Abstract
Allogeneic hematopoietic cell transplantation (HCT) may produce long-term survival in AML after relapse or primary induction failure (PIF). However, outcomes of HCT performed for AML not in remission are historically poor given high relapse rates and transplant-related mortality. Preliminary studies suggest conditioning with clofarabine and myeloablative busulfan (CloBu4) may exert significant anti-leukemic effects without excessive toxicity in refractory hematologic malignancies. A prospective multicenter phase II trial was conducted to determine the efficacy of CloBu4 for patients proceeding directly to HCT with AML not in remission. Seventy-one patients (median age: 56 years) received CloBu4. At day 30 after HCT, 90% achieved morphologic remission. The incidence of non-relapse mortality and relapse at 2 years was 25% and 55%, respectively. The 2-year overall survival (OS) and event-free survival (EFS) were 26% and 20%, respectively. Patients entering HCT in PIF had significantly greater EFS than those in relapse (34% vs 8%; P<0.01). Multivariate analysis comparing CloBu4 with a contemporaneous cohort (Center for International Blood and Marrow Transplantation Research) of AML not in remission receiving other myeloablative conditioning (n=105) demonstrated similar OS (HR: 1.33, 95% confidence interval: 0.92–1.92; P=0.12). HCT with myeloablative CloBu4 is associated with high early response rates and may produce durable remissions in select patients with AML not in remission.
Similar content being viewed by others
Introduction
Approximately 30–40% of patients do not achieve remission with standard induction therapy for AML.1, 2, 3, 4 Once chemotherapy resistance is established, the probability of achieving remission with successive inductions is markedly reduced.5 Similarly, relapse also heralds an increasing likelihood for chemotherapy resistance, particularly in patients with short remission intervals and advanced age.6 In such scenarios, retrospective analyses suggest allogeneic hematopoietic cell transplantation (HCT) with myeloablative conditioning produces long-term survival in ~10–20% of patients.5, 7, 8, 9, 10 Although this is an improvement over conventional chemotherapy, AML not in remission at HCT remains a principle determinant of poor outcome attributable to the risks of high treatment-related mortality (TRM) and relapse.
The problem of AML not in remission is increasing, as HCT is offered to older individuals. Therefore, developing conditioning protocols that are tolerable but effective is a significant need.11, 12, 13 In AML not in remission, retaining myeloablative dosages may be necessary for adequate cytoreduction.14 Current myeloablative regimens such as cyclophosphamide and TBI (CyTBI) or busulfan and cyclophosphamide (BuCy) carry high risks for TRM, particularly in individuals over 50 years of age.15, 16 Toxicity related to HCT conditioning may be exacerbated in AML not in remission, with some studies reporting TRM in excess of 50%.17
Clofarabine has demonstrated significant anti-leukemic effects in relapsed AML with manageable toxicity in older patients.18 We hypothesized that combining clofarabine with myeloablative IV busulfan (CloBu4) would potentiate the anti-leukemic effects of conditioning while retaining sufficient tolerability. In a single-center phase I–II study of hematologic malignancies not in remission at HCT, AML patients receiving CloBu4 demonstrated consistent engraftment, manageable toxicity and high rates of remission.19 Relapse and survival were promising compared with historical outcomes from an earlier treatment era that extensively used CyTBI and BuCy conditioning.7 Others have reported encouraging single-center outcomes with clofarabine–busulfan-based conditioning in high-risk AML.20, 21, 22
To further evaluate the efficacy of myeloablative CloBu4 conditioning, we conducted a prospective multicenter phase II clinical trial to determine survival after HCT exclusively for AML patients not in remission. Outcomes after HCT for study patients were then compared with an external contemporaneous cohort of patients from the Center for International Blood and Marrow Transplantation Research (CIBMTR) who received other myeloablative conditioning regimens for AML not in remission.
Methods
CloBu4 conditioning, patients and donors
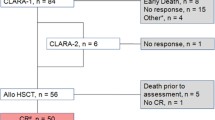
This was a single-arm multicenter prospective clinical trial for relapsed or primary induction failure (PIF) AML not in remission at the time of HCT (NCT01457885). Patients up to 65 years of age were eligible to receive CloBu4 conditioning between November 2011 and December 2013 at twelve HCT centers in the United States and Canada. Patients had bone marrow biopsies demonstrating >5% blasts prior to initiating conditioning. The presence of circulating blasts or extramedullary leukemia (excluding active central nervous system leukemia) was permitted. Patients were required to have chemotherapy resistance as demonstrated by (1) PIF at diagnosis, defined as no remission after two intensive induction regimens or (2) relapsed AML not in remission. For relapse, a minimum of one re-induction attempt was required if the proceeding remission exceeded 6 months. All patients provided written informed consent on an Institutional Review Board-approved protocol (HUM00048709). Unrelated and related donors were required to be high-resolution 8/8 HLA matches at HLA A, B, C and DR.
Conditioning regimen and supportive care
The conditioning regimen consisted of IV busulfan 3.2 mg/kg daily based on adjusted ideal body weight infused over 3 h on 4 consecutive days (−5 to −2). Samples for busulfan pharmacokinetics were obtained after the first busulfan dose and analyzed at the Seattle Cancer Alliance Clinical Pharmacokinetics Laboratory (Seattle, WA, USA) or at a local laboratory. Busulfan dose was adjusted on days −3 and −2, if necessary, to target an area under the curve of 4500–5500 umol-min/day. IV clofarabine 40 mg/m2 was administered over 1 h on days −6 to −2 following completion of the busulfan. Clofarabine dosage was based on actual body weight. Stem cells were administered on day 0. PBSCs or bone marrow were allowed as stem cell sources. Other supportive measures included dexamethasone 12 mg IV on days of clofarabine as an antiemetic and to prevent capillary leak syndrome. Fungal prophylaxis was recommended to be an echinocandin in place of azole antifungals until normalization of liver function enzymes or bilirubin. Acute GvHD (aGvHD) prophylaxis consisted of calcineurin inhibitor with either mycophenolate mofetil (MMF) or methotrexate (MTX). No patients received ATG or other T-cell depleting regimens. No patients received prophylactic donor lymphocyte infusion to prevent relapse.
Comparison cohort: myeloablative conditioning other than CloBu4
Randomization was not performed in part due to difficulties in defining a consensus for standard of care myeloablative conditioning in this setting. Therefore, following completion of the phase II study a planned analysis was conducted to compare overall survival (OS) against patients not in remission who were transplanted in the United States during a contemporaneous time frame. This contemporaneous cohort of patients receiving myeloablative conditioning without clofarabine was identified in collaboration with the CIBMTR. The CIBMTR selected patients with similar eligibility to study patients: age 18–65 years receiving first HLA-identical sibling or 8/8 HLA-matched unrelated donor HCT for AML not in remission between 2008 and 2013. Patients with PIF were defined as no remission after one or two lines of induction. Patients in relapse prior to HCT were without mandate for additional induction attempts. Patients with T-cell depletion were excluded.
Response assessment, definitions and statistical analysis
Remission status for CloBu4 patients was assessed by bone marrow aspirate and biopsy for study patients on days 30, 100 and 365 post HCT or at times of concern for relapse. CIBMTR patients receiving other myeloablative conditioning had disease assessments per local institutional practice. Definition of cytogenetic risk was by ECOG/SWOG classification.23 OS was recorded from the day of HCT (day 0) until death. Event-free survival (EFS) was from the day of HCT until death or post-HCT relapse.
When designing the trial, there was no clear historical precedent for survival given the lack of large prospective data sets in AML not in remission at HCT. Using outcomes from a phase I/II trial of CloBu4 at the University of Michigan and a historical registry cohort (CIBMTR) of other myeloablative regimens,7, 19 we estimated that CloBu4 would result in a 16% improvement in the primary end point of EFS at 1 year. Assuming a type I error rate of 5%, we estimated a sample size of 70 patients would supply 80% power to detect such a difference. The sample size was augmented to 75 patients to allow for subjects who may become non-evaluable. Differences in characteristics between patient groups were assessed with a Wilcoxon rank sum test for continuous values and a χ2-test of association for categorical values. Cumulative incidence and group differences for relapse, non-relapse mortality and GvHD were estimated using proportional hazard models for competing risk.24 OS and EFS were estimated using Kaplan–Meier methods and log-rank tests were used to compare survival between the CloBu4 and CIBMTR cohorts. Multivariate analysis of OS was modeled using Cox regression methods. All analyses were performed in R (R Development Core Team, Vienna, Austria) and SAS statistical packages (SAS Institute, Cary, NC, USA). Final planned analysis was restricted to adult AML defined as age ⩾18 years at the time of enrollment. Three pediatric patients were not included in planned analysis for age <18 years. Among these three patients, two died of progressive AML at 6 and 11 months after HCT. The third remains in remission 2 years after HCT.
Results
Patient characteristics
The characteristics of 71 AML patients aged ⩾18 years who enrolled and received CloBu4 conditioning are shown in Table 1. One patient withdrew after enrolling but prior to initiating study conditioning. The median age was 56 years (range 19–65). Patients had a median of 27% (5–95%) blasts on pre-HCT bone marrow examination and received a median of 2 (range: 1–5) prior inductions. The median time from diagnosis to HCT was 6 months (2–25). For patients with PIF, the median time for diagnosis to HCT was 4 months (range: 2–18). For patients who relapsed, the median time from recurrence of AML to HCT was 2 months (range: <1–11). Re-induction chemotherapy was attempted at relapse in the majority (76%) of patients.
Engraftment, response and relapse
Sixty-seven patients were evaluated by bone marrow aspirate at day 30 post HCT. Four patients were not evaluable as a result of early death (n=3) or lack of study assessments (n=1). Sixty patients (90%) achieved morphologic remission. Of patients achieving remission with available data (n=55), the median days to neutrophil and platelet engraftment were 15 (range: 11–27) and 18 days (10–105), respectively. For the entire cohort (n=71), the 2-year incidence of relapse was 55% (95% confidence interval (CI): 42–66) (Figure 1a). The median time to relapse was 95 days after HCT (range: 41–317). In subset analysis, cytogenetic risk, degree of bone marrow blasts (⩾20% vs <20%) and the presence of circulating blasts were not associated with relapse. Patients known to have FLT3-ITD mutant AML (n=23) had a trend toward higher relapse compared with those with wild-type FLT3-ITD (n=30) (70% vs 43%; P=0.20). A significantly higher relapse rate was observed in patients with relapsed AML (prior CR<6 months=82%; prior CR⩾6 months=65%) compared with patients entering HCT in PIF (38%)(P<0.01).
GvHD
aGvHD prophylaxis consisted of calcineurin inhibitor (CNI) plus MTX in 73% of patients. The cumulative incidence of grade II–IV aGvHD for CloBu4 patients was 38% (95% CI: 26–51). No difference in rates of grade II–IV GvHD was observed according to immunoprophylaxis (38% for CNI+MMF vs 40% CNI+MTX; P=0.84). At 2 years, the cumulative incidence of chronic GvHD was 34% (95% CI: 22–46).
Non-relapse mortality and toxicity
The cumulative incidence of non-relapse mortality (NRM) was 21% (95% CI,12–31) at 1 year and 25% (95% CI, 15–37) at 2 years after CloBu4 (Figure 1b). In subset analysis, NRM did not differ according to performance status, donor type or type of GvHD prophylaxis. At 2 years, a significant increase in NRM was observed in patients experiencing acute GvHD grades II–IV vs grades 0–1 (39% vs 13%; P=0.01). Toxicities involving grade ⩾3 non-hematologic severe adverse events (common terminology criteria for adverse events (CTCAE) Version 4.0) reported from initiation of conditioning to day +30 after HCT are listed in Table 2. One patient required prophylactic intubation due to impending airway compromise from severe mucositis. There were no biopsy confirmed cases of sinusoidal obstruction syndrome. However, one patient developed hepatic failure on day 22 after HCT.
Busulfan pharmacokinetics
Busulfan pharmacokinetics were obtained in the majority (n=63, 89%) of patients after the first busulfan dose (estimated pharmacokinetics were 4258, range 2846–7824). Dose adjustments on days −3 and −2 were performed resulting in a median busulfan area under the curve of 4907. Relapse and NRM did not significantly differ between busulfan exposures greater than or less than the median area under the curve (data not shown).
Overall survival, event-free survival and causes of mortality
The OS for AML patients treated with CloBu4 conditioning at 1 and 2 years was 32% (95% CI, 22–44) and 26% (95% CI, 16–38), respectively (Figure 2a). EFS was 24% (95 CI, 15–34) at 1 year and 20% (95% CI, 11–30) at 2 years (Figure 2b), thus the trial did not meet the primary end point of improved EFS at 1 year.
A trend toward improved OS occurred in patients receiving HCT in PIF status (n=34) vs relapse (n=37) (OS: 34% vs 24%; P=0.09). EFS was significantly improved in patients in PIF status compared with relapse (34% vs 8%; P<0.01) (Figure 3). In PIF patients, a minimum of two inductions prior to HCT were required and EFS did not differ between subjects receiving two inductions (n=24) vs those receiving greater than two inductions (n=10) (P=0.9). In relapsed patients, there were no significant differences in OS according to the duration of prior remission (⩾6 vs <6 months) (28% vs 12%; P=0.3). The presence of peripheral blood blasts, % marrow blasts (⩾20% vs <20%), Karnofsky score (⩾90% vs <90%), FLT-ITD status and karyotype also did not impact OS.
Patients who achieved morphologic remission after HCT (n=60) were analyzed for the impact of aGvHD on OS. Patients developed aGvHD at a median of 29 days (range 9–103). aGvHD was only considered evaluable if occurring before relapse. aGvHD of mild to moderate severity grades was associated with improved OS compared with patients with severe aGvHD (grade 0: 18% vs grade I–II: 38% vs grade III–IV: 0%; P<0.01).
Outcomes of CloBu4 conditioning vs other myeloablative conditioning
This study did not contain randomized controls, and contemporary outcomes for AML not in remission at the time of HCT are poorly understood. Previously, a prognostic scoring model for AML not in remission at HCT treated with myeloablative conditioning was reported by Duval et al.7 The median Duval score for CloBu4 patients was 2 (range: 0–4), which was associated with an OS at 2 years of ~15% in historical cases reported to the CIBMTR between 1995 and 2004. We therefore compared outcomes with CloBu4 to contemporaneous AML patients reported to the CIBMTR. A total of 105 patients were identified with AML not in remission at HCT from 2008 to 2013. Only patients with HLA-matched HCT who received myeloablative conditioning other than CloBu4 were selected (Table 3a). The majority received conditioning with BuCy (34%) or CyTBI regimens (45%), but had similar disease status (PIF vs relapse), Duval scores (median 2; range: 0–4), graft type, donor type, performance status, cytogenetic risk and time to HCT compared with CloBu4 patients. The CIBMTR cohort was significantly younger (48 vs 56 years; P<0.01) with fewer bone marrow blasts at the time of HCT (median 16% vs 27%; P=0.03). Also, some patients in the CIBMTR cohort with CR >6 months did not receive re-induction, whereas the CloBu4 trial mandated re-induction. In univariate analysis, OS was 37% (95% CI, 28–47) for CIBMTR cohort compared with 26% (95% CI, 16–38) for CloBu4 patients at 2 years (P=0.14). The incidence of relapse (58% vs 55%; P=0.69) was similar among groups, but TRM (12% vs 26%; P=0.04) was greater for CloBu4. In multivariate analysis, TRM remained significantly higher for recipients of CloBu4, but no differences in relapse, OS or treatment failure (inverse of leukemia-free survival) were observed (Table 3b). The lack of difference in OS remained after multivariate analysis to adjust for differences in patient characteristics such as age at HCT. The causes of mortality were similar among cohorts with relapse of AML accounting for the majority of treatment failures (Table 4).
Discussion
We performed a multicenter trial utilizing myeloablative conditioning with CloBu4 for relapsed and PIF AML not in remission at the time of HCT. This represents the first prospective multicenter trial designed to improve survival exclusively in AML patients not in remission by increasing the anti-leukemic effects and tolerability of conditioning. The presence of active leukemia at HCT is a dominant predictor of poor outcome with previous survival estimates ranging from 10 to 20%.5, 7, 8, 9, 10 We observed that despite refractoriness to prior chemotherapy, CloBu4 conditioning followed by infusion of allogeneic HCT induces remission in the majority of patients, is tolerable up to 65 years in a cohort traditionally at high risk for TRM, and results in 2-year survivals in a quarter of patients. Unfortunately, a significant number of patients ultimately experience relapse, and it remains unclear whether the selection of specific myeloablative regimens influences the outcome.
AML with PIF or relapse demonstrating resistance to re-induction chemotherapy have uniformly dismal outcomes. In such settings, HCT remains the only therapy with curative potential in a small percentage of patients. The use of CloBu4 conditioning resulted in a 90% CR rate at day 30 post HCT, an effect not anticipated with additional induction therapy. Early response rates for other myeloablative HCT conditioning were not available in the CIBMTR cohort due to the lack of prospective studies. However, one analysis reported CR with CyTBI in 75% of PIF patients,8 implying that CloBu4 possesses anti-leukemic effects at least equivalent to traditional myeloablative regimens.
Of greater importance is whether early remission results in durable responses that improve leukemia-free survival. The relapse incidence with CloBu4 was 55%, which was similar to the 42% incidence reported in an initial phase I/II trial.19 This high rate of relapse after remission suggests the presence of minimal residual disease that was not eliminated or routinely detected by standard assessments. Thus, the primary function of myeloablative conditioning may therefore be to secure an initial remission. Given the high CR rate, CloBu4 could provide a platform for additional strategies to eliminate minimal residual disease that presumably results in subsequent relapse. It is also possible that the dosage of clofarabine administered was insufficient for reaching the maximal anti-leukemic potential of CloBu4. For example, a dosage of 40 mg/m2 as a single agent was initially determined to be the recommended phase II dose for acute leukemia given concerns for potential hepatotoxicity,25 but three other phase I trials combining clofarabine with busulfan or cyclophosphamide/etoposide in HCT have shown tolerability for dosages up to 70 mg/m2 without reaching an maximum tolerated dose (MTD).20, 21, 26 Thus, it is conceivable that higher doses of clofarabine could improve the anti-leukemic potential of conditioning without additional toxicity.
This trial did not demonstrate an improvement in early survival based on a previous single-center phase I/II study of CloBu4 that reported an EFS of 42% at 1 year. However, the 2-year EFS of 20% reported here is similar to the 22% EFS reported in the previous study. Given the lack of large prospective data sets and the historical OS of 10–20% for AML not in remission at HCT,7 an analysis with current CIBMTR data sets was performed to interpret the results of this study. The CIBMTR identified a contemporaneous cohort of AML patients not in remission treated with non-clofarabine myeloablative conditioning. The CIBMTR cohort was analyzed after the prospective phase II study, did not receive protocol-directed therapy, thus this analysis was primarily intended to provide an estimate of contemporary outcomes of similar patients in the modern era, rather than a bonafide control arm. The comparison did not reveal differences in OS, relapse or treatment failure between CloBu4 and CIBMTR patients, suggesting the choice of specific myeloablative conditioning is less critical for maintaining long-term leukemia-free survival. Nonetheless, the lack of difference in OS remained after performing multivariate analysis to adjust for age and other significant pre-HCT differences between cohorts. The inability to identify a precisely matched contemporary comparator cohort suggests comparisons among conditioning regimens are optimally assessed in a prospective randomized trial.
The impact of clinical characteristics on outcomes was examined to determine subgroups likely to benefit from HCT. Previously, the presence of peripheral blasts, degree of HLA match, Karnofsky score, age and remission duration had prognostic value for predicting OS for AML not in remission.7 In CloBu4 study patients, only AML in relapse had a significantly negative prognostic impact on survival. Although resistant to two lines of induction, superior EFS was observed for PIF vs relapsed AML. Because patients with relapsed AML received HCT relatively later in disease, this difference may reflect the emergence of additional biological resistance or selection of subclones, that confer a higher relapse potential after HCT.27
Toxicity from conditioning also has a key role in determining outcomes after HCT. AML not in remission has typically been associated with TRMs of 40–60%, which are higher than anticipated for myeloablative conditioning administered during remission.8, 9, 28, 29 While early TRM was observed in our study, the overall incidence of 25% was relatively low based on historical rates, suggesting CloBu4 is feasible in patients up to 65 years. TRM did not differ according to age, Karnofsky score or from rates observed in our phase I/II experience with CloBu4.
The impressively low TRM of 12% observed in the CIBMTR cohort was an unexpected finding. The CIBMTR cohort utilized several myeloablative regimens, but the majority received BuCy and CyTBI (79%). While this is consistent with TRM reported for BuCy (IV) and CyTBI for younger AML patients in first CR,30 historical TRM rates for oral BuCy have approached 30% for AML greater than first CR31 and higher for ByCy/CyTBI in settings of AML not in remission.8, 17, 28, 29 These differences raise important new questions regarding the contemporary application of myeloablative regimens, such as whether IV dosing of BuCy with pharmacokinetics targeting may facilitate lower TRM than in previous treatment eras, particularly for AML not in remission. These differences may also reflect other improvements in post-HCT care that mitigate conditioning-related complications, but also suggest selection of more fit patients. HCT-CI (comorbidity index) was not available to directly compare comorbidities; however, the median age was significantly greater in CloBu4 patients, potentially explaining these differences in TRM. However, the higher TRM observed in recipients of CloBu4 also raises the possibility that this regimen has greater toxicity than traditional myeloablative regimens (for example, BuCy). Given the heterogeneity of the CIBMTR cohort and the challenges of assessing regimen-related morbidity in AML not in remission at HCT, prospective comparisons between specific myeloablative regimens are needed to optimally assess toxicity.
Patients receiving CloBu4 for AML not in remission appeared particularly vulnerable to aGvHD. The occurrence of grade II–IV aGvHD did not significantly reduce relapse (43% vs 60%, P=0.07), but did correlate with greater TRM (39% vs 13%, P=0.01), which included several deaths related to infection. Among patients who developed severe aGvHD (grades III–IV), there were no survivors. These findings illustrate the importance of maximizing infectious prophylaxis and taking measures that minimize risk for developing severe aGvHD.
Based on historical outcomes, we hypothesized that CloBu4 would be superior to conventional myeloablative conditioning. However, our analysis against a contemporary cohort of AML patients did not demonstrate an OS advantage, even after adjusting for relevant HCT risk factors in multivariate analysis. Since many centers do not offer HCT for patients with AML not in remission, this remains an area of great unmet need. Although we observed significant relapse, CloBu4 resulted in high rates of remission, acceptable tolerability in patients up to 65 years of age and an OS of 26% at 2 years. The early remission states after CloBu4 or other myeloablative regimens may enable the introduction of novel approaches to reduce the risk of relapse after HCT, such as targeting aberrant signaling pathways (FLT3-ITD inhibitors),32 azacitidine33, 34 or approaches that may selectively enhance leukemia antigen presentation to promote GvL effects.35 In conclusion, this study supports the use of CloBu4 or other myeloablative regimens for high-risk AML not in remission at HCT, particularly in settings of PIF.
References
Fernandez HF, Sun Z, Yao X, Litzow MR, Luger SM, Paietta EM et al. Anthracycline dose intensification in acute myeloid leukemia. N Engl J Med 2009; 361: 1249–1259.
Schiller G, Gajewski J, Nimer S, Territo M, Ho W, Lee M et al. A randomized study of intermediate versus conventional-dose cytarabine as intensive induction for acute myelogenous leukaemia. Br J Haematol 1992; 81: 170–177.
Weick JK, Kopecky KJ, Appelbaum FR, Head DR, Kingsbury LL, Balcerzak SP et al. A randomized investigation of high-dose versus standard-dose cytosine arabinoside with daunorubicin in patients with previously untreated acute myeloid leukemia: a Southwest Oncology Group study. Blood 1996; 88: 2841–2851.
Bishop JF, Matthews JP, Young GA, Bradstock K, Lowenthal RM . Intensified induction chemotherapy with high dose cytarabine and etoposide for acute myeloid leukemia: a review and updated results of the Australian Leukemia Study Group. Leuk Lymphoma 1998; 28: 315–327.
Armistead PM, de Lima M, Pierce S, Qiao W, Wang X, Thall PF et al. Quantifying the survival benefit for allogeneic hematopoietic stem cell transplantation in relapsed acute myelogenous leukemia. Biol Blood Marrow Transplant 2009; 15: 1431–1438.
Breems DA, Van Putten WL, Huijgens PC, Ossenkoppele GJ, Verhoef GE, Verdonck LF et al. Prognostic index for adult patients with acute myeloid leukemia in first relapse. J Clin Oncol 2005; 23: 1969–1978.
Duval M, Klein JP, He W, Cahn JY, Cairo M, Camitta BM et al. Hematopoietic stem-cell transplantation for acute leukemia in relapse or primary induction failure. J Clin Oncol 2010; 28: 3730–3738.
Michallet M, Thomas X, Vernant JP, Kuentz M, Socie G, Esperou-Bourdeau H et al. Long-term outcome after allogeneic hematopoietic stem cell transplantation for advanced stage acute myeloblastic leukemia: a retrospective study of 379 patients reported to the Societe Francaise de Greffe de Moelle (SFGM). Bone Marrow Transplant 2000; 26: 1157–1163.
Biggs JC, Horowitz MM, Gale RP, Ash RC, Atkinson K, Helbig W et al. Bone marrow transplants may cure patients with acute leukemia never achieving remission with chemotherapy. Blood 1992; 80: 1090–1093.
Craddock C, Labopin M, Pillai S, Finke J, Bunjes D, Greinix H et al. Factors predicting outcome after unrelated donor stem cell transplantation in primary refractory acute myeloid leukaemia. Leukemia 2011; 25: 808–813.
Appelbaum FR, Gundacker H, Head DR, Slovak ML, Willman CL, Godwin JE et al. Age and acute myeloid leukemia. Blood 2006; 107: 3481–3485.
Pollyea DA, Kohrt HE, Medeiros BC . Acute myeloid leukaemia in the elderly: a review. Br J Haematol 2011; 152: 524–542.
Magenau JM, Braun T, Reddy P, Parkin B, Pawarode A, Mineishi S et al. Allogeneic transplantation with myeloablative FluBu4 conditioning improves survival compared to reduced intensity FluBu2 conditioning for acute myeloid leukemia in remission. Ann Hematol 2015; 94: 1033–1041.
Shimoni A, Hardan I, Shem-Tov N, Yeshurun M, Yerushalmi R, Avigdor A et al. Allogeneic hematopoietic stem-cell transplantation in AML and MDS using myeloablative versus reduced-intensity conditioning: the role of dose intensity. Leukemia 2006; 20: 322–328.
Aoudjhane M, Labopin M, Gorin NC, Shimoni A, Ruutu T, Kolb HJ et al. Comparative outcome of reduced intensity and myeloablative conditioning regimen in HLA identical sibling allogeneic haematopoietic stem cell transplantation for patients older than 50 years of age with acute myeloblastic leukaemia: a retrospective survey from the Acute Leukemia Working Party (ALWP) of the European group for Blood and Marrow Transplantation (EBMT). Leukemia 2005; 19: 2304–2312.
Ringden O, Labopin M, Ehninger G, Niederwieser D, Olsson R, Basara N et al. Reduced intensity conditioning compared with myeloablative conditioning using unrelated donor transplants in patients with acute myeloid leukemia. J Clin Oncol 2009; 27: 4570–4577.
Song KW, Lipton J . Is it appropriate to offer allogeneic hematopoietic stem cell transplantation to patients with primary refractory acute myeloid leukemia? Bone Marrow Transplant 2005; 36: 183–191.
Faderl S, Ferrajoli A, Wierda W, Huang X, Verstovsek S, Ravandi F et al. Clofarabine combinations as acute myeloid leukemia salvage therapy. Cancer 2008; 113: 2090–2096.
Magenau J, Tobai H, Pawarode A, Braun T, Peres E, Reddy P et al. Clofarabine and busulfan conditioning facilitates engraftment and provides significant antitumor activity in nonremission hematologic malignancies. Blood 2011; 118: 4258–4264.
Andersson BS, Valdez BC, de Lima M, Wang X, Thall PF, Worth LL et al. Clofarabine +/- fludarabine with once daily i.v. busulfan as pretransplant conditioning therapy for advanced myeloid leukemia and MDS. Biol Blood Marrow Transplant 2011; 17: 893–900.
Farag SS, Wood LL, Schwartz JE, Srivastava S, Nelson RP Jr., Robertson MJ et al. Phase I trial and pharmacokinetic study of high-dose clofarabine and busulfan and allogeneic stem cell transplantation in adults with high-risk and refractory acute leukemia. Leukemia 2011; 25: 599–605.
Zebrowski AHA, Mangan J, Pinto-Martin J, Luger S, Loren A, Hexner E et al. Clofarabine busulfan conditioning improves outcomes in patients with active acute myelogenous leukemia undergoing allogeneic stem cell transplant. Blood 2014; 124: 1239 (abstract).
Slovak ML, Kopecky KJ, Cassileth PA, Harrington DH, Theil KS, Mohamed A et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood 2000; 96: 4075–4083.
Fine JP, Gray RJ . A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94: 496–509.
Kantarjian HM, Gandhi V, Kozuch P, Faderl S, Giles F, Cortes J et al. Phase I clinical and pharmacology study of clofarabine in patients with solid and hematologic cancers. J Clin Oncol 2003; 21: 1167–1173.
Srivastava S, Jones D, Wood LL, Schwartz JE, Nelson RP Jr., Abonour R et al. A phase I trial of high-dose clofarabine, etoposide, and cyclophosphamide and autologous peripheral blood stem cell transplantation in patients with primary refractory and relapsed and refractory non-Hodgkin lymphoma. Biol Blood Marrow Transplant 2011; 17: 987–994.
Parkin B, Ouillette P, Yildiz M, Saiya-Cork K, Shedden K, Malek SN . Integrated genomic profiling, therapy response, and survival in adult acute myelogenous leukemia. Clin Cancer Res 2015; 21: 2045–2056.
Grigg AP, Szer J, Beresford J, Dodds A, Bradstock K, Durrant S et al. Factors affecting the outcome of allogeneic bone marrow transplantation for adult patients with refractory or relapsed acute leukaemia. Br J Haematol 1999; 107: 409–418.
Wong R, Shahjahan M, Wang X, Thall PF, De Lima M, Khouri I et al. Prognostic factors for outcomes of patients with refractory or relapsed acute myelogenous leukemia or myelodysplastic syndromes undergoing allogeneic progenitor cell transplantation. Biol Blood Marrow Transplant 2005; 11: 108–114.
Copelan EA, Hamilton BK, Avalos B, Ahn KW, Bolwell BJ, Zhu X et al. Better leukemia-free and overall survival in AML in first remission following cyclophosphamide in combination with busulfan compared with TBI. Blood 2013; 122: 3863–3870.
Farag SS, Bolwell BJ, Elder PJ, Kalaycio M, Lin T, Pohlman B et al. High-dose busulfan, cyclophosphamide, and etoposide does not improve outcome of allogeneic stem cell transplantation compared to BuCy2 in patients with acute myeloid leukemia. Bone Marrow Transplant 2005; 35: 653–661.
Chen YB, Li S, Lane AA, Connolly C, Del Rio C, Valles B et al. Phase I trial of maintenance sorafenib after allogeneic hematopoietic stem cell transplantation for fms-like tyrosine kinase 3 internal tandem duplication acute myeloid leukemia. Biol Blood Marrow Transplant 2014; 20: 2042–2048.
de Lima M, Giralt S, Thall PF, de Padua Silva L, Jones RB, Komanduri K et al. Maintenance therapy with low-dose azacitidine after allogeneic hematopoietic stem cell transplantation for recurrent acute myelogenous leukemia or myelodysplastic syndrome: a dose and schedule finding study. Cancer 2010; 116: 5420–5431.
Schroeder T, Czibere A, Platzbecker U, Bug G, Uharek L, Luft T et al. Azacitidine and donor lymphocyte infusions as first salvage therapy for relapse of AML or MDS after allogeneic stem cell transplantation. Leukemia 2013; 27: 1229–1235.
Toubai T, Sun Y, Luker G, Liu J, Luker KE, Tawara I et al. Host-derived CD8+ dendritic cells are required for induction of optimal graft-versus-tumor responses after experimental allogeneic bone marrow transplantation. Blood 2013; 121: 4231–4241.
Acknowledgements
We wish to acknowledge Otsuka Pharmaceutical Co., Ltd. and Sanofi-Aventis U.S. LLC for providing research support to cover the costs of conducting this clinical trial. Sanofi-Aventis also provided clofarabine at no charge.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
HP has received honoraria and has served in as a consultant for Sanofi. The remaining authors declare no conflict of interest. This was an investigator initiated trial with Research Support provided by Otsuka Pharmaceutical Co., Ltd. and Sanofi-Aventis U.S. LLC. The authors are solely responsible for the design, data collection, analysis and decision to publish this trial.
Rights and permissions
About this article
Cite this article
Magenau, J., Westervelt, P., Khaled, S. et al. A multicenter trial of myeloablative clofarabine and busulfan conditioning for relapsed or primary induction failure AML not in remission at the time of allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 52, 59–65 (2017). https://doi.org/10.1038/bmt.2016.188
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2016.188
- Springer Nature Limited
This article is cited by
-
Pretransplantation predictors of survival in nonremission acute myeloid leukemia treated with haploidentical transplantation using steroid-based GVHD prophylaxis
Annals of Hematology (2024)
-
Re-induction therapy in patients with acute myeloid leukemia not in complete remission after the first course of treatment
Annals of Hematology (2023)
-
Myeloablative intravenous busulfan-containing regimens for allo-HSCT in AML or MDS patients over 54 years old: combined results of three phase II studies
International Journal of Hematology (2020)
-
The safety and efficacy of clofarabine in combination with high-dose cytarabine and total body irradiation myeloablative conditioning and allogeneic stem cell transplantation in children, adolescents, and young adults (CAYA) with poor-risk acute leukemia
Bone Marrow Transplantation (2019)
-
Clofarabine followed by haploidentical stem cell transplant using fludarabine, busulfan, and total-body irradiation with post-transplant cyclophosphamide in non-remission AML
International Journal of Hematology (2018)