Abstract
A novel fluorescence-enhanced probe 2-butyl-1,3-dioxoisoindolin-4-yl picolinate (BDIP) has been developed for the detection of Cu2+ based on the excited-state intramolecular proton transfer (ESIPT) process. BDIP utilized a phthalimide derivative as the fluorophore and selected picolinate ester as the recognition site for Cu2+. The probe displayed high selectivity, strong anti-interference ability, and a significant fluorescence enhancement effect for Cu2+ in phosphate buffer saline (PBS, 10 mM, pH 7.4) with the detection limit of 31 nM. BDIP also possesses the advantages of simple synthesis steps, large Stokes shift, and good water solubility. Moreover, BDIP was used for Cu2+ detection in real water samples, with the result being satisfactory.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Copper is the third most abundant transition metal in the human body, widely distributed in human blood and tissues. Cu2+ plays vital roles in physiological processes such as energy production, signal transduction, cellular oxygen transport, and activation [1,2,3]. Abnormal levels of Cu2+ in the human body can cause several diseases such as Menkes disease [4], Wilson’s disease [5], Alzheimer’s disease [6], Parkinson’s disease [7], coronary heart disease [8], amyotrophic lateral sclerosis [9], and myelopathy [10]. In addition, due to its wide range of applications in agriculture and industrial production, Cu2+ is causing serious environmental problems [11, 12]. The US Environmental Protection Agency (EPA) has set the threshold limit of Cu2+ in drinking water to 20 μM [13]. Therefore, the development of sensitive and selective detection methods for Cu2+ is vital for human health and environmental safety.
The current methods for detecting Cu2+ include atomic absorption spectroscopy [14], atomic emission spectroscopy [15], inductively coupled plasma mass spectrometry [16], electrochemical [17], and so on. However, these methods are time-consuming, unsuitable for real-time analysis, and need tedious sample preparation and expensive instruments. Compared with the above methods, fluorescent probes have received more and more attention in detecting heavy metal ions in recent years because of their advantages of low cost, easy operation, real-time analysis, and non-destructive biological imaging [18,19,20]. So far, many Cu2+ fluorescent probes have been developed [21,22,23], but most probes still have some limitations, such as poor selectivity, need for organic solvents, cumbersome synthesis steps, and fluorescence quenching [24,25,26,27,28,29]. Therefore, it is still highly desirable to develop a fluorescent probe for Cu2+ with good water solubility, simple synthesis, and excellent performance.
Organic molecules with excited-state intramolecular proton transfer (ESIPT) property usually have a relatively high Stokes shift due to their unique luminescence mechanism, effectively avoiding self-absorption and inner filter effect [30]. In recent years, they have received extensive attention in the field of fluorescent probes. Recently, a new fluorophore, 3-hydroxyphthalimide, based on the ESIPT mechanism, has been developed and used for the detection of ions and small molecules and biological imaging research [31,32,33]. In addition to a relatively high Stokes shift, it also has the advantages such as high fluorescence quantum yield, high optical stability, and easy synthesis [34]. In this study, a fluorescent probe BDIP for Cu2+was synthesized using N-butyl-3-hydroxyphthalimide (BHIO) as the fluorescent matrix and 2-pyridinecarbonyl as the recognition group, based on the ESIPT mechanism (Scheme 1). We reason that the 2-pyridinecarbonyl group can prohibit the ESIPT process and quench the fluorescent emission of the fluorophore BHIO by esterification. When reacts with Cu2+, the BHIO can be released from probe BDIP by Cu2+-promoted hydrolysis reaction of the picolinate ester and the ESIPT process is recovered, which result in fluorescent enhancement simultaneously. Compared with the representative fluorescent probe that used picolinate ester as the recognition site for Cu2+, BDIP showed a larger Stokes shift, wider linear range and lower detection limit (Table S1, Supporting Information).
Experimental
Instruments and reagents
All reagents and solvents were of analytical grade which purchased from commercial suppliers, used directly in the experiment without further purification. Deionized water was used throughout all experiments. Column chromatography was performed using silica gel 200–300 mesh from Qingdao Haiyang Chemical Co., Ltd. The solutions of Na+, K+, Ca2+, Mg2+, Zn2+, Cd2+, Hg2+, Co2+, Ni2+, Fe3+ and Al3+ were prepared from their chloride salts; the solutions of Cu2+, Mn2+, Cr3+, Fe2+, Ag+ and Pb2+ were prepared from their nitrate salts; the solution of Cu+ was prepared from [Cu(MeCN)4][PF6]. 1H NMR and 13C NMR spectra were measured on a Bruker AV-400 spectrometer with chemical shifts reported in ppm (in DMSO-d6, TMS as internal standard). High-resolution mass spectra (HRMS, ESI) were taken on a 7.0 T FTICR-MS (Varian). Absorption spectra were recorded with a Shimadzu UV-2550 spectrophotometer (Japan). Fluorescence spectra measurement was performed on a Hitachi F-4600 spectrofluorimeter (Japan). The pH was measured with a Model pHs-3C meter (Shanghai, China).
Synthesis of the fluorescent matrix BHIO
A mixture of 3-hydroxyphthalic anhydride (0.50 g, 3.05 mmol) and n-butylamine (0.33 g, 4.58 mmol) in acetic acid (8 mL) was stirred for 6 h at 120 °C. After cooling to room temperature, the reaction solution was poured into 100 mL ice water, and the precipitate was collected by filtration, washed with water, and dried in vacuo. BHIO was obtained as a white solid (yield: 72%). 1H NMR (400 MHz, DMSO-d6) δ: 10.98 (s, 1H), 7.58 (dd, 1H, J = 7.2 Hz, J = 8.4 Hz), 7.25 (d, 1H, J = 6.8 Hz), 7.18 (d, 1H, J = 8.0 Hz), 3.49 (t, 2H, J = 7.2 Hz), 1.49–1.56 (m, 2H), 1.21–1.30 (m, 2H), 0.87 (t, 3H, J = 7.6 Hz); 13C NMR (100 MHz, DMSO-d6) δ: 167.70, 166.65, 155.03, 135.82, 133.50, 123.14, 114.59, 113.86, 36.65, 30.00, 19.45, 13.45; ESI-HRMS (m/z) calculated for C12H13NNaO3[M + Na]+: 242.07876, found, 242.07878.
Synthesis of the fluorescent probe BDIP
A mixture of BHIO (0.10 g, 0.46 mmol), N, N-Diisopropylethylamine (DIPEA, 0.18 g, 1.38 mmol), pyridine-2-carbonyl chloride hydrochloride (0.12 g, 0.69 mmol), and a catalytic amount of 4-Dimethylaminopyridine (DMAP) was dissolved in anhydrous dichloromethane (20 mL), and stirred at room temperature for 10 h. Then the solvent was evaporated under reduced pressure and the obtained residue was purified by silica gel chromatography with petroleum ether/ethyl acetate (2:1, V/V) as eluent to afford the desired product as white solid (yield: 85%). 1H NMR (400 MHz, DMSO-d6) δ: 8.84—8.86 (m, 1H), 8.28—8.30 (m, 1H), 8.10–8.15 (m, 1H), 7.95 (t, 1H, J = 7.5 Hz), 7.84 (d, 1H, J = 7.4 Hz), 7.75—7.80 (m, 2H), 3.50 (t, 2H, J = 7.0 Hz), 1.48–1.55 (m, 2H), 1.20–1.29 (m, 2H), 0.85 (t, 3H, J = 7.4 Hz); 13C NMR (100 MHz, DMSO-d6) δ: 167.02, 165.53, 162.54, 150.21, 146.02, 145.96, 137.87, 136.56, 133.27, 128.73, 128.31, 126.03, 122.34, 121.13, 37.20, 29.85, 19.46, 13.43; ESI-HRMS (m/z) calculated for C18H16N2NaO4 [M + Na]+: 347.10023, found, 347.10040.
Spectroscopy analysis
The probe BDIP was dissolved in DMSO to prepare a 1 mM stock solution. The metal cations (K+, Na+, Ag+, Ca2+, Mg2+, Cu2+, Zn2+, Al3+, Fe3+, Fe2+, Cd2+, Cr3+, Co2+, Hg2+, Ni2+, Pb2+, and Mn2+) were dissolved in deionized water to prepare 2 mM stock solutions, respectively. Cu+ stock solution (2 mM) was prepared in MeCN. 30 μL of the probe BDIP stock solution was mixed with 30 μL of the metal ions stock solutions. Then the resulting solution was diluted to 3 mL with phosphate buffer saline (PBS, 10 mM, pH 7.4) and placed in a cuvette. In the anti-interference experiment, 30 μL of other metal cations stock solutions, 30 μL of the probe BDIP stock solution, and 30 μL Cu2+ stock solution were sequentially added into the PBS. The final volume of the test solution was set at 3 mL and placed in a cuvette. The fluorescence measurements were carried out after reacting for 15 min at room temperature. The following parameters were set for measurement: λex = 344 nm, λem = 515 nm, slit width: 5 nm and 5 nm, and voltage: 700 V.
Application in real water samples
Two real water samples were taken to validate the analytical application of the probe BDIP. One water sample was taken from tap water in the laboratory, and the other was taken from the Jian Lake of Jining Medical University. Before the tests, each sample was filtered to remove the impurities using filter paper. Then the two water samples were used to prepare PBS (10 mM, pH 7.4) and a probe BDIP stock solution (3 mM). Cu2+ was determined by the standard addition method. BDIP stock solutions (30 μL) and Cu2+ stock solution (0, 1, 3 or 5 μL) were placed into a cuvette and diluted to 3 mL with the two water samples, and gave the test solutions. The final mixtures contained BDIP (10 μM) and Cu2+ (0, 1, 3 or 5 μM). The test solution was incubated for 15 min at room temperature, and the fluorescence was measured at 515 nm. For each Cu2+ concentration, water samples were tested three times. Comparing with the standard curve, the concentrations of Cu2+ can be observed.
Results and discussion
Synthesis of probe BDIP
The probe BDIP was prepared with 3-hydroxyphthalic anhydride as the starting material by a two-step reaction, as shown in Scheme 1. First, the condensation reaction of 3-hydroxyphthalic anhydride with n-butylamine was carried out at a high temperature, and the resulting fluorescent matrix BHIO was directly precipitated from the reaction solution. Then, with DIPEA as the acid-binding agent and DMAP as a catalyst, the acylation reaction of BHIO with pyridine-2-carbonyl chloride produced the probe BDIP. The structures of BHIO and BDIP were confirmed by 1H NMR, 13C NMR, and HRMS (see Figs. S1–S6).
Effect of the reaction time of probe BDIP with Cu2+
To obtain the best response time for Cu2+detection, we investigated the effect of the reaction time on the fluorescence intensity. As shown in Fig. 1, in PBS (10 mM, pH 7.4), the fluorescence intensity of the probe BDIP solution (10 μM) was very low. When 2 equivalents of Cu2+ were added to the probe BDIP solution, the fluorescence intensity at 515 nm gradually increased. With the progress of the reaction, the increased rate of the fluorescence intensity gradually decreased and the fluorescence intensity remained unchanged after 15 min. Therefore, in the subsequent experiments, a reaction time of 15 min was used.
Effect of Cu2+ concentration
Through the Cu2+ fluorescence titration experiment, we investigated the sensitivity and linear range of the probe BDIP. As shown in Fig. 2A, the fluorescence emission spectra of probe BDIP (10 μM) in the PBS at different concentrations of Cu2+ from 0 to 50 μM were recorded. Upon excitation at 344 nm, the free BDIP solution showed a very weak emission peak at 515 nm. With an increase in Cu2+ concentration, the fluorescence intensity at 515 nm increased significantly and reached a plateau with an eightfold enhancement at the concentration of 20 μM (Fig. 2B). The Stokes shift of probe BDIP is as high as 171 nm, which can effectively prevent self-absorption and increase the signal-to-noise ratio of fluorescence imaging.
The inset in Fig. 2B indicated a good linear relationship between the fluorescence intensity of BDIP and the concentration of Cu2+, and the regression equation was y = 532.9x + 909.7 (R2 = 0.9938). According to the formula for detection limit (LOD): LOD = 3σ/K (wherein, σ is the standard deviation of the blank fluorescence spectrum and K is the slope of the straight line), the detection limit of the probe BDIP for Cu2+ was 31 nM, lower than the threshold limit (20 μM) set by the US EPA. This indicated that the probe BDIP had relatively good detection sensitivity for Cu2+.
Effect of pH on the detection performance of the probe BDIP
Considering the significance of pH in practical applications, the pH effects on the fluorescent response of probe BDIP (10 μM) in the absence and presence of Cu2+ were investigated (Fig. 3). In the absence of Cu2+, weak fluorescence signal of probe BDIP was observed within a wide pH range from 3.0 to 11.0, indicating that the probe BDIP is relatively stable under acidic and weakly alkaline conditions. On the other hand, after adding 2 equivalents of Cu2+, the fluorescence was gradually increased in the region of pH 3.0–7.4 and reached a maximum at pH 7.4. When pH was further increased, the fluorescence intensity gradually decreased. Therefore, considering the pH experiment results and the biological applications, the physiological conditions (pH 7.4) were selected to detect Cu2+.
Selectivity and anti-interference of probe BDIP
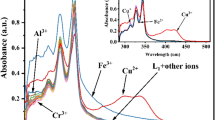
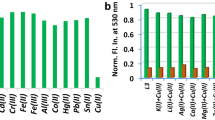
The selectivity of the fluorescent probe is an important parameter for performance evaluation. To investigate the selectivity of the probe BDIP, We examined the fluorescence response of BDIP to various interfering cations including K+, Na+, Ag+, Ca2+, Mg2+, Zn2+, Al3+, Fe3+, Fe2+, Cd2+, Cr3+, Co2+, Hg2+, Ni2+, Pb2+, Cu+ and Mn2+. As shown in Fig. 4A, the fluorescence intensity of the probe BDIP (10 μM) did not change significantly after the addition of potentially interfering cations (20 μM), whereas, after adding two equivalents of Cu2+, the fluorescence intensity increased significantly, indicating that the probe BDIP had specific selectivity for Cu2+ and could be used for the analysis and detection of Cu2+.
To further investigate the Cu2+ detection ability of the probe BDIP in the presence of other competing ions, we also conducted anti-interference experiments. 20 μM different metal ions and 10 μM probe BDIP were mixed in PBS first. And then 20 μM Cu2+ was added. As shown in Fig. 4B, similar fluorescence responses of probe BDIP to Cu2+ were observed regardless of the presence or absence of various other interfering cations. These results indicated that the probe BDIP could be used to detect Cu2+ in a complex environment.
Recognition mechanism of probe BDIP for Cu2+
According to the previously reported work [35, 36], we proposed the possible response mechanism of BDIP for Cu2+ (Scheme 2). Due to the presence of the 2-pyridinecarbonyl group, the ESIPT process based on the keto-enol tautomer in BHIO is inhibited, resulting in weak fluorescence of probe BDIP. When Cu2+ coexists with BDIP, 2-pyridinecarbonyl group in BDIP acts as a “recognition group”, which brings Cu2+ in close proximity to the ester bond. Then the Cu2+ coordinates with nitrogen and neighboring carbonyl oxygen from the 2-picolinic ester. The formation of this complex promotes the hydrolysis of 2-picolinic ester which acts as an “anchoring group” [37, 38]. Once hydrolysis occurs, the fluorescent matrix BHIO is released from BDIP and Cu2+ is regenerated which participates in the hydrolysis process of the remaining ester. Finally, this catalytic sensing cycle causes the fluorescent signal to gradually increase. This transformation was confirmed by MS, UV–vis and emission spectroscopic studies. UV–vis and fluorescence emission spectra of Cu2+-treated BDIP solution were compared with those of fluorescent matrix BHIO. As shown in Fig. S7 and Fig. S8, the spectra of these two compounds were nearly identical. Furthermore, MS spectrum of Cu2+-treated BDIP solution showed a major peak at m/z 218.08215 (see Fig. S9), which was consistent with the deprotonated molecular weight ([M-H]− 218.08227) of BHIO.
Detection of Cu2+ in real water samples
To validate the practical application potential of the probe BDIP, we detected Cu2+ in tap water and lake water under the same above conditions. The probe BDIP (10 μM) and a certain amount of Cu2+ were added to the water samples. No obvious fluorescence enhancement was observed when probe BDIP was added directly to the water samples. After Cu2+ was added to the water sample, significant fluorescence enhancement can be observed. The recovery tests were carried out and the relative standard deviation RSD were calculated. Table 1 indicated that the recoveries of Cu2+ were 95.0–104.0%, and the RSD were 1.35–3.80%. This showed that the probe BDIP could be used for the quantitative detection of Cu2+ in real water samples with high accuracy and precision.
Conclusions
In conclusion, we developed a new ESIPT-based fluorescent probe (BDIP) for Cu2+ based on the specific hydrolysis reaction of the picolinate moiety. The fluorescence intensity of the probe BDIP at 515 nm increased significantly when treated with Cu2+, which indicated a consequence of the ESIPT turn-on. In PBS (10 mM, pH 7.4), probe BDIP displayed high selectivity and sensitivity for Cu2+ with excellent anti-interference ability. Moreover, the probe was successfully utilized for the quantitative detection of Cu2+ in real water samples, and the results indicated that BDIP possessed potential applicability for Cu2+ detection in environmental samples.
References
E.L. Que, D.W. Domaille, C.J. Chang, Chem. Rev. 5, 1517 (2008)
G. Inesi, J. Cell Commun. Signal. 5, 227 (2011)
L. Banci, I. Bertini, S. Ciofi-Baffoni, T. Kozyreva, K. Zovo, P. Palumaa, Nature 465, 645 (2010)
S.G. Kaler, Nat. Rev. Neurol. 7, 15 (2011)
M.E. Del Castillo-Busto, S. Cuello-Nunez, C. Ward-Deitrich, T. Morley, H. Goenaga-Infante, Anal. Bioanal. Chem. 414, 561 (2022)
R. Squitti, M. Ventriglia, I. Simonelli, C. Bonvicini, A. Costa, G. Perini, G. Binetti, L. Benussi, R. Ghidoni, G. Koch, B. Borroni, A. Albanese, S.L. Sensi, M. Rongioletti, Biomolecules 11, 960 (2021)
S. Montes, S. Rivera-Mancia, A. Diaz-Ruiz, L. Tristan-Lopez, C. Rios, Oxid. Med. Cell Longev. 2014, 147251 (2014)
E.S. Ford, Am. J. Epidemiol. 151, 1182 (2000)
J.S. Valentine, P.A. Doucette, S. Zittin-Potter, Annu. Rev. Biochem. 74, 563 (2005)
S.R. Jaiser, G.P. Winston, J. Neurol. 257, 869 (2010)
Y. Wang, J. Shi, H. Wang, Q. Lin, X. Chen, Y. Chen, Ecotoxicol. Environ. Saf. 67, 75 (2007)
X.O. Xiong, Y.X. Li, W. Li, C.Y. Lin, W. Han, M. Yang, Resour. Conserv. Recy. 54, 985 (2010)
Edition of the Drinking Water Standards and Health Advisories (EPA 822-S-12-001). US EPA Office of Science and Technology, Washington, (2012)
D. Citak, M. Tuzen, Food Chem. Toxicol. 48, 1399 (2010)
N. Ozbek, S. Akman, Food Chem. 200, 245 (2016)
J.S. Becker, A. Matusch, C. Depboylu, J. Dobrowolska, M.V. Zoriy, Anal. Chem. 79, 6074 (2007)
G.M. Alves, J.M. Magalhães, P. Salaün, C.M. van den Berg, H.M. Soares, Anal. Chim. Acta 703, 1 (2011)
S. Chowdhury, B. Rooj, A. Dutta, U. Mandal, J. Fluoresc. 28, 999 (2018)
S.H. Park, N. Kwon, J.H. Lee, J. Yoon, I. Shin, Chem. Soc. Rev. 49, 143 (2020)
N. Duan, S. Yang, H. Tian, B. Sun, Food Chem. 358, 129839 (2021)
G. Sivaraman, M. Iniya, T. Anand, N.G. Kotla, O. Sunnapu, S. Singaravadivel, A. Gulyani, D. Chellappa, Coordin Chem. Rev. 357, 50 (2018)
S. Sharma, K.S. Ghosh, Spectrochim. Acta A Mol. Biomol. Spectrosc. 254, 119610 (2021)
S. Chakraborty, V. Ravindran, P.V. Nidheesh, S. Rayalu, ChemistrySelect 5, 10432 (2020)
N.R. Chereddy, S. Janakipriya, P.S. Korrapati, S. Thennarasu, A.B. Mandal, Analyst 138, 1130 (2013)
B. Fang, Y. Liang, F. Chen, Talanta 119, 601 (2014)
B. Tabakci, A. Yilmaz, J. Mol. Struct. 1075, 96 (2014)
C. Baslak, A.N. Kursunlu, Photochem. Photobiol. Sci. 17, 1091 (2018)
Q. Dai, H. Liu, C. Gao, W. Li, C. Zhu, C. Lin, Y. Tan, Z. Yuan, Y. Jiang, New J. Chem. 42, 613 (2018)
J. Pan, J.M. Yu, S.Y. Qiu, A.Y. Zhu, Y. Liu, X.X. Ban, W. Li, H. Yu, L.T. Li, J. Photoch. Photobio. A 406, 113018 (2021)
A.C. Sedgwick, L. Wu, H.H. Han, S.D. Bull, X.P. He, T.D. James, J.L. Sessler, B.Z. Tang, H. Tian, J. Yoon, Chem. Soc. Rev. 47, 8842 (2018)
Y. Wu, Z. Li, Y. Shen, ACS Omega 4, 16242 (2019)
X.T. Pan, Q. Li, Y.Y. Xu, S.L. Hu, J. Chem. Res. 44, 349 (2020)
J. Tang, H. Wu, S. Yin, Y. Han, Tetrahedron Lett. 60, 541 (2019)
X. Liu, L. Yang, L. Gao, W. Chen, F. Qi, F. Song, Tetrahedron 71, 8285 (2015)
D.J. Zhu, A.S. Ren, X.C. He, Y.H. Luo, Z.H. Duan, X.W. Yan, Y.H. Xiong, X. Zhong, Sensor. Actuat. B-chem. 252, 134 (2017)
J. Xu, Z. Wang, C. Liu, Z. Xu, B. Zhu, N. Wang, K. Wang, J. Wang, Anal. Sci. 34, 453 (2018)
T.H. Fife, T.J. Przystas, J. Am. Chem. Soc. 107, 1041 (1985)
R.M. Kierat, R. Kraemer, Bioorg. Med. Chem. Lett. 15, 4824 (2005)
Acknowledgements
This research was financially supported by the Shandong Provincial Natural Science Foundation (ZR2020MB107), the State Key Laboratory of Natural and Biomimetic Drugs (K20180201), the NSFC cultivation project of Jining Medical University (JYP2019KJ11), and the Supporting Fund for Teachers' research of Jining Medical University (JYFC2019KJ042).
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sheng, X., Kong, L., Wang, J. et al. A phthalimide-based ESIPT fluorescent probe for sensitive detection of Cu2+ in complete aqueous solution. ANAL. SCI. 38, 689–694 (2022). https://doi.org/10.1007/s44211-022-00084-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s44211-022-00084-9