Abstract
This phase 1 study (clinical trial NCT00477815) was conducted to determine the maximum tolerated dose (MTD) of yttrium-90 ibritumomab tiuxetan (90Y-Zevalin) with high dose melphalan (HDM) therapy in multiple myeloma (MM) patients undergoing autologous stem cell transplantation (ASCT). In a 3+3 trial design, 30 patients received rituximab 250 mg/m2 with indium-111 ibritumomab tiuxetan (111In-Zevalin) for dosimetry (day −22); rituximab 250 mg/m2 with escalating doses of 90Y-Zevalin (day −14); melphalan 100 mg/m2 (days −2,−1) followed by ASCT (day 0) and sargramostim (GM-CSF, day 0) until neutrophil engraftment. Each patient’s 111In-Zevalin dosimetry data were used to calculate the dose of 90Y-Zevalin (in mCi) to deliver 10, 12, 14, 16, 18 or 20 Gy to the liver. Dose limiting toxicities were seen in 3 patients. The overall response rate was 73% (22/30) with stringent complete response in 2 patients; complete response, 5; very good partial response, 12; and partial response, 3. The median PFS was 16.5 months and the median overall survival was 63.4 months. In MM, the MTD of 90Y-Zevalin with HDM is 18 Gy to the liver. The addition of radiation with novel delivery methods such as radioimmunotherapy combined with standard transplant regimens warrants further study.
Similar content being viewed by others
Introduction
High dose therapy followed by autologous stem cell transplantation (ASCT) is considered the standard of care for patients with multiple myeloma (MM) under the age of 65 years based on randomized clinical trials.1, 2, 3, 4, 5, 6 It is also used selectively for patients older than 65.7, 8 Although ASCT induces a complete response (CR) in the majority of patients, it is not curative and patients invariably relapse with MM. While impressive strides have been made in bringing novel agents into the treatment of MM in induction, consolidation after ASCT, and maintenance phases with improvements in survival of these patients, high dose melphalan (HDM) used at 200 mg/m2 (Mel200) has remained the standard conditioning chemotherapy for patients with MM undergoing ASCT for decades.9
External beam radiation therapy remains a very effective modality in the treatment of patients with painful bone lesions and impending fractures.10 Since MM is almost always a disseminated disease, it is difficult to expand the capabilities of external beam radiation therapy in MM. Radioimmunotherapy offers the potential to expand the effectiveness of radiation therapy given its systemic administration. 90Yttrium (90Y) ibritumomab tiuxetan is a unique radioimmunoconjugate that uses ibritumomab, a murine IgG1 kappa anti-CD20 monoclonal antibody, covalently linked to the MX-DTPA linker-chelator tiuxetan that provides a high-affinity chelation site. Ibritumomab tiuxetan can be conjugated to Indium-111 (111In) to form 111In ibritumomab tiuxetan (111In-Zevalin) used for scanning and dosimetry or 90Y to form 90Y ibritumomab tiuxetan (90Y-Zevalin) for therapy.11 90Y is a high-energy beta-emitting radioisotope with an X90 (a measure of the radius in which the isotope deposits 90% of the energy emitted with beta particle decay) of 5 mm allowing it to target CD20+ cells along with bystander cells within 5 mm with a half-life of 64 h.12 This allows for targeted therapy to areas containing CD20+ cells in contrast to indiscriminate total body irradiation. Zevalin is FDA-approved in the United States for relapsed low grade and follicular non-Hodgkin lymphoma as well as for consolidation after initial chemotherapy in follicular lymphoma.13 It is the only commercially available radioimmunoconjugate currently available for therapy for lymphoma.
In an effort to improve upon the standard HDM conditioning regimen in patients with MM, we added escalating doses of 90Y-Zevalin to the myeloablative dose of melphalan 200 mg/m2 in a phase 1 study. There were three theoretical potential benefits to this approach all based on the known radiosensitivity of myeloma cells. There is a body of literature that would suggest that myeloma cells can express the CD20 antigen,14, 15 and myeloma stem cells have been postulated to be CD20 positive,16 although this remains controversial.17 Any CD20+ cells—malignant or reactive would be directly targeted by the 90Y-Zevalin. The third potential mechanism is that CD20− myeloma cells in proximity to benign marrow CD20+ lymphocytes would receive lethal radiation due to the 5 mm path length of 90Y beta emission. In this paper, we report the results of the completed study.
Material and methods
Objectives
The primary objective of this phase 1 study was to determine the safety of rituximab, 90Y-Zevalin, HDM and ASCT in patients with previously treated MM. The secondary objectives included the determination of response rate and progression factors (time to progression, PFS, duration of response) in patients treated with this regimen.
Patient selection
This trial (NCT00477815) was conducted at the Mayo Clinic, Rochester, MN after approval by the Scientific Review Committee of the Mayo Clinic Cancer Center and the Mayo Institutional Review Board. Patients ⩾18 years old, with a diagnosis of MM and candidates for HDM and ASCT were considered eligible for this trial. Patients were required to have an Eastern Cooperative Oncology Group performance status of 0, 1 or 2; ANC of ⩾1500/mm3, platelet count ⩾100 000/mm3, serum creatinine ⩽twice the upper limit of normal, bilirubin ⩽2 mg/dL, aspartate aminotransferase and alkaline phosphatase ⩽thrice the upper limit of normal, left ventricular ejection fraction ⩾45%, corrected pulmonary diffusion capacity ⩾50%, in the absence of uncontrolled infection, pregnancy or active nursing, HIV infection and other active malignancy requiring therapy. Prior therapy for MM was required to have been completed more than 3 weeks before registration; cyclophosphamide pulsing for stem cell collection was permitted. Patients were not eligible if they were on other cancer therapy or chronic corticosteroids at doses of prednisone>20 mg/day. All patients provided written, informed consent prior to study entry. The study accrued between May 2005 and June 2011 allowing follow-up for all patients of at least 54 months.
Study design and drug administration
Six dose levels (DL1-6) of 90Y Zevalin were tested with standard HDM and stem cell support. Patients received rituximab 250 mg/m2 with 111In-Zevalin over 10 min on day −22 followed by rituximab 250 mg/m2 with escalating doses of 90Y-Zevalin over 10 min on day −14 that were calculated to deliver the phase I Gy dose level to the liver; and melphalan 100 mg/m2 days −2 and −1. The administration of cold rituximab with Zevalin is the approved regimen for lymphoma. This was followed by ASCT on day 0. Sargramostim (GM-CSF) was administered starting day 0 until neutrophil engraftment. There was no maintenance therapy. The six dose levels of 90Y-Zevalin that were tested were individualized doses in mCi predicted to deliver 10, 12, 14, 16, 18 or 20 Gy to the liver. A standard 3+3 study design was used. Three patients were enrolled in each cohort. If no patient experienced a dose limiting toxicity (DLT) by day 90 post transplantation, the next cohort was treated at the next dose escalation. If ⩾2 patients experienced a DLT, the dose was de-escalated by 1. In the event of 1 DLT, three more patients were treated at the same dose; and in the event of 1/6 patients with a DLT a dose escalation was performed, for ⩾2 DLT a dose de-escalation was performed. The maximum tolerated dose (MTD) was considered the highest dose at which one or fewer patients out of six had a DLT.
Definitions
The following were considered as DLT: any non-hematologic grade 4 toxicity, excluding stomatitis, fatigue, anorexia, diarrhea, vomiting or infection; grade 4 pulmonary toxicity for >14 days, any non-hematologic grade 3 toxicity not resolving in 96 h excluding stomatitis, fatigue, anorexia, diarrhea, vomiting or infection; delayed engraftment, defined as an ANC not recovered to ⩾500/mm3 by day 21 post transplant and/or platelet transfusion dependency >35 days. In the evaluation of toxicities meeting the above DLT criteria, we considered day −22 to day −2 as the period of the rituximab and Zevalin and then day −2 forward as the effect of the HDM. DLT events that occurred during day −22 to day −2 were to be considered a DLT secondary to rituxan/Zevalin; those occurring between day −2 and the subsequent follow-up were to be evaluated by the study investigators. statistical team and the bone marrow transplant team to decide on the next dose level.
Responses were measured according to the International Myeloma Working Group criteria.18 A CR was defined as immunofixation-negative in serum and urine with <5% marrow plasma cells. A stringent CR fulfilled criteria for CR but also had a normal serum immunoglobulin free light chain and a negative bone marrow by immunohistochemistry and/or immunofluorescence. A very good partial response was reached if ⩾90% decrease in serum M-protein and a urine M-protein <100 mg/24 h. A partial response included ⩾50% reduction in serum M-protein, ⩾90% reduction in urine M-protein (or<200 mg/24 h) and ⩾50% decrease in soft tissue plasmacytomas.
Statistical analysis
Data were frozen as of February 2016. PFS was measured as the time from ASCT until progression of myeloma or death due to any cause. Overall survival (OS) was the time from ASCT until death due to any cause. Patients were censored at date last known to be alive. Time to next therapy was measured as the time from date of ASCT to time of starting next therapy. The PFS and OS curves were calculated using the Kaplan–Meier method (SAS 9.2, Cary, NC, USa).19
Results
Patient information
A total of 30 patients were enrolled and completed the therapy on this trial. The demographic and baseline clinical characteristics are summarized in Table 1. The treatment assignment by cohort is shown in Table 2. Forty percent (12/30) of patients were treated for relapsed disease with 37% (11/30) having had a prior ASCT.
Determination of MTD
Six dose levels were studied ranging from 10 to 20 Gy to the liver. This resulted in a median dose of 90Y-Zevalin (mCi) that ranged from 76 mCi in DL1 to 185 mCi for DL6 (individual patient dose range, 72–216 mCi; Table 2). There was one DLT at 16 Gy 90Y-Zevalin (dose level 4; DL4). The toxicities in this 73-year-old white male with chemoresistant relapsed MM included CMV viremia (grade 3), delayed engraftment and hepatic failure (grade 5). This adverse event (AE) prompted suspension of the trial and revision to start acyclovir prophylaxis at time of 90Y-Zevalin infusion, rather than waiting until HDM. There were two DLTs at DL6 (20 Gy), making DL5—18 Gy to the liver—the MTD. The first DLT at DL6 was a case of fatal jejunal ischemia/infarction in the setting of Escherichia coli bacteremia that occurred on day +12 in a 67-year-old white female with relapsed resistant MM and history of coronary artery disease, ischemic colitis and diabetes mellitus. The second DLT at DL6 was delayed platelet engraftment in a 65-year-old female with multiple relapsed MM and a prior ASCT 6 years previous; thus, the protocol therapy represented her second ASCT. Her platelets recovered to normal after a second stem cell infusion 2 months after protocol therapy. Of note, a third patient had toxicity at DL6, that may have been related to protocol therapy, but it occurred outside of the DLT toxicity observation window: fatal biopsy proven veno-occlusive disease (grade 5), which began day 91 post ASCT in a 62-year-old white male who presented with primary refractory MM, peri-mobilization staphylococcus aureus bacteremia with septic emboli, and line-associated venous thrombosis.
Safety
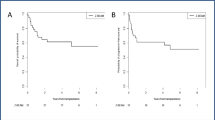
After 90Y Zevalin and rituximab, the most common AEs, regardless of attribution were: leukopenia, neutropenia and lymphopenia. Between days −22 and −2 (prior to HDM) the AEs observed, regardless of attribution, are summarized in Figure 1a. Total protocol AEs encountered are summarized in Figure 1b and Table 3. The most common AE was myelosuppression seen at grade 4 toxicity in 100% of patients. In total, 27% of patients developed a non-hematologic grade 4+ AE. The most common grade 3+ infection events were febrile neutropenia in 80% (24/30) of patients and 43% (13/30) patients had sepsis or bacteremia. As expected, grade 3–4 gastrointestinal AEs and metabolic AEs occurred in 57% (17/30) and 40% (12/30) patients, respectively. The next most common AE grouping was cardiovascular, with the majority being orthostatic hypotension requiring intravenous fluids; 10% (3/30) patients had atrial tachycardia and 3% (1/30) had congestive heart failure.
There have been four deaths not attributed to progressive disease, 3 within 100 days of ASCT. There was one case of DLT in a patient undergoing her second stem cell transplant (SCT). The patient was treated on dose level 4 and experienced jejunal ischemia without perforation that occurred in association with E. coli bacteremia on day 41. The second case, also a second transplant, developed hepatic failure in the setting of CMV infection/viremia. Liver biopsy showed no fibrosis, severe venous congestion consistent with venous outflow obstruction and zone 3 hepatocellular necrosis consistent with drug or toxin effect. The third case, a first SCT, was also treated at dose level 6. He had an uneventful recovery and engraftment and was dismissed from the Transplant Center with a normal total bilirubin. He developed venocclusive disease and was treated with defibrotide without benefit. The patient died of liver failure. The fourth case, a patient treated on dose level 2 engrafted at day +12 and had no DLT during the first 35 days. However, he developed CMV pneumonia during observation and died on day +46.
Engraftment
The median time to neutrophil engraftment in all patients was 11 days (range, 8–17). The median time to platelet engraftment ⩾20 000 was 11 days (range, 7–75) and ⩾50 000 was 15 days (range, 11–89), including the one patient who required a second stem cell infusion. The time to engraftment at individual dose levels is outlined in Table 4.
Response to therapy and survival
The overall response rate was 73% (22/30), with 7% sCR, 17% CR, 40% very good partial response and 10% partial response (Table 5). The median follow-up of surviving patients is 60.5 months (range, 54.1–95). None of the patients received maintenance therapy, and their median PFS from ASCT was 16.5 months (95% confidence interval (CI): 9.2–29.4). The median OS from ASCT was 63.4 months (95% CI: 31.8–not reached); 1-, 2- and 3-year OS from ASCT was 77% (95% CI: 63–93%), 73%, (95% CI: 59–91) and 63% (95% CI: 48–83), respectively. The median OS from diagnosis of the cohort was 93.3 months (95% CI 60.4–127.8). As of January 2016,16 (53%) patients have died; the other 14 remain alive.
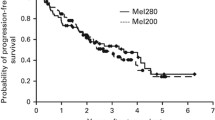
The median PFS for patients proceeding to early and delayed ASCT, respectively (Figure 2), was 29.6 months (95% CI: 9.2–46.1) and 10.8 months (95% CI: 1.9–17.5). The 3-year OS from ASCT for patients proceeding to early and delayed ASCT was 78% (95% CI: 61–100) and 42% (95% CI: 21–81), respectively. The 5-year OS from ASCT for patients proceeding to early and delayed ASCT was 67% (95% CI: 48–92) and 33% (95% CI: 15–74), respectively.
Discussion
High dose melphalan at 200 mg/m2 has remained the standard conditioning regimen for MM for over three decades. Various attempts have been made to improve this conditioning regimen platform. Increasing the dose beyond 200 mg/m2 deepens responses but results in more severe mucosal toxicity.20 Incorporation of novel agents such as bortezomib to HDM has shown promising results.21, 22 The addition of busulphan to HDM is also an area of ongoing research.23 Because of the known sensitivity of myeloma tumor cells to radiation, various radiotherapy strategies have been employed including TBI24 and therapeutic bone-seeking radioisotopes such as 153Samarium-EDTMP,25 and 166Holmium-DOTMP.26 While each of these regimens has shown promise, none has yet replaced single-agent HDM as the standard of care. Radioimmunotherapy builds on the strategy of delivering targeted radiotherapy directly to the tumor and thus avoids the toxicity of TBI to healthy tissue. The utility of 90Y-Zevalin in conditioning regimens such as BEAM (carmustine, etoposide, cytarabine, melphalan) and others has been extensively tested in B-cell lymphomas.27 In this phase 1 study, we have added 90Y-Zevalin in MM conditioning. The rationale for targeting CD20 in MM is based on the knowledge that while CD20 is expressed in up to 49% of myeloma patients and with heterogeneous expression,15, 28, 29 there is evidence that clonal CD19+/CD20+ B cells appear resistant to high dose therapy, and comprise the majority of clonal cells in myeloma patients post-ASCT.30 Moreover, the bone marrow houses polyclonal CD20+ B cells that can be non-specific attractants for the 90Y-Zevalin which then irradiate nearby malignant plasma cells by a crossfire effect.
The risk of myelosuppression from high doses of 90Y-Zevalin is a concern but less so in the context of stem cell salvage. While all patients in our study experienced grade 4 hematologic toxicity, the majority of patients engrafted promptly, and the only platelet engraftment failure occurred at a dose level that in the end was higher than the MTD (DL6—20 Gy to the liver). Fortunately, this patient was salvaged by a second stem infusion. Non-hematologic toxicities were seen, but were manageable in the majority of patients. Four non-myeloma-related deaths occurred, one from ischemic colitis in a patient with multiple risk factors for vascular disease, one from hepatic failure associated with CMV viremia, another from veno-occlusive disease at a dose level of 20 Gy to the liver (DL6), and 1 with CMV pneumonitis 1.6 months after the transplant. While veno-occlusive disease has not been reported in the current medical literature as a toxicity of 90Y-Zevalin, both radiation and HDM are reported risk factors.31, 32, 33 More experience is needed to conclusively attribute veno-occlusive disease to 90Y-Zevalin alone or when combined with HDM in myeloma. To date, none of these patients has developed secondary myelodysplastic syndrome or acute leukemia.
Although disease control and assessment of efficacy was not the primary objective of this study, the findings of an overall response rate of 73%, most of who had a very good partial response or better (63%), is encouraging. Even more impressive, however, were the rates of PFS and OS considering that we did not administer maintenance therapy, the patients enrolled were relatively high risk with 43% either primary refractory or transplanted at relapse, and that more than one-third of the patients received 90Y-Zevalin conditioning as part of a second ASCT.34
Our current study demonstrates that, in patients with MM, the MTD for 90Y-Zevalin in combination with fixed dose of melphalan 200 mg/m2 was 18 Gy to the liver. This dose translated into doses of 90Y-Zevalin ranging from 124 to 182 mCi (median, 168 mCi). This dose of 90Y-Zevalin is markedly higher than the maximum dose of 32 mCi given for standard radioimmunotherapy for lymphoma indications without stem cell support. The regimen was associated with a grade 4+ non-hematologic AE in 27% patients and produced a very good partial response or better in 63% patients. This regimen provides proof of concept of using radiation targeted to the marrow compartment where MM cells reside. The recent demonstration of the effectiveness of unlabeled monoclonal antibodies to CD3835, 36 and signaling lymphocytic activation molecule F7 (SLAMF7)37 provide the rationale to consider these antibodies for radioimmunotherapy for MM. In addition, the demonstration that attenuated oncolytic measles viruses engineered to express the sodium iodide symporter (NIS) can localize to MM deposits provides an alternative method of delivery of radionuclides such as 131Iodine.38 Our study of 90Y-Zevalin plus HDM and ASCT provides important data regarding the toxicity and efficacy of these approaches and provides valuable information regarding the doses that can be safely used with stem cell support in future trials.
References
Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med 1996; 335: 91–97.
Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med 2003; 348: 1875–1883.
Voorhees PM, Usmani SZ . The role of high-dose melphalan and autologous stem cell transplant in the rapidly evolving era of modern multiple myeloma therapy. Clin Adv Hematol Oncol 2016; 14: 719–728.
Attal M, Lauwers-Cances V, Hulin C, Leleu X, Caillot D, Escoffre M et al. Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N Engl J Med 2017; 376: 1311–1320.
Gay F, Oliva S, Petrucci MT, Montefusco V, Conticello C, Musto P et al. Autologous transplant vs oral chemotherapy and lenalidomide in newly diagnosed young myeloma patients: a pooled analysis. Leukemia 2017; 31: 1727–1734.
Palumbo A, Cavallo F, Gay F, Di Raimondo F, Ben Yehuda D, Petrucci MT et al. Autologous transplantation and maintenance therapy in multiple myeloma. N Engl J Med 2014; 371: 895–905.
Wildes TM, Finney JD, Fiala M, Gao F, Vij R, Stockerl-Goldstein K et al. High-dose therapy and autologous stem cell transplant in older adults with multiple myeloma. Bone Marrow Transplant 2015; 50: 1075–1082.
Muchtar E, Dingli D, Kumar S, Buadi FK, Dispenzieri A, Hayman SR et al. Autologous stem cell transplant for multiple myeloma patients 70 years or older. Bone Marrow Transplant 2016; 51: 1449–1455.
Moreau P, Attal M, Harousseau JL . New developments in conditioning regimens before auto-SCT in multiple myeloma. Bone Marrow Transplant 2011; 46: 911–915.
Talamo G, Dimaio C, Abbi KK, Pandey MK, Malysz J, Creer MH et al. Current role of radiation therapy for multiple myeloma. Front oncol 2015; 5: 1–6.
Witzig TE . Radioimmunotherapy for patients with relapsed B-cell non-Hodgkin lymphoma. Cancer Chemother Pharmacol 2001; 48 (Suppl 1): S91–S95.
Witzig TE, Flinn IW, Gordon LI, Emmanouilides C, Czuczman MS, Saleh MN et al. Treatment With ibritumomab tiuxetan radioimmunotherapy in patients with rituximab-refractory follicular non-Hodgkin's lymphoma. J Clin Oncol 2002; 20: 3262–3269.
Witzig TE . Moving radioimmunotherapy forward for follicular lymphoma. J Clin Oncol 2013; 31: 294–296.
Kapoor P, Greipp PT, Morice WG, Rajkumar SV, Witzig TE, Greipp PR . Anti-CD20 monoclonal antibody therapy in multiple myeloma. Br J Haematol 2008; 141: 135–148.
Yavasoglu I, Sargin G, Kadikoylu G, Doger FK, Bolaman Z . Immunohistochemical evaluation of CD20 expression in patients with multiple myeloma. Rev Bras Hematol Hemoter 2015; 37: 34–37.
Matsui W, Wang Q, Barber JP, Brennan S, Smith BD, Borrello I et al. Clonogenic multiple myeloma progenitors, stem cell properties, and drug resistance. Cancer Res 2008; 68: 190–197.
Hajek R, Okubote SA, Svachova H . Myeloma stem cell concepts, heterogeneity and plasticity of multiple myeloma. Br J Haematol 2013; 163: 551–564.
Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K et al. International uniform response criteria for multiple myeloma. Leukemia 2006; 20: 1467–1473.
Kaplan E, Meier P . Nonparametric estimation for incomplete observations. J Am Stat Assoc 1958; 53: 457–481.
Moreau P, Hullin C, Garban F, Yakoub-Agha I, Benboubker L, Attal M et al. Tandem autologous stem cell transplantation in high-risk de novo multiple myeloma: final results of the prospective and randomized IFM 99-04 protocol. Blood 2006; 107: 397–403.
Lonial S, Kaufman J, Tighiouart M, Nooka A, Langston AA, Heffner LT et al. A phase I/II trial combining high-dose melphalan and autologous transplant with bortezomib for multiple myeloma: a dose- and schedule-finding study. Clin Cancer Res 2010; 16: 5079–5086.
Roussel M, Moreau P, Huynh A, Mary JY, Danho C, Caillot D et al. Bortezomib and high-dose melphalan as conditioning regimen before autologous stem cell transplantation in patients with de novo multiple myeloma: a phase 2 study of the Intergroupe Francophone du Myelome (IFM). Blood 2010; 115: 32–37.
Reece D, Song K, LeBlanc R, Mezzi K, Olujohungbe A, White D et al. Efficacy and safety of busulfan-based conditioning regimens for multiple myeloma. Oncologist 2013; 18: 611–618.
Moreau P, Facon T, Attal M, Hulin C, Michallet M, Maloisel F et al. Comparison of 200 mg/m(2) melphalan and 8 Gy total body irradiation plus 140 mg/m(2) melphalan as conditioning regimens for peripheral blood stem cell transplantation in patients with newly diagnosed multiple myeloma: final analysis of the Intergroupe Francophone du Myelome 9502 randomized trial. Blood 2002; 99: 731–735.
Dispenzieri A, Wiseman GA, Lacy MQ, Litzow MR, Anderson PM, Gastineau DA et al. A phase I study of 153Sm-EDTMP with fixed high-dose melphalan as a peripheral blood stem cell conditioning regimen in patients with multiple myeloma. Leukemia 2005; 19: 118–125.
Christoforidou AV, Saliba RM, Williams P, Qazilbash M, Roden L, Aleman A et al. Results of a retrospective single institution analysis of targeted skeletal radiotherapy with (166)Holmium-DOTMP as conditioning regimen for autologous stem cell transplant for patients with multiple myeloma. Impact on transplant outcomes. Biol Blood Marrow Transplant 2007; 13: 543–549.
Stevens PL, Oluwole O, Reddy N . Advances and application of radioimmunotherapy in non-Hodgkin lymphoma. Am J Blood Res 2012; 2: 86–97.
Robillard N, Avet-Loiseau H, Garand R, Moreau P, Pineau D, Rapp MJ et al. CD20 is associated with a small mature plasma cell morphology and t(11;14) in multiple myeloma. Blood 2003; 102: 1070–1071.
Kumar S, Kimlinger T, Morice W . Immunophenotyping in multiple myeloma and related plasma cell disorders. Best Pract Res Clin Haematol 2010; 23: 433–451.
Cremer FW, Goldschmidt H, Moos M . Clonotypic B cells in the peripheral blood of patients with multiple myeloma. Blood 2001; 97: 2913–2914.
Dolai TK, Nataraj KS, Bhattacharya M, Ghosh MK . Veno-occlusive disease following high dose melphalan. Indian j hematol blood transfusion 2012; 28: 62–63.
Labidi SI, Sebban C, Ghesquieres H, Nicolas EV, Biron P . Hepatic veno-occlusive disease after tandem autologous stem cell transplantation conditioned by melphalan. Int J Hematol 2008; 88: 291–293.
Kumar S, DeLeve LD, Kamath PS, Tefferi A . Hepatic veno-occlusive disease (sinusoidal obstruction syndrome) after hematopoietic stem cell transplantation. Mayo Clin Proc 2003; 78: 589–598.
Michaelis LC, Saad A, Zhong X, Le-Rademacher J, Freytes CO, Marks DI et al. Salvage second hematopoietic cell transplantation in myeloma. Biol Blood Marrow Transplant 2013; 19: 760–766.
Lokhorst HM, Plesner T, Laubach JP, Nahi H, Gimsing P, Hansson M et al. Targeting CD38 with daratumumab monotherapy in multiple myeloma. N Engl J Med 2015; 373: 1207–1219.
Lonial S, Weiss BM, Usmani SZ, Singhal S, Chari A, Bahlis NJ et al. Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): an open-label, randomised, phase 2 trial. Lancet 2016; 387: 1551–1560.
Lonial S, Dimopoulos M, Palumbo A, White D, Grosicki S, Spicka I et al. Elotuzumab therapy for relapsed or refractory multiple myeloma. N Engl J Med 2015; 373: 621–631.
Russell SJ, Federspiel MJ, Peng KW, Tong C, Dingli D, Morice WG et al. Remission of disseminated cancer after systemic oncolytic virotherapy. Mayo Clin Proc 2014; 89: 926–933.
Acknowledgements
We thank the RN transplant coordinators—Teresa Miceli, Joan Theuer, Marsha Knudsvig, Michelle Gronseth and Lisa Kaiser for their assistance in patient accrual. We are extremely grateful for the philanthropic support provided by a gift from Hank Asher, which was paramount in our work to advance the treatment of multiple myeloma—an ongoing focus of investigation at Mayo Clinic for over 40 years.
Author contributions
AD, ADS and TEW contributed to the study design, data analysis and collection, care of patients, writing and edited the manuscript. KL contributed to data analysis and collection and the writing and editing of the manuscript. GW assisted with study design, data collection, care of patients and manuscript editing. BL contributed to the study design, data analysis and collection and manuscript editing. MQL, FB, SRH, SKK, DD, WJH, SMA, DAG, DJI, INM, LFP, PBJ, MRL all contributed to data collection, care of patients and editing of the manuscript. MAG provided care of patients and manuscript editing.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work was presented at the 2011 ASH Annual Meeting, San Diego, CA, USA. Blood (ASH Annual Meeting Abstracts) 2011; 118: Abstract 3095. This trial is registered at https://clinicaltrials.gov as NCT00477815.
Rights and permissions
About this article
Cite this article
Dispenzieri, A., D'Souza, A., Gertz, M. et al. A phase 1 trial of 90Y-Zevalin radioimmunotherapy with autologous stem cell transplant for multiple myeloma. Bone Marrow Transplant 52, 1372–1377 (2017). https://doi.org/10.1038/bmt.2017.164
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2017.164
- Springer Nature Limited
This article is cited by
-
Use of imaging-based dosimetry for personalising radiopharmaceutical therapy of cancer
Cancer Imaging (2022)
-
Success of the autologous stem cell boost after autologous graft failure in multiple myeloma and AL amyloidosis
Bone Marrow Transplantation (2022)