm.
-
9.
Silicone and superglue.
-
10.
Soldering equipment, fine file, small hand saw with fine teeth.
-
11.
No. 1 cover glasses 18 × 18 mm.
-
12.
Optical power meter such as PM100D with S120C Photodiode (Thorlabs Sweden AB, Göteborg, Sweden).
-
13.
BNC Female to RCA Male Adapter.
2.5 Ca2+ Sensitive Dyes
-
1.
Leak-resistant Ca2+ sensitive Fura-2 (Teflabs, Blackdog Technical Services Inc., Jackson Springs, NC, USA) or other suitable dye. See Note 2 .
-
To make a stock dissolve a 50 μg vial of leak-resistant Fura-2 in 50 μl DMSO (1μg/μl, 0.8 mM stock). Store in 10 μl aliquots at −20 °C. Each aliquot is sufficient for loading islets in four glass-bottom dishes. Once thawed, do not refreeze.
-
2.6 Ca2+ Imaging Setup
-
1.
Inverted epifluorescence microscope and illumination /detection system with trigger out (TTL signal) port.
-
2.
Fiber-coupled blue LED (WT&T, Pierrefonds, QC, Canada) with an attached miniature collimator (WT&T).
-
3.
YFP and Fura-2 excitation and emission filterset. See Note 2 .
-
4.
Micromanipulator system with head stage holder as used for patch clamp (e.g. MHW-3, Narishige, London, UK) to hold the fiber-coupled LED. See Note 3 .
-
5.
Perfusion pump allowing bi-directional (in/out) fine-tuning of the flow.
-
6.
Heated perfusion tube and temperature control system (Alascience, NY, USA) with temperature control sensor mounted on the imaging chamber perfusion inlet to sense the buffer temperature in real-time.
3 Methods
3.1 Mouse Breeding and Genotyping
To leverage cellular activation by blue light-induced depolarization, the ChR-2(H134R) variant is readily available as part of a (Cre-dependent) CAG promoter driven loxP-STOP-loxP-ChR2(H134R)YFP transgene in a mouse line [9] distributed by The Jackson Laboratory (Strain no. 012569). To achieve expression specifically in beta-cells, breed mice with Ins2Cre (“RipCre”) mice or an alternative cell-specific Cre-driver line. We used an in-house colony, originally created in the Herrera lab [10] but The Jackson Laboratory holds strain numbers 018960 and 003573 for purchase that may be used instead. The presence of Cre recombinase in beta-cells causes the excision of the STOP cassette from the CAG-loxP-STOP-loxP-ChR2(H134R)YFP transgene, leading to cell-specific expression of the transgene (“ChR2-YFP” in the following). All offspring must be genotyped. See Note 4 .
-
1.
At weaning take mouse ear biopsies.
-
2.
Add 300 μl 50 mM NaOH to each biopsy.
-
3.
Incubate for 1 h at 98 °C, vortex and return to heat for 30 min.
-
4.
Vortex again and neutralize by adding 30 μl 1 M Tris-HCl to each biopsy, vortex.
-
5.
Centrifuge for 10 min at >10,000 × g to precipitate remaining tissue.
-
6.
Transfer the supernatant to a new tube.
-
7.
Use 1 μl of the supernatant in the PCR reaction and 10 mM primers.
-
8.
For RipCre PCR, with Taq polymerase, use an annealing temperature of 55 °C and 30 cycles following the polymerase manufacturer’s instructions.
-
9.
For ChR2-YFP PCR use Hot Start polymerase with an annealing temperature of 62 °C and 35 cycles following the polymerase manufacturer’s instructions.
-
10.
Run 10 μl of the products on a 1.5–2 % agarose gel and expect bands of 250 bp for the ChR2-YFP transgene , 300 bp for the WT allele, and a band of 750 bp for RipCre (see Fig. 1).
3.2 Islet Preparations
-
1.
Prepare collagenase working solution after thawing an aliquot on ice and add 9 ml HBSS, which is sufficient for three mice.
-
2.
Prepare mouse islets by collagenase injection into the common bile duct according to established protocols [11, 12] with the following amendments:
-
After incubation of the pancreas at 37 °C, add 30 ml ice-cold HBSS to stop the reaction, shake vigorously for >30 s to dissociate the tissue, and immediately pour 10 ml of the homogenized organ into 11 ml PS centrifugation tubes placed on ice.
-
Instead of centrifugation to remove exocrine tissue, let the islets sink down in the tubes for 4 min and carefully remove the top 9 ml of buffer containing mostly exocrine tissue by pipetting or vacuum. Add 9 ml HBSS, invert the tube several times and incubate again. Repeat 3–5 times until the top buffer appears clear at the end of the 4 min incubation. Handpick several times to remove all exocrine cells and collect islets in a petri dish with HBSS on ice. To limit direct light exposure of the transgenic islets, dim the picking microscope illumination system to the minimum while still enabling comfortable vision. See Notes 5 and 6 .
-
3.3 Preparing Dishes for Whole Islet Imaging
During the collagenase incubation (see Subheading 3.2) coat glass-bottom dishes under a biological work cabinet as follows:
-
1.
To prepare one dish, add 57 μl of 0.1 mM NaHCO3 to the dish, add 2 μl of Cell-Tak, mix by pipetting, add 1 μl of 1 M NaOH (see Subheading 3.2, step 1) and mix once again. Scale up as necessary. Critical: Work swiftly; after adding NaOH, Cell-Tak may start binding to the reaction tube.
-
2.
Incubate for a minimum of 20 min.
-
3.
Remove the solution by vacuum or pipetting and wash 2× with 500 μl ddH2O.
-
4.
Let the dishes dry and use directly or wrap with Parafilm and store at 4 °C for max. 1 week until use.
-
5.
Add 2.5 ml RPMI 1640 medium to each dish and continue work under the picking microscope. Collect >20 islets from the collection petri dish on ice in a volume not exceeding 20 μl and place them carefully close together in the center of the glass-bottom dish. Critical: To avoid that islets stick to the inside of the pipette tip, rinse it in medium by pipetting prior to use.
-
6.
When placing the islets, lift the lid of the dish containing the medium only minimally to avoid contamination. Ideally use a microscope mounted under a biological work cabinet.
-
7.
Place all dishes into a larger 15 cm dish or tray to even out eventual horizontal shaking during transport.
-
8.
Incubate overnight in a humidified incubator at 37 °C, 5 % CO2 to let islets attach properly. Open and close incubator doors carefully to avoid vibrations.
3.4 Preparing a LED Illumination System for Islet Batch Incubations
Caution: If you do not have experience with building and soldering on electrical systems, please consult an electrician and/or your local lab workshop. Exposed electrical connections and mistakes during soldering could lead to fire and serious injury. All soldered unprotected leads must be insulated using electrical tape or by heat shrink tubing.
-
1.
Cut or file small openings into the sides of the plastic housing to accommodate the cables.
-
2.
Cut twin cable into 50 cm long pieces, separate the leads slightly on each end and remove about 1 cm of insulation from both ends.
-
3.
Put drops of silicone into the housing, 3–4 cm apart, two for each resistor.
-
4.
Trim the resistors to length leaving about 2 cm on each end.
-
5.
Place each resistor into a pair of silicone drops and place the resistors parallel to each other. Leave about 1.5 cm space on each side and leave about 1 cm space between each resistor. Let the silicone solidify.
-
6.
Solder one lead of each twin cable to one end of the resistor (−), trim the other end in length and solder to a common pole connecting all secondary poles of the resistor to (+) (see Fig. 2). Connect (+) and (−) leads to each one of the banana plugs.
-
7.
Close the housing properly with screws.
-
8.
Pinch two holes (2.5 mm apart) into the tube caps and push the LEDs through the holes. See Fig. 3.
-
9.
To hold them in place, put tubes into a rack, attach the caps, and fill the open side of the caps with silicone and let solidify overnight. Critical: The LEDs should face straight downwards to deliver the light on to the islets.
-
10.
Put heat shrink tubing sleeves around the unconnected twin cables originating from the plastic housing before they are soldered to the LEDs. The longer leg of the LED marks (+), the shorter one (−). After soldering, heat up the heat shrink tube to properly isolate the exposed electrical contacts.
-
11.
Testing the illumination system: Cut off the round bottom of an Ellerman plastic tube using a small saw and glue the even (noncut) end of the tube to a No. 1 cover glass.
-
12.
Place a cap with LED on the tube and press it in while holding the tube. Placing it on the table while pressing may break the glass.
-
13.
Switch on the power meter and set it to the appropriate wavelength . Switch on the power source driving the LEDs and set it to 6 V. At this setting the voltage at the LEDs will measure 3.2 V. See Note 7 .
-
14.
Place the tube with attached coverslip onto the power meter, note the intensity, and check that it is identical for all the LEDs.
-
15.
Mark the light spot outer diameter with a pen while illuminating on a piece of paper. Switch off the LED and use a scale to measure the diameter and to calculate intensity per area.
3.5 In Vitro Batch Islet Stimulations with LED Light
Caution: Do not look directly into the beam of power LEDs as the bright light may damage the eyes.
-
1.
Freshly prepare KRBB with 2.8 and 20.2 mM d-glucose, bubble for 2 min with carbogen gas (95 % O2, 5 % CO2) using a glass Pasteur pipette and add BSA to 0.1 % [w/v]. Let the BSA sink in for 5 min and mix by turning the tube upside down several times. Keep at RT for the duration of the experiment and discard any leftover.
-
2.
Place 1 ml KRBB with basal glucose concentration (2.8 mM) into Ellerman plastic tubes (see Subheading 2.3) in appropriate racks and place 12 islets into each tube.
-
3.
Pick islets under a picking microscope using a 10 μl pipette and place them centrally at the bottom of the tubes.
-
4.
Gas each tube with carbogen to place a 95 % O2, 5 % CO2 atmosphere above the buffer and quickly close the tube with the cap (without LED). Do not gas the solution.
-
5.
Place the rack into a pre-warmed 37 °C waterbath and pre-incubate for 30 min. See Note 8 .
-
6.
Remove the rack from the waterbath, remove the caps, slowly aspirate 0.8 ml of the solution while observing the islets under the picking microscope.
-
7.
To half of the tubes add 0.8 ml KRBB with 2.8 mM glucose to serve as reference, to the rest add 0.8 ml KRBB containing 20.2 mM glucose (giving a final concentration of 16.7 mM glucose). Critical: Remove and add the buffer slowly (add towards the wall of the tubes) to minimize turbulence.
-
8.
Overlay the solution in each tube with carbogen (see Subheading 4) and immediately close with caps containing the LEDs (or without LED as control) to maintain the 95 % O2, 5 % CO2 atmosphere.
-
9.
Put the rack into the waterbath and incubate for 1 h while stimulating with blue light. Critical: Place control nonilluminated tubes in a separate rack protected by aluminum foil to prevent any blue light reaching the control islets.
-
10.
Switch on the power supply and set to 6 V. This will deliver the maximum of 3.2 V to the LEDs. See Note 9 .
-
11.
Disconnect the power source, remove the rack from the waterbath, remove the caps, and while looking against the light slowly sample 400 μl buffer from each tube by using the lower marked line on the tubes as reference. Critical: The islets should not move.
-
12.
Analyze for hormones directly using ELISA or RIA or freeze the samples.
3.6 Ca2+ Indicator Loading of Islets
Important: Work in low light.
-
1.
Thaw a vial of leak resistant Fura-2 stock and bring to room temperature.
-
2.
Adjust Calcium imaging buffer 1 freshly to 2.8 mM glucose. The volume of buffer needed depends on the duration of the experiment and perfusion speed (e.g. 1 ml/min). Also prepare Calcium imaging buffer 1 with 16.7 mM glucose and Calcium imaging buffer 2 with 2.8 mM glucose, both needed as positive controls.
-
3.
Fura-2 working solution: Dissolve 2.5 μl of 0.8 mM Fura-2 stock in 1 ml of Calcium imaging buffer 1 supplemented with 2.8 mM glucose (final concentration 2 μM Fura-2). Vortex to mix thoroughly.
-
4.
Remove the medium from the side of the plate without emptying the center glass bottom. Wash once with 2 ml of 2.8 mM glucose Calcium imaging buffer 1 by carefully pipetting the buffer to the side of the dish and removing it slowly. Add the 1 ml of prepared Fura-2 to the side of the dish and incubate for 40 min at 37 °C protected from light.
-
5.
Remove the Fura-2 incubation buffer from the side of the dish without emptying the center glass bottom. Wash once with 2 ml of 2.8 mM glucose calcium imaging buffer 1. Add 2 ml of fresh buffer without Fura-2 to de-esterify. Incubate for at least 30 min at 37 °C prior to imaging.
3.7 Preparing for the Light Stimulation and Ca2+ Imaging
Caution: Do not look directly at or through the microscope into the beam of power LEDs as the bright light may damage the eyes.
Important: Do not bend the fiber.
An inexpensive way to stimulate the islets directly on an imaging microscope without the need to purchase an additional mirror-based illumination system operating through the side ports of the microscope is to use a fiber-coupled LED.
Before starting the experiment, the illuminating LED has to be calibrated to deliver the desired power to the biological specimen. This is achieved by detecting the emitted light intensity using a (handheld or mounted) power meter at the imaging plane and by measuring the size of the light spot. Since you must not align the LED while looking into the microscope or while the islets are mounted, as this will already stimulate them, this is done in advance. The instructions given below describe the procedure for an inverted microscope.
-
1.
Fasten the fiber-coupled LED to a mounting post (6 mm diameter and 7 cm length) using tape. Attach the post directly to the micromanipulator holder of the headstage. You may need to thicken the post with tape to make it tightly fit into the holder. See Fig. 4 and Note 3 .
-
2.
Mark a glass bottom dish with a dot in the center using a waterproof marker and place it on the microscope stage.
-
3.
Check under the microscope that the marked dot is directly in the visual center corresponding to the central point of where images are acquired. You may also check in live mode using the camera.
-
4.
Remove the center plate of the microscope stage, maximally wind down the objective and place the power meter probe in the theoretical specimen mounting position/focal plane. Switch on the LED, measure the light intensity, and adjust the power of the LED to desired value. Mark the power knob position for later reference. Switch off the LED.
-
5.
Switch the mirror to the acquisition (camera) position or otherwise block the beampath to the eyepiece.
-
6.
Switch on the fiber-coupled LED and direct it onto the marked center of the glass bottom dish while observing from a lateral position. Lower the intensity and do not look directly into the beam. Fix the LED in this position. Mark the outer diameter of the beam with a fine waterproof marker on the dish. Switch the LED power source to remote control mode and now change neither the LED’s position nor the position of the microscope stage. Connect the LED remote control port to the trigger box (TTL out) of the microscope acquisition system. You will eventually need a BNC Female to RCA Male Adapter.
3.8 Islet Perfusion and Temperature Control
-
1.
Carefully place the plastic perfusion insert (see Fig. 4) into the glass-bottom dish containing the dye-loaded islets.
-
2.
Place the dish on the microscope stage, switch mirror to the visual (eyepiece) position and position the islets in the visual center by moving the dish. Use low transmitted light to visualize. Try to get several islets into the field of view. Critical: Do not move the microscope stage, as this will also change the position of the LED meaning that you will have to re-calibrate the LED’s position.
-
3.
Place the perfusion in- and outlet nozzles into the grooves of the insert. Begin perfusion with 2.8 mM Calcium imaging buffer 1 at a rate ~1 ml/min.
-
4.
Switch on the perfusion pump and temperature control system for the tube heating element. Make sure the temperature sensor is in contact with the buffer.
-
5.
Verify that the temperature is stable at a value below 37 °C to avoid accidental overheating of the islets due to temperature fluctuations. Critical: Whenever switching off the perfusion, turn off the tube-heating element first.
-
6.
Perfuse for 5–10 min until temperature and buffer levels have stabilized.
Critical: The temperature and buffer level must be stable during the experiment.
3.9 Image Acquisition and Light Stimulation
-
1.
Verify a positive YFP signal in your transgenic mouse islets and absence in the control islets. You may actually place and image them simultaneously in the same dish.
-
2.
Take a test image of the islets using the Fura-2 filter set with illumination at 340 and 380 nm settings. Take note of the ideal exposure times and keep them constant for the whole set of experiments.
-
3.
Within the acquisition software create a light trigger protocol [3.5–5 V TTL signal out] in order to remote control the fiber-coupled LED for the desired duration and frequency. Start with between 1 and 10 ms. Take into account the exposure time in order not to stimulate the islets simultaneously with image acquisition. This prevents recording the light emitted by the blue LED light. See Notes 2 and 9 .
-
4.
Mark regions for analysis: Select a background region and regions of interest (ROI) covering one or several islets or parts thereof. Save the region selection.
-
5.
Towards the end of the light stimulation protocol perfuse with Calcium imaging buffer 1 (high glucose) or 2 (high potassium) serving as positive controls. See Note 10 .
3.10 Calibration
Absolute Ca2+ concentrations can be calculated after calibration with cells (islets) and ionophores such as ionomycin or A23187, cell-free using a dilution series of buffers with known Ca2+ concentration or both in combination. Commercially available (Fura-2) Ca2+ imaging calibration kits may be a convenient alternative. The absolute Ca2+ concentration can then be inferred by using the calculations as in [13].
3.11 Data Analysis
The data analysis features of imaging acquisition software differ widely and this is only a short example for processing the data.
Open the data file produced during export from the acquisition software using spreadsheet software. Subtract the background values and calculate the 340/380 nm ratio for each ROI using the following formula: Ratio340/380 (R 340/380) = (Mean intensity340 − Mean intensity Background340)/(Mean intensity380 − Mean intensity background380). Plot against time. To determine the position and intensity of the peak of each calcium oscillation, calculate the % difference in ratio (R 340/380) for each successive pair of points: Percent increase = (R i+1 − R i )/(100 × R i ).
4 Notes
-
1.
As an alternative buffer to KRBB (see Subheading 2.1, item 9), Krebs Ringer’s HEPES buffer (KRBH) with 2 mM NaHCO3 and 25 mM HEPES [14] may be used, not requiring gassing with carbogen. Results may differ.
-
2.
Using a red-shifted calcium indicator instead of Fura-2 (see Subheading 3.6, step 1) and an additional narrowing bandpass emission filter (e.g. FB460-10-12.5-SP and FC/PC collimation package F230FC-aligned to 460 nm, both Thorlabs) for the blue LED (see Subheading 2.6, item 2) may allow for islet LED stimulation and simultaneous, rather than sequential or post-stimulus [Ca2+] i signal recording. However, depending on technical (the imaging system being used, its sensitivity, etc.), and biological influences (dye-loading success, etc.), already the easy-to-build solution presented above, allows for high time resolution (comparable to electrophysiological recordings) as the TTL signal switch, stimulatory illumination (1–10 ms) and image acquisition (10–100 ms) are all happening within 10–100 ms; fast enough to (sequentially) record changes in [Ca2+] i and perhaps membrane potential. That said, to use the [Ca2+] i as physiological readout is only an example. Fluorescent or near-infrared voltage-sensitive dyes may also be a possibility.
-
3.
As alternative to the micromanipulator system holding the LED during Ca2+ imaging (see Subheading 3.7, step 1), miniature mounting posts and clamps (both available from e.g. Thorlabs) can be used to build a stand small enough to fit onto the microscope stage without interfering with its use (see Fig. 5).
-
4.
In your breedings (see Subheading 3.1), keep animals hemizygous for both transgenes as animals that are both homozygous ChR2-YFP and hemizygous for RipCre (ChR2-YFP+/+RipCre+/−) exhibit lower weight gain and may not be fertile [own observations], and should thus not be used. If the original strains have different genetic backgrounds, as is the case for the Herrera lab RipCre strain and the used ChR2-YFP strain (Jax strain no. 012569), mice should be inbred for several generations in order to produce genetically identical animals. For more information on colony management see elsewhere [15].
-
5.
For islets that are to be used in microscopy, handpick islets in HBSS without adding BSA (see Subheading 3.2, step 2). Although BSA may increase yield [16] and facilitates easier and faster picking, it reduces the likelihood of the islets to attach to the glass-bottom dishes. Pick in sterilized glass petri dishes or plastic dishes with anti-stick surface to avoid attachment of islets during the process. You may also rinse the dish surface you pick on shortly in HBSS with BSA while proceeding with actually picking in HBSS without BSA.
-
6.
Although animals have been genotyped, the presence of YFP in the ChR2-YFP islets (beta-cells) should be verified by fluorescence microscopy (see Fig. 1a). Use an YFP or a GFP filter set, the latter should work as good.
-
7.
The voltage applied to the LEDs (see Subheading 3.4, step 13) should be initially tested for by using a multimeter.
-
8.
Instead of incubating the islets in the waterbath during the batch incubations (see Subheading 3.5, step 5), a humidified 37 °C, 5 % CO2 incubator may be used instead but the temperature equilibration is considerably slower and results may thus differ. The wiring length of the illumination system (see Subheading 3.4, step 2) must also be amended accordingly.
-
9.
The optionally available software for many the laboratory power sources allows for remote control of the LEDs. This feature can be used to create pulsed-light protocols rather than using constant illumination (see Subheading 3.5, step 10 and Subheading 3.9, step 2 and Fig. 6). However, the minimum duration is 500 ms long for the PS2000B used here, so you may consider alternative devices that allow more flexible settings.
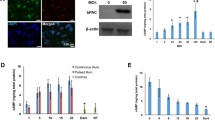
Fig. 6 (a) Example trace of changes in Fura-2 fluorescence ratio (ΔF 340/380) in ChR2-YFP expressing islets stimulated with 500 ms at 1 Hz for different durations (60, 30, and 10 s) with pauses of the same duration in between. Experiment performed at 2.8 mM glucose. (b) Constant illumination for 1 h (LED) compared to control (Ctrl) and effects on glucose-induced insulin release at different glucose concentrations (G, in mM). Adapted from [8]
-
10.
Controlling for the islets to behave normally is essential (see Subheading 3.9, step 4). Healthy islets do exhibit Ca2+ oscillations when stimulated with high glucose [17]. Using high potassium allows estimating if the acquisition settings are within a dynamic range.
-
11.
To more precisely assess Ca2+ signals within single cells of the islet while allowing for stimulation of single cells out of the subpopulation rather than the whole population (see Subheading 3.9, step 4), a confocal laser-scanning microscope to image single cells [18] with combined fluorescence recovery after photobleaching (FRAP) to stimulate them may also be an option, although with a considerably higher price tag than the system presented here.
References
Chow BY, Boyden ES (2013) Optogenetics and translational medicine. Sci Transl Med 5(177):177ps175. doi:10.1126/scitranslmed.3003101
Fenno L, Yizhar O, Deisseroth K (2011) The development and application of optogenetics. Annu Rev Neurosci 34:389–412. doi:10.1146/annurev-neuro-061010-113817
Blouet C, Schwartz GJ (2010) Hypothalamic nutrient sensing in the control of energy homeostasis. Behav Brain Res 209(1):1–12. doi:10.1016/j.bbr.2009.12.024
Kong D, Tong Q, Ye C et al (2012) GABAergic RIP-Cre neurons in the arcuate nucleus selectively regulate energy expenditure. Cell 151(3):645–657. doi:10.1016/j.cell.2012.09.020
Rorsman P, Braun M, Zhang Q (2012) Regulation of calcium in pancreatic alpha- and beta-cells in health and disease. Cell Calcium 51(3-4):300–308. doi:10.1016/j.ceca.2011.11.006
Gromada J, Franklin I, Wollheim CB (2007) Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr Rev 28(1):84–116. doi:10.1210/er.2006-0007
Gopel S, Kanno T, Barg S et al (1999) Voltage-gated and resting membrane currents recorded from B-cells in intact mouse pancreatic islets. J Physiol 521(Pt 3):717–728
Reinbothe TM, Safi F, Axelsson AS et al (2014) Optogenetic control of insulin secretion in intact pancreatic islets with beta-cell-specific expression of Channelrhodopsin-2. Islets 6(1):e28095. doi:10.4161/isl.28095
Madisen L, Mao T, Koch H et al (2012) A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat Neurosci 15(5):793–802. doi:10.1038/nn.3078
Herrera PL (2000) Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development 127(11):2317–2322
Carter JD, Dula SB, Corbin KL et al (2009) A practical guide to rodent islet isolation and assessment. Biol Proced Online 11:3–31. doi:10.1007/s12575-009-9021-0
Li DS, Yuan YH, Tu HJ et al (2009) A protocol for islet isolation from mouse pancreas. Nat Protoc 4(11):1649–1652. doi:10.1038/nprot.2009.150
Grynkiewicz G, Poenie M, Tsien RY (1985) A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260(6):3440–3450
Mahdi T, Hanzelmann S, Salehi A et al (2012) Secreted frizzled-related protein 4 reduces insulin secretion and is overexpressed in type 2 diabetes. Cell Metab 16(5):625–633. doi:10.1016/j.cmet.2012.10.009
Brennan K (2011) Colony management advanced protocols for animal transgenesis. Springer, Heidelberg. doi:10.1007/978-3-642-20792-1
Bertera S, Balamurugan AN, Bottino R et al (2012) Increased yield and improved transplantation outcome of mouse islets with bovine serum albumin. J Transpl 2012:856386. doi:10.1155/2012/856386
Tengholm A, Gylfe E (2009) Oscillatory control of insulin secretion. Mol Cell Endocrinol 297(1-2):58–72
Hodson DJ, Mitchell RK, Bellomo EA et al (2013) Lipotoxicity disrupts incretin-regulated human beta cell connectivity. J Clin Invest 123(10):4182–4194. doi:10.1172/JCI68459
Acknowledgements
The work described here was made possible through financial support by the Albert Påhlsson foundation, Fredrik and Ingrid Thurings foundation, Royal Physiographic Society, and the Swedish Diabetes Association.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media New York
About this protocol
Cite this protocol
Reinbothe, T.M., Mollet, I.G. (2016). Optogenetic Control of Pancreatic Islets. In: Kianianmomeni, A. (eds) Optogenetics. Methods in Molecular Biology, vol 1408. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-3512-3_8
Download citation
DOI: https://doi.org/10.1007/978-1-4939-3512-3_8
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-3510-9
Online ISBN: 978-1-4939-3512-3
eBook Packages: Springer Protocols