Abstract
This research investigates the environmental impact of cement production by exploring eco-friendly geopolymer binders as alternatives. Geopolymer concrete, developed using silica and alumina-rich precursors such as pozzolanic materials, achieves high compressive strength, up to 43.6 N/mm2 with a 16 M concentration and integrated steel fibers. Utilizing manual mixing and industrial by-products, the study pioneers cast-in-situ geopolymers with innovative curing techniques. The paper presents experimental results on the engineering properties of geopolymer concretes of 40 MPa, cured at 100 °C and 60 °C. The study systematically varies binder content, examining proportions of fly ash, GGBS, metakaolin, and silica fume, along with different mix ratios and molar concentrations. Key findings include increased compressive strength with higher NaOH concentration, peaking at 35.2 N/mm2 and 34.22 N/mm2 for 14 M mixes at 7 and 28 days, and 40.29 N/mm2 for 16 M mixes at 7 days. Optimal results were observed at higher curing temperatures, especially with 14 M and 16 M compositions at 100 °C. The study recommends mechanized mixing for efficiency and calls for further investigation into the microstructure and chemistry of geopolymers to advance sustainable construction practices. This research represents a significant step towards eco-conscious building materials, reducing the environmental impact of the construction industry.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The production of Ordinary Portland Cement (OPC) significantly contributes to greenhouse gas emissions, as it releases a substantial amount of carbon dioxide (CO2) into the atmosphere. For every ton of OPC manufactured, one ton of CO2 is emitted, making OPC production a major environmental concern [1]. As OPC ranks as the second most commonly used material globally, just after water, the development of sustainable cement substitutes is imperative. This can be achieved by combining natural resources like kaolin with the cementitious qualities of industrial byproducts such as fly ash and ground granulated blast furnace slag (GGBS) [2, 3]. Geopolymer concrete (GPC) emerges as a promising alternative to traditional cement due to its environmentally benign nature. Named for Davidovits, "geopolymer" refers to an alternate cementitious material that resembles ceramic. Unlike OPC, the polymerization process of geopolymers does not release greenhouse gases. Geopolymers are produced by mixing alkaline solutions with pozzolanic compounds or aluminosilicate sources [4]. Common materials like fly ash and GGBS, which are rich in silica and alumina, can replace cement in GPC, thereby reducing CO2 emissions and enhancing mechanical strength and durability [5, 6].
Numerous studies have explored the properties and performance of GPC made from supplementary cementitious materials (SCMs): Palomo et al. [7] Investigated GPC using Class F fly ash and tested various ratios of alkaline activator to fly ash. The mixtures activated with sodium hydroxide and sodium silicate achieved compressive strengths exceeding 60 MPa after curing for 24 hours at 65 °C. Xu and van Deventer [8] found that an ideal ratio of 0.33 between alkali solution and alumina-silicate yielded a maximum compressive strength of 19 MPa after a 72-hour curing period at 35°C. Hardjito and Rangan [9] studied GPC composition by varying sodium hydroxide concentrations from 8M to 16M and adjusting the sodium hydroxide to sodium silicate ratio. Higher NaOH molarity and Na2SiO3 to NaOH ratio enhanced compressive strength, reaching 67 MPa after 24 hours of curing at 60 °C. Januarti Jaya Ekaputri et al. [10] examined the mechanical properties of GPC made from Jawa Power Paiton fly ash. The highest compressive strength of 48.59 MPa was achieved with a 10 M activator solution and a sodium silicate to sodium hydroxide ratio of 1.5. Tabassum et al. [11] found that different sodium hydroxide solution concentrations have distinct effects on geopolymer concrete mixtures. Rovnanik [12] investigated how curing temperature and duration affect metakaolin-based geopolymer, finding higher temperatures hasten dense structure formation and geopolymerization. Liew et al. [13] explored curing conditions' impact on metakaolin geopolymer pastes, emphasizing heat curing's necessity. Therefore, Geopolymer concrete requires heat curing. Low temperatures hinder geopolymerization, reducing mechanical properties. Geopolymer concrete's strength qualities were shown to be enhanced by an ideal NaOH content of 12 M. A maximum compressive strength of 40.21 MPa was reached by the concrete after 28 days [14]. Achieving sufficient strength also necessitates proper curing. Various studies have demonstrated that the compressive strength of fly ash-based geopolymer concrete (GPC) specimens cured in an oven is higher than that of those cured under ambient conditions [15]. The majority of research has demonstrated that geopolymer concrete is typically produced using sodium hydroxide solution molarities within the range of 8 M to 16 M. Optimal strengths, as indicated by various studies, is generally noted within the concentration range of 12 M to 16 M. [16,17,18].
Despite the extensive research on GPC, there is a need to consolidate and build upon the existing knowledge to develop more practical and cost-effective formulations. The core problem addressed in this study is to identify the optimal mix designs and curing conditions that maximize the strength of GPC. The research aims to fill gaps in understanding the interplay between different SCMs and alkaline activators, as well as the influence of curing regimes on the mechanical properties of GPC. The promise of GPC made by SCMs is found in both its enhanced compressive strength and environmental advantages. This research aims to improve the development of high-performance and sustainable GPC, thereby reducing carbon emissions in the construction sector, by drawing on and expanding upon the findings of earlier studies.
This experimental research focused on the process of making the geopolymer concrete, which is notable for its exceptional characteristics, including strong adhesion, uniformity during mixing, and high slump levels. Initially, the workability of geopolymer concrete decreases during manual mixing due to its high viscosity, primarily because the alkaline-to-geopolymer solids ratio drops according to the mix design. However, by adjusting the alkaline/binder (a/b) ratio to 0.42 and incorporating additional alkaline liquid, the workability of the concrete can be significantly improved. Moreover, a slight increase in the alkali solution content during mixing enhances the desired slump value across different mix proportions of geopolymer concrete. Introducing extra activator to the mix results in a higher concentration of alkali solution, reducing viscosity and cohesion during manual mixing, ultimately facilitating the achievement of necessary workability and strength, especially in higher grades of concrete. Previous studies suggest that sufficient strength also requires curing, as various investigations have demonstrated that the compressive strength of fly ash-based geopolymer concrete (GPC) specimens cured in an oven is higher than that of those cured under ambient conditions. This underscores the importance of controlled curing processes to optimize the mechanical properties of geopolymer concrete.
The primary objective of this study is to explore the compressive strength characteristics of geopolymer concrete by examining how adjustments in the composition of binders impact its performance. The study systematically investigates various combinations of fly ash and GGBS at different proportions, ranging from 10% to 20% for fly ash and 40% to 60% for GGBS, while keeping metakaolin constant at 10%. Silica fume is also included in proportions ranging from 18% to 28%, with the curing procedure involving oven temperatures between 60°C and 100°C. To comprehensively explore the effects on compressive strength, the study considers three different mix proportions and two different molar concentrations (14 and 16 M). By analyzing these different compositions, the researchers aim to gain insights into optimizing the properties of geopolymer concrete for enhanced compressive strength. The implications of this study are significant for advancing the field of geopolymer concrete technology, contributing to the development of more efficient and effective mix designs that exceed current performance standards and providing a deeper understanding of the interactions between different binder components for more sustainable and high-performance concrete solutions.
2 Experimental work
2.1 Materials used
This study utilized various types of binders as the primary alumina-silicon source materials for geopolymer concrete. These binders included class F fly ash (in accordance with IS 3812-2003 [19]) and ground granulated blast furnace slag (GGBS) (in accordance with IS 16714:2018 [20]), with specific gravities of 2.00 and 2.87, respectively, sourced from Suyog Elements Pvt Ltd in Baruch, Gujarat, India. Additionally, metakaolin from AJ Corporation in Mumbai, India, and silica fume from Astra Chemicals in Chennai, Tamil Nadu, India, with specific gravities of 2.6 and 2.64, respectively, were used. The chemical compositions of the fly ash, GGBS, metakaolin, and silica fume are detailed in Table 1. Coarse aggregate with a maximum size of 4.75 mm, a specific gravity of 2.53, and a 24-hour water absorption rate of 4.32% was employed. Fine aggregate, with a maximum size of 600 microns, a specific gravity of 2.63, a 24-hour water absorption rate of 0.6%, and a fineness modulus of 2, was also used. Both aggregates conform to the standards outlined in IS 383-2016 [21] and meet the criteria for zone II.
This investigation utilized commercially available sodium hydroxide (NaOH) pellets with a purity of 98%. Additionally, we utilized liquid sodium silicate (Na2SiO3), commonly known as Waterglass, which is easily obtainable in the market. The detailed chemical composition of sodium silicate is presented in Table 2. It is essential to note that the choice of both sodium hydroxide and sodium silicate was made based on their availability and well-established properties in relevant applications. Demineralized (DM) water is recommended for diluting sodium hydroxide (NaOH). The use of DM water in the mixing process eliminates mineral impurities, resulting in a cleaner and more effective final sodium hydroxide solution.
In this experimental study, a unique variety of fiber known for its outstanding resilience and lasting quality, namely brass-coated micro steel fiber, is employed. This fiber adheres to the standards outlined in ASTM A-820 Type-1 [22]. The dimensions of the fiber utilized in this research are 0.26 mm in diameter and 13 mm in length, exhibiting a straight configuration. Further specifications of the steel fiber are provided in Table 3.
The Conplast SP550, known for its exceptional water-reducing properties, is widely used, especially in micro silica concrete applications. It adheres to the IS: 9103:1999(2007) [23], and ASTM-C-494 Type 'G' [24] standards. Characterized by its brown liquid form and easy dispersal in water, it possesses a specific gravity of 1.24. This additive, containing Sulphonated Naphthalene Superplasticizer, plays a vital role in the current study. Its adaptability and adherence to industry norms make it a valuable choice for enhancing concrete performance. Here, fig. 1 presents photographs of the materials used in the experiment.
2.2 Alkaline liquid
Alkaline liquids are typically formulated by blending a solution of sodium hydroxide with sodium silicate at room temperature. As these two solutions combine, they undergo a reaction known as polymerization, resulting in the release of a substantial amount of heat. It is advisable to allow the mixture to stand for approximately 24 hours. This resting period ensures that the alkaline liquid, serving as a binding agent, is fully prepared [25].
2.3 Preparation of alkaline liquid
2.3.1 Sodium hydroxide
Two separate concentrations of sodium hydroxide pellets are dissolved in water, specifically at 14 and 16 molars. It's strongly recommended to prepare the sodium hydroxide solution at least 24 hours before use. Moreover, if this preparation exceeds a 36-hour duration, the solution tends to transition into a semi-solid state. Thus, it's crucial to utilize the prepared solution within this prescribed time limit [26,27,28].
2.3.2 Molarity calculation
Consider two concentrations of sodium hydroxide (NaOH) solution: 14 and 16 mol per liter. This translates to 560 g (14 × 40) and 640 g (16 × 40) of NaOH solids per liter of water for each molarity, with 40 representing NaOH's molecular weight. It's important to emphasize that water remains the primary constituent in both alkaline solutions. Notably, the NaOH concentration directly influences the quantity of solid NaOH in each solution, with the 16 Molar solution containing a greater mass compared to the 14 Molar solution.
The correct method involves adding 560 g of sodium hydroxide solids gradually to a specified amount of water, such as 500 ml. After ensuring complete dissolution, the volume of Sodium Hydroxide Solution (SHS) is measured to confirm it reaches one liter. If the solution falls short of this volume, additional water is added to reach exactly one liter. Conversely, if the SHS exceeds one liter, 560 g of sodium hydroxide solids are added to a smaller volume of water than previously used, and the process is repeated [29].
2.4 Mixing, casting and curing
A framework and code of practice exist for conventional concrete mixes, but not for geopolymer concrete. Thus, creating a geopolymer concrete mix must be based on conventional mix design concepts. Various mix proportioning methods are used to achieve the necessary concrete strength, considering the task, material properties, availability, field conditions, and requirements for durability and workability. Rangan [25] proposed a fly ash-based technique for geopolymer concrete, while Anuradha et al. [26] provided updated guidelines based on the Indian standard code. In this experimental geopolymer concrete mix was created utilizing the mix design technique specified in IS 10262-2019 [30]. In this study, the geopolymer mixing procedure encompassed five sequential stages. Initially, an alkaline solution was prepared by dissolving sodium hydroxide (NaOH) solids in demineralized water to achieve the desired concentration. This solution was then blended with sodium silicate solution before a 24-hour period prior to casting. Secondly, coarse and fine aggregates were meticulously mixed with the binder manually to create a well-blended dry mixture. The third step involved combining the prepared alkaline solution with a superplasticizer. In the fourth step, the liquid component was gradually incorporated into the dry mix, and the mixing process persisted for approximately 10-15 minutes until a uniform concrete mix was obtained. Finally, steel fibers were introduced and continuously mixed for 3-5 minutes. The workability of the freshly mixed concrete was assessed using the slump cone test, similar to that used for cement concrete. After the flow test, the fresh concrete was placed in the mold according to IS 1199-1959 [31]. Then the concrete was promptly poured into 150mm x 150mm x 150mm molds, followed by compaction using a tampering rod with approximately 45-50 blows to ensure proper compaction. After a 24-hour curing period, the specimens were demolded and subjected to further curing in a hot air oven for an additional 24 hours at various temperatures, alongside being maintained at ambient temperature (25–27°C) until testing. The curing temperatures differ according to the raw material utilized for fly ash-based geopolymer concrete, curing occurs at 60°C [26, 32]. Furthermore, to determine the compressive strengths, geopolymer concrete cubes were tested according to the guidelines specified in IS 516-1959 [33].
Table 4 provides a detailed breakdown of the compositions of the binder mixes, expressed as percentages, and specifies the molar concentrations for three distinct mix designations: Geopolymer -1 (GP1), Geopolymer -2 (GP2), and Geopolymer -3 (GP3). Each mix designation is characterized by concentrations of 14 M and 16 M. In the experimental phase, a total of 72 cubes were cast, with twelve specimens allocated to each mix designation. These cubes underwent curing at various temperatures, as depicted in fig 2. Following an initial 24-hour curing period in an oven, the casted cube specimens were transferred to a laboratory environment and left at room temperature until the conclusion of the testing day, in accordance with the procedure outlined in Fig 3. Subsequently, compression tests were conducted on the cube specimens of geopolymer concrete using a testing machine with a capacity of 2000 KN shown in fig 4. Notably, the results reported represent the average strength derived from three cube measurements. Furthermore, Table 5 presents a summary of the proportions of binder mix designs, detailing the quantities in kilograms per cubic meter (Kg/m3). This comprehensive overview aids in understanding the precise formulation of the geopolymer concrete and its experimental parameters.
3 Results and discussion
3.1 Workability
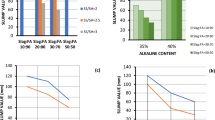
The changes in workability for the different mixes are shown in Fig. 5. The data reveal that the slump value decreased with an increase in GGBS content, consistent with previous studies [34]. Mix no. GP1 exhibited the highest workability with a slump value of 55 mm. Mixes GP2 and GP3 showed the smallest variation in workability values for both molars of GPC. There was no additional water added to the alkaline solution during the GPC casting process. However, adding more GGBFS affected the workability of the trial mixes [16]. Das et al. [35] has identified that the low workability is due to irregular shapes of fly ash and angular GGBFS. These shapes lead to significant particle interlocking, thereby decreasing workability. Greater amounts of CaO in GGBS accelerate hydration and the formation of C-A-S-H/C-S-H gel, leading to quicker setting times and decreased workability [36,37,38]. When metakaolin was added or replaced by other materials, the slump value decreased due to its plate-like shape, which required more water or superplasticizers. The increased surface area and high fineness of slag also contributed to this need for additional water or superplasticizers to maintain workability [39]. With higher silica fume content, the slump in geopolymer concrete decreased. Their high viscosity results in low workability, making them more cohesive and viscous than OPC concrete [40, 41].
3.2 Compressive strength
Following a thorough examination conducted over a period of 7 days, it was discerned that the highest levels of strength were attained under specific conditions. For instance, in the case of the 14 M concentration, the GP3 mixture displayed a remarkable strength of 35.2 N/mm2 after 7 days, while the GP2 mixture exhibited a slightly lower but still notable strength of 34.22 N/mm2 after 28 days. The findings suggest that increasing the GGBS content in the GPC mixtures resulted in higher compressive strength. This improvement is due to the significant production of calcium silicate hydrate gel [42]. Similarly, for the 16 M concentration, the GP2 mixture demonstrated a notable compressive strength of approximately 40.29 N/mm2 after 7 days, while the GP1 mixture showcased an even higher strength of 43.6 N/mm2 after 28 days. Okoye et al., conducted research on the impact of silica fume on the compressive strength of geopolymer concrete. The study revealed that the strength consistently increased with the addition of silica fume, achieving the maximum improvement at a 40% addition, which was the highest amount tested in the experiment [43]. It is crucial to note that all these mixtures underwent initial curing at a temperature of 100°C, indicating a standardized initial condition. This controlled environment ensures consistency in the experimental setup, allowing for accurate comparison and analysis of the results. The compressive strength results for 14 molar mixtures at 7 and 28 days are shown in Fig. 6, illustrating the early-stage performance of each mixture. Meanwhile, Fig. 7 present the compressive strength results for 16 molar mixtures at 7 and 28 days, providing insights into the longer-term performance of the mixes. By encompassing data from the three provided mixes (GP1, GP2, and GP3) across two different molar concentrations (14M and 16M), these figures provide a comprehensive overview of the experimental findings. This comprehensive approach facilitates a deeper understanding of how varying factors such as mix composition and molar concentration impact the compressive strength of the materials over time.
When the GP2 blend with a molarity of 16 was subjected to a curing temperature of 60°C, it exhibited exceptional performance characteristics. Specifically, it achieved an impressive compressive strength of 29.65 N/mm2 within a remarkably short period of just 7 days. This rapid development of strength highlights the effectiveness of the curing process at this temperature. Furthermore, even after the initial 7-day period, the GP2 mixture, still maintained at a molarity of 16, continued to display its strength. Over the course of a 28-day curing period, it further improved its compressive strength, eventually reaching a peak value of 31.48 N/mm2. This sustained enhancement in strength suggests that the curing process not only initiates rapid development but also facilitates continued improvement in the material's mechanical properties over time.
Conversely, the GP1 mix, despite sharing the same molarity (16 Molar), displayed notably lower performance, registering a compressive strength of only 10.44 N/mm2 within the initial 7 days. This value notably lagged behind the corresponding results for both different molarities during the same period. Moreover, for the GP1 mix with a slightly lower molarity of 14 M, the compressive strength recorded after 28 days was similarly inferior, reaching only 16.23 N/mm2. In summary, the GP2 blend, particularly with a molarity of 16, exhibited superior compressive strength characteristics compared to the GP1 mix under similar conditions, showcasing its potential for robust performance in concrete applications.
Figures. 5, 6 illustrate the changes in compressive strength for 14 M and 16 M NaOH concentration solutions. Both figures indicate that increasing the molarity of the NaOH solution leads to a slight rise in compressive strength. Notably, the 16 M mixture, containing 40% GGBFS and 28% silica fume, shows higher compressive strength compared to the 14 M mixture. While the compressive strength of 16 M NaOH solution mixtures increase steadily, the 14 M NaOH mixtures exhibit erratic increments. The higher molarity may facilitate more Al atoms receiving electrons from Na atoms, leading to increased sialate bond formation [44, 45].
4 Conclusions
4.1 Findings
This research focused on developing eco-friendly geopolymer concrete (GPC) using fly ash, GGBS, metakaolin, and silica fume. The study identified optimal mix designs and curing conditions to maximize the compressive strength of GPC. Specifically, the highest compressive strengths were achieved under certain conditions: for 14 M NaOH, the GP3 mixture reached 35.2 N/mm2 after 7 days, and the GP2 mixture reached 34.22 N/mm2 after 28 days. For 16 M NaOH, the GP2 mixture achieved 40.29 N/mm2 after 7 days, and the GP1 mixture reached 43.6 N/mm2 after 28 days. The GP2 blend, with 16 M NaOH and a 60 °C curing temperature, achieved 29.65 N/mm2 in 7 days and 31.48 N/mm2 in 28 days, while the GP1 mix performed poorly, reaching only 10.44 N/mm2 in 7 days and 16.23 N/mm2 in 28 days. Initial curing at 100 °C was essential for consistency. The research demonstrated that GPC offers significant environmental benefits by reducing carbon emissions and supports sustainable construction practices.
4.2 Research Limitations
The study was conducted under controlled laboratory conditions, which may not fully capture real-world applications. It primarily addressed mechanical properties, environmental impacts, and practical scalability. The specific focus on high molarity NaOH solutions and selected binder contents may not represent the full spectrum of possible formulations.
4.3 Recommendations for Future Research
Future studies should focus on consolidating and expanding existing knowledge to develop more practical and cost-effective GPC formulations. Investigations should include a broader range of SCMs, mix proportions, and curing methods, such as ambient curing. Further research should explore the long-term durability and environmental benefits of GPC, optimizing formulations for high-performance and sustainable construction applications. Understanding the microstructural and chemical interactions in GPC will further enhance its development and practical use.
4.4 Implications
Despite the extensive research on GPC, there is a need to consolidate and build upon the existing knowledge to develop more practical and cost-effective formulations. The core problem addressed in this study is to identify the optimal mix designs and curing conditions that maximize the strength of GPC. The research aims to fill gaps in understanding the interplay between different SCMs and alkaline activators, as well as the influence of curing regimes on the mechanical properties of GPC. This study highlights the potential of GPC to significantly reduce carbon emissions in the construction sector, offering a viable alternative to traditional Portland cement. The findings underscore the promise of GPC, particularly in its enhanced compressive strength and environmental advantages, thus contributing to the development of high-performance, sustainable GPC. This work lays the foundation for further advancements in eco-friendly building materials, promoting a more sustainable approach in the construction industry.
Data availability
The data supporting this research is enclosed within this paper and does not need to be referred from an external source.
References
Davidovits J. Geopolymers: man-made rock geosynthesis and the resulting development of very early high strength cement. J Mater Educ. 1994;16:91–91.
Nath P, Sarker PK. Effect of GGBFS on setting, workability and early strength properties of fly ash geopolymer concrete cured in ambient condition. Const Buil Mater. 2014;66:163–71. https://doi.org/10.1016/j.conbuildmat.2014.05.080.
Deb PS, Nath P, Sarker PK. The effects of ground granulated blast-furnace slag blending with fly ash and activator content on the workability and strength properties of geopolymer concrete cured at ambient temperature. Mater Design. 2014. https://doi.org/10.1016/j.matdes.2014.05.001.
Davidovits J. Geopolymers: inorganic polymeric new materials. J Therm Anal Calorim. 1991;37(8):1633–56. https://doi.org/10.1007/BF01912193.
Davidovits J. Global warming impact on the cement and aggregates industries. World Res Rev. 1994;6(2):263–78.
Motorwala A, Shah V, Kammula R, Nannapaneni P, Raijiwala DB. Alkali activated fly-ash based geopolymer concrete. Int J Emerg Technol Adv Eng. 2013;3(1):159–66.
Palomo A, Grutzeck MW, Blanco MT. Alkali-activated fly ashes. Cement Conc Res. 1999. https://doi.org/10.1016/s0008-8846(98)00243-9.
Xu, Hua. "The geopolymerisation of alumino-silicate minerals [thesis]." University of Melbourne (2002).
Hardjito, Djwantoro, and B. Vijaya Rangan. "Development and properties of low-calcium fly ash-based geopolymer concrete.", provided by Curtin University of Technology. (2005). http://hdl.handle.net/20.500.11937/5594
Ekaputri JJ, Damayanti O. Sifat mekanik beton geopolimer berbahan dasar fly ash jawa power paiton sebagai material alternatif. J Pondasi. 2007;13(2):124–34.
Tabassum RK, Khadwal A, Ash F. Effect of sodium hydroxide concentration on various properties of geopolymer concrete. IJETR. 2015;3(10):2454–4698.
Rovnaník P. Effect of curing temperature on the development of hard structure of metakaolin-based geopolymer. Const Buil Mater. 2010. https://doi.org/10.1016/j.conbuildmat.2009.12.023.
Liew YM, Hussin K, Al Bakri Abdullah MM, Binhussain M, Musa L, Khairul Nizar I, Ghazali CMR, Heah CY. Effect of curing regimes on metakaolin geopolymer pastes produced from geopolymer powder. Adv Mater Res. 2013;626:931–6.
Djobo JNY, Tchakouté HK, Ranjbar N, Elimbi A, Tchadjié LN, Njopwouo D. Gel composition and strength properties of alkali-activated oyster shell-volcanic ash: effect of synthesis conditions. J Am Ceram Soc. 2016. https://doi.org/10.1111/jace.14332.
Bakria AMMA, Kamarudin H, BinHussain M, Nizar IK, Zarina Y, Rafiza AR. The effect of curing temperature on physical and chemical properties of geopolymers. Phys Procedia. 2011. https://doi.org/10.1016/j.phpro.2011.11.045.
Aliabdo AA, Abd Elmoaty AEM, Salem HA. Effect of water addition, plasticizer and alkaline solution constitution on fly ash based geopolymer concrete performance. Const Buil Mater. 2016. https://doi.org/10.1016/j.conbuildmat.2016.06.062.
Bidwe SS, Hamane AA. Effect of different molarities of sodium hydroxide solution on the strength of geopolymer concrete. Am J Eng Res. 2015;4(3):139–45.
Raijiwala DB, Patil HS. Geopolymer concrete: a concrete of next decade. J Eng Res Stud. 2011;2(1):19–25.
IS 3812–1: 2003. Specification for Pulverized Fuel Ash, Part 1: For Use as Pozzolana in Cement, Cement Mortar and Concrete (2003).
IS 16714, Specification for Ground Granulated Blast Furnace Slag for Use in Cement, Mortar & Concrete 2018.
Bureau of Indian Standard, DS. "Coarse and Fine Aggregate for Concrete Specification." (2016).
ASTM A820–01. Specification for steel fibers for fiber-reinforced concrete. ASTM International.
IS: 9103:1999, specification for admixtures for concrete (2007).
ASTM-C-494 Type 'G' standard specification for admixtures for concrete.
Rangan BV. Mix design and production of flyash based geopolymer concrete. Indian Conc J. 2008;82(5):7–15.
Anuradha RV, Sreevidya R, Venkatasubramani, B Vijaya Rangan. Modified guidelines for geopolymer concrete mix design using Indian standard. Asian Journal of Civil Engineering. 357–368. 2012
Aisheh YIA, Atrushi DS, Akeed MH, Qaidi S, Tayeh BA. Influence of steel fibers and microsilica on the mechanical properties of ultra-high-performance geopolymer concrete (UHP-GPC). Case Stud Const Mater. 2022. https://doi.org/10.1016/j.cscm.2022.e01245.
Abdellatief M, Alanazi H, Radwan MKH, Tahwia AM. Multiscale characterization at early ages of ultra-high performance geopolymer concrete. Polymers. 2022. https://doi.org/10.3390/polym14245504.
Rajamane NP, Jeyalakshmi R. Quantities of sodium hydroxide solids and water to prepare sodium hydroxide solution of given molarity for geopolymer concrete mixes. Indian Concrete Institute Technical Paper: SRM University, India; 2014.
IS 10262–2019 Recommended guidelines for concrete mix design.
IS 1199–1959. Methods of Sampling and Analysis of Concrete. Bureau of Indian Standard: New Delhi, India (2004)
Bernal SA, Mejía de Gutiérrez R, Provis JL. Engineering and durability properties of concretes based on alkali-activated granulated blast furnace slag/metakaolin blends. Const Buil Mater. 2012. https://doi.org/10.1016/j.conbuildmat.2012.01.017.
IS 516–1959. Methods of Tests for Strength of Concrete. New Delhi, India: Bureau of Indian Standards. 1999.
Nath P, Sarker PK. Effect of GGBFS on setting, workability and early strength properties of fly ash geopolymer concrete cured in ambient condition. Const Buil Mater. 2014. https://doi.org/10.1016/j.conbuildmat.2014.05.080.
Das SK, Shrivastava S. Siliceous fly ash and blast furnace slag based geopolymer concrete under ambient temperature curing condition. Struct Conc. 2020. https://doi.org/10.1002/suco.201900201.
Kumar S, Kumar R, Mehrotra SP. Influence of granulated blast furnace slag on the reaction, structure and properties of fly ash based geopolymer. J Mater Sci. 2010. https://doi.org/10.1007/s10853-009-3934-5.
Koenig A, Herrmann A, Overmann S, Dehn F. Resistance of alkali-activated binders to organic acid attack: assessment of evaluation criteria and damage mechanisms. Const Buil Mater. 2017. https://doi.org/10.1016/j.conbuildmat.2017.06.117.
Li X, Ma X, Zhang S, Zheng E. Mechanical properties and microstructure of class c fly ash-based geopolymer paste and mortar. Materials. 2013. https://doi.org/10.3390/ma6041485.
Ali A, Al-Attar T, Abbas WA. Mechanical performance of blended fly ash-based geopolymer concrete with GGBS and metakaolin. Eng Technol J. 2022. https://doi.org/10.30684/etj.2022.132647.1135.
Rangan BV. Low-calcium, fly-ash-based geopolymer concrete. In: Nawy E, editor. Concrete construction engineering handbook. Florida: CRC Press; 2008. p. 26–31.
Chindaprasirt P, Chareerat T, Sirivivatnanon V. Workability and strength of coarse high calcium fly ash geopolymer. Cement Concrete Comp. 2007. https://doi.org/10.1016/j.cemconcomp.2006.11.002.
Yip CK, Lukey GC, van Deventer JSJ. The coexistence of geopolymeric gel and calcium silicate hydrate at the early stage of alkaline activation. Cement Conc Res. 2005. https://doi.org/10.1016/j.cemconres.2004.10.042.
Okoye FN, Durgaprasad J, Singh NB. Effect of silica fume on the mechanical properties of fly ash based-geopolymer concrete. Ceram Int. 2016. https://doi.org/10.1016/j.ceramint.2015.10.084.
Al Bakri Abdullah MM, Kamarudin H, Abdulkareem OAKA, Ghazali CMR, Rafiza AR, Norazian MN. Optimization of alkaline activator/fly ash ratio on the compressive strength of manufacturing fly ASH-BASED geopolymer. Appl Mecha Mater. 2011. https://doi.org/10.4028/www.scientific.net/amm.110-116.734.
Abdullah MMAB, Kamarudin H, Bnhussain M, Ismail KN, Rafiza AR, Zarina Y. The relationship of NaOH molarity, Na2SiO3/NaOH ratio, fly ash/alkaline activator ratio, and curing temperature to the strength of fly ash-based geopolymer. Adv Mater Res. 2011;328:1475–82. https://doi.org/10.4028/www.scientific.net/amr.328-330.1475.
Acknowledgements
I extend my heartfelt thanks to Dr. Suhasini Kulkarni and Dr. Hardik Solanki for their invaluable support and guidance throughout this academic endeavor, without which its successful completion would not have been possible. I also wish to acknowledge the assistance provided by Parul Universi-ty, Vadodara, in facilitating and supporting this research project. Additionally, I am sincerely grate-ful to Suyog Elements Pvt Ltd, Bharuch, Gujarat, India, and Mangalmurti Conchem Pvt Ltd, Vadoda-ra, Gujarat, for supplying essential materials such as Fly ash, GGBS, and Fosroc Conplast SP550 for my study. Finally, I would like to express my appreciation to Mr. Punit Patel, Mr. Joy Amit Sanghavi, Mr. Jenish Patel, and Mr. Roshan Badadwal, undergraduate students at Parul University, for their valuable assistance in sample preparation.
Author information
Authors and Affiliations
Contributions
Conceptualization, Sandeep Thapa; Methodology, Sandeep Thapa; Validation, Sandeep Thapa, Investigation, Sandeep Thapa, Resource, Sandeep Thapa; Writing Orginal Draft Preperation, Sandeep Thapa; Supervision, Dr. Suhasini Kulkarni and Dr. Hardik Solanki.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Thapa, S., Debnath, S., Kulkarni, S. et al. Mechanical properties of geopolymer concrete incorporating supplementary cementitious materials as binding agents. Discov Civ Eng 1, 62 (2024). https://doi.org/10.1007/s44290-024-00064-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44290-024-00064-0