Abstract
Background/Objectives:
The primary aim was to examine the daily glycemic response to normal-protein (NP) vs higher-protein (HP) breakfasts in overweight adolescents who habitually skip breakfast (H-BS). The secondary aim examined whether the glycemic response to these meals differed in H-BS vs habitual breakfast consumers (H-BC).
Subjects/Methods:
Thirty-five girls (age: 19±1 year; body mass index: 28.4±0.7 kg/m2) participated in the semi-randomized crossover-design study. The participants were grouped according to habitual breakfast frequency. H-BS (n=20) continued to skip breakfast (BS) or consumed a NP (12 g protein) or HP (32 g protein) breakfast for 3 days, whereas the H-BC (n=15) completed the NP and HP breakfast conditions for 3 days. On day 4 of each pattern, an 8 h testing day was completed. The respective breakfast and a standard lunch meal were provided, and plasma was collected to assess morning, afternoon, and total glucose and insulin area under the curves (AUC).
Results:
In H-BS, the addition of a HP breakfast increased total glucose AUC vs BS (P<0.05), whereas NP breakfast increased total insulin AUC vs BS (P<0.05). In H-BC, the HP breakfast reduced morning, afternoon and total glucose AUCs vs NP (all, P<0.05). No differences in insulin were detected. When comparing the HP–NP differential glycemic responses between groups, H-BS experienced greater afternoon and total glucose AUCs following HP vs NP breakfasts (both, P<0.05). No differences in insulin responses were observed between groups.
Conclusions:
Novel differences in the glucose response to HP vs NP breakfasts were observed and were influenced by the frequency of habitual breakfast consumption in overweight adolescents.
Similar content being viewed by others
Introduction
Skipping breakfast has typically been associated with increased body mass index, weight gain and obesity; however, recent evidence also illustrates a strong association with poor glucose control and an increased risk for developing type 2 diabetes (T2D).1, 2, 3 Several acute, controlled feeding studies further support these associations.3, 4 Specifically, according to Jovanovic et al.,3, 4 the consumption of breakfast led to a significantly lower post-lunch rise in plasma glucose in healthy, normal weight and obese, diabetic individuals.3 Thus, these data suggest that the consumption of breakfast improves the glycemic response at the next eating occasion.3, 4
A key dietary factor to consider within the breakfast meal includes the macronutrient composition of the meal. Postprandial glucose concentrations are altered by both the amount and type of carbohydrate (CHO) consumed within the meal.5 Jenkins et al. reported improvements in afternoon CHO metabolism following the consumption of low-glycemic index breakfast meals containing either fiber or slowly absorbed CHO.6, 7 Blunted glucose and insulin responses were observed following the consumption of low-glycemic index breakfast meals compared with a high-glycemic index breakfast meals.6, 7
Increased dietary protein has also been proposed to reduce postprandial glucose concentrations, as protein consumption is known to stimulate insulin secretion.8, 9 However, the data is inconclusive with some,10, 11, 12, 13, 14, 15, 16 but not all studies,17, 18, 19, 20, 21, 22 reporting blunted postprandial glucose and insulin responses following the consumption of higher-protein (HP) vs normal-protein (NP) breakfast meals. These conflicting findings may be due to several key dietary factors including, but not limited to protein type (animal vs plant) or food form (beverages vs solids), which varied within and across studies. Furthermore, the majority of the studies did not screen and/or control for the frequency of breakfast consumption; thus, it is possible that habitual breakfast skippers (H-BS) may respond differently to a breakfast meal than habitual breakfast consumers (H-BC). For example, Farshchi et al. found that breakfast skippers were more likely to have poor glycemic control compared with breakfast consumers.23 Lastly, it is also important to note that none of these studies assessed the effect of protein at breakfast on the glucose and insulin responses at the subsequent lunch meal.
Thus, the primary aim of this study was to examine the effects of consuming a NP vs HP breakfast meal on the daily glucose and insulin responses in overweight/obese adolescents who habitually skip breakfast (BS). In addition, we also sought to examine whether the glycemic responses to these meals differed in H-BS compared with H-BC.
Subjects and methods
Experimental design
Thirty-five overweight and obese adolescent girls participated in the following semi-randomized crossover-design breakfast study. The participants were grouped according to habitual breakfast frequency. The H-BS group randomly completed the following breakfast patterns at home for 3 days: (1) BS; (2) consumption of NP breakfast meals; and (3) consumption of HP breakfast meals. The H-BC group randomly completed the following breakfast patterns at home for 3 days: (1) consumption of NP breakfast meals and (2) consumption of HP breakfast meals. On the 4th day of each pattern (for both groups), the participants came to the University of Missouri in the morning to complete the respective 8-h testing day. The participants began the testing day by completing their respective breakfast pattern for a 4th morning. At 4-h post breakfast, a NP lunch was provided. Blood samples were collected at specific times throughout the 8-h testing day for assessment of plasma insulin and glucose responses.
Study participants
Adolescent girls were recruited from the Columbia, MO, USA area through advertisements, flyers and email list serves to participate in the study. Eligibility was determined through the following inclusion criteria: (1) age range of 13–20 years; (2) overweight to obese (body mass index: 25–34.9 kg/m2); (3) no metabolic or neurological diseases or other health complications; (4) not been clinically diagnosed with an eating disorder; (5) not currently or previously on a weight loss or other special diet in the past 6 months; (6) documented regular menstrual cycles between 21–36 days in duration for the past 6 months. In addition, a dietary questionnaire was completed to document weekly breakfast habits and/or specific foods consumed. The H-BS group infrequently consumed breakfast (⩽2 breakfast occasions/week), whereas the H-BC group frequently consumed a CHO-rich (⩾80% of energy content as CHO as assessed using Nutritionist Pro, Axxya Systems; Redmondrevise, WA, USA) breakfast (⩾5 eating occasions/week). Thus, those that did not consume a CHO-rich breakfast were excluded from participation. There was a 20% dropout rate from the study because of time constraints and non-compliance to the breakfast patterns.
Three hundred and fifty (350) teens were interested in participating in the study. Twenty-five (25) H-BS and thirty-one (31) H-BC met the screening criteria, were available for the 8-h testing days, and began the study. Twenty (20) H-BS and fifteen (15) H-BC completed all study procedures. Both groups included overweight, late adolescent girls that showed normal fasting glucose and insulin concentrations (Table 1).
All participants and their parents (if participant was <18 years of age) were informed of the study purpose, procedures and risks, and signed the consent/assent forms. The study was approved by the MU Health Sciences institutional review board. The participants received a stipend of $150/testing day.
Breakfast patterns
The participants completed each breakfast pattern for a total of 4 consecutive days/pattern (three acclimation days/pattern and one testing day/pattern). For the BS pattern (for the H-BS only), the participants continued to skip the morning meal. For the NP and HP patterns (for both groups), the participants were provided with specific breakfast meals and asked to consume these at home (before school) between 7–9:30 am for 3 days. Throughout this period, the participants were permitted to eat ad libitum throughout the remainder of each day. On Day 4, they completed the respective testing day. There was a 7-day washout period in between each of the breakfast patterns in which all participants returned to their previous habitual breakfast behavior.
The dietary characteristics of the breakfast and lunch meals are shown in Table 2. The breakfast meals were 350 kcals, which was ~18% of the total energy intake estimated from the energy expenditure equations specific for adolescents.24 The macronutrient composition of the NP breakfast contained 15% protein, 65% CHO and 20% fat, whereas the HP breakfast contained 40% protein, 40% CHO and 20% fat. In addition to being matched for fat content, the breakfast meals were similar in energy density, dietary fiber and sugar content. The HP meals included granola and fruit-topped yogurt and a breakfast burrito comprised of a whole-grain wrap, eggs, beef, cheese and salsa. The NP meals included a combination of whole-grain cereals with milk, granola and fruit-topped yogurt, and a mini bagel with cinnamon butter.
The NP lunch meal, provided in each testing day (for both groups) was 500 kcal, which was ~25% of the total energy intake.24 The macronutrient composition of the lunch meals contained 15% protein, 65% CHO and 20% fat.
Testing day procedures
The participants reported to the research facility between 6 and 9 am after an overnight fast to complete the 8-h testing day. Each participant was seated in a reclining chair and, for the next 30 min, was acclimated to the room and became familiarized with the testing day procedures. A catheter was then inserted into the antecubital vein of the non-dominant arm and kept patient by saline drip throughout the remainder of the testing day. At time −15 min, a baseline (fasting) blood sample was drawn. At time 0 min, a meal including water was provided during the NP and HP days and only water during the BS day (for the H-BS only). The participants consumed the meal and/or water within 30 min. Blood sampling was completed throughout the next 8-h. At +240 min (that is, 4-h post breakfast), the NP lunch was provided. Throughout the testing day, the participants were permitted to use the restroom but otherwise remained seated in the reclining chair. While they were reclining, they were permitted to watch videos on the study laptop, read or do homework.
Repeated blood sampling and hormonal analyses
Nineteen (19) blood samples (4 ml/sample; 76 ml/testing day) were collected throughout each 8-h testing day. Specifically, blood was collected at −15, +0, +30, +45, +60, +90, +120, +150, +180, +210, +240, +270, +285, +300, +330, +360, +390, +420, +450 min, with meals consumed at +0 min (breakfast) and +240 min (lunch). The samples were collected in test tubes containing ethylenediaminetetraacetic acid. Within 10 min of collection, the samples were centrifuged at −4 °C for 10 min. The plasma was separated and stored in microcentrifuge tubes at −80 °C for future analysis. Plasma glucose was measured through an in-house glucose oxidase assay (Thermo Fisher Scientific, Waltham, MA, USA). Plasma insulin was measured using the Milliplex MAP magnetic bead-based multi-analyte assay (Millipore Corporation; St Charles, MO, USA; HMHMAG-34K).
Data and statistical analyses
The following outcomes were determined for the plasma glucose and insulin responses following each condition and within each group: 4 h morning (0–4 h), 4 h afternoon (4–8 h) and total 8-h (0–8 h) net incremental Area Under the Curve (AUC) were calculated from the fasting (baseline) time point and the remaining points for each outcome. To compare the between-group glycemic responses following the HP and NP meals, the HP—NP difference scores were calculated for the morning, afternoon and total glucose and insulin AUCs.
To address the primary aim, repeated measures analysis of variances were performed to compare the main effects of breakfast condition (BS, NP, HP) in the morning, afternoon, and total glucose and insulin responses in the H-BS. When main effects were detected, pairwise comparisons, using Fisher’s least significant difference, were applied.
To address the secondary aim, mixed factor analysis of variances were performed to compare the main effects of breakfast condition (NP, HP), group (H-BS, H-BC), and condition × group interactions for the glucose and insulin AUC responses. When an interaction was detected, an independent t-test was performed on the HP—NP difference scores for the glucose and insulin responses between the habitual breakfast skippers vs breakfast consumers.
Analyses were conducted using the Statistical Package for the Social Sciences (SPSS; version 21.0; Chicago, IL, USA). P<0.05 was considered statistically significant. All data are reported at mean±s.e.m.
Results
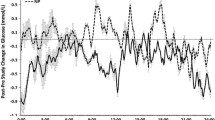
The glucose and insulin responses throughout each of the breakfast patterns for the H-BS and the H-BC are shown in Figure 1. Figure 2 illustrates the HP—NP difference scores between the H-BS vs H-BC. The line graphs illustrate the time course of change, whereas Table 3 includes the morning, afternoon, and total AUC measures.
Within-group comparisons
In the H-BS, a main effect of breakfast was detected for morning and total glucose AUC (P<0.05). Post hoc pairwise comparisons revealed that although both breakfasts led to a twofold increase in morning glucose AUC vs BS (both, P<0.05), only the HP breakfast increased total glucose AUC vs BS (P<0.05). No other differences were detected. A main effect of breakfast was also detected for morning and total insulin AUC (P<0.05). Post hoc pairwise comparisons revealed that although both breakfasts led to a 12-fold increase in morning insulin AUC vs BS (both, P<0.05), the HP breakfast led to lower morning insulin AUC vs NP (P<0.05). In addition, the NP breakfast led to a twofold increase in total insulin AUC vs BS (P<0.05), whereas the HP breakfast did not.
In the H-BC, the HP breakfast meal led to a twofold reduction in morning, afternoon and total glucose AUCs vs NP (all, P<0.05). However, no differences in morning, afternoon or total insulin AUCs were detected between breakfast meals.
Between-group HP—NP differences
Although no main effects of condition or group were detected, condition × group interactions were detected for afternoon and total glucose AUCs (both, P<0.05). As shown in Table 3, when compared with the breakfast consumers, the breakfast skippers experienced greater afternoon and total glucose AUC following the HP breakfast vs the NP breakfast (both, P<0.05). This is illustrated by the greater afternoon and total glucose HP—NP differences (Table 3 and Figure 2). No main effects of condition, group or interactions were detected for any of the insulin outcomes.
Discussion
We sought to examine the effects of increased dietary protein at breakfast on the glucose and insulin responses throughout the day in overweight/obese adolescent girls who habitually skip breakfast as well as those who habitually eat breakfast. We demonstrated that the addition of breakfast, regardless of macronutrient content, had no effect on the post-lunch glucose and insulin responses in habitual breakfast skippers. However, habitual breakfast frequency (whether the participants frequently consumed or skipped) significantly modulated the post-lunch and total glucose responses to the normal-protein vs higher-protein breakfast meals. Specifically, compared with the habitual breakfast consumers, the habitual breakfast skippers experienced greater afternoon and daily glucose responses following the higher-protein breakfast vs the normal-protein breakfast. Collectively, these data illustrate novel differences in the glucose response to increased dietary protein at breakfast, which were influenced by the frequency of habitual breakfast consumption in overweight adolescents.
Acute, postprandial hyperglycemia, or glycemic excursions, elicit detrimental effects on key indices of cardiovascular function25, 26 and is associated with an increased risk for the development of T2D27 and cardiovascular complications.28, 29, 30, 31 Thus, strategies to reduce postprandial hyperglycemia might be beneficial in preventing the manifestation of T2D and cardiovascular disease. One such strategy includes the addition of breakfast, particularly one rich in protein. Breakfast skipping has been negatively associated with poor glucose control and an increased risk of developing T2D and cardiovascular disease.2, 3 Thus, the daily consumption of breakfast has been postulated to beneficially modulate blood glucose control throughout the day.32
Jovanovic et al.3, 4 conducted two randomized crossover-design studies in normal-weight, healthy adults and in obese individuals with T2D to examine the effects of skipping breakfast on markers of glucose control and T2D risk factors. In the first study,3 post-lunch metabolic responses were compared following the consumption of a ~650 kcal breakfast containing 15 g protein, 106 g CHOs and 18 g fat vs no breakfast in obese adults with T2D. In the second study,4 post-lunch metabolic responses were compared following the consumption of a larger meal (that is, 850 kcal) containing 44 g protein, 103 g CHOs and 29 g fat vs no breakfast in healthy, normal-weight adults. Compared with skipping breakfast, the consumption of breakfast lowered the post-lunch rise in plasma glucose by 73% in healthy, normal-weight subjects and 95% in those with T2D.3, 4 The reduced glycemic control during a subsequent meal when the prior meal is skipped has been termed ‘the second meal phenomenon’3, 4 and has been postulated to occur as a result of an increased glucose conversion into muscle glycogen after lunch.4 Specifically, Jovanovic et al.4 reported a 50% increase in postprandial plasma glucose conversion to muscle glycogen after lunch following breakfast compared with when breakfast was skipped.
Unlike Jovanovic et al.,3, 4 the current study did not detect improvements in post-lunch glucose or insulin concentrations following the addition of the normal-protein (high CHO) breakfast in individuals who habitually skip breakfast. This discrepancy may be due to several factors including age, habitual breakfast habits and the varied breakfast characteristics. For example, the average ages of the participants in the Jovanovic et al.3, 4 studies were 56±3 and 47±4 years, whereas the current study was 19±1 years of age. This large difference in age could result in different physiological responses in glycemic response.33, 34 In addition, it is unknown whether the participants in the Jovanovic et al. studies were habitual breakfast skippers or habitual breakfast consumers. In the current study, we observed that habitual breakfast skippers experience lower circulating afternoon and daily glucose concentrations after the consumption of a normal-protein (high CHO) breakfast compared with the breakfast consumers. Thus, frequency of breakfast consumption appears to be an important factor in glycemic responses to CHO-rich breakfast meals. More studies are required to identify the mechanism-of-action by which habitual breakfast patterns influence the physiological responses to CHO-rich meals. Lastly, the Jovanovic et al. studies contained large breakfasts (650–850 kcals) with ~100 g of CHOs. The current study included only 350 kcal and 38–59 g CHOs. The effect of energy and CHO content on these outcomes is supported by the continued elevation in glucose (at 2 h post breakfast) in the Jovanovic et al. studies, whereas the glucose responses in the current study returned to baseline levels within 2 h. Collectively, these data suggest that age, habitual breakfast habits and the size and quality of the breakfast meal have a significant impact on the second meal phenomenon.
The replacement of CHO with protein has been shown to decrease the postprandial glucose and/or insulin responses (of that meal) in some,10, 11, 12, 13, 14, 15, 16 but not all studies.17, 18, 19, 20, 21, 22 However, glucose and insulin responses were not assessed at the subsequent meal in any of these studies. Thus, the current study was the first to examine whether this response would extend into and contribute to the ‘second meal phenomenon’. No differences in subsequent meal glucose and insulin responses were observed with the higher-protein breakfast in habitual breakfast skippers. However, those that habitually consumed a CHO-rich breakfast exhibited greater reductions in post-lunch glucose following the higher-protein breakfast compared with the normal-protein breakfast. Although speculative, these data would suggest an increased inability to optimally metabolize and/or utilize a large quantity of protein, at breakfast, in those who do not typically eat the morning meal. Alternatively, it is possible that the metabolic differences observed when eating a higher-protein vs normal-protein breakfast meal might drive the breakfast-skipping behavior. Further research is needed to test these concepts and explore potential mechanisms-of-action.
Limitations
Several study limitations have been identified. One limitation involves the investigation of only two selected factors, plasma glucose and insulin. Other various modulators such as circulating free fatty acids, ketones and/or hepatic glucose production may also be involved in the altered glycemic response to higher-protein breakfast meals.
Another study limitation included the varying sources of protein and CHOs within the breakfast meals. Previous studies in a meta-analysis have reported that insulin concentrations are blunted after a meal containing slow-digesting protein, like casein, compared with fast-digesting whey protein.13, 35 Although the higher-protein meals contained similar quantities of beef and egg protein between the breakfast groups in the current study, the normal-protein breakfast meals were void of egg and beef and primarily contained dairy and wheat protein. Thus, the absence of significant insulin increases with the higher-protein breakfast meals might have been due to the varied protein qualities within and between the breakfast meals. With respect to the CHO sources, a combination of whole grains and enriched flour were included in both the normal-protein and higher-protein breakfasts. However, we do not have the specific nutrient breakdown of these sources. Thus, due to the varied glycemic responses following different types of CHOs,36 it is possible that the glycemic response to these meals may have been influenced by the CHO source.
At last, although both groups were acclimated to the breakfast meals prior to the testing day, it is unclear as to whether the consumption of these meals over the long-term (weeks–months) would improve the glycemic response to the high-protein meals, particularly in those who habitually skip breakfast.
Conclusions
In summary, these data suggest that the addition of breakfast, regardless of macronutrient content, has minimal effects on the daily glycemic responses in individuals who habitually skip the morning meal. However, novel differences in the glycemic response to higher vs normal-protein breakfast meals were detected and appear to be influenced by habitual breakfast consumption in overweight/obese adolescent girls.
References
Deshmukh-Taskar P, Nicklas TA, Radcliffe JD, O'Neil CE, Liu Y . The relationship of breakfast skipping and type of breakfast consumed with overweight/obesity, abdominal obesity, other cardiometabolic risk factors and the metabolic syndrome in young adults. The National Health and Nutrition Examination Survey (NHANES): 1999–2006. Public Health Nutr 2012; 16: 1–10.
Mekary RA, Giovannucci E, Willett WC, van Dam RM, Hu FB . Eating patterns and type 2 diabetes risk in men: breakfast omission, eating frequency, and snacking. Am J Clin Nutr 2012; 95: 1182–1189.
Jovanovic A, Gerrard J, Taylor R . The second-meal phenomenon in type 2 diabetes. Diabetes Care 2009; 32: 1199–1201.
Jovanovic A, Leverton E, Solanky B, Ravikumar B, Snaar JE, Morris PG et al. The second-meal phenomenon is associated with enhanced muscle glycogen storage in humans. Clin Sci (Lond) 2009; 117: 119–127.
Arai H, Mizuno A, Sakuma M, Fukaya M, Matsuo K, Muto K et al. Effects of a palatinose-based liquid diet (Inslow) on glycemic control and the second-meal effect in healthy men. Metabolism 2007; 56: 115–121.
Jenkins DJ, Wolever TM, Nineham R, Sarson DL, Bloom SR, Ahern J et al. Improved glucose tolerance four hours after taking guar with glucose. Diabetologia 1980; 19: 21–24.
Jenkins DJ, Wolever TM, Taylor RH, Griffiths C, Krzeminska K, Lawrie JA et al. Slow release dietary carbohydrate improves second meal tolerance. Am J Clin Nutr 1982; 35: 1339–1346.
Blachier F, Mourtada A, Sener A, Malaisse WJ . Stimulus-secretion coupling of arginine-induced insulin release. Uptake of metabolized and nonmetabolized cationic amino acids by pancreatic islets. Endocrinology 1989; 124: 134–141.
Floyd JC Jr., Fajans SS, Pek S, Thiffault CA, Knopf RF, Conn JW . Synergistic effect of essential amino acids and glucose upon insulin secretion in man. Diabetes 1970; 19: 109–115.
Veldhorst MA, Nieuwenhuizen AG, Hochstenbach-Waelen A, Westerterp KR, Engelen MP, Brummer RJ et al. Comparison of the effects of a high- and normal-casein breakfast on satiety, 'satiety' hormones, plasma amino acids and subsequent energy intake. Br J Nutr 2009; 101: 295–303.
Belza A, Ritz C, Sorensen MQ, Holst JJ, Rehfeld JF, Astrup A . Contribution of gastroenteropancreatic appetite hormones to protein-induced satiety. Am J Clin Nutr 2013; 97: 980–989.
Blom WA, Lluch A, Stafleu A, Vinoy S, Holst JJ, Schaafsma G et al. Effect of a high-protein breakfast on the postprandial ghrelin response. Am J Clin Nutr 2006; 83: 211–220.
Acheson KJ, Blondel-Lubrano A, Oguey-Araymon S, Beaumont M, Emady-Azar S, Ammon-Zufferey C et al. Protein choices targeting thermogenesis and metabolism. Am J Clin Nutr 2011; 93: 525–534.
Al Awar R, Obeid O, Hwalla N, Azar S . Postprandial acylated ghrelin status following fat and protein manipulation of meals in healthy young women. Clin Sci 2005; 109: 405–411.
Ratliff J, Leite JO, de Ogburn R, Puglisi MJ, VanHeest J, Fernandez ML . Consuming eggs for breakfast influences plasma glucose and ghrelin, while reducing energy intake during the next 24 hours in adult men. Nutr Res 2010; 30: 96–103.
Agus MS, Swain JF, Larson CL, Eckert EA, Ludwig DS . Dietary composition and physiologic adaptations to energy restriction. Am J Clin Nutr 2000; 71: 901–907.
Leidy HJ, Armstrong CLH, Tang M, Mattes RD, Campbell WW . The influence of higher protein intake and greater eating frequency on appetite control in overweight and obese men. Obesity 2010; 18: 1725–1732.
Veldhorst MA, Nieuwenhuizen AG, Hochstenbach-Waelen A, Westerterp KR, Engelen MP, Brummer RJ et al. Effects of high and normal soyprotein breakfasts on satiety and subsequent energy intake, including amino acid and 'satiety' hormone responses. Eur J Nutr 2009; 48: 92–100.
Leidy HJ, Mattes RD, Campbell WW . Effects of acute and chronic protein intake on metabolism, appetite, and ghrelin during weight loss. Obesity 2007; 15: 1215–1225.
Makris AP, Borradaile KE, Oliver TL, Cassim NG, Rosenbaum DL, Boden GH et al. The individual and combined effects of glycemic index and protein on glycemic response, hunger, and energy intake. Obesity (Silver Spring) 2011; 19: 2365–2373.
Clifton PM, Keogh JB, Noakes M . Long-term effects of a high-protein weight-loss diet. Am J Clin Nutr 2008; 87: 23–29.
Noakes M, Keogh JB, Foster PR, Clifton PM . Effect of an energy-restricted, high-protein, low-fat diet relative to a conventional high-carbohydrate, low-fat diet on weight loss, body composition, nutritional status, and markers of cardiovascular health in obese women. Am J Clin Nutr 2005; 81: 1298–1306.
Farshchi HR, Taylor MA, Macdonald IA . Deleterious effects of omitting breakfast on insulin sensitivity and fasting lipid profiles in healthy lean women. Am J Clin Nutr 2005; 81: 388–396.
Rodriguez G, Moreno L, Sarria A, Fleta J, Bueno M . Resting energy expenditure in children and adolescents: agreement between calorimetry and prediction equations. Clin Nutr 2002; 21: 255–260.
Standl E, Schnell O, Ceriello A . Postprandial hyperglycemia and glycemic variability: should we care? Diabetes Care 2011; 34 (Suppl 2), S120–S127.
Marfella R, Verrazzo G, Acampora R, La Marca C, Giunta R, Lucarelli C et al. Glutathione reverses systemic hemodynamic changes induced by acute hyperglycemia in healthy subjects. Am J Physiol 1995; 268: E1167–E1173.
Lorenzo C, Okoloise M, Williams K, Stern MP, Haffner SM . The metabolic syndrome as predictor of type 2 diabetes: the San Antonio heart study. Diabetes Care 2003; 26: 3153–3159.
Smith NL, Barzilay JI, Shaffer D, Savage PJ, Heckbert SR, Kuller LH et al. Fasting and 2-hour postchallenge serum glucose measures and risk of incident cardiovascular events in the elderly: the Cardiovascular Health Study. Arch Intern Med 2002; 162: 209–216.
Meigs JB, Nathan DM, D'Agostino RB Sr., Wilson PW . Fasting and postchallenge glycemia and cardiovascular disease risk: the Framingham Offspring Study. Diabetes Care 2002; 25: 1845–1850.
Qiao Q, Pyorala K, Pyorala M, Nissinen A, Lindstrom J, Tilvis R et al. Two-hour glucose is a better risk predictor for incident coronary heart disease and cardiovascular mortality than fasting glucose. Eur Heart J 2002; 23: 1267–1275.
Brunner EJ, Shipley MJ, Witte DR, Fuller JH, Marmot MG . Relation between blood glucose and coronary mortality over 33 years in the Whitehall Study. Diabetes Care 2006; 29: 26–31.
Pereira MA, Erickson E, McKee P, Schrankler K, Raatz SK, Lytle LA et al. Breakfast frequency and quality may affect glycemia and appetite in adults and children. J Nutr 2011; 141: 163–168.
Reaven G . Age and glucose intolerance: effect of fitness and fatness. Diabetes Care 2003; 26: 539–540.
Reaven GM, Chen N, Hollenbeck C, Chen YD . Effect of age on glucose tolerance and glucose uptake in healthy individuals. J Am Geriatr Soc 1989; 37: 735–740.
Dangin M, Boirie Y, Garcia-Rodenas C, Gachon P, Fauquant J, Callier P et al. The digestion rate of protein is an independent regulating factor of postprandial protein retention. Am J Physiol Endocrinol Metab 2001; 280: E340–E348.
Willett W, Manson J, Liu S . Glycemic index, glycemic load, and risk of type 2 diabetes. Am J Clin Nutr 2002; 76: 274S–280S.
Acknowledgements
We thank Laura Ortinau and Tyler Lasley for assisting with the glucose and insulin data collection. Regarding the author contributions: AYA and HJL designed the research, conducted the research and analyzed the data. AYA developed the first draft of the paper. AYA, JPT and HJL had primary responsibility for manuscript revisions and final content. All authors substantially contributed to the completion of the manuscript and all have read and approved the final manuscript. The Beef Checkoff and the Egg Nutrition Center supplied funds to complete the study but were not involved in the design, implementation, analysis or interpretation of data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Alwattar, A., Thyfault, J. & Leidy, H. The effect of breakfast type and frequency of consumption on glycemic response in overweight/obese late adolescent girls. Eur J Clin Nutr 69, 885–890 (2015). https://doi.org/10.1038/ejcn.2015.12
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2015.12
- Springer Nature Limited