Abstract
Extracellular vesicles (EVs) are cell-derived membrane-bound vesicles with heterogenous contents, including genetic materials, proteins, lipids and small metabolites. The classic EVs are exosomes, which originate from endosomal systems, and microvesicles, which are shed from the plasma membrane. Newly discovered organelle migrasome, once released from cells, adds another player to the EV realm. EVs are present in biological fluids and are important in multiple physiological and pathological processes, including immune regulation and cancer metastasis. Knowledge of EV biology is essential to promote the clinical application of EVs as potential candidates for non-invasive liquid biopsy and drug delivery vehicles. This is a fast-expanding field, but more attention should be paid to the fundamental biology of EVs in order to keep up with the explosive growth of translational needs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Communication from one cell to another, or between a cell and its microenvironment, is crucial in both physiological and pathological conditions. Such crosstalk is achieved by direct cell-cell contact and canonical secretion as well as by membrane-bound extracellular vesicles (EVs). Almost all tested cells are capable of secreting various types of EVs, containing biologically active cargos such as DNA, RNA, protein and metabolites, that vary in response to the microenvironment. Although released externally, these vesicles resemble their cell origin to some extent, and reflect the real-time state of the parent cell. EVs end up in most bodily fluids, including blood, urine, saliva and breast milk. This makes EVs perfect candidates for non-invasive liquid biopsy. By virtue of their cellular origin, EVs have high biocompatibility and low immunogenicity, which means they have potential as drug delivery vehicles and therapeutic reagents. Rapid advances in our understanding of the fundamental biology of EVs clearly demonstrate the potential of EVs as cancer biomarkers and drug delivery vehicles, while technical advances accelerate progress towards clinical application.
This review aims to discuss the biological properties of the two major EV classes, exosomes and microparticles, as well as newly discovered organelles called migrasomes which are specifically released from migrating cells. We will highlight the utility of EVs for the development of disease diagnostics and therapeutics, which is rooted in basic EV biology and promoted by advanced integrated multidisciplinary technologies.
2 EV biology
2.1 EV classes, biogenesis, and cargos
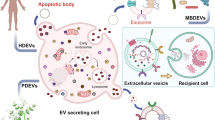
The secretion of extracellular vesicles was initially described as a means of eliminating unneeded compounds from the cell (Trams et al. 1981). In recent years, EVs have been recognized as membrane-protected vesicles containing bioactive agents which can execute certain functions such as signal communication. Although the classification of EVs is continuously evolving, based on our current knowledge of their biogenesis, they can be broadly divided into two main categories, exosomes and microvesicles. The newly discovered organelles called migrasomes, once released extracellularly, become a type of EV (Fig. 1). Our understanding of these vesicles is largely based on evidence provided by transmission and immunoelectron microscopy, fluorescence imaging, genetic approaches and biochemical means. Each EV has unique characteristics, such as size, biogenesis, and biomarkers (Table 1). Exosomes have received more attention than other types of EVs. In recent years, however, research interests have shown some tendency to focus on larger EVs rather than exosomes since their distinct functions and wide range of cargos have gradually been revealed (Lee et al. 2011; Cocucci et al. 2009; Jiao et al. 2021).
Features of EVs. EV biogenesis and signal transmission. The right side shows a schematic representation of exosome, microvesicle and migrasome biogenesis. The left side shows signal transmission which can be achieved by direct activation of surface receptors for outside-in signaling, fusion with target cells or complete engulfment of EVs. After uptake by cells, EVs enter early endosomes which subsequently fuse with other organelles in the endocytic compartment. Steps of EV biogenesis are denoted by blue arrows. Steps of EV uptake and cargo release are denoted by purple arrows. “Internalized EVs” can be self-generated (cell-autonomous) or generated by other cells (cell-nonautonomous)
Exosomes, which have diameters of 30–150 nm, were initially identified as membrane vesicles released by reticulocytes, an observation subsequently extended to a wide variety of cell types (Johnstone et al. 1987). Exosomes originate from endocytic pathways. The inward budding of endosomal membranes forms intraluminal vesicles (ILVs) in the lumen of a multivesicular body (MVB). ILVs are released as exosomes by fusion of the MVB membrane with the plasma membrane (Harding et al. 1984; Pan et al. 1985). The whole process involves particular sorting machineries, which ensure cargo specificity in exosomes. The discovery of the ESCRT (endosomal sorting complexes required for transport) machinery in ILV formation and cargo sorting was the first breakthrough (Hurley, 2008; Tamai et al. 2010; Colombo et al. 2013). There are four different ESCRTs, ESCRT 0, I, II, and III, which act in a stepwise manner (Hurley, 2008; Henne et al. 2011). ESCRT 0 recognizes ubiquitinated proteins, which is a signal for segregation of cargos into ILVs, on the outside of the endosomal membrane (Tamai et al. 2010; Raiborg & Stenmark, 2009). ESCRT I and II are subsequently recruited to initiate and drive the budding of ILV membranes (Shields et al. 2009; Razi & Futter, 2006; Katzmann et al. 2001). In turn, ESCRT III is recruited for the scission of the ILVs into the MVB lumen (Lin et al. 2005). This process is finalized by removal of the ubiquitin tag from the cargo proteins via recruitment of a deubiquitinating enzyme (McCullough et al. 2006; Agromayor & Martin-Serrano, 2006; Ma et al. 2007), and disassembly of ESCRT-III by the ATPase Vps4 (Stuchell-Brereton et al. 2007; Azmi et al. 2006). An ESCRT-independent pathway also exists, in which ceramide mediates membrane deformation in ILV and MVB formation (Trajkovic et al. 2008). Tetraspanin family proteins regulate the cargo sorting for exosomes (Theos et al. 2006; van Niel et al. 2011). These tetraspanin proteins form clusters and dynamic membrane macrodomains that mediate budding (Charrin et al. 2014). Additional mechanisms, for example co-sorting of chaperones or proteins with high affinity for certain kinds of lipids, also contribute to sorting. Apart from proteins, RNAs are important cargos of exosomes (Geminard et al. 2004). Sorting of RNAs is achieved by sorting of RNA-binding proteins with specific motifs, which bind and sort RNAs depending on their sequence (Villarroya-Beltri et al. 2013). The sorting machinery and its regulation is undoubtedly important and our knowledge is far from complete. The exosome proteomic database, available from the “ExoCarta” website”, shows that exosome contents are highly heterogeneous between different cell types and body fluids. The nature and abundance of exosome cargos are influenced by the physiological or pathological state of the donor cell. How these stimuli regulate the cargo sorting machinery in order to achieve the highly heterogeneous features of exosomes is largely unknown. Endosome dynamics and sorting machineries should be considered when investigating exosomes as biomarkers or when manipulating exosomes.
Microvesicles, initially known as “platelet dust”, were first identified as subcellular material originating from platelets and have long been studied mainly for their role in blood coagulation (Wolf, 1967; Sims et al. 1988; Satta et al. 1994). Microvesicles range from 500 nm to several microns in diameter. They are formed by outward budding of vesicles from the plasma membrane (Tricarico et al. 2017). Generation of microvesicles requires membrane lipid rearrangement and plasma membrane deformation, in which cytoskeleton elements are widely involved. Sorting of cargos into microvesicles is achieved through plasma membrane anchoring (Al-Nedawi et al. 2008). Microvesicles have received much less attention than exosomes and the detailed mechanisms of their biogenesis remain to be elucidated.
Migrasomes, first discovered in 2015, are micron-scale vesicles which contain numerous small internal vesicles. During cell migration, migrasomes grow on the tips or intersections of retraction fibers, which mark the path of cell migration on the extracellular matrix (ECM) (Ma et al. 2015). Migrasomes remain active communications with cell body before being detached from retraction fibers and released as extracellular vesicles. Migrasome formation requires cell migration. Thus, the first study to document the physiological function of migrasomes was in zebrafish embryonic development, which involves massive cell migration (Jiang et al. 2019). Migrasomes, enriched in a combination of ligands including chemokines, morphogens and growth factors, deliver signaling cues and function in organ morphogenesis and positioning (Jiang et al. 2019). Detached migrasomes have also been identified in multiple body fluids, expanding the range of their potential functional scenarios (Zhao et al. 2019). Unlike other extracellular vesicles, migrasomes maintain contact with the parent cells through retraction fibers for quite a long period of time before their eventual extracellular release. Large-scale plasma membrane deformation is observed in migrasome formation. Tetraspanin family proteins, especially TSPAN4, together with cholesterol, form micron-scale membrane macrodomains to accomplish the membrane shaping for retraction fiber extension and migrasome biogenesis (Zhang et al. 2020; Huang et al. 2019). Integrins play a role in providing the force that adheres migrasomes to the ECM (Wu et al. 2017). Thus, TSPAN4 and integrins are markers of migrasomes. Migrasomes are a medium for intercellular lateral transfer of RNAs and proteins. Interestingly, distinct from exosomes, migrasomes contain membrane-bound organelles and large amounts of mRNA derived from the parent cell (Zhu et al. 2021). Recently, Yu’s group showed that damaged mitochondria in migrating cells are transported into migrasomes and subsequently disposed of. This process, known as mitocytosis, requires the damaged mitochondria to be positioned at the cell periphery for sorting into migrasomes. Mitocytosis not only maintains mitochondrial quality for cell viability, but is also likely to be involved in sending out mitochondrial stress information (Jiao et al. 2021). At present, it is largely unknown whether other membrane-bound organelles and mRNAs are sorted into migrasomes. This is being actively explored.
2.2 EV destinations
Once released into the microenvironment, EVs reach the recipient cells and trigger functional responses or promote phenotypic changes. Signal transmission can be achieved by direct activation of surface receptors for outside-in signaling, fusion with target cells or complete engulfment of EVs, all of which require vesicle recognition and docking (Fig. 1). The specificity is determined by recognition between ligands enriched at the surface of EVs and receptors on the plasma membrane of the recipient cells, which depend on the origin and subpopulation of EVs and on the identity of the recipient cells. In terms of specific enrichment of surface molecules, plasma membrane-derived EVs might be different from endocytic pathway-derived EVs. So far, several ligand-receptor pairs have been reported. Protein-glycan interactions include lectins and glycan moieties (Hao et al. 2007; Saunderson et al. 2014; Barres et al. 2010; Shimoda et al. 2017), and heparan sulfate proteoglycans and fibronectin (Christianson et al. 2013). Lipid-protein recognition can occur between phosphatidylserine (PS) that is flipped to the outside of the membrane and T cell immunoglobulin mucin (TIM) family proteins (Sims et al. 2017). Adhesion molecules include tetraspanins, integrins, ICAM-1, ECM components, etc (Hao et al. 2007; Yuan et al. 2017). Specifically, the interaction of integrins with ECM proteins, mostly fibronectin and laminin, has been shown to have important roles in ensuring that exosomes interact with the right recipient (Purushothaman et al. 2016). In in vivo situations, integrin heterodimers drive EVs towards specific target organs, possibly through the cell-associated ECM.
EVs can directly activate cell surface receptors via protein and bioactive lipid ligands (Polgar et al. 2005). EVs can also deliver contents through membrane fusion with the plasma membrane. In addition, EVs can be internalized, which requires release of their contents inside the target cells to actually elicit a downstream response. The destiny of internalized EVs may be lysosome degradation to provide metabolites. For EVs with a signaling mission, their contents should retain their original function within the recipient cell. This functional competence is supported by mounting evidence showing targeted conversion of recipient cells. Interestingly, EVs can release their contents into the cytosol of the parent cell through back fusion with the MVB membrane (Bissig & Gruenberg, 2014) (Fig. 1).
2.3 EVs in immune modulation
The role of EVs in diseases, the onset and progression of which require cell-cell signal transduction, has been widely documented. The study of exosomes in immune responses and tumors has progressed at a rapid pace compared with other functions in embryonic development, neurodegeneration, metabolic diseases and cardiovascular diseases. Regardless of the system or disease being studied, the same basic cell biology questions should be answered, such as uncovering the identities of the donor and recipient cells, the candidate molecules, the sorting machinery and the functional response of recipient cells. For example, regulation of the immune response by exosomes is accomplished through direct antigen presentation by MHC molecules on the exosome surface. Exosomes derived from B lymphocytes induce antigen-specific MHC class II-restricted T cell responses, suggesting a role for exosomes in antigen presentation in vivo (Raposo et al. 1996). Exosomes derived from ovalbumin (OVA)-treated dendritic cells elicited OVA-specific CD8+ T cell activation (Wahlund et al. 2017). This sets up a foundation for studying exosomes as cancer vaccines. Modulation of immune responses by exosomes also involves presentation of immunoregulatory surface molecules on exosomes. Presentation of immunoregulatory molecules such as PD-L1 (programmed cell death ligand 1) on the exosome surface contributes to suppression of T cells and promotion of tumor growth (Chen et al. 2018a; Poggio et al. 2019). Exosomes may also regulate the immune response by influencing gene expression and signaling pathways in recipient cells, principally by the transfer of microRNAs (miRNAs) and proteins, which is well documented in other reviews. Exosomes can either eliminate or promote innate or adaptive immune responses under different pathological conditions (Robbins & Morelli, 2014).
2.4 EVs in cancer
Exosomes have been associated with neoplasia, tumor growth and metastasis, the hallmark features of cancer. Neoplastic reprogramming and tumor growth can be promoted by exosome cargos including miRNAs, as well as HRas and Kras mRNAs (Abd Elmageed et al. 2014). Another study showed that cancer cell exosomes induced random mutations and initiated malignant cell transformation, and the transformed cells could form tumors in vivo (Stefanius et al. 2019). Metastatic ability can be transferred through exosomes to promote the epithelial-to-mesenchymal transition (EMT) of poorly metastatic cells (Le et al. 2014).
Exosomes are detected in the tumor microenvironment, which is composed of cancer cells, stromal cells and stromal elements such as ECM components. Modulation of the immune response by tumor-derived exosomes involves a prometastatic inflammatory response (Le et al. 2014), which influences dendritic cell maturation and immunosuppression. The reciprocal exchange of exosomes between the stroma and cancer cells has been reported to enhance fitness for tumor growth. This exosomal exchange results in metabolic changes, PTEN suppression or autocrine Wnt-PCP signaling through transfer of metabolites, miRNAs or proteins (Zhang et al. 2015; Luga et al. 2012). Exosomes have been implicated in the angiogenic and ECM remodelling of the tumor microenvironment. In this context, suppression of endothelial tight junction proteins, reduced integrity of blood vessel endothelial cells, and degradation of the ECM through matrix metalloproteases (MMPs) are involved in promoting tumor growth and dissemination (Zhou et al. 2014; Kucharzewska et al. 2013; Genschmer et al. 2019; Yokoi et al. 2017).
Apart from creating their own in situ microenvironment for tumorigenesis, tumors induce formation of microenvironments in distant organs to support the survival and outgrowth of tumor cells even before colonization. These distant microenvironments are named pre-metastatic niches (PMNs) (Kaplan et al. 2005). When mice were pre-injected with exosomes from highly metastatic melanoma cells, they developed many more metastatic tumors than mice injected with exosomes from weakly metastatic melanoma cells. This proves that EVs carry important factors for PMN formation (Peinado et al. 2012). Follow-up studies showed that EVs induce a series of events during PMN formation to create a supportive microenvironment for metastasis, including vascular leakiness, attracting and educating bone marrow-derived cells (BMDCs), and reprogramming of local resident cells to induce proinflammatory responses and metabolic fitness (Costa-Silva et al. 2015). Moreover, the integrin pairs on exosomes predict the site of tumor metastasis (Hoshino et al. 2015). More interestingly, at the very early stage of tumorigenesis, tumor cells have the potential to metastasize by dormancy in pre-formed PMNs before undergoing activation and growth. This explains why some tumors produce metastases long after the primary tumor is removed (Peinado et al. 2017). Although there is still some debate about the role of tumor-derived exosomes in shaping PMNs, there is hope that this feature of exosomes could be used to predict whether a patient’s cancer will metastasize.
2.5 Techniques for studying EV biology
Progress in the EV field requires understanding of the underlying fundamental cell biology mechanisms. Advanced biochemistry approaches, imaging methods and omics techniques have been very important in advancing the EV field. High-throughput, large-scale knockdown or knockout screening has greatly accelerated the discovery of the protein machinery network. Super-resolution omics makes identification and quantification of EV contents increasingly accurate. Breakthroughs in live-imaging methods have improved the temporal and spatial resolution when analyzing cultured cells or animal models. These advances will surely aid in the understanding of the mechanisms and function of EVs (Wu et al. 2021).
3 Research milestones in the development of exosomes for clinical translation
After they were first discovered in 1980, EVs were largely dismissed for a couple of decades as cell debris or “waste carriers” of the cell. A series of landmark events both in cell biology and technology development support the potential of EVs as cancer biomarkers (Fig. 2). Jan Lötvall’s group was the first to report that exosomes could serve as a novel mechanism of genetic exchange between cells, through the transfer of functional RNA molecules, including mRNA and miRNA (Valadi et al. 2007). Johan Skog et al. reported that exosomes specifically promote tumor growth through the transport of mRNA, miRNA and angiogenic proteins. These tumor-derived exosomes have the potential to provide diagnostic information through a blood test (Skog et al. 2008). This was followed by the work of Lydia Alvarez-Erviti et al. who were the first to deliver siRNA with targeted exosomes in vivo (Alvarez-Erviti et al. 2011). Another important study introduced proteins into the diagnostic story. Glypican-1 (GPC1), a cell surface proteoglycan, was identified to be specifically enriched in exosomes harvested from the blood of patients with pancreatic cancer. The level of GPC1 distinguished benign pancreatic disease from pancreatic cancer with high sensitivity and specificity (Melo et al. 2015). The role of exosomes in pre-metastatic niche formation, and the organ-seeking nature of exosomes through integrin zip coding, may make it possible to predict whether and where metastasis will occur (Costa-Silva et al. 2015; Hoshino et al. 2015). Recently, the discovery of migrasomes, which are formed and released via completely new mechanisms, has steered the EV field in broader directions (Jiao et al. 2021; Ma et al. 2015) The integration of microfluidics and various sensors has made it possible to isolate and detect EVs on a chip, with significantly improved accuracy and sensitivity (Kanwar et al. 2014). Simultaneously, exosomes have been explored for delivery of a miRNA or siRNA payload to facilitate anticancer treatment. Clinical-grade mesenchymal stem cell (MSC)-derived exosomes harbouring KrasG12D siRNA (iExosomes) have been used to treat pancreatic cancer in multiple animal models (Kamerkar et al. 2017). It is interesting to see from these milestones that the interplay between basic scientific discoveries and technological developments has led to huge advances in the EV field. Though there are still many debated ideas and technical limitations in the newly emerging areas of the EV field, it is clear that EVs are implicated in many facets of disease development and progression, and therefore they are ideal candidates as biomarkers and/or therapeutic tools.
Milestones in EV research. Schematic representation of key milestones in EV research covering advances in EV biology and technology, and eventually culminating in clinical applications: 2007: First report of EV-mediated RNA transfer as a novel mechanism of genetic exchange between cells (Valadi et al. 2007). 2008: Early studies document that transfer of EVs to tumor cells promotes tumor growth (Skog et al. 2008). 2011: First study to use exosomes harboring siRNA to achieve gene knockdown in vivo. The accomplishment of in vivo transmission of RNA through EVs is the foundation of using EVs as bio-delivery vehicles (Alvarez-Erviti et al. 2011). 2014: A chip-based method is established to study EVs with high sensitivity and specificity (Kanwar et al. 2014). 2015: EVs carrying specific protein biomarkers of disease are reported, which steers the focus of EV research towards proteomics and protein functions (Melo et al. 2015). 2015: The concept is raised of roles for EVs in the PMN, including vascular leakiness, inflammation, ECM remodeling and tumor cell recruitment and survival (Peinado et al. 2017). 2015: The discovery of migrasomes, with completely new mechanisms of biogenesis and release (Ma et al. 2015). 2017: Clinical grade “iExosomes” are used to treat pancreatic cancer, a milestone in the use of EVs for clinical treatment (Kamerkar et al. 2017)
4 EVs as diagnostic biomarkers
4.1 EVs as the target of liquid biopsy
Nowadays, the analysis of cancer characteristics is central to patient management and treatment decisions. Liquid biopsy provides the opportunity of detecting, analyzing and monitoring cancer in various body fluids such as blood or urine instead of a fragment of cancer tissue. EVs have many advantages as targets of non-invasive liquid biopsy. The biggest advantage of EV analysis over other blood-based liquid biopsy targets such as circulating tumor cells (CTCs) is the access to a larger population of biomarkers in EVs. EVs harvested from a patient’s blood contain enough materials to analyze and use as a diagnostic indicator. The heterogeneity of EV cargos in various disease conditions provides an opportunity to establish EV-related disease specificity. Tumors contain a heterogeneous mix of cancer cells which generate different cancer cell clones within tumor tissues. Thus, tissue biopsy might not be able to provide an accurate landscape of the entire tumor. EVs from patients’ blood offer comprehensive information about tumor heterogeneity. In addition to genetic information, analysis of EVs can also be used to monitor proteins and metabolites associated with the tumor state. Longitudinal sampling to monitor disease progression is informative and much easier to carry out, since repeated sampling of body fluids is more acceptable than repeated biopsies.
4.2 Technologies used for analysis of EV biomarkers
Techniques for isolating and characterizing EVs are the key to connecting the mounting evidence for EV cargos in different diseases with clinical applications using EVs as diagnostic biomarkers. Conventional methods to isolate EVs from plasma or other samples include ultracentrifugation, density-gradient centrifugation, filtration, polymer-based precipitation, size exclusion chromatography (SEC) and immunoaffinity purification (Doyle & Wang, 2019; Coumans et al. 2017). To increase the purity of EVs, immunoaffinity purification can be performed using tissue-specific exosome markers to enable extraction of subgroups of EVs. This allows tracing of EV origins and avoids contamination from other EV sources. Such a strategy has been actively adapted for purification of brain-derived exosome due to the presence of brain tissue-specific exosome markers (Shi et al. 2014; Yu et al. 2020; Deng & Miller, 2019). Other strategies include 2-step purification with ultracentrifugation followed by immunoaffinity purification to further increase the purity (Shurtleff et al. 2016). In summary, the performance of different exosome isolation methods varies significantly due to contamination, poor yield, intensive labor, low throughput or high equipment demands, and there are various trade-offs between parameters. There are several different characterization techniques. Analysis of morphological features relies on electron microscopy (EM), fluorescence imaging and nanoparticle tracking analysis (NTA). Targeted detection of either RNAs or proteins relies on PCR- or antibody-based detection. Most recently, unbiased multi-omics approaches have been applied to investigate the molecular composition of EVs. These approaches combine next-generation sequencing, metabolomics and mass spectrometry-based proteomics. Advanced super-resolution detection methods demand high quality samples. These methods have limited tolerance of contamination and protein/chemical labelling, and they require small sample volumes and fairly high enrichment. Low throughputs are another concern. Thus, there is an urgent need to develop advanced isolation technologies compatible with these increasingly refined analysis tools. The introduction of microfluidics has facilitated chip-based isolation procedures, a big step forward which will significantly improve the accuracy of exosome isolation from biological samples (Vaidyanathan et al. 2018). Microfluidic isolation methods are typically rapid and efficient and require small starting volumes. They allow for the development of innovative separation mechanisms based on the acoustic, electrophoretic, and electromagnetic properties of the exosomal vesicles. Most recently, in 2021, Liu’s group designed a device to impose periodic negative pressure oscillations (NPOs) on a nanoporous membrane to achieve separation of label-free exosomes from small particles (free proteins or nucleic acids) (Chen et al. 2021). The isolation methods are compatible with downstream omics studies which provide deep and unbiased characterization of EV cargos. This is a valuable resource for identifying biomarkers.
4.3 Challenges and opportunities in the clinical translation of EVs as biomarkers
Though much of the clinical data suggests important links between EVs and diseases, these studies are at an early stage and the results are only correlative. Fundamental questions about the biological mechanisms underlying exosome structure and function remain unanswered, although landmark discoveries have been made. Further investigations are required to fully resolve the functional capabilities of these vesicles.
The function of EV RNA cargos has received more attention than protein cargos, due to the fact that proteomics techniques lag behind sequencing techniques in terms of both depth and throughput. However, protein cargos have equally important functions and should receive greater focus. Recently, there have been multiple attempts using proteomics approaches to revisit the cargos of exosomes from both cell culture and clinical samples (Shi et al. 2014; Hoshino et al. 2020; Jeppesen et al. 2019; Kugeratski et al. 2021). These studies for the first time involved samples from large clinical cohorts to reveal the high level of heterogeneity in clinical samples and to shed some light on EV proteins which are consistently and significantly enriched under disease conditions. In addition, the proteins present on the surface of EVs provide invaluable information about the physiological states of the parental cells of EVs. A multicomponent, combinatorial approach using a combination of EV markers will provide the most accurate indication of disease. At present it is challenging to isolate desired EV subpopulations with high purity from the mixture of EVs in body fluids. There is a clear need to establish better exosomal markers for this purpose. Therefore, the EV surface proteome should be put on the agenda.
Large-scale unbiased discovery of exosome biomarkers requires high quality clinical cohorts and standardized isolation and characterization protocols. Currently, variation in isolation strategies and analytical techniques has made multi-centre integrated analysis difficult, especially for proteomic analysis. This adds another drawback to these already low-throughput techniques.
Regarding clinical application scenarios, most of the isolation approaches are still not compatible with clinical analysis due to issues of scalability, standardization and validation. Furthermore, several of the approaches are time-consuming and require large sample amounts and extensive pre-treatment steps.
5 EVs as therapeutic agents and bio-delivery vehicles
5.1 EVs as therapeutic agents
Targeting extracellular vesicles to exploit their innate therapeutic potential or using them for drug delivery are important emerging strategies for therapy. Due to their bioactive cargos, EVs on their own have therapeutic potential. In myocardial infarction or other models of injury, it is confirmed that exosomes are responsible for the beneficial outcomes of transplanted MSCs rather than the MSCs themselves (Bruno et al. 2009; Lai et al. 2010). This pioneering discovery boosted research into the application of EVs derived from MSCs in driving tissue regeneration. The success of therapy based on MSC-derived exosomes is reflected by the growing number of ongoing clinical trials for various diseases (Zipkin, 2020).
5.2 EVs as bio-delivery vehicles
Exosomes derived from B cells present antigens and stimulate T cells in vivo. Dendritic cells (DCs) secrete exosomes expressing MHC-I/II molecules and T cell co-stimulatory molecules, which suppress tumor growth depending on T cell function (Raposo et al. 1996; Zitvogel et al. 1998). Although it is not exactly clear whether the antigen presentation by exosomes is direct or indirect in vivo, these milestone discoveries promote the investigation of exosomes as immune modulators (Fig. 3). The resulting immune activation or suppression can be exploited in different disease scenarios like cancer or autoimmune diseases.
EVs as therapeutic agents. Exploitation of EVs as therapeutic agents. a. EV-based therapeutics can be achieved by direct activation of surface ligands or by transfer of growth factors, soluble proteins, bioactive lipids and genetic materials. b, c. Extracellular vesicles can be engineered to contain certain contents or to present ligands on the surface by exogenous (b) or endogenous (c) loading. b. Post-purification EVs are exogenously loaded with the purified cargo of interest. The resulting EVs retain their original cargo. c. EV-producing cells are modified to overexpress fusion proteins in which a targeted cargo is joined with a canonical EV membrane protein
A study in 2007 was the first to show that exosomes facilitate lateral RNA transfer between cells (Valadi et al. 2007). Later, another study successfully delivered siRNA to mouse brain by injection of exosomes (Alvarez-Erviti et al. 2011). This was the first time a desired cargo was loaded into self-generated exosomes to achieve delivery in vivo. The inherent ability of EVs to cross biological barriers, even the blood-brain barrier, makes them more attractive than other delivery mediators (Zhuang et al. 2011). These pioneering studies established the foundation of exosomes as bio-delivery vesicles and promoted investigations into clinical-grade exosomes derived from stem cells (Fig. 3).
There are two main strategies to exploit extracellular vesicles for cargo delivery, endogenous loading or exogenous loading (Fig. 3). Endogenous loading can be achieved by engineering the parental cells so that they express a fusion protein consisting of a targeted cargo protein joined to a canonical EV membrane protein. For exogenous loading, EVs are first isolated and then loaded with a purified RNA of interest through co-incubation, sonication or electroporation. The resulting EVs retain their original cargo (Zickler & El Andaloussi, 2020). It should be noted that other intracellular components will also be actively or passively loaded into the engineered EVs, which may influence the therapeutic efficacy. Importantly, engineering of parental cells and post-purification loading can be used together, thereby taking advantage of both specific biodistribution and increased anti-tumor efficacy. Other than that, EV surface proteins can be modified by optical tags and radioactive isotope labelling for monitoring purposes (Salunkhe, 2020).
5.3 Challenges
Although multiple discoveries have paved the way to potential clinical applications of EVs, the clinical translation of EVs is still at a very early stage and major challenges need to be overcome. For example, MSC-derived exosomes have been shown to both inhibit and promote tumor growth (Zhu et al. 2012; Bruno et al. 2013). Such discrepancies are probably a consequence of uncertainty about which EV cargos have bioactive effects, variability in the cell culture conditions which produce EVs, unstandardized purification protocols, and a lack of EV quality control markers. Therefore, suitable cell sources for clinical-grade EV production are urgently needed to increase yield, purity and safety. In addition, standardized protocols for EV purification should be strictly established. Last but not least, knowledge of the fundamental biology of EVs should keep up with the explosive growth of translational needs.
6 Conclusions and perspectives
Much progress has been made in recent years in understanding the basic biology of extracellular vesicles. However, despite the enormous therapeutic potential of EVs, this field still needs a systematic understanding of the basic mechanisms involved in EV biogenesis, cargo sorting and release. Over the past decades, scientists have characterized the involvement of endocytic pathways in EV generation. The subsequent explorations of EV function in immune regulation or tumor metastasis and EV engineering are mostly based on endocytic pathways. However, the potential of EVs should not be limited only to one certain pathway. The discovery of migrasomes has steered the field towards broader applications. Migrasomes remain strong connection with cell body as cellular organelles before being released extracellularly as a type of EV. The unique biogenesis mechanisms of migrasomes allows cells to release a wider range of molecules, which brings new opportunities to exploit EVs as biomarkers, therapeutic agents and bio-delivery vehicles. The discovery of cellular organelles in migrasomes, which function in organelle quality control and reflect cellular stress conditions, provides another exciting example of EV functions beyond our current knowledge of exosomes.
Advanced technologies like imaging methods or omics methods should be developed for deep analysis of small amounts of EVs or even individual EVs. The in-depth identification, accuracy and reproducibility of quantification achieved by advanced omics techniques will guarantee new discoveries about the basic mechanisms of EV biogenesis and function, and will promote the clinical translation of EVs.
The EV field is highly interdisciplinary. Cell biologists, physicians and engineers should work together on the basic functions of extracellular vesicles and on their translation from the bench to the bedside.
Availability of data and materials
Not applicable.
References
Abd Elmageed ZY, Yang Y, Thomas R, Ranjan M, Mondal D, Moroz K, et al. Neoplastic reprogramming of patient-derived adipose stem cells by prostate cancer cell-associated exosomes. Stem Cells. 2014;32(4):983–97. https://doi.org/10.1002/stem.1619.
Agromayor M, Martin-Serrano J. Interaction of AMSH with ESCRT-III and deubiquitination of endosomal cargo. J Biol Chem. 2006;281:23083–91. https://doi.org/10.1074/jbc.M513803200.
Al-Nedawi K, et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol. 2008;10(5):619–24. https://doi.org/10.1038/ncb1725.
Alvarez-Erviti L, Seow Y, Yin HF, Betts C, Lakhal S, Wood MJA. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29(4):341–5. https://doi.org/10.1038/nbt.1807.
Azmi I, Davies B, Dimaano C, Payne J, Eckert D, Babst M, et al. Recycling of ESCRTs by the AAA-ATPase Vps4 is regulated by a conserved VSL region in Vta1. J Cell Biol. 2006;172(5):705–17. https://doi.org/10.1083/jcb.200508166.
Barres C, et al. Galectin-5 is bound onto the surface of rat reticulocyte exosomes and modulates vesicle uptake by macrophages. Blood. 2010;115(3):696–705. https://doi.org/10.1182/blood-2009-07-231449.
Bissig C, Gruenberg J. ALIX and the multivesicular endosome: ALIX in wonderland. Trends Cell Biol. 2014;24(1):19–25. https://doi.org/10.1016/j.tcb.2013.10.009.
Bruno S, Collino F, Deregibus MC, Grange C, Tetta C, Camussi G. Microvesicles derived from human bone marrow mesenchymal stem cells inhibit tumor growth. Stem Cells Dev. 2013;22(5):758–71. https://doi.org/10.1089/scd.2012.0304.
Bruno S, et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol. 2009;20:1053–67. https://doi.org/10.1681/ASN.2008070798.
Charrin S, Jouannet S, Boucheix C, Rubinstein E. Tetraspanins at a glance. J Cell Sci. 2014;127(Pt 17):3641–8. https://doi.org/10.1242/jcs.154906.
Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018a;560(7718):382–6. https://doi.org/10.1038/s41586-018-0392-8.
Chen Y, Li Y, Ma L, Yu L. Detection of Migrasomes Methods Mol Biol. 2018b;1749:43–9. https://doi.org/10.1007/978-1-4939-7701-7_5.
Chen Y, Zhu Q, Cheng L, Wang Y, Li M, Yang Q, et al. Exosome detection via the ultrafast-isolation system: EXODUS. Nat Methods. 2021;18(2):212–8. https://doi.org/10.1038/s41592-020-01034-x.
Christianson HC, Svensson KJ, van Kuppevelt TH, Li JP, Belting M. Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proc Natl Acad Sci U S A. 2013;110(43):17380–5. https://doi.org/10.1073/pnas.1304266110.
Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol. 2009;19(2):43–51. https://doi.org/10.1016/j.tcb.2008.11.003.
Colombo M, Moita C, van Niel G, Kowal J, Vigneron J, Benaroch P, et al. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci. 2013;126(Pt 24):5553–65. https://doi.org/10.1242/jcs.128868.
Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol. 2015;17(6):816–26. https://doi.org/10.1038/ncb3169.
Coumans FAW, Brisson AR, Buzas EI, Dignat-George F, Drees EEE, el-Andaloussi S, et al. Methodological guidelines to study extracellular vesicles. Circ Res. 2017;120(10):1632–48. https://doi.org/10.1161/CIRCRESAHA.117.309417.
Deng F, Miller J. A review on protein markers of exosome from different bio-resources and the antibodies used for characterization. J Histotechnol. 2019;42(4):226–39. https://doi.org/10.1080/01478885.2019.1646984.
Doyle LM, Wang MZ. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells. 2019;8. https://doi.org/10.3390/cells8070727.
Geminard C, De Gassart A, Blanc L, Vidal M. Degradation of AP2 during reticulocyte maturation enhances binding of hsc70 and Alix to a common site on TFR for sorting into exosomes. Traffic. 2004;5(3):181–93. https://doi.org/10.1111/j.1600-0854.2004.0167.x.
Genschmer KR, et al. Activated PMN exosomes: pathogenic entities causing matrix destruction and disease in the lung. Cell. 2019;176:113–126 e115. https://doi.org/10.1016/j.cell.2018.12.002.
Gyorgy B, et al. Detection and isolation of cell-derived microparticles are compromised by protein complexes resulting from shared biophysical parameters. Blood. 2011;117(4):e39–48. https://doi.org/10.1182/blood-2010-09-307595.
Hao S, Bai O, Li F, Yuan J, Laferte S, Xiang J. Mature dendritic cells pulsed with exosomes stimulate efficient cytotoxic T-lymphocyte responses and antitumour immunity. Immunology. 2007;120(1):90–102. https://doi.org/10.1111/j.1365-2567.2006.02483.x.
Harding C, Heuser J, Stahl P. Endocytosis and intracellular processing of transferrin and colloidal gold-transferrin in rat reticulocytes: demonstration of a pathway for receptor shedding. Eur J Cell Biol. 1984;35(2):256–63.
Henne WM, Buchkovich NJ, Emr SD. The ESCRT pathway. Dev Cell. 2011;21(1):77–91. https://doi.org/10.1016/j.devcel.2011.05.015.
Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527(7578):329–35. https://doi.org/10.1038/nature15756.
Hoshino A, et al. Extracellular vesicle and particle biomarkers define multiple human cancers. Cell. 2020;182:1044–61 e1018. https://doi.org/10.1016/j.cell.2020.07.009.
Huang Y, Zucker B, Zhang S, et al. Migrasome formation is mediated by assembly of micron-scale tetraspanin macrodomains. Nat Cell Biol. 2019;21(8):991–1002. https://doi.org/10.1038/s41556-019-0367-5.
Hurley JH. ESCRT complexes and the biogenesis of multivesicular bodies. Curr Opin Cell Biol. 2008;20(1):4–11. https://doi.org/10.1016/j.ceb.2007.12.002.
Jeppesen DK, et al. Reassessment of exosome composition. Cell. 2019;177:428–45 e418. https://doi.org/10.1016/j.cell.2019.02.029.
Jiang D, Jiang Z, Lu D, Wang X, Liang H, Zhang J, et al. Migrasomes provide regional cues for organ morphogenesis during zebrafish gastrulation. Nat Cell Biol. 2019;21(8):966–77. https://doi.org/10.1038/s41556-019-0358-6.
Jiao H, et al. Mitocytosis, a migrasome-mediated mitochondrial quality-control process. Cell. 2021;184:2896–910. e13. https://doi.org/10.1016/j.cell.2021.04.027.
Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987;262(19):9412–20. https://doi.org/10.1016/S0021-9258(18)48095-7.
Kamerkar S, LeBleu VS, Sugimoto H, Yang S, Ruivo CF, Melo SA, et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546(7659):498–503. https://doi.org/10.1038/nature22341.
Kanwar SS, Dunlay CJ, Simeone DM, Nagrath S. Microfluidic device (ExoChip) for on-chip isolation, quantification and characterization of circulating exosomes. Lab Chip. 2014;14(11):1891–900. https://doi.org/10.1039/c4lc00136b.
Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438(7069):820–7. https://doi.org/10.1038/nature04186.
Katzmann DJ, Babst M, Emr SD. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell. 2001;106(2):145–55. https://doi.org/10.1016/s0092-8674(01)00434-2.
Kucharzewska P, Christianson HC, Welch JE, Svensson KJ, Fredlund E, Ringnér M, et al. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc Natl Acad Sci U S A. 2013;110(18):7312–7. https://doi.org/10.1073/pnas.1220998110.
Kugeratski FG, Hodge K, Lilla S, McAndrews KM, Zhou X, Hwang RF, et al. Quantitative proteomics identifies the core proteome of exosomes with syntenin-1 as the highest abundant protein and a putative universal biomarker. Nat Cell Biol. 2021;23(6):631–41. https://doi.org/10.1038/s41556-021-00693-y.
Lai RC, Arslan F, Lee MM, Sze NSK, Choo A, Chen TS, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4(3):214–22. https://doi.org/10.1016/j.scr.2009.12.003.
Le MT, et al. miR-200-containing extracellular vesicles promote breast cancer cell metastasis. J Clin Invest. 2014;124(12):5109–28. https://doi.org/10.1172/JCI75695.
Lee TH, D’Asti E, Magnus N, al-Nedawi K, Meehan B, Rak J. Microvesicles as mediators of intercellular communication in cancer--the emerging science of cellular 'debris'. Semin Immunopathol. 2011;33(5):455–67. https://doi.org/10.1007/s00281-011-0250-3.
Lin Y, Kimpler LA, Naismith TV, Lauer JM, Hanson PI. Interaction of the mammalian endosomal sorting complex required for transport (ESCRT) III protein hSnf7–1 with itself, membranes, and the AAA+ ATPase SKD1. J Biol Chem. 2005;280:12799–809. https://doi.org/10.1074/jbc.M413968200.
Luga V, Zhang L, Viloria-Petit AM, Ogunjimi AA, Inanlou MR, Chiu E, et al. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell. 2012;151(7):1542–56. https://doi.org/10.1016/j.cell.2012.11.024.
Ma L, Li Y, Peng J, Wu D, Zhao X, Cui Y, et al. Discovery of the migrasome, an organelle mediating release of cytoplasmic contents during cell migration. Cell Res. 2015;25(1):24–38. https://doi.org/10.1038/cr.2014.135.
Ma YM, et al. Targeting of AMSH to endosomes is required for epidermal growth factor receptor degradation. J Biol Chem. 2007;282:9805–12. https://doi.org/10.1074/jbc.M611635200.
McCullough J, Row PE, Lorenzo Ó, Doherty M, Beynon R, Clague MJ, et al. Activation of the endosome-associated ubiquitin isopeptidase AMSH by STAM, a component of the multivesicular body-sorting machinery. Curr Biol. 2006;16(2):160–5. https://doi.org/10.1016/j.cub.2005.11.073.
Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523(7559):177–82. https://doi.org/10.1038/nature14581.
Pan BT, Teng K, Wu C, Adam M, Johnstone RM. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J Cell Biol. 1985;101(3):942–8. https://doi.org/10.1083/jcb.101.3.942.
Peinado H, Alečković M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18(6):883–91. https://doi.org/10.1038/nm.2753.
Peinado H, Zhang H, Matei IR, Costa-Silva B, Hoshino A, Rodrigues G, et al. Pre-metastatic niches: organ-specific homes for metastases. Nat Rev Cancer. 2017;17(5):302–17. https://doi.org/10.1038/nrc.2017.6.
Piccin A, Murphy WG, Smith OP. Circulating microparticles: pathophysiology and clinical implications. Blood Rev. 2007;21(3):157–71. https://doi.org/10.1016/j.blre.2006.09.001.
Poggio M, et al. Suppression of exosomal PD-L1 induces systemic anti-tumor immunity and memory. Cell. 2019;177:414–27 e413. https://doi.org/10.1016/j.cell.2019.02.016.
Polgar J, Matuskova J, Wagner DD. The P-selectin, tissue factor, coagulation triad. J Thromb Haemost. 2005;3(8):1590–6. https://doi.org/10.1111/j.1538-7836.2005.01373.x.
Purushothaman A, et al. Fibronectin on the surface of myeloma cell-derived exosomes mediates exosome-cell interactions. J Biol Chem. 2016;291:1652–63. https://doi.org/10.1074/jbc.M115.686295.
Raiborg C, Stenmark H. The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature. 2009;458(7237):445–52. https://doi.org/10.1038/nature07961.
Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183(3):1161–72. https://doi.org/10.1084/jem.183.3.1161.
Razi M, Futter CE. Distinct roles for Tsg101 and Hrs in multivesicular body formation and inward vesiculation. Mol Biol Cell. 2006;17(8):3469–83. https://doi.org/10.1091/mbc.e05-11-1054.
Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14(3):195–208. https://doi.org/10.1038/nri3622.
Salunkhe S. Dheeraj, Basak, M., Chitkara, D. & Mittal, A. surface functionalization of exosomes for target-specific delivery and in vivo imaging & tracking: strategies and significance. J Control Release. 2020;326:599–614. https://doi.org/10.1016/j.jconrel.2020.07.042.
Satta N, et al. Monocyte vesiculation is a possible mechanism for dissemination of membrane-associated procoagulant activities and adhesion molecules after stimulation by lipopolysaccharide. J Immunol. 1994;153:3245–55.
Saunderson SC, Dunn AC, Crocker PR, McLellan AD. CD169 mediates the capture of exosomes in spleen and lymph node. Blood. 2014;123(2):208–16. https://doi.org/10.1182/blood-2013-03-489732.
Sellam J, Proulle V, Jüngel A, Ittah M, Miceli Richard C, Gottenberg JE, et al. Increased levels of circulating microparticles in primary Sjogren's syndrome, systemic lupus erythematosus and rheumatoid arthritis and relation with disease activity. Arthritis Res Ther. 2009;11(5):R156. https://doi.org/10.1186/ar2833.
Shi M, Liu C, Cook TJ, Bullock KM, Zhao Y, Ginghina C, et al. Plasma exosomal alpha-synuclein is likely CNS-derived and increased in Parkinson's disease. Acta Neuropathol. 2014;128(5):639–50. https://doi.org/10.1007/s00401-014-1314-y.
Shields SB, Oestreich AJ, Winistorfer S, Nguyen D, Payne JA, Katzmann DJ, et al. ESCRT ubiquitin-binding domains function cooperatively during MVB cargo sorting. J Cell Biol. 2009;185(2):213–24. https://doi.org/10.1083/jcb.200811130.
Shimoda A, Tahara Y, Sawada SI, Sasaki Y, Akiyoshi K. Glycan profiling analysis using evanescent-field fluorescence-assisted lectin array: importance of sugar recognition for cellular uptake of exosomes from mesenchymal stem cells. Biochem Biophys Res Commun. 2017;491(3):701–7. https://doi.org/10.1016/j.bbrc.2017.07.126.
Shurtleff MJ, Temoche-Diaz MM, Karfilis KV, Ri S, Schekman R. Y-box protein 1 is required to sort microRNAs into exosomes in cells and in a cell-free reaction. Elife. 2016;5. https://doi.org/10.7554/eLife.19276.
Sims B, et al. Role of TIM-4 in exosome-dependent entry of HIV-1 into human immune cells. Int J Nanomedicine. 2017;12:4823–33. https://doi.org/10.2147/IJN.S132762.
Sims PJ, Faioni EM, Wiedmer T, Shattil SJ. Complement proteins C5b-9 cause release of membrane vesicles from the platelet surface that are enriched in the membrane receptor for coagulation factor Va and express prothrombinase activity. J Biol Chem. 1988;263(34):18205–12. https://doi.org/10.1016/S0021-9258(19)81346-7.
Skog J, Würdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10(12):1470–6. https://doi.org/10.1038/ncb1800.
Stefanius K, et al. Human pancreatic cancer cell exosomes, but not human normal cell exosomes, act as an initiator in cell transformation. Elife. 2019;8 https://doi.org/10.7554/eLife.40226.
Stuchell-Brereton MD, Skalicky JJ, Kieffer C, Karren MA, Ghaffarian S, Sundquist WI. ESCRT-III recognition by VPS4 ATPases. Nature. 2007;449(7163):740–4. https://doi.org/10.1038/nature06172.
Tamai K, Tanaka N, Nakano T, Kakazu E, Kondo Y, Inoue J, et al. Exosome secretion of dendritic cells is regulated by Hrs, an ESCRT-0 protein. Biochem Biophys Res Commun. 2010;399(3):384–90. https://doi.org/10.1016/j.bbrc.2010.07.083.
Theos AC, Truschel ST, Tenza D, Hurbain I, Harper DC, Berson JF, et al. A lumenal domain-dependent pathway for sorting to intralumenal vesicles of multivesicular endosomes involved in organelle morphogenesis. Dev Cell. 2006;10(3):343–54. https://doi.org/10.1016/j.devcel.2006.01.012.
Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol Chapter 3, Unit 3 22. 2006. https://doi.org/10.1002/0471143030.cb0322s30.
Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319(5867):1244–7. https://doi.org/10.1126/science.1153124.
Trams EG, Lauter CJ, Salem N Jr, Heine U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim Biophys Acta. 1981;645(1):63–70. https://doi.org/10.1016/0005-2736(81)90512-5.
Tricarico C, Clancy J, D'Souza-Schorey C. Biology and biogenesis of shed microvesicles. Small GTPases. 2017;8(4):220–32. https://doi.org/10.1080/21541248.2016.1215283.
Vaidyanathan R, Soon RH, Zhang P, Jiang K, Lim CT. Cancer diagnosis: from tumor to liquid biopsy and beyond. Lab Chip. 2018;19:11–34. https://doi.org/10.1039/c8lc00684a.
Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–9. https://doi.org/10.1038/ncb1596.
van Niel G, Charrin S, Simoes S, Romao M, Rochin L, Saftig P, et al. The tetraspanin CD63 regulates ESCRT-independent and -dependent endosomal sorting during melanogenesis. Dev Cell. 2011;21(4):708–21. https://doi.org/10.1016/j.devcel.2011.08.019.
Villarroya-Beltri C, Gutiérrez-Vázquez C, Sánchez-Cabo F, Pérez-Hernández D, Vázquez J, Martin-Cofreces N, et al. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat Commun. 2013;4(1):2980. https://doi.org/10.1038/ncomms3980.
Wahlund CJE, Güclüler G, Hiltbrunner S, Veerman RE, Näslund TI, Gabrielsson S. Exosomes from antigen-pulsed dendritic cells induce stronger antigen-specific immune responses than microvesicles in vivo. Sci Rep. 2017;7(1):17095. https://doi.org/10.1038/s41598-017-16609-6.
Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol. 1967;13(3):269–88. https://doi.org/10.1111/j.1365-2141.1967.tb08741.x.
Wu D, Xu Y, Ding T, Zu Y, Yang C, Yu L. Pairing of integrins with ECM proteins determines migrasome formation. Cell Res. 2017;27(11):1397–400. https://doi.org/10.1038/cr.2017.108.
Wu J, et al. Iterative tomography with digital adaptive optics permits hour-long intravital observation of 3D subcellular dynamics at millisecond scale. Cell. 2021;184:3318–32 e17. https://doi.org/10.1016/j.cell.2021.04.029.
Yokoi A, Yoshioka Y, Yamamoto Y, Ishikawa M, Ikeda SI, Kato T, et al. Malignant extracellular vesicles carrying MMP1 mRNA facilitate peritoneal dissemination in ovarian cancer. Nat Commun. 2017;8(1):14470. https://doi.org/10.1038/ncomms14470.
Yu Z, Shi M, Stewart T, Fernagut PO, Huang Y, Tian C, et al. Reduced oligodendrocyte exosome secretion in multiple system atrophy involves SNARE dysfunction. Brain. 2020;143(6):1780–97. https://doi.org/10.1093/brain/awaa110.
Yuan D, Zhao Y, Banks WA, Bullock KM, Haney M, Batrakova E, et al. Macrophage exosomes as natural nanocarriers for protein delivery to inflamed brain. Biomaterials. 2017;142:1–12. https://doi.org/10.1016/j.biomaterials.2017.07.011.
Yuana Y, Bertina RM, Osanto S. Pre-analytical and analytical issues in the analysis of blood microparticles. Thromb Haemost. 2011;105(03):396–408. https://doi.org/10.1160/TH10-09-0595.
Zhang L, Zhang S, Yao J, Lowery FJ, Zhang Q, Huang WC, et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature. 2015;527(7576):100–4. https://doi.org/10.1038/nature15376.
Zhang Y, Wang J, Ding Y, Zhang J, Xu Y, Xu J, et al. Migrasome and tetraspanins in vascular homeostasis: concept, present, and future. Front Cell Dev Biol. 2020;8:438. https://doi.org/10.3389/fcell.2020.00438.
Zhao X, Lei Y, Zheng J, Peng J, Li Y, Yu L, et al. Identification of markers for migrasome detection. Cell Discov. 2019;5(1):27. https://doi.org/10.1038/s41421-019-0093-y.
Zhou W, Fong MY, Min Y, Somlo G, Liu L, Palomares MR, et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell. 2014;25(4):501–15. https://doi.org/10.1016/j.ccr.2014.03.007.
Zhu W, Huang L, Li Y, Zhang X, Gu J, Yan Y, et al. Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth in vivo. Cancer Lett. 2012;315(1):28–37. https://doi.org/10.1016/j.canlet.2011.10.002.
Zhu M, Zou Q, Huang R, et al. Lateral transfer of mRNA and protein by migrasomes modifies the recipient cells. Cell Res. 2021;31(2):237–40. https://doi.org/10.1038/s41422-020-00415-3. Epub 2020 Sep 29).
Zhuang X, Xiang X, Grizzle W, Sun D, Zhang S, Axtell RC, et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther. 2011;19(10):1769–79. https://doi.org/10.1038/mt.2011.164.
Zickler AM, El Andaloussi S. Functional extracellular vesicles aplenty. Nat Biomed Eng. 2020;4(1):9–11. https://doi.org/10.1038/s41551-019-0507-z.
Zipkin M. Big pharma buys into exosomes for drug delivery. Nat Biotechnol. 2020;38(11):1226–8. https://doi.org/10.1038/s41587-020-0725-7.
Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, et al. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med. 1998;4(5):594–600. https://doi.org/10.1038/nm0598-594.
Acknowledgements
Not applicable.
Funding
This work is supported by the Ministry of Science and Technology of the People’s Republic of China (2018YFA0507102) and National Natural Science Foundation of China (91754108) to Y.C.; National Natural Science Foundation of China (92054301, 31790401, and 32030023), Beijing Municipal Science & Technology Commission (Z201100005320019), and the Ministry of Science and Technology of the People’s Republic of China (2017YFA0503404) to L. Y.
Author information
Authors and Affiliations
Contributions
Y. C and L. Y wrote the manuscript. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The author Li Yu is a member of the Editorial Board for Current Medicine. The paper was handled by the other journal Editor and has undergone rigorous peer review process. Author Li Yu was not involved in the journal’s review of, or decisions related to, this manuscript. The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, Y., Yu, L. Extracellular vesicles: from bench to bedside. Curr Med 1, 3 (2022). https://doi.org/10.1007/s44194-022-00001-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44194-022-00001-2