Abstract
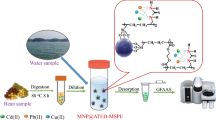
Environmental contamination caused by heavy metals is a significant global concern. The presented study investigated the efficiency of iron PAMAM-OH encapsulated magnetic nanoparticles (Fe-MNP-G2-OH) as sorbent for the preconcentration of copper and cobalt from tea samples. High metal-chelating ethylenediamine core polyamidoethanol generation-2 (PAMAM-G2-OH) was encapsulated with iron oxide (Fe3O4) to synthesize the sorbent. Limit of detection (LOD) values for copper and cobalt extracted and detected by the developed Fe-MNP-G2-OH -SPE-FAAS method were 0.52 and 1.1 μg L−1, respectively. There were 230- and 101-fold improvement in detection limits for copper and cobalt, respectively, when compared to direct FAAS measurement. The percent recovery results for the analytes in green and black tea samples ranged from 93 to 107%, with low relative standard deviation (%RSD) values. The synthesis of nanoparticle was carried out through a unique method, which was characterized by thermogravimetric analysis (TGA), scanning electron microscope (SEM), and Fourier transform infrared spectroscopy (FTIR) methods. The analytical results demonstrated the applicability and effectiveness of Fe-MNP-G2-OH nanoparticles on the preconcentration of copper and cobalt from tea samples and the developed method is suitable for the trace detection of heavy metals by FAAS method. To the best our knowledge, this is the first study where copper and cobalt in green and black tea samples were extracted by Fe-MNP-G2-OH adsorbent and precipitation of the adsorbent during its synthesis was carried out in acetone medium rather than aqueous one.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pollution of natural resources is a global problem that affects the environment and living organisms. Despite the fight against pollution, the increasing population leads to increase the sources of pollution day by day [33]. Toxic metal pollution, which can be cumulative in the environment, impacts living things from the smallest organism to the more advanced ones [22, 33]. The non-stop release of heavy metals into the environment and the dissolution of these chemicals in water medium make them more difficult to remove. Some common heavy metals in the environment include Hg2+, Cd2+, Pb2+, Cr3+, As3+, Cu2+, Ni2+, and Co2+. The toxicity levels of these metals and their maximum permissible limits in water, soil, or foods were declared by various regulatory authorities [28]. For these reasons, it is very important to detect these heavy metals accurately and precisely in various environmental matrices at trace levels.
Analytical techniques such as inductively coupled plasma-mass spectrometry (ICP-MS), inductively coupled plasma-optical emission spectrometry (ICP-OES), graphite furnace atomic absorption spectrometry (GFAAS), and anodic stripping voltammetry (ASV) have been used for the determination of several elements at trace levels [11, 20, 21, 23]. These instruments have high precision but need high operational cost. In contrast, flame atomic absorption spectrometry (FAAS) is more accessible and relatively inexpensive than other instruments. However, auxiliary techniques are carried out to overcome the main disadvantages of FAAS systems. It is known that FAAS system has low sensitivity for most metals, resulting from low nebulization efficiency and rapid exit of atoms from the atomization region. Various preconcentration methods such as liquid-phase microextraction (LPME), cloud point microextraction (CPE), solidified floating organic drop microextraction (SFODME) and solid-phase extraction (SPE) have been applied to enrich the analyte(s) to a concentration level measurable by the FAAS system [5, 7, 27, 32]. The use of modified magnetic sorbents in SPE methods to preconcentrate different analytes from various sample matrices has been one of the popular applications in recent years. Magnetic nanoparticles (MNPs) have been widely used in analyte preconcentration processes and there are several studies to maximize their potentials in preconcentration processes. MNPs are useful materials for SPE applications because their adsorption surface areas are significantly higher than other materials, and can collect analytes even from complex environmental matrices [27].
MNPs can be modified with various structures that selectively or more effectively bind to the target analyte and separate the analyte from the bulk sample. Dendrimers are highly branched and functional macromolecules with a strong heavy metal binding capacity [31]. Dendrimeric molecules have been evaluated as strong metallic chelators/sorbents due to their inner cages and nitrogen functional groups [24]. After dendrimers have been modified, they can be conveniently used as SPE sorbents.
The synthesis of magnetic dendrimers using Fe3O4 needs the Michael addition and amidation reactions. These magnetic dendrimers have analytical applications for the extraction of various analytes in the literature [37, 38]. In addition, some studies on the production of magnetic sorbents by dendrimer encapsulation are available in some studies [6, 14, 18]. Since encapsulated and core-produced dendrimeric nanoparticles are completely different in terms of their structures, these materials tend to behave differently. Yuan et al. presented a magnetic sorbent prepared by growing third-generation poly(amido)amine on the surface of superparamagnetic microspheres. It was observed that the synthesized sorbent provided a better maximum adsorption capacity (395.2 mg g−1) for U(VI) than many reported studies. In addition, it was observed that the sorbent could be reused 5 times without any significant change in the U(VI) removal efficiency [36]. Yuan et al. synthesized magnetic PAMAM nanoparticles for use in a magnetic solid-phase extraction (MSPE) method to preconcentrate cadmium and mercury ions. Diethyldithiocarbamate (DDTC-Na) was selected as the chelator and high-performance liquid-phase chromatography and ultraviolet variable wavelength detector (HPLC-VWD) was utilized in the separation and detection of the analytes. Under the optimal conditions of the proposed analytical method, the detection limits for Cd2+ and Hg2+ were recorded as 0.016 and 0.040 μg L−1, respectively [37]. In another work conducted by Ma et al., PAMAM dendrimers (MGO-PAMAM) up to the fourth generation were synthesized using graphene-oxide-supported Fe3O4 nanoparticles [18]. MGO-PAMAM structures are characterized by various techniques such as transmission electron microscopy (TEM), X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), elemental analysis, X-ray photoelectron spectroscopy (XPS), and thermogravimetric analysis (TGA). Further, MGO-PAMAM can be evaluated as a sorbent for the extraction of various analytes. Results have shown that the MGO-PAMAM third-generation sorbent is an effective sorbent for Hg(II), with a maximum adsorption capacity of 113.71 mg g−1 [18].
This study proposed an unconventional procedure for the synthesis of Fe-MNP-G2-OH sorbent and an accurate and sensitive analytical method for copper and cobalt at trace levels in tea samples. To the best our knowledge, this is the first study that Fe-MNP-G2-OH sorbent was synthesized in acetone medium by the precipitation reaction and used as sorbent for the extraction of cobalt and copper from tea samples. Encapsulated PAMAM-OH MNP was synthesized using iron salts, and characterization studies were performed using TGA, SEM and FTIR. The synthesis procedure was optimized in terms of iron salts/dendrimer molar ratio. The encapsulated magnetic particles were used as an effective sorbent for the preconcentration of copper and cobalt from tea samples (green and black tea). Substantial parameters such as pH, buffer solution volume, sorbent amount, dissolvent type and volume, mixing type and volume were optimized, and the analytical performance of the method was evaluated under optimum conditions.
Experimental
Materials and methods
Analytically grade chemicals and reagents (PAMAM-OH G2, 10% in methanol, iron (II) sulfate heptahydrate, iron (III) chloride, acetone, sodium hydroxide, pH 3–10 buffer solutions, nitric acid, and hydrochloric acid) used in the synthesis of the nanoparticles and analytical method development were of analytical grade and purchased from Sigma Aldrich (Darmstadt-Germany). Standard stock solutions of copper and cobalt with approximate concentrations of 1000 mg/L were purchased from High-Purity Standards (Charleston, SC—USA) and diluted to prepare working and calibration standard solutions for analytical method development.
Instrumentation
A Shimadzu AA-7000 model (Kyoto, Japan) FAAS system was used for the measurement of absorbance values. A Photron multi-hollow cathode lamp (Cr–Cu–Co–Fe–Ni–Mn) (Victoria, Australia) was employed as a light source and operated at 10 mA and 20 mA to determine cobalt and copper at their analytical wavelengths of 240.7 and 324.8 nm, respectively. A neodymium magnet was used to separate the sorbent from the matrix. Ultrapure deionized water was obtained from a Merck Millipore Direct-Q 3 UV water purification system (Merck KGaA, Darmstadt, Germany). FTIR spectra were obtained using a Thermo Scientific Nicolet IS10 FTIR spectrophotometer. For the morphological characterization of the nanoparticles, SEM analyses were employed using ZEISS Sigma 300 (Oberkochen, Germany). TGA analysis was implemented using Seiko Exstar 6000 (Chiba, Japan).
Preparation of iron PAMAM-G2-OH encapsulated magnetic nanoparticle (Fe-MNP-G2-OH)
PAMAM-G2-OH was purchased from Sigma Aldrich and used after removing its methanol content. PAMAM-G2-OH (0.25 g, 0.07 mmol) was dissolved in 15 mL of ultrapure deionized water and 1000 eq. of iron (III) chloride (12.5 g, 17 mmol) and 500 eq. of iron (II) sulfate heptahydrate (9.47 g, 0.85 mmol) salts were added to the PAMAM-G2-OH solution. The solution was heated for an hour at 60 °C under a nitrogen atmosphere with a constant stirring at 250 rpm. Sodium hydroxide solution (0.75 M) was added to the reaction medium until the pH was fixed to 7.0 to initiate the reduction process. Black colored bare nanoparticles were formed after the solution was kept at pH 7.0 and stirred for one hour. The nanoparticles were separated from the solution by centrifugation and decantation of the supernatant liquid. When the supernatant was added to excess acetone solution (1000 mL) at + 4.0 °C, iron-encapsulated PAMAM-G2-OH (Fe-MNP-G2-OH) precipitates were formed [2, 17]. The precipitated Fe-MNP-G2-OH was separated from the solution after centrifugation and decantation were performed. The synthesized nanoparticles were placed in a vacuum oven and dried for about 5.0 h at 70–80 °C.
Preparation of bare iron (II,III) oxide nanoparticles (bare MNP)
The iron (II,III) oxide nanoparticle (bare MNP) was prepared by the reaction of only iron salts without using PAMAM-G2-OH. Here, 2.0 eq. iron (III) chloride (0.56 g, 3.4 mmol) and 1.0 eq. iron (II) sulfate heptahydrate (0.47 g, 1.7 mmol) were mixed in 50 mL of deionized water and heated to 60 °C. After 1.0 h, sodium hydroxide (0.75 M) was added to the reaction mixture until the pH value reached to 8.0. Black precipitates were formed when the base solution was added. After a 30-min mixing period, the precipitate was separated from the solution with a magnet, washed, and dried in a vacuum oven ready to use [9].
Extraction procedure
Sample and standard solutions (15 mL) were pipetted into 50 mL centrifuge tubes and buffered with 1.0 mL of pH 9.0 buffer solutions for each analyte. The solution was vortexed for 30 s before adding 20 mg of the Fe-MNP-G2-OH nanoparticles and followed by vortex mixing (45 s for cobalt and 60 s for copper) to enhance the interaction between the analytes in the solution and the sorbent. The analyte adsorbed onto the Fe-MNP-G2-OH sorbent was collected at the bottom of the centrifuge tube using a neodymium magnet and the aqueous supernatant was carefully discarded. Next, 0.40 mL of concentrated nitric acid (%65, w/v) was added to the Fe-MNP-G2-OH sorbent and vortexed for 15 s. The sorbent was completely dissolved in concentrated nitric acid. Next, the acidic solution was aspirated to the FAAS system for absorbance measurements.
Preparation of the real samples
The applicability and reliability of the developed method were determined by performing recovery experiments on green and black tea samples. Different tea brands produced in Turkey were purchased from local markets (İstanbul, Turkey). The tea samples were prepared by boiling in drinking water at 95 °C. Specifically, 10 g of green and black tea was separately added to 100 mL of drinking water in a teapot and left to brew for 2.0 h [4]. At the end of the brewing period, the samples were filtered through 125 mm filter papers. The filtered sample solutions were spiked to desired concentration values and then diluted with distilled water. After this process, there was a 100-fold dilution for both samples.
Results and discussion
To determine the optimum parameters of the analytical method, experiments were carried out by keeping all parameters constant while testing only one parameter at different variables. The parameter that gave the highest absorbances was selected as the optimum value for subsequent experiments.
Characterization of Fe-MNP-G2-OH sorbent
PAMAM-G2-OH, including 16 terminal-OH functional groups on its surface, was reacted with iron salts, and the reduction reaction was carried out in a basic medium. After the precipitation of the filtrate phase with acetone, Fe-MNP-G2-OH nanoparticle was obtained. TGA, SEM and FTIR techniques were used for the characterization of the synthesized magnetic nanoparticle. In the last step of the synthesis procedure of Fe-MNP-G2-OH, it was stated that the MNP was obtained by precipitating the filtrate part in acetone [2, 17]. PAMAM-G2-OH is soluble in solvents such as acetone, methanol, and water and it is not possible to precipitate. However, Fe-MNP-G2-OH was precipitated using acetone in the procedure presented in this study. Figure 1 shows the comparative FTIR spectra of the structures.
Accordingly, it was observed that the specific vibration peaks of the PAMAM-G2-OH molecule overlapped with the spectrum of the Fe-MNP-G2-OH. The characteristic absorption band at 1635 cm−1 and 1549 cm−1 belongs to C=O stretching and N–H bending vibrations of amide groups and overlapped in the spectra of the PAMAM-G2-OH [3, 12] and the Fe-MNP-G2-OH. In addition, the O–H group stretching was observed as a broad peak at 3279 cm−1 and 3265 cm−1 in FTIR spectra of the PAMAM-G2-OH and the Fe-MNP-G2-OH. It was also deduced that the intense peaks around 620 cm−1 and 1136 cm−1 were due to the vibration of − CO–O–Fe stretching [3, 12] and the vibration bands at 636 cm−1 corresponding to Fe–O bond. These results proved the success of Fe-MNP-G2-OH synthesis.
The TGA analyses were conducted to investigate the temperature degradation characteristics of PAMAM-G2-OH and Fe-MNP-G2-OH. The TGA curves provided valuable information about the organic and inorganic contents of these structures. It was observed that PAMAM-G2-OH did not leave any residue under a nitrogen atmosphere even at a high temperature of 800 °C. However, the introduction of Fe-MNP-G2-OH, which contains Fe3O4 nanoparticles, resulted in a high char yield of 57% under the same conditions. These results indicate that the incorporation of Fe3O4 nanoparticles significantly affected the thermal degradation characteristics of the Fe-MNP-G2-OH structure.
In the SEM analysis (Fig. 2) performed to examine the particle size distribution of Fe-MNP-G2-OH, Fig. 2 displays the presence of spheroids composed of Fe3O4 nanoparticles. These nanoparticle-sized spheroids were observed and provided a detailed insight into the morphology and structure of the Fe-MNP-G2-OH nanoparticles.
Although many studies that include the synthesis of the dendrimeric nanoparticle have been reported in the literature [1, 36], the MNP precipitation process was performed in acetone in this study. In the procedure, the molar ratio of total iron salts molar equivalent to dendrimer was 3000. It was observed that, as the salt content was increased, the yield, magnetization rate, and the total Fe amount in the synthesized MNPs increased. According to these literature findings, the total salts/dendrimer molar ratio was optimized by testing ratios of 30, 100, 500, 1500, 3000, and 4500. Experiments show Fe content increase with the molar total salts/dendrimer ratio. To measure the total amount of Fe in the synthesized MNP, 40 mg of the MNP was dissolved in 600 μL of concentrated nitric acid and sent to the FAAS system for absorbance measurement. The total Fe content in MNPs achieved saturation after 3000-fold, hence the optimal total salts/dendrimer ratio was chosen as 3000.
Optimization of SPE parameters
Effect of pH and volume of buffer solution
The pH value of the extraction medium affects the interaction between the terminal -OH groups and interior functional groups of the Fe-MNP-G2-OH with the analyte. Therefore, pH value was an important parameter for the developed SPE method. Figures 3 and 4 show the absorbance values recorded for copper and cobalt standard solutions extracted in the pH range of 1.0–12.
Acidic region up to pH 6.0 recorded very low/no absorbance values, and this could result from the protonation of the functional groups on the nanoparticles. In addition, the high amounts of ionic species in the acidic region could compete with copper and cobalt for adsorption sites on the nanoparticles, resulting in low absorbance values. According to the results obtained, the optimum pH media for copper and cobalt were selected as pH 9.0 for both MNPs. In addition, the tendency of metals to form organometallic complexes at high pH may increase their binding/adsorption to the MNP surface [39]. To find the optimum volume of the buffer solutions, experiments were carried out with 0.5, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5 and 4.0 mL volumes of buffer solution. As can be seen in Fig. S1, the optimum value was found to be 1.0 mL for both analytes due to its high absorbance values for both analytes.
Effect of sorbent amount
It is extremely important to determine the optimum amount of nanoparticles in SPE process because it directly affects the availability of active surface sites for analyte adsorption. It is known that the efficiency of elution decreases when more than the optimum amount of the MNP is used if the relationship between the amount of MNP and the volume of eluent is considered. MNP amounts below the optimum value lead to less active sites for efficient adsorption of analytes and cause a decrease in the analytical performance. In addition, it is important to keep the eluent volume as low as possible to achieve the high preconcentration factor. For these reasons, different MNP amounts including 20, 30, 60, and 80 mg were tested and the optimum value according to the highest absorbance value was chosen as 30 mg for Fe-MNP-G2-OH and 20 mg for the bare MNP, given in Figs. S2 and S3. No absorbance signal was recorded for the bare MNP amount under 20 mg for both analytes. A linear decrease in absorbance values for the bare MNP was recorded after 20 mg, and this can be ascribed to the eluent volume (0.40 mL of concentrated HNO3), which was not sufficient to elute the adsorbed analytes from the surface of the relatively large amounts of MNP.
Effect of dissolvent type and volume
In most SPE methods, the adsorbed analytes are desorbed from the surface of the sorbent material with an appropriate dissolvent. In some cases, sorbent materials tend to completely dissolve in the selected dissolvent and it was also observed in this study. In order to achieve a high enrichment/preconcentration factor, the difference between the initial and final volumes should be high. For this purpose, the dissolvent used should have a high dissolution capacity for the analyte even at its low volumes. It is crucial to find a proper dissolvent type for the desorption of analyte without causing significant noise in the absorbance signal of the analyte. For this purpose, concentrated nitric acid, hydrochloric acid and acetic acid were tested for their dissolution capabilities for both analytes. As can be seen in Fig. S4, concentrated nitric acid was chosen as the optimum dissolvent for both sorbent types. The average absorbance values recorded for concentrated nitric acid were 1.2-fold for Cu2+ and 1.3-fold for Co2+ higher than hydrochloric acid. The lowest absorbance values were recorded for the sorbents dissolved in acetic acid. The possible organic species in the acetic acid could interfere with the beamline and cause the decrease in absorbance values. In addition, 0.10, 0.20, 0.30, 0.40, 0.60, 0.70 and 0.80 mL volumes of the selected dissolvent were tested, and the optimum value obtained for both analytes was 0.40 mL of concentrated nitric acid, given in Fig. S5. The lowest absorbance values were recorded for the lowest two (0.10 and 0.20 mL) and the highest two (0.70 and 0.80 mL) dissolvent volumes, and this observation can be attributed to insufficient dissolution and high dilution for the lowest and highest volumes, respectively.
Effect of mixing type and period
The interaction between analytes and sorbent materials in a solution can be enhanced by employing an appropriate sample mixing method with a certain period. Manual mixing, mechanical shaker, ultrasonication, and vortex mixing types were tested for three replicate samples of the two analytes. All mixing types were applied for a 30 s period. The absorbance values recorded for the mixing types were compared to an extraction performed without any mixing process to ascertain the effect of mixing. According to the results given in Fig. S6, the highest average absorbance was recorded for vortex mixing, which was about 3.7 and 5.1 times higher than that of no mixing for copper and cobalt, respectively. Different vortex mixing periods including 15, 30, 45, 60, and 90 s were then tested. As can be seen in Fig. S7, the optimum mixing periods determined were 45 s for cobalt and 60 s for copper.
The efficiency and practicality of the vortex mixer was used to facilitate the dissolution process and obtain a homogenous solution for aspiration into the FAAS system. After testing different vortex periods, 30 s emerged as the optimum one due to high and repeatable absorbance values.
Analytical figures of merit for the Fe-MNP-G2-OH-SPE-FAAS method
Analytical performance of the Fe-MNP-G2-OH-SPE-FAAS method was carried out by analyzing aqueous standard solutions (2.5–250 μg L−1 for Cu2+ and 5.0–250 μg L−1 for Co2+) under the optimum SPE conditions given in Table 1. The contribution of the dendrimeric structure in the MNP was evaluated by comparing Fe-MNP-G2-OH with bare MNPs. According to the results given in Table 2, the bare-MNP-SPE-FAAS method enhanced the detection sensitivity of the conventional FAAS system by about 31 and 14 times for copper and cobalt, respectively.
In the Fe-MNP-G2-OH-SPE-FAAS method, a linear range was determined for copper between 2.5 and 250 μg L−1 with a correlation coefficient of 0.9995, and the limits of detection (LOD) and quantification (LOQ) were found to be 0.52 and 1.7 μg L−1, respectively. Therefore, the Fe-MNP-G2-OH-SPE-FAAS method was about 230 times more sensitive than the FAAS method for copper. Percent relative standard deviation values (%RSD) were calculated using the lowest concentration in the calibration plot for both analytes. As for cobalt, the Fe-MNP-G2-OH-SPE-FAAS method was linear between 5.0 and 250 μg L−1 with a correlation coefficient of 0.9991, and LOD and LOQ values of 1.1 and 3.7 μg L−1, respectively. Hence, the detection limit of FAAS for cobalt was decreased about 101 times by the Fe-MNP-G2-OH-SPE-FAAS method. The performance of Fe-MNP-G2-OH was about 4.4- (copper) and 4.6-fold (cobalt) higher than the bare-MNP as can be seen in Table 2.
The analytical performance of the method reported in this study was compared to similar studies that have been reported in the literature based on LOD values. In the related literature, the LODs reported for copper and cobalt fall in the range of 1.5–220 μg L−1 and 1.2–2.4 μg L−1, respectively. According to these results, it can be said that the performance of our proposed study is comparable or relatively better for copper [19, 27, 34, 35] and cobalt [8, 15, 16, 25]. In addition, other published studies reported for the determination of copper and cobalt are given in Table 2. These results demonstrated that LOD, LOQ and %RSD values for the developed method are lower or relatively close to that of the reported methods in the literature.
Recovery tests
Recovery experiments are extremely important in the analytical method development because these experiments ensure the applicability and reliability of the proposed analytical method to various sample matrices. Real tea samples (green and black tea) were prepared by brewing with drinking water in a teapot. Since no analyte was detected in the blank tea samples, the optimum Fe-MNP-G2-OH-SPE-FAAS method was applied to the sample solutions spiked to final concentrations of 50, 75, and 200 μg L−1 of the two analytes. Since tea samples are known to contain various components such as polyphenols, caffeine, amino acids, and vitamins, the calibration standards were prepared using the spiked tea samples. Here, green tea and black tea extracts, which did not contain the analytes of interest, were used to prepare 6-point matrix-matched calibration standards (25–250 μg L−1), and the calibration plots given in Figs. S8 and S9 were used to quantify the analytes found in the spiked tea samples. The percent recovery results calculated for copper and cobalt in both samples were in the range of 97–101% and 93–107%, respectively. The percent recovery results summarized in Table 3 were satisfactory meaning that the developed analytical method can be used to accurately quantify copper and cobalt in green and black tea samples.
Conclusions
In the presented study, PAMAM-G2-OH was encapsulated through a specific synthesis procedure to yield Fe-MNP-G2-OH sorbent and its structural characterization was achieved using SEM, TGA and FTIR. In addition, optimization experiments related to total iron and dendrimer molar ratios were carried out to effectively synthesize and characterize the Fe-MNP-G2-OH sorbent. The synthesized magnetic nanoparticle was used as a sorbent for Fe-MNP-G2-OH-SPE method. The parameters of the SPE method were systematically optimized to enhance the extraction efficiency and absorbance signals for both analytes. The Fe-MNP-G2-OH-SPE-FAAS method was found to be 230 and 101 times more sensitive for Cu2+ and Co2+, respectively, if compared to the FAAS method. In addition, the applicability and reliability of the method were tested using different tea samples spiked to different concentrations and satisfactory results close to 100% were obtained. As a result, the developed Fe-MNP-G2-OH-SPE-FAAS method increased the sensitivity of the FAAS method and overcame interference from components of the complex tea samples. The method presented in this study is a simple, rapid, inexpensive, efficient, and green analytical approach for the determination of copper and cobalt at trace levels. The developed method can be extended to other heavy metals in tea samples. In addition, the synthesized sorbent can be used for the extraction of other organic and inorganic species to obtain trace determination of the target analyte(s).
Data availability
Not applicable.
Code availability
Not applicable.
References
E.A. Afshar, M.A. Taher, New fabrication of CuFe2O4/PAMAM nanocomposites by an efficient removal performance for organic dyes: Kinetic study. Environ. Res. 204, 112048 (2022)
S. Ahsan, C. Rao, The role of surface charge in the desolvation process of gelatin: implications in nanoparticle synthesis and modulation of drug release. Int. J. Nanomedicine 12, 795–808 (2017)
Akbari, H., Gholami, M., Akbari, H., Adibzadeh, A., Taghavi, L., Hayati, B., & Nazari, S. (2020). Poly (amidoamine) generation 6 functionalized Fe3O4@SiO2/GPTMS core–shell magnetic NPs as a new adsorbent for Arsenite adsorption: kinetic, isotherm and thermodynamic studies. Journal of Environmental Health Science and Engineering, 18.
A.S. Alnaimat, M.C. Barciela-Alonso, P. Herbello-Hermelo, R. Domínguez-González, P. Bermejo-Barrera, In vitro assessment of major and trace element bioaccessibility in tea samples. Talanta 225, 122083 (2021)
S.A. Arain, T.G. Kazi, H.I. Afridi, M.S. Arain, A.H. Panhwar, N. Khan, J.A. Baig, F. Shah, A new dispersive liquid–liquid microextraction using ionic liquid based microemulsion coupled with cloud point extraction for determination of copper in serum and water samples. Ecotoxicol. Environ. Saf. 126, 186–192 (2016)
S. Cholimi, A. Mutalib, R. Soetikno, E. Anwar, M. Radji, A. Pujiyanto, P. Purnamasari, D. Joshita, H.G. Adang, Synthesis of gold nanoparticles with polyamidoamine (PAMAM) generation 4 dendrimer as stabilizing agent for CT scan contrast agent. Macromol. Symp. 353, 96–101 (2015)
de S. Dias F, Peixoto LB, Meira LA, Barreto JA (2018) Ultrasound-assisted emulsification of solidified floating organic drop microextracted for pre-concentration of cadmium in food and water samples. Anal Methods, 10(35): 4257-4263
S.G. Elci, Determination of cobalt in food by magnetic solid-phase extraction (MSPE) preconcentration by polyaniline (PANI) and polythiophene (PTH) coated magnetic nanoparticles (MNPs) and microsample injection system—flame atomic absorption spectrometry (MIS-FAAS). Instrum. Sci. Technol. 49(3), 258–275 (2021)
K.R. Gopidas, J.K. Whitesell, M.A. Fox, Nanoparticle-cored dendrimers: synthesis and characterization. J. Am. Chem. Soc. 125(21), 6491–6502 (2003)
A.A. Gouda, R. El Sheikh, H.M. El Sayed, A.M. Khedr, S.A. Al Ezz, W. Gamil, M. Hamdy, Ultrasound-assisted dispersive microsolid-phase extraction for preconcentration of trace cobalt and nickel in environmental samples prior to their determination by flame atomic absorption spectrometry. J. Appl. Spectrosc. 89(3), 567–578 (2022)
J.P. Goullé, L. Mahieu, J. Castermant, N. Neveu, L. Bonneau, G. Lainé, D. Bouige, C. Lacroix, Metal and metalloid multi-elementary ICP-MS validation in whole blood, plasma, urine and hair. Reference values. Forensic Sci Int 153(1), 39–44 (2005)
Y. Harinath, D.H.K. Reddy, L.S. Sharma, K. Seshaiah, Development of hyperbranched polymer encapsulated magnetic adsorbent (Fe3O4@SiO2–NH2-PAA) and its application for decontamination of heavy metal ions. J. Environ. Chem. Eng. 5(5), 4994–5001 (2017)
M. Imamoglu, Novel determination of copper (II) in natural waters by solid-phase extraction (SPE) flow-injection (FI) flame atomic absorption spectrometry (FAAS). Anal. Lett. 56(3), 517–529 (2023)
V.A. Jiménez, K. Marrugo, C.H. Campos, J.B. Alderete, C.C. Torres, Copper metallic nanoparticles capped with PEGylated PAMAM-G3 dendrimers for the catalytic reduction of low solubility nitroarenes of pharmaceutical interest. Catal. Today 372, 27–35 (2021)
G. Karimipour, M. Ghaedi, R. Sahraei, A. Daneshfar, M.N. Biyareh, Modification of gold nanoparticle loaded on activated carbon with Bis(4-methoxysalicylaldehyde)-1,2-phenylenediamine as new sorbent for enrichment of some metal ions. Biol. Trace Elem. Res. 145(1), 109–117 (2012)
M. Khajeh, E. Sanchooli, Synthesis and evaluation of silver nanoparticles material for solid phase extraction of cobalt from water samples. Appl. Nanosci. 1(4), 205–209 (2011)
G. López-Téllez, P. Balderas-Hernández, C.E. Barrera-Díaz, A.R. Vilchis-Nestor, G. Roa-Morales, B. Bilyeu, Green method to form iron oxide nanorods in orange peels for chromium(VI) reduction. J. Nanosci. Nanotechnol. 13(3), 2354–2361 (2013)
Ma, Y.-X., Xing, D., Shao, W.-J., Du, X.-Y., & La, P. (2017). Preparation of Polyamidoamine Dendrimers Functionalized Magnetic Graphene Oxide for the Adsorption of Hg(II) in Aqueous Solution. Journal of Colloid and Interface Science, 505.
D. Meng, S. Ju, Q. Hu, X. Li, Flame atomic absorption spectrometric determination of Cu and Ni in tobacco samples after Preconcentration using ammonium pyrrolidine dithiocarbamate modified magnetic nanoparticles. Int. J. Environ. Anal. Chem. 95(15), 1435–1449 (2015)
M. Moradnia, H. Movahedian Attar, Z. Heidari, F. Mohammadi, R. Kelishadi, Monitoring of urinary arsenic (As) and lead (Pb) among a sample of pregnant Iranian women. J. Environ. Health Sci. Eng. 19(2), 1901–1909 (2021)
B. Ninwong, S. Chuanuwatanakul, O. Chailapakul, W. Dungchai, S. Motomizu, On-line preconcentration and determination of lead and cadmium by sequential injection/anodic stripping voltammetry. Talanta 96, 75–81 (2012)
Y. Niu, R. Qu, H. Chen, L. Mu, X. Liu, T. Wang, Y. Zhang, C. Sun, Synthesis of silica gel supported salicylaldehyde modified PAMAM dendrimers for the effective removal of Hg(II) from aqueous solution. J. Hazard. Mater. 278, 267–278 (2014)
K.B.S. Perelonia, K.C.D. Benitez, R.J.S. Banicod, G.C. Tadifa, F.D. Cambia, U.M. Montojo, Validation of an analytical method for the determination of cadmium, lead and mercury in fish and fishery resources by graphite furnace and Cold Vapor Atomic Absorption Spectrometry. Food Control 130, 108363 (2021)
J. Płotka-Wasylka, M. Marć, N. Szczepańska, J. Namieśnik, New polymeric materials for solid phase extraction. Crit. Rev. Anal. Chem. 47(5), 373–383 (2017)
Sadıqov, E., Elyas Sodan, N., Siyal, A. N., Elçi, A., & Elçi, L. (2021). Determination of cobalt and copper in water, plant, and soil samples by magnetite nanoparticle-based solid-phase microextraction (SPME) coupled with microsample injection system-flame atomic absorption spectrometry (MIS-FAAS). Instrumentation Science & Technology, 1–19.
F. Salimi, M. Shamsipur, E. Koosha, M. Ramezani, A new dispersive micro-solid phase extraction based on rejection property method combined with FAAS for the simultaneous determination of cobalt and copper after optimisation by Box-Behnken design. Int. J. Environ. Anal. Chem. 102(4), 872–884 (2022)
A. Samadi, M. Amjadi, Magnetic Fe3O4@C nanoparticles modified with 1-(2-thiazolylazo)-2-naphthol as a novel solid-phase extraction sorbent for preconcentration of copper (II). Microchim. Acta 182(1), 257–264 (2015)
I.M. Sayre, International standards for drinking water. J. Am. Water Works Ass. 80(1), 53–60 (1988)
S. Seidi, L. Alavi, Novel and rapid deep eutectic solvent (DES) homogeneous liquid–liquid microextraction (HLLME) with flame atomic absorption spectrometry (FAAS) detection for the determination of copper in vegetables. Anal. Lett. 52(13), 2092–2106 (2019)
N. Sohrabi-Gilani, A. Ghayournezhad, S. Rostamzadeh Mansour, Determination of ultratrace levels of cobalt (II) and chromium (III) by magnetic dispersive solid-phase extraction (SPE) using urea-formaldehyde polymer/magnetite nanoparticles with flame atomic absorption spectrometry (FAAS). Anal. Lett. 55(16), 2650–2667 (2022)
D.A. Tomalia, H. Baker, J. Dewald, M. Hall, G. Kallos, S. Martin, J. Roeck, J. Ryder, P. Smith, A new class of polymers: starburst-dendritic macromolecules. Polym. J. 17(1), 117–132 (1985)
J.S. Trindade, V.A. Lemos, U.M.F. Mata Cerqueira, C.G. Novaes, S.A. Araujo, M.A. Bezerra, Multivariate optimization of a dispersive liquid-liquid microextraction method for determination of copper and manganese in coconut water by FAAS. Food Chem. 365, 130473 (2021)
Y. Wang, H. Xu, X. Zhao, H. Meng, Y. Lu, C. Li, Alkynyl functionalized MoS2 mesoporous materials with superb adsorptivity for heavy metal ions. J. Hazard. Mater. 424, 127579 (2022)
E. Yavuz, Ş Tokalıoğlu, Ş Patat, Core-shell Fe(3)O(4) polydopamine nanoparticles as sorbent for magnetic dispersive solid-phase extraction of copper from food samples. Food Chem. 263, 232–239 (2018)
E. Yavuz, Ş Tokalıoğlu, H. Şahan, Ş Patat, Nanosized spongelike Mn3O4 as an adsorbent for preconcentration by vortex assisted solid phase extraction of copper and lead in various food and herb samples. Food Chem. 194, 463–469 (2016)
Yuan, D., Chen, L., Xiong, X., Zhang, Q., Liao, S., Yuan, L., & Wang, Y. (2016). Synthesis of PAMAM dendron functionalized superparamagnetic polymer microspheres for highly efficient sorption of uranium(VI). Journal of Radioanalytical and Nuclear Chemistry, 309.
Y. Yuan, Y. Wu, H. Wang, Y. Tong, X. Sheng, Y. Sun, X. Zhou, Q. Zhou, Simultaneous enrichment and determination of cadmium and mercury ions using magnetic PAMAM dendrimers as the adsorbents for magnetic solid phase extraction coupled with high performance liquid chromatography. J. Hazard. Mater. 386, 121658 (2020)
Zarei, A., Saedi, S., & seidi, F. (2018). Synthesis and Application of Fe3O4@SiO2@Carboxyl-Terminated PAMAM Dendrimer Nanocomposite for Heavy Metal Removal. Journal of Inorganic and Organometallic Polymers and Materials, 28(6), 2835-2843
Y. Zhang, H. Zhang, Z. Zhang, C. Liu, C. Sun, W. Zhang, T. Marhaba, pH Effect on Heavy Metal Release from a Polluted Sediment. J. Chem. 2018, 7597640 (2018)
Funding
Financial support from Yildiz Technical University (Yildiz Teknik Üniversitesi, Grant/Award Number: FDK-2020–3926) is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
OY (PhD. O. Y. Author 1): data curation; formal analysis; investigation; methodology; validation; visualization; roles/writing—original draft. İK (Prof. Dr. I. K. Author 2): conceptualization; funding acquisition; project administration; supervision; validation; visualization. All the authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yılmaz, Ö., Koyuncu, İ. Determination of copper and cobalt in different tea samples at trace levels by FAAS after preconcentration with a novel iron PAMAM-OH-encapsulated magnetic nanoparticle as SPE sorbent. ANAL. SCI. 40, 633–641 (2024). https://doi.org/10.1007/s44211-023-00495-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s44211-023-00495-2