Abstract
Polysaccharides are inspiring and valuable molecules to the development of novel drug delivery systems owing to their natural availability, non-toxicity, biocompatibility, good biological performance, and chemical similarity to the physiological environment, besides their noticeable use for tailored-materials assembly. Biodegradable hydrogels based on polysaccharides have been widely studied as potential pharmaceutical forms due to their controlled release properties, which improve drug bioavailability, therapeutic efficacy, and patient compliance. Despite these advantages, polysaccharide materials present insufficient mechanical properties or processability, thus, to overcome these drawbacks, feasible and suitable crosslinking methods are employed to improve polysaccharide hydrogels strength and stability. Therefore, this review presents recent advances in crosslinking methods of polysaccharide hydrogels, including chitosan, cellulose, hyaluronic acid, and alginate, providing examples of manufacturing processes with emphasis in their use as carriers in drug delivery. Polysaccharide-based hydrogels represent a sustainable, biocompatible, and appreciable alternative to obtain novel drug delivery systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Drug delivery systems may allow an active compound to reach its target site with minimized adverse side effects, in addition to the maintenance of a controlled release rate. These requirements can be accomplished by the entrapment of a drug in polymers hydrogels systems, which are able to release the drug, essentially, as a result of the polymer solubility modification, biodegradation, deaggregation, conformational changes, modification of the drug-system affinity, or even cleavage of the drug-system linkages (Fig. 1) [1,2,3]. The mechanism of drug release is highly affected by the morphology of hydrogels, as porosity and swelling degree [2, 4]. Therefore, the release strategy will depend on the design of the drug delivery system and on the physical and chemical features of the target cells or tissue.
Hydrogels are three-dimensional polymeric networks with hydrophilic character, which can absorb large amounts of water or biological fluids without dissolving or losing their morphology [5, 6]. They are assembled from hydrophilic polymers crosslinking, and the swelling is one of the main properties of these materials, very desirable for drug delivery. The presence of hydrophilic groups, such as hydroxyl (–OH), carboxyl (–COOH), amine (–NH2), amide (–CONH2), and sulfonate (–SO3H), confers swelling abilities to hydrogels, besides reactive capability to produce new chemical features and tailored-materials [7,8,9]. These three-dimensional networks can be divided according to the origin of the polymer: natural, when employing biopolymers such as alginate, cellulose, and chitosan; synthetic, namely polyvinyl alcohol, polyacrylamide, poly (sodium acrylate), poly (acrylic acid), and polyvinylpyrrolidone; and semi-synthetic, when composed by mixtures of natural and synthetic polymers [10].
Biodegradable hydrogels as drug delivery systems have attracted keen interest considering the limitations of conventional delivery systems, namely tablets, capsules, granules, ointments, syrups, and suppositories, which present poor absorption and bioavailability, lack/burst of drug release, oscillations in plasma drug level, early excretion, repeated dosing, and eventual adverse effects [2, 11, 12]. The incorporation of drugs in biodegradable polymeric networks minimizes overdose problems, facilitates drug delivery in long treatments, and brings benefits in toxicity, different routes of administration, and efficient bioavailability [13, 14]. As a consequence, the specific and controlled release rates lead to the maintenance of drug in therapeutic level, which reduces daily dose, improves treatment efficacy, and ensures patient compliance [1].
Smart hydrogels undergo physical and chemical changes depending on external stimuli, which boosts their application in medical and pharmaceutical fields [15]. These hydrogels responses can be modulated by changes in pH, temperature, salt concentration, glucose, electric field, enzymes activity, biomarkers, cells permeability, and others [16]. Thermosensitive hydrogels exhibit volume and structure changes according to temperature variation [14, 15, 17], while pH-sensitive hydrogels can undergo morphological expansion and/or contraction as a result of the attraction of positive or negative ions [16]. The more sensitive the hydrogel with respect to the target environment the more accurate the drug delivery system. Therefore, new approaches in therapeutics and new strategies of action are at the forefront of research for novel drug delivery systems, including the use of hydrogels as carriers for countless drugs.
According to polymerization and preparation techniques, manufactured hydrogels can include membranes, films, scaffolds, microgels, nanogels, or microspheres [10, 18]. Noteworthy, the administration routes, namely oral, transdermal, topical, inhalation, and intravenous, affect the efficiency of drug delivery [1,2,3,4,5,6,7,8,9,10,11,12,13, 15]. Therefore, due to its scale and structure features, macroscopic and injectable hydrogels may be suitable for transdermal administration and tissue engineering, respectively, while microgels are promising options for oral delivery systems, and nanogels (10 to 100 nm) appropriate for systemic delivery (Fig. 2) [2, 3, 19–35].
The transdermal route is one of the most explored for hydrogels drug release, mainly because it is a minimally invasive, painless, and self-administered method [36, 37], and allows different application devices, such as patches, gels, creams, and lotions [38]. The drug is absorbed through subcutaneous tissues into the bloodstream, where circulates until reaches the target [39]. Hydrogels confer greater permeability, are washable, flexible, viscoelastic, hydrate the skin, and allow longer contact between drug and skin surface [37].
Injectable hydrogels are relatively low viscosity polymeric solutions, which once injected should gel in situ, although can be significantly influenced by internal environment of the organism [16, 40]. The gelation rate is an extremely critical parameter, as it must be slow to allow injection and fast to avoid leakage [41].
Oral drug administration exhibits gastrointestinal (GI) tract as barrier for drug absorption, namely, the great acidic gastric environment, digestive enzymes, and continuous intestinal mucus secretion. Although, this is the most preferred route for patients. In this context, microgels, which are colloidal hydrogels, crosslinked polymers networks with diameter between 100 nm and 10 μm, bring interesting results for oral drug administration, like burst release for gastric wounds or even sustained drug uptake onto intestinal cellules. Therefore, hydrogels properties can be tailored towards the requirements for specific drug delivery approaches [3, 27, 42].
Hydrogels properties are greatly influenced by polymers’ features, as concentration, molecular weight, chemical composition, types of polymer linkage, and available functional groups. Additionally, to build three-dimensional polymeric networks, manufacturing techniques significantly depend on the crosslinking methods, which allow to obtain tailor-made materials optimized for drug delivery applications. Thus, this review presents recent advances in crosslinking methods of polysaccharide hydrogels, including chitosan, cellulose, hyaluronic acid, and alginate, providing examples of their manufacturing processes, emphasizing their use as carriers in drug delivery systems.
Polysaccharide-Based Polymeric Hydrogels for Drug Delivery Systems
Polysaccharides are high molecular weight carbohydrates, formed by several monosaccharide units linked by glycosidic bonds in linear or branched chains. Their chemical structure depends on the configuration of monosaccharides and the type of glycosidic bonds, characteristics which determine the natural roles and potential applications of these polymers. Noteworthy, polysaccharides have a wide range of physical, chemical, and biological properties [13, 43,44,45].
Polysaccharide-based materials are especially appealing because these natural polymers are polyfunctional, biodegradable, and abundant [10, 39, 44,45,46]. Besides stability upon storage, polysaccharides are non-toxic and biocompatible, given their natural availability. They are present in all living cells, mainly as cell wall and cell membrane components, which also explain its good biological performance and enzyme-controlled biodegradability [43, 44, 47]. In addition to its chemical similarity to the physiological environment, polysaccharides are renewable and sustainable raw materials, features which add environmental and resource-saving benefits regarding manufacturing processes and supply chains that depend on polysaccharides as primal matter [5, 12, 15].
Although, water solubility and poor mechanical properties, polysaccharides are polyfunctional molecules. The presence of free hydrophilic functional groups, as carboxyl, amino, and hydroxyl, among others, brings many possibilities of physical and chemical modifications, allowing to build required attributes in tailor-made polysaccharides, including drug conjugation and functionalization [9]. Therefore, as value-added material, polysaccharides encourage studies on alternative synthesis routes and use of different polymers to obtain new hydrogels formulations.
Several polysaccharides have been reported as potential materials for the obtainment of efficient drug delivery systems, including chitosan, cellulose, hyaluronic acid, and alginate, which will be discussed in this review. Some recent examples of hydrogels applied in drug delivery systems are summarized in Table 1, which describes the hydrogel components, the crosslink strategy, and the improved properties of the polysaccharide-based materials after hydrogel manufacturing.
Alginate
Alginate is derived from brown algae [48] or produced by some bacteria. This polysaccharide consists of → 4)-β-d-mannopyranuronic acid-(1 → (M) and → 4)-α-l-gulopyranuronic acid-(1 → (G) units, disposed as homogeneous MM- and GG-blocks, interspersed with heterogeneous MG-blocks. Blocks proportion and spatial distribution directly influence the physicochemical properties. The G-blocks are critical for hydrogel formation, since they participate in intermolecular crosslinking with Ca2+ [7, 25, 49].
Alginate is used to encapsulate drugs, cells, and growth factors [10]. Considering the several hydroxyl and carboxyl groups in its backbone, their low toxicity, and high gelling capacity, alginate hydrogels are very promising materials to integrate countless delivery systems. Therefore, these properties expand alginate relevance for biomedical and pharmaceutical applications [7, 12, 19].
Conventionally, alginate gelling process occurs during the exchange of Na+ ions from sodium alginate with divalent cations, such as Ca2+, which is the most widely used to produce alginate gels [7, 12]. However, pure alginate gels commonly present low mechanical strength and mucoadhesive properties. Hence, some crosslinking strategies are able to circumvent these problems. For instance, increased mucoadhesive property in oral gels for insulin release was achieved by modifying alginate with cysteine (Table 1) [3].
Another strategy to improve its properties is the use of nanocellulose as a reinforcing agent. Alginate hydrogel and magnetic nanocellulose beads (isolated from rice husk) were produced as a potential ibuprofen release system by Supramaniam et al. [50]. The hydrogels demonstrated a gradual release of the drug, avoiding burst effect (immediate release). This result was attributed to the presence of nanocellulose, which restricted the release of ibuprofen during dissolution due to the high physical entanglement (Table 1).
Ma et al. [51] proposed an interpenetrating hydrogel of sodium alginate and nanocellulose for sustained release of aspirin. The polymeric network was double crosslinked by coagulation with acetic acid followed by treatment with CaCl2 solution. A significant increase in mechanical performance (~ 55%) was achieved after the second crosslinking with Ca2+. In addition, thermogravimetric results indicated that the presence of nanocellulose improved thermal stability, as well as prolonged the release of aspirin up to 80 h (Table 1).
An alginate injectable hydrogel was developed for the treatment of myocardial infarction. Loaded with agents encoding vascular endothelial growth, the hydrogels demonstrated high storage modulus and immediate self-repair after stress testing, which was explained by the ionic crosslinks between Ca2+ and α-l-guluronic blocks of alginate (Table 1) [52].
Cellulose
Cellulose is the most abundant biopolymer on the planet and the main component of plants cell wall. It is a linear homopolysaccharide formed by → 4)-β-d-glucopyranose-(1 → [53, 54]. They are obtained from bacteria [55, 56], and green plants, including alternative sources, such as lignocellulosic residues [57, 58].
Its structural feature allied to its low cost, good biocompatibility, and biodegradability, made cellulose an excellent material for hydrogels manufacturing. However, cellulose presents low solubility, restricting its use to obtain new pharmaceutical forms [47, 59].
In this context, soluble cellulose derivatives are accomplished by chemical modification, such as esterification, etherification, or oxidation [60]. The use of cellulose and its modified derivatives, such as cellulose acetate [61, 62] and carboxymethyl cellulose [63, 64], in hydrogels favor the porous structure due to the repulsive forces of intramolecular carboxyl groups, promoting positive effect in the swelling capacity of the hydrogels [8, 65].
At nanoscale, nanocrystalline and nanofibrillated celluloses are also widely explored. These nanomaterials have a large contact surface, which allows better interaction with other polymers, resulting in high strength and stability, mainly due to the complexity of the network of hydrogen interactions [66, 67]. Thus, nanocellulose hydrogels are exciting materials to obtain efficient drug delivery systems [18].
Chitosan
Chitosan is the second most abundant natural polysaccharide on the planet. It is obtained by alkaline or enzymatic deacetylation of chitin [10, 25], which is found in the exoskeleton of invertebrates, crustaceans, and insects, as well as in the cell wall of fungi. Chitosan is a cationic polysaccharide comprising of → 4)-2-amino-2-deoxy-β-d-glucopyranose-(1 → (β-d-glucosamine) and → 4)-2-acetamide-2-deoxy-β-d-glucopyranose-(1 → (N-acetyl-β-d-glucosamine) units [13, 25, 45].
Chitosan hydrogels have good mucoadhesive properties, swelling reversibility, flexible adaption to external triggers, and drug loading competence [68]. Additionally, they present antibacterial, anti-inflammatory, and hemostatic properties. Therefore, these features make chitosan an attractive matter to accelerate wound healing processes [69]. According to Nguyen et al. [68], several in vitro and in vivo studies have shown that chitosan hydrogels loaded with anti-inflammatory drugs promoted important decrease in pro-inflammatory cytokines activity. Consequently, chitosan is considered a promising anti-inflammatory therapeutic carrier.
Saifullah et al. [28] developed a mucoadhesive emulsion based on lipids and acetylated chitosan for oral administration. The emulsion, loaded with Cefixime®, a water insoluble antibacterial drug, showed optimal stability in different pH of gastrointestinal fluids, increasing plasma concentration levels of Cefixime® up to 15 µg/mL compared to free form of commercial drug (6 µg/mL). They conclude that the bioavailability of the drug was favored by the mucoadhesive property of chitosan (Table 1).
Cysteine-conjugated chitosan hydrogels, embedded with zinc oxide nanoparticles and loaded with naringenin, presented great improvement in the swelling degree. The sustained drug release rate of conjugated chitosan hydrogels improved bioavailability in comparison to native chitosan hydrogels (Table 1) [70].
Patches of chitosan and polyvinyl alcohol loaded with vitamin B12 were developed by Yekrang et al. [39]. In vivo studies showed a slow and continuous release of the vitamin and increased mechanical properties of the adhesives. The cytocompatibility assay revealed low toxicity and no lesions or tissue damage were observed in the skin of the animal models (Table 1).
Injectable thermosensitive hydrogels based on chitosan and polygalacturonic acid were developed for bone tissue engineering, showing excellent sol–gel transition [41]. Ghanavi et al. [71] reported the development of thermoresponsible mucoadhesive hydrogels for ocular drug delivery purposes. The dual crosslinked chitosan-based hydrogel presented sustained-release profile for vancomycin and prednisolone, and appropriate antibacterial activity in vitro. Consequently, mucoadhesive property of chitosan may favor interactions with human eye cornea (Table 1).
Hyaluronic Acid
Hyaluronic acid is a linear polysaccharide consisting of repeating units of → 4)β-d-glucopyranuronic acid-(1 → (β-d-glucuronic acid) and → 3)-2-acetamide-2-deoxy-β-d-glucopyranose-(1 → (N-acetyl-β-d-glucosamine) units [72, 73]. This glycosaminoglycan is naturally produced by human body, as important part of the extracellular matrix, and plays a vital role in multiple biological activities [73, 74]. It can be obtained from animal sources, like rooster combs, or from microbial sources, as Streptococcus [75, 76] and Bacillus genera [77]. Depending on the source, hyaluronic acid can present high or low molecular weight, which directly influences its applications [52, 78].
According to Attia et al. [75], the high viscoelasticity of hyaluronic acid results from the presence of a single carboxyl group per disaccharide unit. The carboxyl group dissociates at physiological pH, giving a polyanionic character to the polymer chains. These negatively charged chains can expand and tangle at low concentrations, contributing to the viscoelastic properties of the polymer network. Because of its high-water holding capacity, viscoelasticity, biocompatibility, non-immunogenicity, and biodegradability, hyaluronic acid is considered a suitable material for uses in pharmaceutical area to obtain efficient drug delivery systems, noteworthy, as wound dressing [72, 73, 79, 80].
Polymeric matrices based on hyaluronic acid present significant benefits when employed as drug carriers, such as optimum drug concentration maintenance, enhanced therapeutic effects, improved treatment efficiency, and prolonged in vivo release rates [79, 81]. Hyaluronic acid also presents high stretchability, flexibility, and permeability [73].
Optimal properties of adhesive dressings were reported by Liu et al. [82] in a hydrogel based on modified hyaluronic acid and ε-polylysine. Histological studies revealed that the hydrogel was effective in eliminating Gram (+) and (−) bacteria on the surface of wounds, accelerating the healing process.
A promising pH-sensitive hydrogel based on hyaluronic acid and hydroxyethyl cellulose loaded with an antibacterial agent was developed for transdermal delivery systems (Table 1) [83]. El-Aassar et al. [24] observed that hyaluronic acid increased the entanglements among polyvinyl alcohol (PVA) chains, reinforcing the PVA based polymeric matrix developed to deliver silver nanoparticles. Pan et al. [80] also reported that the addition of hyaluronic acid on a PVA polymeric matrix increased thermal stability, solubility, swelling index, water vapor permeability, and elongation.
Thereby, add-valued renewable polysaccharides as alginate, cellulose, chitosan, and hyaluronic acid, among others, allow to manufacture reinforced hydrogel matrices using countless strategies, which depend upon the chemical nature of the polysaccharides. Therefore, the process of synthesizing polysaccharide-based hydrogels is directly related to the crosslinking reactions and the type of interactions to which the polymeric matrices are subjected.
Crosslinking Methods of Hydrogels: Recent Advances
Crosslinking processes are generally classified into three categories: chemical, physical, and enzymatic (Fig. 3). Chemical e physical processes are the most common discussed in the literature. In chemical crosslinked hydrogels, covalent bonds among the polymeric chains sustain the polymeric network, and physical hydrogels are maintained by mechanical chain entanglements, van der Waals interactions, hydrogen interactions, hydrophobic, and electronic associations [4, 47]. Enzymatic crosslinks produce covalent bonds, although, linkages are specific, according to enzyme type and, usually, demand mild reaction conditions [85].
Chemically Crosslinked Hydrogels
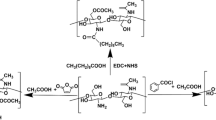
Chemically crosslinked hydrogels are those in which the three-dimensional network is formed from covalent bonds mediated by chemical reactions, presenting a strong interaction between the polymeric chains, which confers them chemical, mechanical, and dimensional stabilities when compared to physical hydrogels [86]. Consequently, changes in the external environment usually do not cause the breakage of these bonds, only affecting the absorption and release capacity of the active compound [46]. Due to their highly reactive groups, polysaccharides can be modified with the introduction of functional groups before being chemically crosslinked, favoring crosslinking reactions [87], like represented in Fig. 4.
Schematic representation of chemical crosslinking method. Inspired by Muhammad et al. [88]
Chemical crosslinks occur between polysaccharides bearing reactive groups, which can form covalent bonds, or by the addition of synthetic or organic molecules that act as crosslinkers, which introduce linkages and remain linked to polymers chains. Glutaraldehyde, phosphate groups, genipin, epoxy, acrylamides, polycarboxylic acids, and formaldehyde are common crosslinkers employed for polysaccharides [46, 89, 90].
Glutaraldehyde is a low-cost, easily available, readily reactive, and strong stabilizer crosslinker agent. It is a bifunctional aldehyde that is the most frequently used crosslinker in biodegradable hydrogels, however, there is important evidence of glutaraldehyde cytotoxicity [89, 91, 92].
Genipin is an expensive natural crosslinker agent, a bicyclic molecule that can hydrolyze in aqueous solutions to give two reactive aldehydes, which easily link to polysaccharides under mild conditions to form stable products [27, 93, 94]. Increased genipin as linker for chitosan hydrogels brought stability in acidic pH, in addition, wound healing and antibacterial chitosan-based biomaterial crosslinked with genipin was developed to treat and effectively heal ulcer wounds [93, 95]. Nevertheless, its safety is debated since genipin induces instant apoptosis of liver and dermal cells. Moreover, genipin induces neurite PC12 cells differentiation, supporting neurite development in a dose dependent manner [89].
Polycarboxylic acids, including citric acid, are low-cost alternative crosslinkers able to overcome the toxicity and costs associated with other agents, such as glutaraldehyde and genipin, and considered efficient crosslinkers for polysaccharides, such as cellulose. The crosslinking occurs due to the attachment of the polycarboxylic acid carboxyl group via esterification with cellulosic hydroxyl, followed by additional esterification with another cellulosic hydroxyl, resulting in cellulose chains covalently linked [29, 96].
Several studies have reported the use of other crosslinkers, including sodium trimetaphosphate (STMP), which is considered a low-cost, safe, and low-toxicity agent with no reported adverse effects on humans, suitable for polysaccharide-based hydrogels [97, 98].
Natural crosslinkers are preferred over the toxic and high-cost ones, since green options are trend in chemical reactions [46]. Moreover, there are crosslinking reactions that dismiss crosslinkers. Naturally reactive polysaccharide groups or functionalized polysaccharides are able to form covalent bonds in suitable approaches and great biocompatibility. For instance, amino sugars are promptly to form amide bonds with uronic acids via carbodiimide chemistry, or to form amines via reductive amination of aldehydes [99]. Polysaccharide modifications to introduce reactive groups are also a very common crosslinking strategy, which lead to interesting hydrogel properties, including periodate oxidation followed by Schiff base reaction [88].
Amidation Reaction
Amide bonds, formed from condensation between carboxylic acid and amine groups, are ubiquitous in organic molecules in biology, organic synthesis, and pharmaceutical compounds [100, 101]. Coupling via carbodiimide (N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride, EDC), a water soluble and non-toxic compound, is the most common way to form amide bonds [102,103,104]. In aqueous solution, carboxyl group is activated by carbodiimide resulting in O-acylisourea ester intermediate, which reacts with amine groups via a nucleophilic attack at carbonyl carbon [105].
Polysaccharide-based hydrogels obtained by carbodiimide chemistry, are potentially biodegradable and biocompatible [106]. Rodríguez-Félix et al. [107] prepared chitosan hydrogels using EDC and l-glutamic acid. The crosslink coupled glutamic acid carboxyl groups to chitosan amino groups. Similarly, Chang et al. [108] synthesized in a temperature-controlled environment a hydrogel by mixing hyaluronic acid and gelatin to form a colloid, where gelatin amine groups formed amide bonds with hyaluronic (HA) acid carboxyl groups. Hsu et al. [103] manufactured hydrogels in PBS solution by mixing a gelatin solution with HA and different concentrations of EDC solution. Higher EDC content led to more compact structures, decreasing the absorption capacity of the hydrogels.
Carbodiimide chemistry reactions commonly includes N-hydroxysuccinimide (NHS) to favor the ester intermediate stabilization and promote amide bonds, in aqueous media under mild conditions of temperature and pH [109]. In order to improve the therapeutical efficacy of insulin delivery via oral release, a multifunctional drug delivery system was developed [3]: arginine-insulin electrostatic complexes were encapsulated in liposomes, which were enfolded by alginate and modified alginate hydrogels. Cysteine-grafted alginate was prepared by EDC/NHS amidation to contain insulin loaded liposomes. Both systems presented continuous hypoglycemic effect, however, compared to alginate system, cysteine-grafted alginate hydrogel system presented prolonged release in addition to effective hypoglycemic effect. Therefore, bio-efficacy of insulin via oral delivery was enhanced due to pH-sensitive property and adhesive ability of modified alginate hydrogel [3].
Schiff Base Reaction
In most bonds of covalent nature, the connection formed between atoms requires a significant amount of energy to be broken [87]. However, Schiff bases form transient (dynamic) covalent bonds that can be dissociated and reassociated naturally or in response to external stimuli changes in their three-dimensional network, providing self-healing [110], and biodegradability [111]. According to Zhou et al. [32], Schiff base hydrogels can exhibit self-healing properties and enhanced injectability due to the reversibility of Schiff base formation, especially in an aqueous environment. These materials are also pH-sensitive and undergo hydrolysis reaction at accelerated rates in acidic environments.
A Schiff base is defined as a compound that contains a double bond linking carbon and a nitrogen atom (R1 = N-R2, R ≠ H) [112]. Despite commonly used to form complexes with transition metals [113,114,115], its versatility and promptly reactive, combined with the ability to stabilize functional reactive groups of biopolymers, Schiff base reactions favor the synthesis of biodegradable hydrogels. The reaction between an electrophilic carbon of an aldehyde/ketone and an amine forms covalent bonds such as imines and its derivatives, depending on the precursors [116].
The most common method to modify and introduce aldehyde or ketone groups into polysaccharides is via oxidative cleavage of vicinal diols driven by sodium periodate (NaIO4) [117, 118]. Muhammad et al. [88] evaluated the effect of reaction time and sodium periodate (NaIO4) concentration on the degree of oxidation of alginate and hyaluronic acid. These polysaccharides can yield in situ gelling hydrogels to be used in drug delivery systems by imine bond formation. This covalent reaction occurs between aldehyde groups of oxidized polysaccharides and amino groups of carboxymethyl chitosan. The studies pointed out that NaIO4 content is the main parameter that influences the final concentration of aldehyde groups, which impacts the physicochemical properties of the hydrogel. Furthermore, the results suggest that milder conditions cannot significantly oxidize polymers that form intermolecular interactions. Thus, polysaccharides such as cellulose require harsher conditions or an assisted process, as shown by Dang et al. [119].
Qian et al. [120] developed a self-healing, injectable, and pH-responsive hydrogel based on N-carboxyethyl chitosan. The modification was carried out through Michael reaction with acrylic acid under neutral conditions, followed by oxidation of hyaluronic acid with different concentrations of NaIO4 at PBS solution. The increased aldehyde hyaluronic content enhanced the crosslinked networks, which reflected in smaller pore size and improved mechanical strength. Maiz-Fernández et al. [90] conducted a similar study evaluating the stoichiometric ratio between N-succinyl-chitosan and aldehyde hyaluronic acid hydrogel manufactured by the same method, mixing both in PBS solution. The results showed that higher crosslinking density resulted in greater mechanical resistance, shorter degradation, and swelling time.
In order to increase stability and develop a smart delivery system triggered by external magnetic field and acid medium, Jahanban-Esfahlan et al. [121] incorporated by co-precipitation Fe3O4 magnetic nanoparticles in alginate-gelatin matrix. The hydrogel was synthesized via reductive amination, reacting gelatin amino groups and NaIO4 oxidized alginate in aqueous media, followed by NaBH4 reduction.
Mechanical strength can be improved in many ways, for instance, nanoparticles incorporation, increased crosslinking density, or development of dual or multi-crosslinked hydrogel. In this context, Zhang et al. [122] developed an injectable in situ hydrogel based on biofunctionalized hyaluronic acid and oxidized alginate. Carbodiimide chemistry was used to incorporate 3,3′-dithiobis(propionohydrazide) and carbohydrazide into HA backbone, resulting in the production of thiol and hydrazide groups, respectively. The dual crosslinked hydrogel synthesis took place in PBS medium by disulfide and hydrazone bond formation.
Due to the ionic character of some natural polymers, doubly crosslinked hydrogels can be easily manufactured by combining physical and chemical interactions. Zhang et al. [123] prepared a chemical and physical reticulated hydrogel derived from gellan gum and carboxymethyl chitosan. NaIO4 oxidized gellan gum containing CaCl2 was mixed with carboxymethyl chitosan aqueous solution in the presence of gellan gum microspheres. The reaction resulted in a complex gel embedded with microspheres in which oxidized gellan gums were simultaneously crosslinked by Ca2+ and imine bond with carboxymethyl chitosan.
Shi et al. [34] employed double crosslinking (Schiff base and electrostatic interaction) in the synthesis of injectable microgels of chitosan and polyethylene glycol, which were cytocompatible and provided a favorable environment for cell survival and proliferation. Zhou et al. [32] obtained oxidized hydroxypropyl cellulose via oxidation in acidic conditions for further hydrogel synthesis with carboxymethyl chitosan. Ngwabebhoh et al. [124] synthesized dialdehyde carrageenan gum through a H2O2 and CuSO4 redox reaction.
Polysaccharide chemical modification and functionalization allow to obtain three-dimensional polymer networks for drug delivery applications via suitable and classical aqueous media reactions, namely Schiff base, amidation and reductive amination. These reactions are effective and low cost. In addition, carbodiimide chemistry and reductive amination are commonly used at large scale processes, including to manufacture pharmaceutical ingredients, which could leverage industrial employment of polysaccharide-based hydrogels [125, 126].
Physical Crosslinked Hydrogels
Hydrogels in which the polymer matrix is formed through weak interactions such as hydrogen bonds, hydrophobic interactions, ionic/electrostatic bonds, crystallization, and the entanglement of the polymer chains are called physically crosslinked (Fig. 5). Due to the types of interactions formed, they usually generate transient junctions that can be reversed by changes in the environment (e.g., pH and temperature). Unlike chemical hydrogels, physical hydrogels can be prepared by simply dissolving the polymer in water or aqueous solutions, without the need of crosslinkers or initiators [127].
Schematic representation of physical crosslinking method. Inspired by Qin et al. [128]
Hydrogen Bond Formation
Hydrogen bond hydrogels can be formed using polymers with hydrogen-bonding groups such as amides, carboxyl, and hydroxyl groups, which can act as hydrogen bond donors or acceptors. These groups can form hydrogen bonds with each other, leading to the formation of physical crosslinking between the polymer chains [129]. The strength and density of hydrogen bonds can be controlled by the choice of polymer, pH, and ionic strength of the solution [130].
Hydrogen bonding can also be used to stabilize drugs in formulations by forming intermolecular hydrogen bonds between drug molecules or between the drug and other excipients in the formulation. This can help to prevent the drug from degrading or undergoing chemical reactions that could decrease its effectiveness [131, 132]. Furthermore, hydrogen bonding can influence the release of drugs from delivery systems by affecting the strength of the interactions between the drug and the delivery system [133, 134].
Ma et al. [135] synthesized hydrogen bonding hydrogels based on oxidized nanocellulose and nanochitin, and the synthesis was performed at an acidic medium to allow the formation of hydrogen bonding between carboxyl groups from polymers. Wu et al. [79] also prepared hydrogen bonding hydrogels based on an oxidized hyaluronic acid-Konjac glucomannan, resulting a in porous structure that had a rich storage space for drug loading.
Physical techniques can also be used to induce and control hydrogen bond formation in hydrogels [136]. Qin et al. [128] synthesized hydrogen-bond-based hydrogels by mixing chitosan and pullulan in aqueous solutions by electrospinning, they reported that the main interactions occurred between amine groups from chitosan and hydroxyl groups from pullulan, consequently, an increase in chitosan content resulted in improved thermal stability.
Long et al. [137] developed a 3D printable hydrogel based on chitosan and pectin. The hydrogel scaffold was produced by both biopolymer solutions in acidic medium and then extruded without heat treatment. Using the same technique (direct ink writing), Baniasadi et al. [138] printed polyvinyl alcohol and TEMPO-cellulose nano fibers hydrogels previously mixed using a digital homogenizer. The abundance of -OH groups in both polymers leads to the formation of high stable structure.
Electrostatic Interactions
Electrostatic interactions refer to the attractive or repulsive forces between electrically charged particles. Biopolymers that contain ionizable groups, such as carboxyl or amine groups, can undergo electrostatic interactions in hydrogel matrices [139]. These polymers can become charged when they ionize in water and can interact with oppositely charged counterions. Furthermore, to make polysaccharides ionizable, chemical groups such as carboxyl [140], sulfate [141], and amino [142] groups can be introduced into the polysaccharide structure.
A polyelectrolyte complex hydrogel can be formed from polysaccharides with opposite charges through polyelectrolyte complexation, which is a process in which the electrostatic interaction between the positively and negatively charged polysaccharide chains leads to the formation of a stable hydrogel [143, 144]. Alternatively, charged biopolymers can form hydrogels via ionotropic gelation, which is a process in which the polysaccharide chains are crosslinked by cations such as calcium [145], zinc [146], or iron ions [147]. The electrostatic interaction between the negatively charged carboxylate groups in the polysaccharide chains and the positively charged cations is mainly responsible for the gelation process [147, 148].
As well as in hydrogen bonding-based hydrogels, the pH and temperature are important factors to consider for electrostatic crosslinking materials, mainly because pH and temperature affect the degree of ionization of functional groups and the strength of interaction between them [149].
Hamedi et al. [150] synthesized a hydrogel by mixing and stirring a chitosan solution dissolved in acetic acid and an alginate aqueous solution. The tridimensional network structure is generated from polyanion and polycation interactions derived from alginate and chitosan, respectively. Similarly, Maiz-Fernández et al. [151] developed PEC hydrogels to be used as a self-healing and controlled drug release system based on hyaluronic acid and chitosan.
To improve the mechanical properties, Deng et al. [152] incorporated cellulose nanofibers into an alginate-chitosan polymeric matrix. The addition of nanocellulose resulted in an interpenetrating hydrogel, where the protonated groups between chitosan and alginate are electrostatically bonded, in which they are interconnected with nanocellulose through hydrogen bonding. Furthermore, reinforced hydrogels can effectively mitigate the burst release behavior.
Omer et al. [153] developed a pH-responsive polyelectrolyte complex hydrogel based on sodium alginate, carboxymethyl chitosan, and aminated chitosan. The hydrogel was synthesized by ionic gelation and coating process. The dual polyelectrolyte complex enhanced the hydrogel stability, drug encapsulation and prevented the immediate release profile.
Khan et al. [154] prepared a metal ion-polysaccharide complex hydrogel by mixing chitosan and alginate solutions dissolved separately followed by dropwise addition of CaCl2. The addition of hydrophilic drugs in alginate solution leads to, concurrently, formation of hydrogel network and encapsulation of the drug(s). In a similar way, Goodarzi et al. [155] developed an alginate-chitosan nanoparticles-based hydrogel dropping CaCl2 and chitosan-loaded solution into alginate. The anionic groups of nano-structured-loaded synthesized by ionotropic gelation are crosslinked with Ca2+, while the cationic portion entrap the anionic character drug.
Calcium chloride is the most used crosslinking agent for ionic crosslinked polysaccharide-based hydrogels [145, 156, 157]. Kim et al. [158] synthesized an injectable hydrogel based on calcium and hyaluronic acid, forming a complex via acid–base reaction between hyaluronic acid and calcium acetate.
Potiwiput et al. [159] synthesized a double crosslinked hydrogel combining ionic crosslinking and electrostatic interaction. Alginate carboxyl group can form a chelate complex with divalent Ca2+. Carboxymethyl cellulose, with cationic charge was employed to enable physical crosslinking with the anionic charge of alginate. The dual crosslinking approach enhanced rheological properties in swelling behavior.
Enzymatic Crosslinked Hydrogels
Enzymes catalyze biological reactions generating covalent bonds and linkages in a powerful and highly specific way (Fig. 6). Enzymatic-mediated crosslinking reactions have become popular due to their capability of forming mechanically stable hydrogels under mild conditions, including physiological temperature and pH. Moreover, enzymes may be recycled and reused, in free or immobilized forms, making this class of materials ideal to obtain drug delivery systems [88, 160]. In addition, they can be tailored to achieve in situ gelation, injectability, desired mechanical properties, and degradability [160, 161]. Noteworthy, due to enzymes chemoselectivity, enzymatic reactions only occur in the presence of a substrate that owns a specific functional group [162]. Efforts in molecular biology and biotechnology have revealed a plethora of possibilities concerning enzymes isolation, production, and optimization, aiming enzymes evolution in a high-throughput manner [163]. Therefore, advances in this field could make enzymes manufacturing processes more affordable.
Transglutaminases are widely spread thiol enzymes that form an amide bond by catalyzing an acyl transfer, mainly between a γ-carboxamide group of the glutamine residue and a primary amine [164]. The efficiency of the catalyst is related to the abundance of amino groups, the spatial conformation, and its molecular chain flexibility [165]. Sun et al. [166] synthesized an alginate-gelatin antibiotic-loaded hydrogel through transglutaminase crosslinking that prolonged drug release. Furthermore, with no effect on the crosslinking degree, a higher concentration of transglutaminase resulted in fastest gelation.
Peroxidases are a family of enzymes that oxidizes substrate molecules through hydrogen peroxide (H2O2) consumption that act as an electron acceptor [165]. Among them, horseradish peroxidase is a single-chain β-type hemeprotein capable of catalyzing the crosslinking of phenol- or aniline-rich derivatives through three consecutive oxidation reactions in the presence of H2O2, generating covalent bonds [164, 167]. Tran et al. [88] developed a chitosan gallic acid-conjugated hydrogel with different mechanical properties and microstructures by changing H2O2 content. Jung et al. [161] manufactured a chitosan–polyethylene glycol hydrogel using the enzymatic crosslinking reaction of horseradish peroxidase.
Liu et al. [82] reported the development of a hydrogel based on modified hyaluronic acid and ε-polylysine. Enzymatic crosslinking with peroxidase and Schiff base reaction promoted rapid sol–gel transition, favoring mechanical strength, and preserving hydrogel morphology. Histological studies revealed that this hydrogel was effective in eliminating Gram (+) and (−) bacteria on the surface of the wounds, accelerating the healing process.
Enzymatic crosslinking methods promote covalent bonds exploring specific sites in polysaccharides, which bring the opportunity to add chemo- and regioselectivity to hydrogels manufacturing processes. Therefore, in general, chemical and physical crosslinking strategies allow to obtain a plethora of polysaccharide-based materials, stable, malleable, and injectable three-dimensional networks with transient features, or even permanent ones with large ranges of storage modules. Furthermore, besides solving the poor mechanical properties of polysaccharide hydrogels, crosslinking methods allow to tailor polysaccharide-based materials according to applications requirements.
The development of drug delivery sciences demands numerous systems to carry and deliver, smartly, small drugs, peptides, proteins, nucleic acids, monoclonal antibodies, or even cells. Moreover, multiple drugs delivery systems are also required, usually for cancer treatments [168, 169]. Consequently, to keep up with these limitless requirements, there must be tailorable countless options, which are the polysaccharide-based materials. These natural and renewable polymers represent suitable tools that allow drug delivery systems expansion, inasmuch as polysaccharide sciences co-evolve with the new demands. Given that, sustainable and ecofriendly processes that promote polysaccharides modification are in evidence. Noteworthy, hydrogels based on xanthan gum and cellulose were obtained by reactive extrusion and thermopressing processes, without crosslinkers, using glycerol as plasticizer. The designed biopolymers matrices presented smooth surface with a pH-dependent degree of swelling and increased thermal stability and tensile strength [170].
In this context, a new paradigm for industrial supply chains could be make more efforts to value natural and renewable prime matter face the necessity of sustainable processes. Hence, it is imperative to make polysaccharide-based raw materials more affordable, a substantial offer would encourage sustainability, besides favoring the drug delivery development.
Conclusion and Perspectives
Polysaccharide-based hydrogels have unique properties to be used as carriers in drug delivery systems, becoming important due to the need for more sustainable practices in this field, making them highly value-added products. The main advantages of these materials include the controlled delivery of drugs, non-toxicity, biodegradability, biocompatibility, good biological performance, easy availability, and chemical similarity to the physiological environment. Additionally, polysaccharides present a wide range of physical, chemical, and biological properties considering the presence of free functional groups that can be easily modified for expanding applications and overcoming limitations.
Depending on crosslinking methods, the properties of the hydrogels can change. Covalent junctions result in more chemical, mechanical, and dimensional stable hydrogels. However, chemical crosslinking may require pre-functionalizing reactions and the use of chemicals of varying toxicity. Additionally, it is important to highlight that are alternative crosslinkers including sodium trimetaphosphate, polycarboxylic acids, like citric acid, and linkage strategies, as carbodiimide chemistry, reductive amination, among others, which are considered safe and low toxic, suitable for polysaccharide-based hydrogels. Physically crosslinked hydrogels can be considered a simple and environmentally friendly strategy to obtain hydrogels with non-toxicity to be used in drug delivery systems. Enzymatic crosslinking reactions have become popular due to their capability of forming mechanically stable hydrogels under mild conditions, resulting in suitable materials to be used as carriers in drug delivery systems.
In order to design polysaccharide-based hydrogels for drug delivery systems key considerations might be made regarding drug, target/route, hydrogel features, crosslinking method, and biocompatibility evaluation. Drugs must be considered about its molar mass, solubility, stability, toxicity, reactivity, since it determines interactions between drugs, target, and hydrogels. Different type of cells or tissues constitute the targets, which presents specific attributes that must be taken account, like pH, mucus secretion, biomarkers, among others.
Therefore, target characteristics also establish the requirements for polysaccharide-based hydrogels design and crosslink strategy selection. In general, the greater the crosslinking degree the higher the hydrogels mechanical strength. Hence, considering the drug rout of administration, the polysaccharides and crosslinking methods can be selected. Due to the countless tailoring possibilities for polysaccharides, there is not an established protocol to follow, researchers use creativity to deceive the barriers until the target. The utilization of natural crosslinkers, low-cost reactions, and ecofriendly processes are tendencies to contribute to sustainability and affordable materials. Afterwards, physicochemical and physicomechanical characterization are able to predict drug release data and outline biological experiments, for which in silico, in vitro, and ex vivo trials are preferable to avoid animal models.
Finally, polysaccharide base-hydrogels represents a sustainable, feasible, and biocompatible tool to obtain novel drug delivery systems, and it can be considered a technological solution for replacing conventional materials. Although some hydrogels can be produced by one-pot synthesis, most are designed for laboratory scale and do not meet the needs of the pharmaceutical industry, thus, despite excellent laboratory results, efforts must be taken to bring new information about the large-scale production through sustainable and high‐throughput approaches. In a short time, industrial-scale manufacturing could favor amidation and reductive amination strategies to produce polysaccharide hydrogels. Noteworthy, reactive extrusion represents a promptly industrial available method to produce polysaccharide-based hydrogels in a sustainable and low-cost way, corresponding to an appealing trend for the establishment of automated manufacturing platforms.
References
C. Alvarez-Lorenzo, A. Concheiro, Smart drug delivery systems: from fundamentals to the clinic. RSC 50, 7743–7765 (2014)
D.E. Ciolacu, R. Nicu, F. Ciolacu, Cellulose-based hydrogels as sustained drug-delivery systems. Materials 13, 5270 (2020)
H. Wu, J. Nan, L. Yang, H.J. Park, J. Li, Insulin-loaded liposomes packaged in alginate hydrogels promote the oral bioavailability of insulin. J. Control. Release 353, 51–62 (2023)
S. Simões, A. Figueiras, F. Veiga, Modular hydrogels for drug delivery. J. Biomater. Nanobiotechnol. 3, 85–199 (2012)
B. Tian, J. Liu, Smart stimuli-responsive chitosan hydrogel for drug delivery: a review. Int. J. Biol. Macromol. 235, 123902–123902 (2023)
S. Uman, A. Dhand, J.A. Burdick, Recent advances in shear-thinning and self-healing hydrogels for biomedical applications. J. Appl. Polym. Sci. 137, 48668 (2019)
D. Das, H.T.T. Pham, S. Lee, I. Noh, Fabrication of alginate-based stimuli-responsive, non-cytotoxic, terpolymers semi-IPN hydrogel as a carrier for controlled release of bovine albumin serum and 5-amino salicylic acid. Mater. Sci. Eng. C. 98, 42–53 (2019)
S.M.-J. Kabir, P.P. Sikdar, B. Haque, M.A.R. Bhuiyan, A. Ali, M.N. Islam, Cellulose-based hydrogel materials: chemistry, properties and their prospective applications. Prog. Biomater. 7, 153–174 (2018)
M. Al-Muhanna, N. Anwar, Md.S. Hasmin, A.K. Nayak, Synthesis of tailor-made polysaccharides: an overview, in Tailor-Made Polysaccharides in Drug Delivery. ed. by A. Nayak, Md.S. Hasnain (Academic Press, Cambridge, 2022), pp.1–20
S. Yang, F. Wang, H. Han, H.A. Santos, Y. Zhang, H. Zhang, J. Wei, Z. Cai, Fabricated technology of biomedical micro-nano hydrogel. Biomed. Technol. 2, 31–48 (2023)
S. Adepu, S. Ramakrishna, Controlled drug delivery systems: current status and future directions. Molecules 26, 5905 (2021)
M. Dattilo, F. Patitucci, S. Prete, O.I. Parisi, F. Puoci, Polysaccharide-based hydrogels and their application as drug delivery systems in cancer treatment: a review. J. Funct. Biomater. 14, 55 (2023)
Y.K. Sung, S.W. Kim, Recent advances in polymeric drug delivery systems. Biomater Res. 24, 150–161 (2020)
N.N. Ferreira, L.M.B. Ferreira, V.M.O. Cardoso, F.I. Boni, A.L.R. Souza, M.P.D. Gremião, Recent advances in smart hydrogels for biomedical applications: from self-assembly to functional approaches. Eur. Polym. J. 99, 117–133 (2018)
Q. Chen, Y. He, Q. Li, K. Yang, L. Sun, H. Xu, R. Wang, Intelligent design and medical applications of antimicrobial hydrogels. Colloids Interface Sci. Commun. 53, 100696 (2023)
E. Hasanzadeh, A. Seifalian, A. Mellati, J. Saremi, S. Asadpour, S.E. Enderami, H. Nekounam, N. Mahmoodi, Injectable hydrogels in central nervous system: unique and novel platforms for promoting extracellular matrix remodeling and tissue engineering. Mater. Today. Biol. 20, 100614 (2023)
Y. Xiao, Y. Gu, L. Qin, L. Chen, X. Chen, W. Cui, F. Li, N. Xiang, X. He, Injectable thermosensitive hydrogel-based drug delivery system for local cancer therapy. Colloids Surf. B. 200, 111581 (2021)
J. Mantovan, J.F. Pereira, B.M. Marim, V.G. Resta, G.A. Gil-Giraldo, S. Mali, Nanocellulose hydrogels, in Industrial Applications of Nanocellulose and Its Nanocomposite, vol. 1, ed. by S.M. Sapuan, M.N.F. Norrrahim, R.A. Ilyas (Elsevier, Kidlington, 2022), pp.1–524
M. Zhang, Z. Fan, J. Zhang, Y. Yang, C. Huang, X. Zhang, D. Ding, G. Liu, N. Cheng, Multifunctional chitosan/alginate hydrogel incorporated with bioactive glass nanocomposites enabling photothermal and nitric oxide release activities for bacteria-infected wound healing. Int. J. Biol. Macromol. 232, 123445–123445 (2023)
L. Deng, B. Wang, W. Li, Z. Han, S. Chen, H. Wang, Bacterial cellulose reinforced chitosan-based hydrogel with highly efficient self-healing and enhanced antibacterial activity for wound healing. Int. J. Biol. Macromol. 217, 77–87 (2022)
L.C. Wong, J.H. Poh, W.T. Tan, B.-K. Khor, V. Murugaiyah, C.P. Leh, C.F. Goh, Cellulose hydrogel development from unbleached oil palm biomass pulps for dermal drug delivery. Int. J. Biol. Macromol. 224, 483–495 (2023)
J. Radwan-Pragłowska, L. Janus, M. Piątkowski, A. Sierakowska, D. Matysek, ZnO nanorods functionalized with chitosan hydrogels crosslinked with azelaic acid for transdermal drug delivery. Colloids Surf. B. 194, 111170 (2020)
H. Wei, S. Liu, Z. Tong, T. Chen, M. Yang, Y. Guo, H. Sun, Y. Wu, Y. Chu, L. Fan, Hydrogel-based microneedles of chitosan derivatives for drug delivery. React. Funct. Polym. 172, 105200 (2020)
M.R. El-Aassar, O.M. Ibrahim, M.M.G. Fouda, N.G. El-Beheri, M.M. Agwa, Wound healing of nanofiber comprising polygalacturonic/hyaluronic acid embedded silver nanoparticles: in-vitro and in-vivo studies. Carbohydr. Polym. 238, 116175 (2018)
J. Li, H. Wu, K. Jiang, Y. Liu, L. Yang, H.J. Park, Alginate calcium microbeads containing chitosan nanoparticles for controlled insulin release. Appl. Biochem. Biotechnol. 193, 463–478 (2021)
R. Rakhshaei, H. Namazi, H. Hamishehkar, M. Rahimi, Graphene quantum dot cross-linked carboxymethyl cellulose nanocomposite hydrogel for pH-sensitive oral anticancer drug delivery with potential bioimaging properties. Int. J. Biol. Macromol. 150, 1121–1129 (2023)
G. Kaufmann, M.P. Klein, M.I. Goettert, T.A.S. Aguirre, Development and cytotoxicity evaluation of a cylindrical pH-responsive chitosan-genipin hydrogel for the oral delivery of diclofenac sodium. Eur. Polym. J. 181, 111649 (2022)
S. Saifullah, T. Kanwal, S. Ullah, M. Kawish, S.M. Habib, I. Ali, A. Munir, M. Imran, M.R. Shah, Design and development of lipid modified chitosan containing mucoadhesive self-emulsifying drug delivery systems for cefixime oral delivery. Chem. Phys. Lipids. 235, 105052 (2021)
M. Pereira, F.C. Silva, S. Simões, H.M. Ribeiro, A.J. Almeida, J. Marto, Innovative, sugar-free oral hydrogel as a co-administrative vehicle for pediatrics: a strategy to enhance patient compliance. AAPS PharmSciTech 23, 107 (2022)
Y.H. Lee, B.-W. Lee, Y.C. Jung, B.-I. Yoon, H.-M. Woo, B.-J. Kang, Application of alginate microbeads as a carrier of bone morphogenetic protein-2 for bone regeneration. J. Biomed. Mater. Res. 107, 286–294 (2018)
G. Shi, Y. Zhou, W. Liu, C. Chen, Y. Wei, X. Yan, L. Wu, W. Wang, L. Sun, T. Zhang, Bone-derived MSCs encapsulated in alginate hydrogel prevent collagen-induced arthritis in mice through the activation of adenosine A2A/2B receptors in tolerogenic dendritic cells. Acta Pharmacol. Sin. B. 13, 2778–2794 (2023)
Y. Zhou, Z. Zhai, Y. Yao, J.C. Stant, S.L. Landrum, M.J. Bortner, C.E. Frazier, K.J. Edgar, Oxidized hydroxypropyl cellulose/carboxymethyl chitosan hydrogels permit pH-responsive, targeted drug release. Carbohydr. Polym. 300, 120213 (2023)
J. Huang, Y. Deng, J. Ren, G. Chen, G. Wang, F. Wang, X. Wu, Novel in situ forming hydrogel based on xanthan and chitosan re-gelifying in liquids for local drug delivery. Carbohydr. Polym. 186, 54–63 (2018)
T. Shi, D. Niu, J. You, S. Li, G. Li, K. Ren, S. Yan, G. Xu, J. Yin, Injectable macro-porous chitosan/polyethylene glycol-silicotungstic acid double-network hydrogels based on “smashed gels recombination” strategy for cartilage tissue engineering. Int. J. Biol. Macromol. 233, 123541 (2023)
N.-G. Kim, P. Chandika, S.-C. Kim, D.-H. Won, W.S. Park, I.-W. Choi, S.G. Lee, Y.-M. Kim, W.-K. Jung, Fabrication and characterization of ferric ion cross-linked hyaluronic acid/pectin-based injectable hydrogel with antibacterial ability. Polumey. 271, 125808 (2023)
Z. Karim, P. Karwa, S.R.R. HireMath, Polymeric microneedles for transdermal drug delivery—a review of recent studies. J. Drug. Deliv. Sci. Technol. 77, 103760 (2022)
R. Maji, C.A. Omolo, Y. Jaglal, S. Singh, N. Devnarain, C. Mocktar, T. Govender, A transferosome-loaded bigel for enhanced transdermal delivery and antibacterial activity of vancomycin hydrochloride. Int. J. Pharm. 607, 120990–120990 (2021)
D. Mazhar, N.U. Haq, M. Zeeshan, Q.U. Ain, H. Ali, S. Khan, S.A. Khan, Preparation, characterization, and pharmacokinetic assessment of metformin HCl loaded transfersomes co-equipped with permeation enhancer to improve drug bioavailability via transdermal route. J. Drug. Deliv. Sci. Technol. 84, 104448 (2023)
J. Yekrang, N.G. Shahbazi, F. Rostami, M. Ramyar, Electrospun chitosan/polyvinyl alcohol nanofiber patches loaded with vitamin B12. Int. J. Biol. Macromol. 230, 123187–123187 (2023)
H. Wang, H. Zhang, Z. Xie, K. Chen, M. Ma, Y. Huang, M. Li, Z. Cai, P. Wang, H. Shen, Injectable hydrogels for spinal cord injury repair. Eng. Regen. 3, 407–419 (2022)
G.K. Wasupalli, D. Verma, Thermosensitive injectable hydrogel based on chitosan-polygalacturonic acid polyelectrolyte complexes for bone tissue engineering. Carbohydr. Polym. 294, 119769 (2022)
F. Scheffold, Pathways and challenges towards a complete haracterization of microgels. Nat. Commun. 11, 4315–4315 (2020)
J. Pushpamalar, P. Meganathan, H.L. Tan, N.A. Dahlan, L.T. Ooi, B.N.H.M. Neerooa, R.Z. Essa, K. Shameli, S.Y. Teow, Development of a polysaccharide-based hydrogel drug delivery system (DDS): an update. Gels. 7, 153 (2021)
K. Ganguly, K. Chaturvedi, U.A. More, M.N. Nadagouda, T.M. Aminabhavi, Polysaccharide-based micro/nanohydrogels for delivering macromolecular therapeutics. J. Control. Release 193, 162–173 (2014)
E. Madivoli, Polysaccharide based hydrogels in drug delivery systems, wound healing, and agriculture. Chem. Afr. (2023)
A.C. Alavarse, E.C.G. Frachini, R.L.C.G. Silva, V.H. Lima, A. Shavandi, D.F. Crosslinkers for polysaccharides and proteins: synthesis conditions, mechanisms, and crosslinking efficiency: a review. Int. J. Biol. Macromol. 202, 558–596 (2022)
J. Pushpamalar, A.K. Veeramachineni, C. Owh, X.J. Loh, Biodegradable polysaccharides for controlled drug delivery. Chem. Plus. Chem. 81, 504–514 (2016)
H. López-Hortas, H. Domínguez, M.D. Torres, Valorisation of edible brown seaweeds by the recovery of bioactive compounds from aqueous phase using MHG to develop innovative hydrogels. Process. Biochem. 78, 100–107 (2019)
J. Tan, Y. Luo, Y. Guo, Y. Zhou, X. Liao, D. Li, X. Lai, Y. Liu, Development of alginate-based hydrogels: crosslinking strategies and biomedical applications. Int. J. Biol. Macromol. 239, 124275–124275 (2023)
J. Supramaniam, R. Adnan, N.H.M. Kaus, R. Bushra, Magnetic nanocellulose alginate hydrogel beads as potential drug delivery system. Int. J. Biol. Macromol. 118, 640–648 (2018)
H. Ma, J. Zhao, Y. Liu, L. Liu, J. Yu, Y. Fan, Controlled delivery of aspirin from nano cellulose-sodium alginate interpenetrating network hydrogels. Ind. Crops. Prod. 192, 116081 (2023)
C. Wu, Y. Zhang, Y. Xu, L. Long, X. Hu, J. Zhang, Y. Wang, Injectable polyaniline nanorods/alginate hydrogel with AAV9-mediated VEGF overexpression for myocardial infarction treatment. Biomaterials 296, 122088–122088 (2023)
N.D. Rusli, A.A.A. Ghani, K. Mat, M.T. Yusof, M. Zamri-Saad, H.A. Hassim, The potential of pretreated oil palm frond in enhancing rumen degradability and growth performance: a review. Adv. Anim. Vet. Sci. 9, 881–822 (2021)
J.K. Saini, H. Hemansi, A. Kaur, A. Mathur, Strategies to enhance enzymatic hydrolysis of lignocellulosic biomass for biorefinery applications: a review. Bioresour. Technol. 360(2022), 127517–127517 (2022)
A.P.C. Almeida, J.N. Saraiva, G. Cavaco, R.P. Portela, C.R. Leal, R.G. Sobral, P.L. Almeida, Crosslinked bacterial cellulose hydrogels for biomedical applications. Eur. Polym. J. 177, 111438 (2023)
E.M. Zyl, M.A. Kennedy, W. Nason, S.J. Fenlon, E.M. Young, L.J. Smith, S.R. Bhatia, J.M. Coburn, Structural properties of optically clear bacterial cellulose produced by Komagataeibacter hansenii using arabitol. Biomater. Adv. 148, 213345 (2023)
R. Freixo, F. Casanova, A.B. Ribeiro, C.F. Pereira, E.M. Costa, M.E. Pintado, Ó.L. Ramos, Extraction methods and characterization of cellulose fractions from a sugarcane by-product for potential industry applications. Ind. Crops. Prod. 197, 116615 (2023)
J.F. Pereira, B.M. Marim, S. Mali, Chemical modification of cellulose using a green route by reactive extrusion with citric and succinic acids. Polysaccharides. 3, 292–305 (2022)
T.A. Debele, S.L. Mekuria, H.-C. Tsai, Polysaccharide based nanogels in the drug delivery system: application as the carrier of pharmaceutical agents. Mater. Sci. Eng. C. 68, 964–981 (2016)
L.-H. Fu, C. Qi, M.-G. Ma, P. Wan, Multifunctional cellulose-based hydrogels for biomedical applications. J. Mater. Chem. 7, 1541–1562 (2019)
M. Abbaszadeh, S.M. Meybodi, A. Zeri, E.M. Khorasgani, H.M. Heravi, N. Kasaiyan, Cellulose acetate nanofibrous wound dressings loaded with 1% probucol alleviate oxidative stress and promote diabetic wound healing: an in vitro and in vivo study. Cellulose 29, 5359–5374 (2022)
S. Farzamfar, M. Naseri-Nosar, A. Vaez, F. Esmaeilpour, A. Ehterami, H. Sahrapeyma, H. Samadian, A.-A. Hamidieh, S. Ghorbani, A. Goodarzi, A. Azimi, M. Salehi, Neural tissue regeneration by a gabapentin-loaded cellulose acetate/gelatin wet-electrospun scaffold. Cellulose 25, 1229–1238 (2018)
M.V. Konovalova, D.S. Tsaregorodtseva, A.N. Venzhik, R.A. Poltavtseva, E.V. Svirshchevskya, Antiadhesion effect of materials based on carboxymethylchitosan and carboxymethylcellulose. Appl. Biochem. Microbiol. 58, 155–160 (2022)
P. Nidhi, K. Dev, P. Negi, A. Sourirajan, Development and evaluation of hydrogel formulation comprising essential oil of Mentha longifolia L. for oral candidiasis. Adv. Tradit. Med. (2022). https://doi.org/10.1007/s13596-022-00636-4
A. Mahmood, D. Patel, B. Hickson, J. DesRochers, X. Hu, Recent progress in biopolymer-based hydrogel materials for biomedical applications. Int. J. Mol. Sci. 23, 1415 (2022)
H. Bai, Z. Li, S. Zhang, W. Wang, W. Dong, Interpenetrating polymer networks in polyvinyl alcohol/cellulose nanocrystals hydrogels to develop absorbent materials. Carbohydr. 200, 468–476 (2018)
H. Luo, R. Cha, J. Li, W. Hao, Y. Zhang, F. Zhou, Advances in tissue engineering of nanocellulose-based scaffolds: a review. Carbohydr. Polym. 224, 115144 (2019)
H.T.T. Nguyen, N.H.N. Do, H.D. Lac, P.L.N. Nguyen, P.K. Le, Synthesis, properties, and applications of chitosan hydrogels as anti-inflammatory drug delivery system. J. Porous Mater. 30, 655–670 (2023)
G. Bajaj, W.G.V. Alstine, Y. Yeo, Zwitterionic Chitosan derivative, a new biocompatible pharmaceutical excipient Prevents Endotoxin-Mediated Cytokine. Release. PLoS ONE 7, e30899 (2012)
D. George, P.U. Maheswari, K.M.M.S. Begum, Cysteine conjugated chitosan based green nanohybrid hydrogel embedded with zinc oxide nanoparticles towards enhanced therapeutic potential of naringenin. React. Funct. Polym. 148, 104480 (2020)
M. Ghanavi, A. Khoshandam, S. Aslzad, M. Fathi, A. Barzegari, E.D. Abdolahinia, K. Adibkia, J. Barar, Y. Omidi, Injectable thermosensitive PEG-g-chitosan hydrogel for ocular delivery of vancomycin and prednisolone. J. Drug. Deliv. Sci. Technol. 83, 104385 (2023)
S. Tiwari, P. Bahadur, Modified hyaluronic acid based materials for biomedical applications. Int. J. Biol. Macromol. 121, 556–571 (2019)
R. Ucm, M. Aem, Z. Lhb, V. Kumar, M.J. Taherzadeh, V.K. Garlapati, A.K. Chandel, Comprehensive review on biotechnological production of hyaluronic acid: status, innovation, market and applications. Bioengineered 13, 9645–9661 (2022)
A. Manzoor, A.H. Dar, V.K. Pandey, R. Shams, S. Khan, P.S. Panesar, J.F. Kennedy, U. Fayaz, S.A. Khan, Recent insights into polysaccharide-based hydrogels and their potential applications in food sector: a review. Int. J. Biol. Macromol. 213, 987–1006 (2022)
Y.A. Attia, A.M.A. Nazawi, H. Elsayed, M.W. Sadik, Carbon nanotubes catalyzed UV-trigger production of hyaluronic acid from Streptococcus equi. Saudi J. Biol. Sci. 28, 84–491 (2021)
Y. Zhang, J. Dong, G. Xu, R. Han, J. Zhou, Y. Ni, Efficient production of hyaluronic acid by Streptococcus zooepidemicus using two-stage semi-continuous fermentation. Bioresour. Technol. 377, 128896 (2023)
Y. Ma, Y. Qiu, C. Yu, S. Li, H. Xu, Design and construction of a Bacillus amyloliquefaciens cell factory for hyaluronic acid synthesis from Jerusalem artichoke inulin. Int. J. Biol. Macromol. 205, 410–418 (2022)
R.M.M. Melero-Fernandez, U.T. Arasu, R. Kärnä, S. Oikari, K. Rilla, D. Vigetti, A. Passi, P. Heldin, M.I. Tammi, A.J. Deen, Effects of mutations in the post-translational modification sites on the trafficking of hyaluronan synthase 2 (HAS2). Matrix Biol. 80, 85–103 (2019)
H. Wu, N. Bu, J. Chen, Y, Chen, R, Sun, C. Wu, J. Peng, Construction of Konjac glucomannan/oxidized hyaluronic acid hydrogels for controlled drug release. Polymers 14, 927 (2022)
N.C. Pan, G.T. Bersaneti, S. Mali, M.A.P.C. Celligoi, Films based on blends of polyvinyl alcohol and microbial hyaluronic acid. Braz. Arch. Biol. Technol. 63, e20190386 (2020)
I.S. Bayer, Hyaluronic acid and controlled release: a review. Molecules 6, 2649 (2020)
S. Liu, X. Liu, Y. Ren, P. Wang, Y. Pu, R. Yang, X. Wang, X. Tan, Z. Ye, V. Maurizot, B. Chi, Mussel-inspired dual-cross-linking hyaluronic acid/ε-polylysine hydrogel with self-healing and antibacterial properties for wound healing. ACS Appl Mater. Interfaces. 12, 27876–27888 (2020)
S.S. Kwon, B.J. Kong, S.N. Park, Physicochemical properties of pH-sensitive hydrogels based on hydroxyethyl cellulose–hyaluronic acid and for applications as transdermal delivery systems for skin lesions. Eur. J. Pharm. Biopharm. 92, 146–154 (2015)
J. Zhang, T. Liu, Z. Liu, Q. Wang, Facile fabrication of tough photocrosslinked polyvinyl alcohol hydrogels with cellulose nanofibrils reinforcement. Polymer 173, 103–109 (2019)
D.L. Tran, P.L. Thi, T.T.H. Thi, K.D. Park, Novel enzymatically crosslinked chitosan hydrogels with free-radical-scavenging property and promoted cellular behaviors under hyperglycemia. Prog. Nat. Sci. 30, 661–668 (2020)
W. Hu, Z. Wang, Y. Xiao, S. Zhang, J. Wang, Advances in crosslinking strategies of biomedical hydrogels. Biomater. Sci. (2019). https://doi.org/10.1039/c8bm01246f
J. Xu, Y. Liu, Hydrogels based on Schiff base linkages for. Molecules 19, 1–21 (2019)
M. Muhammad, C. Willems, J. Rodríguez-Fernández, G. Gallego-Ferrer, T. Growth, Synthesis and characterization of oxidized polysaccharides for in situ forming hydrogels. Biomolecules 10, 1–18 (2020)
G.S. Krishnakumar, S. Sampath, S. Muthusamy, M.A. John, Importance of crosslinking strategies in designing smart biomaterials for bone tissue engineering: a systematic review. Mater. Sci. Eng. C. 96, 941–954 (2019)
S. Maiz-Fernández, L. Pérez-Álvarez, U. Silván, J.L. Vilas-Vilela, S. Lanceros-Méndez, Dynamic and self-healable chitosan/hyaluronic acid-based in situ-forming hydrogels. Gels. 8, 477 (2022)
D.M. Casali, M.J. Yost, M.A. Matthews, Eliminating glutaraldehyde from crosslinked collagen films using supercritical CO2. J. Biomed. Mater. Res. A. 106, 86–94 (2018)
T. Takigawa, Y. Endo, Effects of glutaraldehyde exposure on human health. J. Occup. Health. 48, 75–87 (2006)
I. Kondratowicz, I. Shalayel, M. Nadolska, S. Tsujimura, Y. Yamagata, I. Shitanda, A. Zebda, Impact of lactic acid and genipin concentration on physicochemical and mechanical properties of chitosan membranes. J. Polym. Environ. 31, 221–123 (2023)
Y. Yu, S. Xu, S. Li, H. Pan, Genipin-cross-linked hydrogels based on biomaterials for drug delivery: a review. Biomater. Sci. (2021). https://doi.org/10.1039/d0bm01403f
J.A. Olmo, L. Pérez-Álvarez, V. Sáez-Martínez, S. Benito-Cid, L. Ruiz-Rubio, R. Pérez-González, J.L. Vilas-Vilela, J.M. Alonso, Wound healing and antibacterial chitosan-genipin hydrogels with controlled drug delivery for synergistic anti-inflammatory activity. Int. J. Biol. Macromol. 203, 679–694 (2022)
T. Tan, J. Zhou, X. Gao, X. Tang, H. Zhang, Synthesis, characterization and water absorption behavior of tartaric acid-modified cellulose gel from corn stalk pith. Ind. Crop. Prod. 169, 113641 (2021)
N.S.M. Sechi, P.T. Marques, Preparation and physicochemical, structural and morphological characterization of phosphorylated starch. Mater Res. 20, 174–180 (2017)
N. Maaloul, P. Oulego, M. Rendueles, A. Ghorbal, M. Diaz, Enhanced Cu(II) adsorption using sodium trimetaphosphate-modified cellulose beads: equilibrium, kinetics, adsorption mechanisms, and reusability. Environ. Sci. Pollut. Res. 28, 446523–446539 (2021)
F.G. Colodi, D.R.B. Ducatti, M.D. Noseda, M.M. Carvalho, S.M.B. Winnischofer, M.E.R. Duarte, Semi-synthesis of hybrid ulvan-kappa-carrabiose polysaccharides andevaluation of their cytotoxic and anticoagulant effects. Carbohydr. Polym. 267, 118161 (2021)
X. Wang, Challenges and outlook for catalytic direct amidation reactions. Nat. Catal. 2, 98–102 (2019)
M. Todorovic, D.M. Perrin, Recent developments in catalytic amide bond formation. Pept. Sci. 112, e24210 (2020)
M.T. Sabatini, L.T. Boulton, H.F. Sneddon, T.D. Sheppard, A green chemistry perspective on catalytic amide bond formation. Nat. Catal. 2, 10–17 (2019)
Y.Y. Hsu, K.L. Liu, H.H. Yeh, H.R. Lin, H.L. Wu, J.C. Tsai, Sustained release of recombinant thrombomodulin from cross-linked gelatin/hyaluronic acid hydrogels potentiate wound healing in diabetic mice. Eur. J. Pharm. Biopharm. 135, 61–71 (2019)
C.H. Cheng, Y.S. Chen, H.T. Chang, K.C. Chang, S.M. Huang, S.M. Liu, W.C. Chen, In vitro evaluation of antibacterial activity and biocompatibility of synergistically cross-linked gelatin-alginate hydrogel beads as gentamicin carriers. J. Drug Deliv. Sci. Technol. 79, 104078 (2023)
N. Nakajima, Y. Ikada, Mechanism of amide formation by carbodiimide for bioconjugation in aqueous media. Bioconjugate. Chem. 6, 123–130 (1995)
A. Sannino, S. Pappadà, M. Madaghiele, A. Maffezzoli, L. Ambrosio, L. Nicolais, Crosslinking of cellulose derivatives and hyaluronic acid with water-soluble carbodiimide. Polymer 46, 11206–11212 (2005)
D.E. Rodríguez-Félix, D. Pérez-Caballero, T. del Castillo-Castro, M.M. Castillo-Ortega, Y. Garmendía-Diago, J. Alvarado-Ibarra, M. Plascencia-Jatomea, A.S. Ledezma-Pérez, S.E. Burruel-Ibarra, Chitosan hydrogels chemically crosslinked with L-glutamic acid and their potential use in drug delivery. Polym. Bull. 80, 2617–2636 (2023)
K.C. Chang, W.-C. Chen, C.-H. Chen, C.-L. Ko, S.-M. Liu, J.-C. Chen, Chemical cross-linking on gelatin-hyaluronan loaded with hinokitiol for the preparation of guided tissue regeneration hydrogel membranes with antibacterial and biocompatible properties. Mater. Sci. Eng. C 119, 111576 (2021)
I. Aranaz, A.R. Alcántara, M.C. Civera, C. Arias, B. Elorza, A.H. Caballero, N. Acosta, Chitosan: an overview of its properties and applications. Polymers (Basel). 13 (2021)
S. Huang, X. Kon, Y. Xiong, X. Zhang, H. Chen, W. Jiang, Y. Niu, W. Xu, C. Ren, An overview of dynamic covalent bonds in polymer material and their applications. Eur. Polym. J. 141, 110094 (2020)
D.-Q. Li, S.-Y. Wang, Y.-J. Meng, J.-F. Li, J. Li, An injectable, self-healing hydrogel system from oxidized pectin/chitosan/γ-Fe2O3. Int. J. Biol. Macromol. 164, 4566–4574 (2020)
E. Raczuk, B. Dmochowska, J. Samaszko-Fiertek, J. Madaj, Different Schiff bases—structure, importance and classification. Molecules 27, 787 (2022)
P. Dubey, S. Gupta, A.K. Singh, Complexes of Pd(II), η6-C6H6Ru(II), and η5-Cp*Rh(III) with Chalcogenated Schiff bases of Anthracene-9-carbaldehyde and base-free catalytic transfer hydrogenation of aldehydes/ketones and N-alkylation of amines. Organometallics 38, 944–961 (2019)
F. Ghobakhloo, D. Azarifar, M. Mohammadi, H. Keypour, H. Zeynali, Copper(II) Schiff-base complex modified UiO-66-NH2(Zr) metal-organic framework catalysts for knoevenagel condensation-Michael addition-cyclization reactions. Inorg. Chem. 61, 4825–4841 (2022)
M. Skrodzki, V. Patroniak, P. Pawluć, Schiff base Cobalt(II) complex-catalyzed highly markovnikov-selective hydrosilylation of alkynes. Org. Lett. 23, 663–667 (2021)
J.L. Pratihar, P. Mandal, C.K. Lai, S. Chattopadhyay, Tetradentate amido azo Schiff base Cu(II), Ni(II) and Pd(II) complexes: synthesis, characterization, spectral properties, and applications to catalysis in C-C coupling and oxidation reaction. Polyhedron 161, 317–324 (2019)
J. Liu, J. Li, F. Yu, Y. Xin, Zhao, X. Mei, Mo, J. Feng, Pan, In situ forming hydrogel of natural polysaccharides through Schiff base reaction for soft tissue adhesive and hemostasis. Int. J. Biol. Macromol. 147, 653–666 (2020)
X. Peng, L. Li, J. Xing, C. Cheng, M. Hu, Y. Luo, S. Shi, Y. Liu, Z. Cui, X. Yu, Cross-linking porcine peritoneum by oxidized konjac glucomannan: a novel method to improve the properties of cardiovascular substitute material. J. Leather Sci. Eng. 5, 1–17 (2023)
X. Dang, P. Liu, M. Yang, H. Deng, Z. Shan, W. Zhen, Production and characterization of dialdehyde cellulose through green and sustainable approach. Cellulose 26, 9503–9515 (2019)
C. Qian, T. Zhang, J. Gravesande, C. Baysah, X. Song, J. Xing, J, Injectable and self-healing polysaccharide-based hydrogel for pH-responsive drug release. Int. J. Biol. Macromol. 123, 140–148 (2019)
R. Jahanban-Esfahlan, H. Derakhshankhah, B. Haghshenas, B, Massoumi, M, Abbasian, M. Jaymand, A bio-inspired magnetic natural hydrogel containing gelatin and alginate as a drug delivery system for cancer chemotherapy. Int. J. Biol. Macromol. 156, 438–445 (2020)
Y. Zhang, X. Li, N. Zhong, Y. Huang, K. He, X. Ye, Injectable in situ dual-crosslinking hyaluronic acid and sodium alginate based hydrogels for drug release. J. Biomater. Sci. Polym. Ed. 30, 995–1007 (2019)
X. Zhang, Y. Pan, S. Li, L. Xing, S. Du, G. Yuan, J. Li, T. Zhou, D. Xiong, H. Tan, Z. Ling, Y. Chen, X. Hu, X. Niu, Doubly crosslinked biodegradable hydrogels based on gellan gum and chitosan for drug delivery and wound dressing. Int. J. Biol. Macromol. 164, 2204–2214 (2020)
F.A. Ngwabebhoh, R. Patwa, O. Zandraa, N. Saha, P. Saha, Preparation and characterization of injectable self-antibacterial gelatin/carrageenan/bacterial cellulose hydrogel scaffolds for wound healing application. J. Drug Deliv. Sci. Technol. 63, 102415 (2021)
E. Stoll, T. Tongue, K.G. Andrews, D. Valette, D.J. Hirst, R.M. Denton, A practical catalytic reductive amination of carboxylic acids. Chem. Sci. 11, 9494–9500 (2020)
J. Magano, Large-scale amidations in process chemistry: practical considerations for reagent selection and reaction execution. Org. Process Res. Dev. 26, 1562–1689 (2022)
M.L. Pita-López, G. Fletes-Vargas, H. Espinosa-Andrews, R. Rodríguez-Rodríguez, Physically cross-linked chitosan-based hydrogels for tissue engineering applications: a state-of-the-art review. Eur. Polym. J. 145, 110176 (2021)
Z.-Y. Qin, X.-W. Jia, Q. Liu, B.-H. Kong, H. Wang, Fast dissolving oral films for drug delivery prepared from chitosan/pullulan electrospinning nanofibers. Int. J. Biol. Macromol. 137, 224–231 (2019)
Z. Li, Z. Lin, Recent advances in polysaccharide-based hydrogels for synthesis and applications. Aggregate. 2, e21 (2021)
V.V. Khutoryanskiy, A.V. Dubolazov, G.A. Mun, pH- and ionic strength effects on interpolymer complexation via hydrogen-bonding. Hydrogen. Interpolymer complexes form. Struct. Appl. 53, 1–22 (2009)
Z. Shariatinia, A. Mazloom-Jalali, Chitosan nanocomposite drug delivery systems designed for the ifosfamide anticancer drug using molecular dynamics simulations. J. Mol. Liq. 273, 346–367 (2019)
S. Ata, A. Rasool, A. Islam, I. Bibi, M. Rizwan, M.K. Azeem, A.R. Qureshi, M. Iqbal, Loading of Cefixime to pH sensitive chitosan based hydrogel and investigation of controlled release kinetics. Int. J. Biol. Macromol. 155, 1236–1244 (2020)
S. Saboo, U.S. Kestur, D.P. Flaherty, L.S. Taylor, Congruent release of drug and polymer from amorphous solid dispersions: insights into the role of drug-polymer hydrogen bonding, surface crystallization, and glass transition. Mol. Pharm. 17, 1261–2127 (2020)
Z. Luo, C. Liu, P. Quan, D. Yang, H. Zhao, X. Wan, L. Fang, Mechanistic insights of the controlled release capacity of polar functional group in transdermal drug delivery system: the relationship of hydrogen bonding strength and controlled release capacity. Acta Pharm. Sin. B 10, 928–945 (2020)
H. Ma, J. Yu, L. Liu, Y. Fan, An optimized preparation of nanofiber hydrogels derived from natural carbohydrate polymers and their drug release capacity under different pH surroundings. Carbohydr. Polym. 265, 118008 (2021)
Y. Chen, S.Y.H. Abdalkarim, H.-Y. You, Y. Li, J. Xu, J. Marek, J. Yao, K.C. Tam, Double stimuli-responsive cellulose nanocrystals reinforced electrospun PHBV composites membrane for intelligent drug release. Int. J. Biol. Macromol. 155, 330–339 (2020)
J. Long, A.E. Etxeberria, A.V. Nand, C.R. Bunt, S. Ray, A. Seyfoddin, A 3D printed chitosan-pectin hydrogel wound dressing for lidocaine hydrochloride delivery. Mater. Sci. Eng. C. 104, 109873 (2019)
H. Baniasadi, Z. Madani, R. Ajdary, O.J. Rojas, J. Seppälä, Ascorbic acid-loaded polyvinyl alcohol/cellulose nanofibril hydrogels as precursors for 3D printed materials. Mater. Sci. Eng. C. 130, 112424 (2021)
G. Janarthanan, I. Noh, Recent trends in metal ion based hydrogel biomaterials for tissue engineering and other biomedical applications. J. Mater. Sci. Technol. 63, 35–53 (2021)
M. Diener, J. Adamcik, J. Bergfreund, S. Catalini, P. Fischer, R. Mezzenga, R. Fibrillar, Quaternary structures induced by divalent ions in a carboxylated linear polysaccharide. ACS Macro Lett. 9, 115–121 (2020)
A.S. Kazachenko, N.Y. Vasilieva, V.S. Borovkova, O.Y. Fetisova, N. Issaoui, Y.N. Malyar, E.V. Elsuf’ev, A.A. Karacharov, A.M. Skripnikov, A.V. Miroshnikova, A.S. Kazachenko, D.V. Zimonin, V.A. Ionin, Food xanthan polysaccharide sulfation process with sulfamic acid. Foods 10, 2571 (2021)
Y. Cohen, R. Rutenberg, G. Cohen, B. Veltman, R. Gvirtz, E. Fallik, D. Danino, E. Eltzov, E. Poverenov, Aminated polysaccharide-based nanoassemblies as stable biocompatible vehicles enabling crossing of biological barriers: an effective transdermal delivery of diclofenac medicine. ACS Appl. Bio Mater. 3, 2209–2217 (2020)
M. Ishihara, S. Kishimoto, S. Nakamura, Y. Sato, H. Hattori, Polyelectrolyte complexes of natural polymers and their biomedical applications. Polymers (Basel). 11, 1–12 (2019)
X. Hu, Y. Wang, L. Zhang, M. Xy, Construction of self-assembled polyelectrolyte complex hydrogel based on oppositely charged polysaccharides for sustained delivery of green tea polyphenols. Food Chem. 306, 125632 (2020)
S. Tang, J. Yang, L. Lin, K. Peng, Y. Chen, S. Jin, W. Yao, Construction of physically crosslinked chitosan/sodium alginate/calcium ion double-network hydrogel and its application to heavy metal ions removal. Chem. Eng. J. 393, 124728 (2020)
J. Huang, Y.-L. Wang, X.-D. Yu, Y.-N. Zhou, L.-Q. Chu, Enhanced fluorescence of carboxymethyl chitosan via metal ion complexation in both solution and hydrogel states. Int. J. Biol. Macromol. 152, 50–56 (2020)
W.N. Sharratt, C.G. Lopes, M. Sarkis, G. Tyagi, R. O’Connell, S.E. Rogers, J.T. Cabral, Ionotropic gelation fronts in sodium carboxymethyl cellulose for hydrogel particle formation. Gels. 7, 1–21 (2021)
S. Pedroso-Santana, N. Fleitas-Salazar, Ionotropic gelation method in the synthesis of nanoparticles/microparticles for biomedical purposes. Polym. Int. 69, 443–447 (2020)
M.C. Cortez-Trejo, J.D. Figueroa-Cárdenas, D. Quintanar-Guerrero, D.K. Baigts-Allende, J. Manríquez, S. Mendoza, Effect of pH and protein-polysaccharide ratio on the intermolecular interactions between amaranth proteins and xanthan gum to produce electrostatic hydrogels. Food Hydrocoll. 129, 107648 (2022)
H. Hamedi, S. Moradi, A.E. Tonelli, S.M. Hudson, Preparation and characterization of chitosan–alginate polyelectrolyte complexes loaded with antibacterial thyme oil nanoemulsions. Appl. Sci. 9, 3933 (2019)
S. Maiz-Fernández, N. Barroso, L. Pérez-Álvarez, U. Silván, J.L. Vilas-Vilela, S. Lanceros-Mendez, 3D printable self-healing hyaluronic acid/chitosan polycomplex hydrogels with drug release capability. Int. J. Biol. Macromol. 188, 820–832 (2021)
W. Deng, Y. Tang, J. Mao, Y. Zhou, T. Chen, X. Zhu, Cellulose nanofibril as a crosslinker to reinforce the sodium alginate/chitosan hydrogels. Int. J. Biol. Macromol. 189, 890–899 (2021)
A.M. Omer, M.S. Ahmed, G.M. El-Subruiti, R.E. Khalifa, A.S. Eltaweil, Ph-sensitive alginate/carboxymethyl chitosan/aminated chitosan microcapsules for efficient encapsulation and delivery of diclofenac sodium. Pharmaceutics 13, 338 (2021)
Y.A. Khan, K. Ozaltin, A. Bernal-Ballen, A. Di Martino, Chitosan-alginate hydrogels for simultaneous and sustained releases of ciprofloxacin, amoxicillin and vancomycin for combination therapy. J. Drug Deliv. Sci. Technol. 61, 102126 (2021)
A. Goodarzi, M. Khanmohammadi, S. Ebrahimi-Barough, M. Azami, A. Amani, A. Baradaran-Rafii, N.L. bakhshaiesh, A. Ai, A. Farzin, J. Ai, Alginate-based hydrogel containing taurine-loaded chitosan nanoparticles in biomedical application. Arch. Neurosci. 6, e86349 (2019)
J. Chalitangkoon, M. Wongkittisin, P. Monvisade, Silver loaded hydroxyethylacryl chitosan/sodium alginate hydrogel films for controlled drug release wound dressings. Int. J. Biol. Macromol. 159, 194–203 (2020)
N. Pilipenko, O.H. Gonçalves, E. Bona, I.P. Fernandes, J.A. Pinto, G.D. Sorita, F.V. Leimann, M.F. Barreiro, Tailoring swelling of alginate-gelatin hydrogel microspheres by crosslinking with calcium chloride combined with transglutaminase. Carbohydr. Polym. 223, 115035 (2019)
H.-S. Kim, J. Yang, K. Kim, U.S. Shin, Biodegradable and injectable hydrogels as an immunosuppressive drug delivery system. Mater. Sci. Eng. C 223, 472–481 (2019)
S. Potiwiput, H. Tan, G. Yuan, S. Li, T. Zhou, J. Li, Y. Jia, D. Xiong, X. Hu, Z. Ling, Y. Chen, Dual-crosslinked alginate/carboxymethyl chitosan hydrogel containing in situ synthesized calcium phosphate particles for drug delivery application. Mater. Chem. Phys. 241 (2020)
L. Wang, J. Li, D. Zhang, S. Ma, J. Zhang, F. Gao, F. Guan, M. Yao, Dual-enzymatically crosslinked and injectable hyaluronic acid hydrogels for potential application in tissue engineering. RSC Adv. 1, 2870–2876 (2020)
H.Y. Jung, P.L. Thi, K.-H. HwangBo, J.W. Bae, K.D. Park, Tunable and high tissue adhesive properties of injectable chitosan based hydrogels through polymer architecture modulation. Carbohydr. Polym. 261, 117810 (2021)
E. Badali, M. Hosseini, M. Mohajer, S. Hassanzadeh, S. Sepideh, J. Hilborn, M. Khanmohammadi, Enzymatic crosslinked hydrogels for biomedical application. Polym. Sci. Ser. A 63, S1–S22 (2022)
R.G. Rosenthal, X.D. Zhang, K.I. Đurđić, J.J. Collins, D.A. Weitz, Controlled continuous evolution of enzymatic activity screened at ultrahigh throughput using drop-based microfluidics. Angew. Chem. Int. 62, e202303112 (2023)
X. Wang, C. Guo, C. Gao, Y. Song, J. Zhang, J. Huang, M. Hui, Synthesis of casein-γ-polyglutamic acid hydrogels by microbial transglutaminase-mediated gelation for controlled release of drugs. J. Biomater. Appl. 36, 237–245 (2021)
T. Wu, C. Liu, X. Hu, Enzymatic synthesis, characterization and properties of the protein-polysaccharide conjugate: a review. Food Chem. 372, 131332 (2022)
C.-K. Sun, C.-J. Ke, Y.-W. Lin, F.-H. Lin, T.-H. Tsai, J.-S. Sun, Transglutaminase cross-linked gelatin-alginate-antibacterial hydrogel as the drug delivery-coatings for implant-related infections. Polymers 13, 414 (2021)
F.W. Krainer, A. Glieder, An updated view on horseradish peroxidases: recombinant production and biotechnological applications. Appl. Microbiol. Biotechnol. 99, 1611–1625 (2015)
J. Gao, J.M. Karp, R. Langer, N. Joshi, The future of drug delivery. Chem. Mater. 35, 359–363 (2023)
S. Fumoto, K. Nishida, Co-delivery systems of multiple drugs using nanotechnology for future cancer therapy. Chem. Pharm. Bull. 68, 603–612 (2020)
J.F. Pereira, B.M. Marim, B.M. Simões, F. Yamashita, S. Mali, Hydrogels based on gelatin, xanthan gum, and cellulose obtained by reactive extrusion and thermopressing processes. Prep. Biochem. Biotechnol. (2023). https://doi.org/10.1080/10826068.2022.2162921
Funding
Funding was provided by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Capes-DS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Data Availability
The statements are properly cited in the paper and no additional data is available.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Paiva, M.T.P., Kishima, J.O.F., Silva, J.B.M.D. et al. Crosslinking Methods in Polysaccharide-Based Hydrogels for Drug Delivery Systems. Biomedical Materials & Devices 2, 288–306 (2024). https://doi.org/10.1007/s44174-023-00118-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s44174-023-00118-4