Abstract
Mucormycosis is the third most frequent invasive mycosis, following candidiasis and aspergillosis. It is frequently neglected due to its rare occurrence; but recently attend the status of notifiable disease due to its higher incidence in both developed and developing nations. India has received global notice since its estimated instances were greater than the global estimated figures. Mucormycosis has several clinical manifestations, including rhino-orbital-cerebral (ROCM), pulmonary, gastrointestinal, cutaneous, renal, and diffuse Mucormycosis. ROCM is the most frequent clinical manifestation in India, although pulmonary mucormycosis is prevalent worldwide. This review also discusses host defenses, pre disposing risk factors and fungal virulence factors that impair host's ability to prevent fungus invasion and disease establishment. The diagnosis of the disease depends on clinical interventions, histological or microbiological procedures along with molecular methods to obtain timely results. But there are still unmet challenges for rapid diagnosis of the disease. Treatment of the disease is achieved by multimodal approaches such as reversal of underlying predisposing factors, rapid administration of antifungals in optimal doses and surgical procedures to remove infected tissues. Liposomal Amphotericin B, Posaconazole and Isavuconazoles are preferred as the first line of treatment procedures. clinical trials. Different studies have improved the existing drug and under clinical trials while several studies predicted the new potential targets as CotH and Ftr1 as shown in infection and in vitro models. Therefore, current scenario demands a multidisciplinary approach is needed to investigate the prevalence, pathogenesis which is highly important for the advancement of rapid diagnosis and effective treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microbes are the true ancestor of all living beings and we co-evolved with the microbe to sustain in the biosphere. These tiny and efficient players, as microbiota are providing digestive aids to humans, establishing the immune system and also prevent the disease progression. Paradoxically, it is also true that microbes are the major player to threat human, animal and plant health. Taking the current situations into consideration, infectious disease is the leading cause of death worldwide. In early December 2019, China got few cases of atypical pneumonia infections; which was found to be caused by severe acute respiratory syndrome corona virus 2 (SARS-CoV-2). This Corona virus disease 2019 (COVID-19) transmitted quickly and affected 113 countries and affecting 1 million lives; thus got a pandemic status on 11 march 2020. To date, there is more than 6.3 million death across globe [1]. The mortality and morbidity rate are high in this disease due to its rapid transmission, complex infection process, and associated co-lateral complications such as diabetes, cardiovascular disease, lung diseases, and hypertension. Patient experience a range of symptoms during COVID 19 infection which could be mild to severe. The mortality due to COVID 19 Severe symptoms need medical assistance and, in some cases, ICU stay is also suggested. Long term stay in ICU increases the chances of worse outcomes due to hospital associated nosocomial infection by pathogenic or opportunistic bacterial and fungal species [2]. Co-infection with COVID 19 increases the mortality rates in affected populations. Mucormycosis stand in the frontline due to its high incidence rates, differential clinical presentation, delayed diagnosis and resistant to antifungal treatment [3]. Mucormycosis is not a new disease that suddenly arise in the pandemic situation rather, it has a long history behind. Mucormycosis got much attention in India due to its high prevalence and death rates and declared as a notifiable disease as per Epidemic Disease Act of 1897 on June 2021. [4]
Global prevalence of Mucormycosis
Prevalence of Mucormycosis is increasing steadily across globe [5, 6]. The rate of prevalence is higher in China and India as they are on the top list for diabetes; one of the predisposing factor for mucormycosis [5, 7]. From the disease toll in pre-pandemic time (2000–2017) states the burden is higher on Europe (34%) than in Asia (31%); followed by north and south America (28%), Africa (3%), Australia (3%) [6, 8]. An autopsy-based study from hematological malignancies cases from USA reported 0.006 cases 100 autopsies 1989 to 1993 to 0.018 cases in 2004–2008 [9]. Another study on prevalence of mucormycosis and hospitalization in USA showed 0.12 cases per 10,000 discharge from 2005–2012 [10, 11]. Multicentric study from Spain estimated 0.43 cases per million/year and 0.62 cases hospitalization million per year [12]. Another study from Spain reported 1.2 cases per 100,000 admissions (1988–2006) to 3.3 cases per 100,000 admissions (2007–2015) [5, 6, 13]. A nationwide population-based study in Spain estimated the disease prevalence rose 0.7cases per million in1997 to 1.2 cases per million in 2006 [14]. Switzerland had seen 0.57cases per 100,000 hospitalizations per year before 2003 to 6.3 cases per 100,000 hospitalizations after 2003 [5]. They found that the increased prevalence is due to overuse of voriconazole and caspofungin as prophylaxis in HSCT populations [5, 15]. Study from Belgium also reported increased prevalence of the disease from 0.019 cases per 10,000 patients in 2000 to 0.148 cases per 10,000cases in 2009 [16]. Iran had noticed a rise in mucor infection i.e., 9.7% in 2008 to 23.7% in 2014 [17]. Japan had observed 0.01% of cases in 1969, while it substantially increased to 0.16% in 1989. [18]
India on Focus
Although global disease toll is also upsurging in recent two decades, but the rise in India is phenomenal. India has certain risk factors, clinical forms and causative agents are prevalent. The form of disease, its causative agent and risk factors are also unique. Globally, Rhizopus arrhizus is the most common agent to cause mucormycosis, also predominant in Indian sub-continent [5, 19]. The Apophysomyces is the second cause for the fatal disease; as compared to Lichtheimia species in developed countries [20]. Rhizopus homothallicus, Thamnostylum lucknowense, Mucor irregularis and Saksenaea erythrospora are also emerging in India. [6, 7, 21] An ecological survey in Indian soil and air reported the presence of such pathogenic species. Rhizopus, Lichtheimia, Cunninghamella, Rhizomucor and Apophysomyces are predominating Indian soil sample [5, 22]. The clinical presentation is also validating the survey where ROCM (47–74%) is the commonest type of mucormycosis in India involving Rhizopus. Cutaneous mucormycosis (10–30%) is the second most common in India and caused by Apophysomyces (60%) via nosocomial infection [23]. Pulmonary infection represents 3–22%, where hematological malignancy, CKD, SOT and diabetes mellitus are considered as conventional risk factors while post pulmonary tuberculosis stands as emerging risk for Indian population [7]. Renal mucormycosis represents 0.5–9% where CKD and hemodialysis is the major underlying conditions. But studies in India also shown that renal infection cases are also from immunocompetent populations. Gastrointestinal mucormycosis represents 2–8% and disseminated infections reported about 0.5–9% [5]. The Immunocompetent population is also affected and it is well differentiated when compared to global data. In India, 3–26% of mucormycosis cases are immunocompetent, while 18–19% are globally [20]. Even before COVID 19 pandemic, India is having increased prevalence of Mucormycosis compared to global data. Disease statistics shown 12.9cases 1990 to1999, while a significant increment i.e., 35.6 cases per year is seen from 2000 to 2004. Further, 50 cases per year had reported in just 18 months (2006–2007). Study from a single tertiary hospital showed 24.7(1990–2007) cases per year to 89 cases /year (2013–2015) [5]. Leading International Fungal Education (LIFE) has estimated that prevalence of mucormycosis across the globe rages from 0.6–3 cases per million while India alone accounted for 140 cases per million. [5, 6]
After SARS COV2 infection, the number has been spurred across all countries. As of July 2021, the epidemiological studies in India states the disease toll gets twofold increments in mucormycosis from 2019 to 2020. Government of India declared mucormycosis as notifiable disease on 10th May 2021 due to significant increase in disease toll (Fig. 1) [24]. According to the Health Ministry of India, as on 29th Nov 2021, there have been 51,775 instances (Fig. 1) of the fungal mucormycosis, with 4,332 fatalities. Maharashtra and Gujrat are India's worst-affected states with 1129 and 656 death reports respectively.
The Surge of COVID 19 associated Mucormycosis in India is ascribed to the overuse of steroids, poor glycemic management, usage of industrial grade oxygen, inappropriate humidification and cleanliness, and unique mutant virus strains. These factors have all led to significant immunosuppression and help in the fungal growth. over use of steroids, immunomodulatory drugs, antibiotics, during COVID 19, overuse of Zn supplements to boost immunity also increase the growth of fungi [25,26,27]. Viewing at pre-pandemic times in India, the incidence of mucormycosis is quite high compared to other countries. Underlying reasons for the higher incidence in India could be due to increasing number of diabetic population, warm, humid conditions, use of cow dungs for the purpose of fuel and daily life also increase the incidence of mucormycosis. [25,26,27]
The pathogens and their virulence factors
In 1885, the German pathologist Patalauf reported the first case of mucormycosis in human and described it as mycosis mucorina. Later American pathologist R.D. Baker coined the term Mucormycosis [28]. Previously, it is also known as Zygomycosis. Which was earlier defined as an infection caused by fungal species under Mucorales and zygomycotic species. This is actually including two clinicopathologically varied diseases. Again, reclassification based on molecular phylogenetic analysis confirmed that Zygomycota is to be polyphyletic and excluded from mucormycosis group of infections. Thus mucormycosis refers to the disease caused by the members of order Mucorales, under the phylum Glomeromycota and subphylum Mucoromycotina [28]. The Mucorales encompasses 55 genera with 261 species. Among them, 39 species from 12 genera viz. Actinomucor, Apophysomyces, Cunninghamella, Lichtheimia, Mycotypha, Mucor, Rhizomucor, Rhizopus, Saksenaea, Syncephalastrum, Thamnostylum and Cokeromyces have been reported to be involved in human infections [29]. As the disease is caused by the number of species from 12 genera and with varied clinical presentation involving ranges of tissues [5, 29, 30]. The pathogenesis could be different for species or genus level and it’s difficult to target for antifungal development. Thus, makes it a rare and complicated disease till date. Pathogenesis is a function of virulence factors of pathogen and the host responses. Differences in virulence factors and host invasion process across the species in order Mucorales; make it a complicated disease complex.
Species under genus Rhizopus, Lichtheimia and Mucor are common causative agents for Mucormycosis. R.oryzae is the most prevalent species across the globe [31] while Cunninghamella bertholletiae carries the highest mortality rates among Mucorales infection [32]. The aggressiveness and high mortality rate have not extensively studied in the species. But in the animal studies, and in vitro growth analysis of Cunninghamella suggest the accelerate growth of sporangiospores germination, hyphal growth, increase metabolic rate could help in the rapid organismal growth for disease progression and aggressive tissue injury [32].Rhizopus is well associated with ROCM form of mucor infection while Cunninghamella species are responsible for pulmonary and disseminated clinical form. Apophysomyces and Saksenaea species are reported from cutaneous Mucormycosis. Underlying disease also influence the type of disease form and species involved; as an instance, Rhizopus get a preferable environment in ketoacidosis while corticosteroid therapy provides the environment for Lichtheimia. The route of infection, thermotolerance properties, rate of germination could also influence the disease status. For example, Rhizopus and Rhizomucor produce dry spores and capable for spreading in air while Mucor and Lichtheimia having wet sporangia releasing the spores in small droplets. The difference in the sporangia property decides the infection mode; The prior groups prefer airborne route of infection while the later infect in contact mode of infection [33]. Clinically relevant organisms are thermotolerant and monophyletic, with greater pathogenicity than non-clinically relevant species [34]. Experimental studies on mesophilic strains in Galleria mellonella cultured at permissive temperatures, indicating the presence of additional components playing roles in the pathogenesis of clinical Rhizopus species [34]. Molecular pathogenesis is not well understood till date in Mucorales. Several studies on R. delemar and in M. circinelloides deciphered few virulent factors by transcriptomics and genomics analysis. These virulence factors can be possible targets for management of Mucormycosis (Table 1). Comparative genomics study of virulent and avirulent strains from M. circinelloides identified 800 genes which are present in virulent strain but absent or truncated or discontinuous in avirulent strain [35]. Transcriptomics studies further revealed that nutrition assimilation and metabolism gene expression is at higher fold in virulent strain that indicates the germination and survival strategy of pathogen in phagosome environment. [35]
Ftr1 gene
Iron is a crucial element for all organisms to survive, like wise Mucorales require Fe inside host for growth and survival. Ftr1 is a gene, expressed as high affinity Fe uptake and transport. This is expressed at higher fold in R. oryzae during iron starvation in immune deficient mice infection models [36]. Knock down of this particular gene results into reduced virulence in the experimented strain. Passive immunization with anti Ftr1 results into enhanced survival. [31, 36]
Spore Coat family Protein (CotH)
Spore coat family protein (CotH) is atypical protein kinases are the structural component of human pathogens. CotH protein is responsible for integrity of the spore and help in germination as mutants of CotH in Bacillus subtilis, shows deficient germination [37]. Studies found CotH protein are not present in non-invasive pathogen exhibiting that they have role in disease progression and tissue invasion. CotH protein is highly expressed and present in high copy number in pathogenic strain than to non-virulent strains [38, 39]. CotH, the signature protein of Mucorales, absent in other fungus such as Aspergillus and Candida. [40] For host interaction and invasion, they rather use agglutinin like proteins and thaumatin like proteins [41]. Studies from R.delemar showing CotH2 and CotH3 are important for host invasion process and they have specific motif exposed to surface for interaction with host endothelium [38]. CotH interacts with specific GRP 78 ligand present in the host endothelium. GRP 78 is a heat shock protein expressed in endoplasmic reticulum of endothelial cell. On stressed condition, the ligand protein is induced and transported to endothelial surface. The interaction between CotH and GRP78 is well studied in DKA mice model. The condition of Fe overload and hyperglycemia induces GRP expression on cell surfaces and enhances disease progression (Fig. 3) [40]. CotH specific PCR on mucormycosis diagnosis and therapeutics as anti-CotH monoclonal antibody could be strategy for better and early management of the disease. [40, 42]
Proteases
Proteases are important factors for virulence in fungal pathogens as they have the ability to cause tissue destruction and necrosis [43].Secreted proteases such as Aspatryl, Serine proteases and carbohydrate active enzymes could be the possible potential virulence factors for the mucorales. Studies on A. variabilis showed, it contains 488 predicted proteases; similar to R. arrhizus and L. corymbifera. Serine and aspartic proteases are the major constituents while carbohydrate active enzymes are the major part of secretory protein in the species [44]. Alkali Rhizopus Protease enzymes are detected in the clinical isolates of R. microspores; has a role in enhancement of coagulation process in mucor infections [45]. Comparative genomic analysis from several mucor species concluded that M. velutinosus possess unique aspartyl proteases that cause skin dissemination in host. [46, 47]
Secondary metabolites
Along with these, secondary metabolites and toxins can be a detrimental factor for the pathogenesis. Secondary metabolites including Polyketide synthase, non-ribosomal peptide synthetase and L-tryptophan dimethyl allyl transferase could progress to endothelial injury. Genes specifying to these lytic enzymes are present in the high copy number in R. oryzae than other fungal pathogens. [42]
Calcineurin
Likewise, Calcineurin is another vital factor concerning virulence of Mucorales [39, 48, 49]. Calcineurin is a serine-threonine phosphatase that is reliant on calcium and calmodulin. It is made up of a complex of two subunits: catalytic subunit A, which has phosphatase activity, and regulatory subunit B, which binds calcium-bound calmodulin and activates the enzymatic complex [50]. Three catalytic A subunits and one regulatory B subunit (CnbR) make up Mucorales (CnaA, CnaB and CnaC). The dimorphic transition adds to the pathogenicity of this fungus, as shown by the mutants' trapped in the yeast phase when the CnbR gene is disrupted or when calcineurin inhibitors are added [49, 50]. RNA interference studies explored different virulence factors of M. circinelloides in infection models such as in G. mellonella and in Murine model. [45]
Mcpld and McMyo5
Mcpld gene is another essential virulence factor for the organism [51]. Knocking out the above gene results into reduced virulence and deficient growth rate. This particular gene is well conserved and encodes for phospholipase D which is involved in germination and hyphal growth of M. circinelloides. It is well studied in different pathogens like Corynebacterium pseudotuberculosis and Aspergillus fumigatus. This protein is involved in macrophage death, tissue invasion and systemic dissemination [39]. McMyo5 gene encodes for a cargo transported in the organism and constantly involved in the transport of organelles, mRNA, secretory vesicles, lipid and protein vesicles. On mutant analysis, it was found that a delayed germination reduced polarity and also diminished virulence. [51]
Mucoricin
Mucoricin is a known virulence factor taking part in tissue necrosis and invasion [52]. This can be a potential target as polyclonal antibodies against this toxin decrease its ability to harm human cells in vitro and protect mice with Mucormycosis from hypovolemic shock, organ damage, and death [40]. Silencing the toxin in R. delemar by RNA interference reduces the fungus's capacity to damage host cells and decreases pathogenicity in mice [52, 53]. M. circinelloides that germinating within macrophages, requires the expression of genes involved nutrition digestion and metabolism.
Transcription Factors
These genes were found to be more strongly expressed in the virulent strain than in the avirulent strain, allowing it to survive and germinate within a phagosome. These mechanisms appear to be regulated by the transcription factors Aft1 and Atf2 [54]. These are few studies on different Mucorales species deciphering number of virulence factors involved in the complex pathogenesis of Mucormycosis and also represent novel antifungal targets to combat the deadly pathogens. In the next section we are going to discuss how the disease progress in the host and how associated risk factor influence disease progression under pathogenesis heading.
Pathogenesis
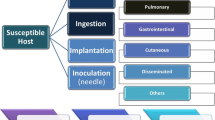
Mucormycosis is a rare but a fatal invasive fungal infection; not taken in the serious note yet. It is caused by the saprophytic group of molds within order Mucorales. It is classically categorized as an opportunistic infection and targeting immune-compromised group of populations. The risk always remains at the populations with diabetes mellitus with or without ketoacidosis, neutropenia, chronic renal failure, haemochromatosis, organ transplantation, haemopoietic transplantation, acquired immune deficiency syndrome, long term ICU stay, taking assistance of ventilation and having several drugs such steroid, deferoxamine (Fig. 2). COVID 19 infection facilitates the growth of mucormycosis by weakening the immune system and airways epithelium. Immunocompetent (with trauma patients) have the evidences of mucormycosis; infection is associated with contaminated soil and water. It is the 3rd most invasive disease after candidiasis and aspergillosis with high mortality rates (20%-50%). With delayed surgical and antifungal therapy, it can go up to 70–90% of mortality rate [58].
Major clinical representaions of Mucormycosis: Pulmonary, Rhinoorbito-cerebral, cutaneous, gastrointestinal and disseminated mucormycosis(Upper Panel). Lower panel displays potential risk factors that associated with development of mucormycosis including the existence of underlying diseases such as renal insufficiency, diabetes mellitus, and haematological malignancies. Voriconazole, broad-spectrum antibiotics, and steroids have all been linked to an increased risk of mucormycosis. Furthermore, a rise in blood iron levels, neutropenia, and immunosuppressive therapies such as stem cell therapy and organ transplant render people more susceptible to Mucormycosis
Pathogenesis of mucormycosis begins from the ingestion or inhalation or percutaneous inoculation of sporangiospores (Fig. 3). The spore encounter; but they are not always able to invade the immunocompetent host. The host response begins after their invasion to lungs cells or into the subcutaneous tissue, then the spores face the challenges from mononuclear and polynuclear phagocytes [59]. The oxidative stress and defensins produced by phagocytotic cells able to kill the pathogens and the spores. But in immunocompromised such as in neutropenic or people with phagocytic dysfunction slipped into Mucormycosis. For a full-blown disease, spore has to defeat immune system, germinate into hyphae and cause angio-invasive condition. Body offers resistance by innate immune system such as mucosal barrier; loaded with antimicrobial agents and also play the nutritional immunity strategy to kill the invaded spores [60, 61]. Fe is a crucial factor for the growth of Mucorales but host traps the iron by serum proteins to create crisis for the survival of pathogens which is a universal defense mechanism [62]. But potential pathogens use different strategies to host defense to grow inside host.
Pathogenesis of Mucormycosis. It depicts the difference of Mucorales invasion in healthy individual in comparison to patients with underlying disease conditions or immunocompromised population. A. displays the healthy individual with no sign of disease. (i) First with the exposure to the spores of Mucorales to host; macrophages getting activated and phagocytosed the spores. The germination process recruits neutrophils and kills the Mucorales. (ii) Bood vessels showing no sign of invasion with active immune system, maintained blood glucose, balanced acid base system and well-proportioned Fe pool with active transferrin system. B. Explains the tissue invasion due to mucormycosis in presence of different predisposing factors. (i) Exposure of spores to the unhealthy or immunocompromised individual. Due to lack of active immune system, the spores getting germinated and invade into the endothelium. (ii) In presence of high glucose concentration; iron binding proteins and storage proteins get glycated and become non-functional. When there is acidosis, the pH is disturbed, and the functionality of transferrin is also affected. Increase in the Fe pool does augment growth and germination. The CotH present on the spores get interacted to over expressed GRP78 and helps in tissue invasion. Siderophore mediated transport of Fe is increased in deferoxamine treated individuals. The expression of Ftr1 and Fob1/2 is increased to assimilate Fe to grow and invade. Further growth of Mucorales cause thrombosis and tissue necrosis
After entering into the host, macrophages come into force, they engulf the spores and kills by the oxidative stress. In case, the spores escape the killing strategy of macrophage and able to germinate; the process induce the chemotaxis of neutrophils to damage the germinated hyphae by reactive oxygen species, perforins, cationic peptides, peptidases. Neutrophils also attract other inflammatory cells by secreting TNF alpha, interferon gamma and interleukins1b [63]. They inhibit the germination of spores of R. oryzae in human macrophages [64]. Expression of toll like receptors are enhanced in mucormycosis infection and identify the bound pathogen by pathogen-associated molecular pattern; thus, induces immunological reaction. [65]
Natural killer cells usually kill the hyphal structure by its direct cytotoxic effect or by immunomodulatory action by secreting tumor necrosis factor alpha, interferon gamma and granulocyte macrophage colony stimulating factor [66, 67]. Platelets are also secreting fungicidal cytokines and chemokines to contribute its effectiveness towards killing the pathogen. It also activates the other immunomodulatory cells to cause immunological reaction cascade [39, 45]. Platelets also suppress hyphal growth by aggregation and clot formation. Platelet derived growth factor receptor has been shown to induce endocytosis of fungal pathogen. This platelet derived growth factor pathway is induced in different disease conditions such as in candidiasis. Treatment with inhibitors for platelet derived growth factors reduces endothelial damage and angioinvasion. [68] At certain conditions, the host immunity is not enough and thus pathogens escape the barriers to cause the deadly outcome. These factors and conditions that induce disease progression are known as risk factors. Individual risk factors are described in details in the later part.
The spore size (3–11 micron) is the determinant factor for the site and disease progression. The bigger size spores entrap in the paranasal sinuses or else it invades to lungs and the invasive disease occurs [42]. The spores also secrete lytic enzymes and metabolites such as alkaloids and mycotoxins causes’ tissue invasion and lower the host response [61]. The composition of the spore wall appears to affect phagocyte recognition and intracellular killing. Rhizopus spp. suppress mouse alveolar macrophage phagosome maturity via melanin on the spore surface and iron metabolism, and both of these have vital functions in immune defense modulation [45]. Spores adhere to the extra cellular matrix (ECM) protein in the basement membrane, laminin and collagen –IV. Host invasion by the spores is due to the role of multiple lipolytic or glycosidic enzymes such as proteases and subtilase etc. [31] Mucorales fungi promote fungal penetration into airway epithelial cells by activating EGFR signaling. After R. arrhizus var. delemar infection in mice, survival was markedly improved by inhibiting EGFR signaling using already FDA-approved medications. [69]
Hemolysis and invasion are the signature symptoms of the disease. Pathogens utilize CotH protein to interact with endothelial receptor GRP78 (Glucose Receptor Protein). Mucorales now get entered into endothelial cells after binding to GRP78 protein [61, 70, 71]. The CotH protein is expressed in higher copy numbers in potential pathogenic species such as Rhizopus, Mucor, Lichtheimia etc. than in other species [35]. In Mucormycosis, the binding of CotH to GRP78 protein facilitates endocytosis of the fungus into the endothelial cells and causes injury (Fig. 3). [70, 71] Mucorales use this specific strategy that results in tissue invasion and destruction as opposed to other organisms that cause invasive diseases such as Candidiasis and Aspergillosis. The CotH protein is the determinant factor for pathogen invasion and clinical representation. Those with hematologic malignancies can develop lung infections from inhaling Rhizopus spores, whereas DKA patients are far more likely to develop rhino orbital/cerebral mucormycosis. R. delemar interacts with GRP78 and CotH3 to invade and damage nasal epithelial cells, potentially leading to rhino orbital /cerebral mucormycosis. These two proteins are highly expressed during uncontrolled DM [71]. But in case of hematological malignancies, Epidermal growth factor receptor (EGFR) is stimulated and host cell invasion results when R. delemar CotH7 identifies integrin 1 as a receptor on alveolar epithelial cells [72]. Suppression of GRP78 by short hair pin RNA (ShRNA) or by antibodies leads to block the fungal invasion. R. oryzae mutant with reduced CotH expression was impaired for invading and damaging endothelial cells and CHO cells overexpressing GRP78 [42, 70, 72]. Treatment with anti-CotH Abs abolished the ability of R. oryzae to invade host cells and protected DKA mice from mucormycosis [40, 56]. Anti-CotH3 antibodies were administered passively, which increased neutrophil infiltration and caused R. delemar to be killed through Fc receptor-mediated accelerated opsonophagocytic or by increasing macrophage killing ability [40, 73]. Immunosuppressed mice were also shielded against R. delemar and other Mucorales by monoclonal antibodies produced against the CotH3 peptide, which likewise worked in concert with antifungal medications to shield DKA animals from infection. [40, 56]
Further, Fe availability is a crucial factor for the progress of pathogenesis. Fe is a double-edged sword as its higher concentration could lead to tissue injury by Fenton / Weiss reaction [74]. Hence, in the mammalian host, iron concentration is maintained in a narrow range. Mucorales obtain Fe from host via several mechanisms. Patients with a high Fe pool in their blood are more susceptible to R. oryzae and other zygomycetes, but not candida or aspergillus [42]. R. oryzae needs exogenous iron supplements for growth and survival in vitro. [75, 76] Iron starvation created by chelators such as deferiprone or by deferasirox became lethal to the pathogen in vitro as well as in Rhizopus-infected mice [76]. The treatment has improved the survival, reduced fungal burden and induced the host response. Further it was found that Fe starvation leads to apoptosis in R. oryzae. [77, 78] Thus, Iron starvation could be an adjuvant therapy for this infection. In general, three major systems exist in fungi to transport and assimilate Fe. These systems are as follows: 1. A reductive iron uptake system, which involves the conversion of ferric to ferrous and transport via permeases. 2. A siderophore permease: aids in Fe assimilation via siderophore. 3. An assimilation system for Fe from haemin. [31]
In iron abundance, fungi obtain Fe by using low-affinity iron reductase and permease complex while in the Fe depletion, the fungi assimilate this major nutrient by high affinity Fe reductase-permease complex. The system comprises of 1. surface reductase Fre (a plasma membrane ferric reductase encoded by FRE genes) which reduce Fe3+ to Fe2+, (2) Fet3 is an iron transport multicopper ferroxidase encoded by FET3 genes which oxidizes Fe2+ to Fe3+, and (3) Ftr1 is a high affinity iron permease encoded by FTR1 gene which transport Fe3+ inside the fungal cell [31, 36, 71]. High affinity iron permease (FTR1) is highly expressed in R. oryzae during murine infection model [36, 79]. Inhibition of FTR1 expression by RNA interference or gene disruption leads to decrease in the virulence property of fungus in the animal model [31]. Anti-Ftr1p passive immunotherapy protected the DKA mice from mucormycosis [31, 36]. Siderophore mediated iron assimilation is seen in the Fe starvation environment. Siderophore mediated Fe acquisition occurs by different mechanisms, i) Direct transfer of Fe across the membrane of fungus without entrance of siderophore to the cell. ii) Direct transfer of Fe through plasma membrane after reducing ferric to ferrous; occurs in xenosiderophores. iii) Uptake of whole siderophore into the fungus cell [75]. After entering the complex, iron is reduced and stored, whereas siderophores readily enter the environment to obtain more iron for the pathogen [80, 81]. Rhizopus is known to secrete rhizoferrin, a siderophore that supplies iron through a receptor-mediated and energy-dependent process [81]. Studies revealed R. oryzae has 13 possible siderophore permeases which could act as receptors for siderophores, including rhizoferrin or deferoxamine [80]. A third mechanism by which fungi can obtain iron from the host via heme. Rhizopus has 2 homologous of heme oxygenase; probably assist the organism to obtain iron from the host and also evidence for angioinvasion. Indirect evidence suggests that Ftr1 gene expression promotes Fe uptake in R. oryzae for direct Fe assimilation from Heme or by siderophore mediated transport. [36, 71]
In Diabetic keto acidosis patients, the blood pH reduces due to acidosis which impairs the ability of Fe binding protein to bind the free iron (Fig. 2). In presence of high glucose concentration; iron binding proteins and storage proteins get glycated and become non-functional. Again, in acidosis the pH is disturbed, and the functionality of transferrin is also affected [19, 82]. Increase in the Fe pool does augment growth and germination. Iron chelation could somehow manage the disease status, for that Deferoxamine was first taken as therapeutics but later was found to be ineffective. Deferoxamine actually increases the fungi's ability to absorb iron as it acts as pathogens own siderophores [80]. Deferasirox, an iron chelator that was recently licensed by the American Food and Drug Administration to treat transfusion-related iron overload, also demonstrated significant in vitro activity and in vivo efficacy in mice against clinical isolates of Mucorales. [83]
The CotH present on the spore germlings get interacted to over expressed GRP78 and helps in tissue invasion. The expression of Ftr1 and Fob1/2 is increased to assimilate Fe to grow and invade. Further growth of Mucorales cause thrombosis and tissue necrosis. Thus, Fe availability increases for the pathogen to survive and grow. Hyperglycemia and ketoacidosis are two important predisposing factors for the mucormycosis infection. Globally, diabetes population is increasing and India is getting the 2nd position from top, hence the risk and rate of infection is increasing which is seen in the COVID 19 super infection [5, 19]. Mucormycosis has become a significant invasive fungal infection in the past 20 years in solid organ transplant (SOT) and hematopoietic stem cell transplant (HSCT) recipients, where it frequently has an aggressive clinical course and high mortality rates [5]. The potent T cell-depleting medications and/or antibodies used for immunosuppression in transplant recipients are to blame for the groups' elevated risk of invasive fungal infection, despite the presence of multiple risk factors in these two populations [84]. The rate of mucormycosis infection global Vs India is discussed in the epidemiology section. Other than these, organ transplantation, cancer, immunosuppressive drugs, long term ICU stay and treatment with deferoxamine also predisposes to Mucormycosis infection (Fig. 2).
Clinical features in Mucormycosis
The hallmark of the disease is tissue necrosis from angioinvasion and subsequent thrombosis. Based on the affected tissue sites, clinical forms are basically divided into 6 forms (Fig. 2).
Rhino-Orbito-Cerebral Mucormycosis (ROCM)
This is the most common form of disease associated with diabetes mellitus [85]. It is also reported in the patients with malignancies, recipients of hematopoietic stem cells or solid organ transplantation. Rarely, protracted neutropenia patients or those who had solid organ transplants have also had rhino-cerebral mucormycosis. The use of steroids for graft-versus-host disease has been substantially linked to such disease conditions. Infection develops after the inhalation of fungal spores that allows the fungal spores to paranasal sinuses. Then the infection spreads to adjacent tissue sites like palate, cavernous and sphenoid sinuses, orbits and brain tissue. Necrosis of the tissue represents a black eschar which is a hallmark of ROCM. Initial signs of rhino cerebral mucormycosis include eye or face discomfort and facial numbness that are similar to those of sinusitis, then conjunctival suffusion, blurred vision, and soft tissue edema appear. If left untreated, infection often moves from the ethmoid sinus to the orbit, where it can cause proptosis and a loss of extraocular muscle function. Intracranial spread is indicated from cavernous sinus thrombosis, hemiparesis, altered mentation and focal seizures [86]. Magnetic Resonance (MR) imaging and Nasal endoscopy are quite essential for the diagnosis of disease and its progression [86]. ROCM is the commonest form seen in Indian population. The progressive ROCM invades to cranium increases the mortality rate. Diabetic population with sudden visual impairment with headache will be sign for mucormycosis and with the help of imaging studies, the earliest diagnosis can be done to improve survival. [87]
Pulmonary Mucormycosis
It occurs mostly in neutropenic or in cancer population undergoing chemotherapy and population with HSCT. The overall mortality rate is much higher globally; estimated around 76% [63]. Inhalation of spores or hematogenous or lymphatic spread can cause pulmonary mucormycosis [87]. The diagnosis procedure is mostly shadowed by invasive aspergillosis. The air crescent sign noticed in aspergillosis is also shared by mucormycosis infection. The symptoms of the disease are high grade fever, nonproductive cough or hemoptysis, pleuritic chest pain and dyspnea. Endobronchial and tracheal lesions are found in diabetes with pulmonary mucormycosis [88]. It causes blocked airways, lungs collapsing, invasion with hilar blood vessels and resulted hemoptysis. It also invades the adjacent tissue area such as media sternum, pericardium and chest wall. [85, 87]
Cutaneous Mucormycosis
It results from the direct inoculation of fungal spores into the skin through trauma or wounds [89]. The entry of the fungi into skin could be intravenous catheter, insulin injection sites, contaminated wounds, burns, or surgical sites [90]. It could be natural or hospital acquired. The common affected areas for the disease are arms and legs. Other areas that can be affected includes back, scalp, face, thorax, abdomen, breast, perineum and gluteal region. It affects skin or subcutaneous tissue, deeply to muscle, tendons or bones and it also disseminated when involves other noncontiguous organs [63]. The onset of primary cutaneous mucormycosis is gradual, slowly progressing disease leading to gangrene and dissemination. Initially plaques are noticed. Then these plaques are necrotic and erythematous halo to eschar formation accompanied with surrounding erythema and induration. [63]
Gastrointestinal Mucormycosis
It is a rare form of the disease, accounting for only 7% of cases. However, it has a high mortality rate (85%) if the diagnosis or antifungal therapy is delayed [63]. This is most common in neonates, malnourished children, and patients receiving corticosteroid therapy. The infection is spread through the consumption of contaminated food. It can affect any part of the GIT, but the stomach is the most commonly infected site, followed by the colon and ileum [63, 91]. The disease can cause necrotizing enterocolitis in neonates and mass-like appendicitis or illeal lesions in neutropenic patients. Mucormycosis of the gastrointestinal tract can spread to the liver, spleen, or pancreas. Mucorales infiltrate the bowel walls and blood vessels, causing bowel perforation, peritonitis, sepsis, and gastrointestinal hemorrhage. Radiological modalities such as CT scan or MRI of the abdomen revealing mucosal wall thickening and reactive lymphadenopathy not specifically indicating gastric mucormycosis. Additional investigation such as Endoscopic or surgical biopsy of the lesions and EGD require to establish the diagnosis procedures. The ulcerated patchy mucosal lesions with greenish or greyish overlying exudate confirms the infection. A biopsy report of the lesion is essential to differentially diagnose the disease from gastric malignancy. [91]
Disseminated Mucormycosis
When mucor infection spread from one organ to other hematogenously is known as disseminated mucormycosis. Patients with iron overload, undergoing treatment with deferoxamine, patients with immune suppression/ neutropenia and leukemia are under the risk for dissemination. The incidence of disseminated mucormycosis is highest in pulmonary mucormycosis with neutropenic group, while dissemination less commonly originated from gastrointestinal tract, sinuses, or from cutaneous lesions. Brain is the common site for dissemination. Other common sites are listed as liver, spleen and heart. Disseminated mucormycosis has diverse symptoms as it involves range of tissue and organs. The mortality rate of this clinical form is high as 96%. [87, 89]
Uncommon form of Mucormycosis
It includes endocarditis, osteomyelitis, peritonitis and pyelonephritis. Endocarditis occurs primarily on and around the prosthetic valves and lead to aortic thrombosis. Osteomyelitis usually occurs after traumatic inoculation or in surgical methods. Renal mucormycosis is a common complication of disseminated mucormycosis. Isolated renal infection is common in immunocompromised hosts such as allograft recipients, HIV patients, patients with uncontrolled diabetes, cancer patients, and intravenous (IV) drug users. The symptoms are flank pain and fever. Isolated Renal mucormycosis in immunocompetent individual is extremely rare and has high mortality up to 77%. Few cases 11 cases in 10 year of time period were published by Chakrabarti et al. from India. China also showing these cases in different population. Isolated mucormycosis in mastoid, oral mucosa, bone, bladder, trachea, media sternum or in ear is also reported. [5, 63]
Diagnosis and Challenges
Early detection of pathogen could help in the timely treatment and manage the outcome of the disease. Diagnosis is a real challenge in mucormycosis as clinical presentation is over shadowed by other fungal and bacterial infections. It raises suspicion and initiates laboratory diagnosis; which includes identification of risk factors, evaluation of clinical symptoms, analysis of imaging modalities, and classical diagnostic procedures such as histology, cultures, and cutting-edge molecular technologies.
Imaging
For suspected pulmonary mucormycosis, pulmonary CT scan is recommended for the detection of the reversed halo sign, in diabetic population complaining about facial pain, swelling and sinusitis, proptosis or with ophthalmoplegia, both cranial CT and MRI is highly recommended. Nasal endoscopy is also recommended if sinusitis is present in the same population. [92]
Histopathology
It demonstrates the presence of fungus or its structure in the biopsies of affected tissues or BAL fluids with mucormycosis [93]. Grocott methenamine-silver and Periodic acid Schiff PAS stains are used for visualization of pathogen. It is a vital method as it detects the fungus as pathogen in the specimen and define the invasion stage of the disease. It also further unravels the presence of co infection in the given sample [6, 92]. The positron emission tomography-computer tomography with [18F]-fluorodeoxyglucose is an emerging imaging method for the early management and diagnosis. Endobronchial ultrasound-guided fine needle aspiration is also a potential method to detect mucormycosis in suspected individuals. [94]
Direct Microscopy
For a direct microscopy, KOH wet mounts are used. Staining with blankophor or calcofluor white with KOH, enhances the visualization of fungal structure [95]. It is an inexpensive but a productive method used for rapid diagnosis when used along with histopathology; this is strongly recommended by European Confederation of Medical Mycology/Mycoses Study Group Education & Research Consortium ECMM/MSGERC [94, 96, 97]. Histochemistry methods preferably using monoclonal Ab against mucoromycetes to aid diagnosis procedure and also differentiated the infections from invasive aspergillosis.
Culture Method
This method identifies the pathogen at its genus and species level. After the growth of particular culture organism; the pathogen is identified by its colonial, growth temperature profile and by microscopic characterization. MALDI-TOF/TOF is a promising method for the identification of the cultured pathogen. This is a potential method to identify the mold by species specific approach; it is a simple, reproducible and cost-effective method to diagnose the disease at earliest. The databases which are used should be more expanded and validated. [94, 98]
These are few methods are used for the identification of the Mucorales in the infection. There are several pitfalls in the above methods for the accurate identification. But molecular methods are recommended for high specificity and accuracy and remains as gold standard for diagnosis.
Emerging molecular Methods
Molecular methods are emerging as a robust diagnostic tool for the identification of pathogen at species level. Molecular detection of Mucorales is now up to the DNA based approach. PCR based methods for amplification of specific target sequence and identification is getting the priority. This includes PCR, nested PCR, real time PCR, nested PCR combined with RFLP, the use of high-resolution melt analysis in qPCR use of micro arrays and ESI MS coupled to PCR are increasing specificity and reduces false positive results. Most of the molecular tests target the 18S ribosomal RNA genes, 28SrDNA, mitochondrial gene rnl, cytochrome b gene and CotH gene. Further, use of pan fungal primers that target ITS genomic region with the PCR amplified DNA, or multiplex PCR using specific primers targeting a restricted number of Mucorales genus and species are identifying exact species with accuracy. It is reliable and recommended as first line method for species detection among Mucorales. [98, 99]
Few evolutionary methods are on its way for development and under investigations. Whole genome sequencing has been useful for molecular typing and early diagnosis by using cell free DNA in blood [100]. Recognition of fucomannan from clinical sample such as serum, BAL fluid or from urine acts a biomarker for an early detection of disease. A lateral flow assay has been developed for the detection of the fucomannan in mucormycosis infections; and successfully detected the same in DKA mice model infected with various Mucorales [101]. Dihexasaccharide in serum (MS-DS) is also detected in mucormycosis affected cases via MALDI-TOF method and can be utilized as a biomarker for early diagnosis [101]. CotH which is a key protein present in mucoromycetes and absent in other invasive fungi and can be taken as a biomarker for the detection of mucormycosis infection [42]. Breath volatile metabolites assay by thermal desorption gas chromatography or by tandem mass spectrometry detects exogenous fungal volatile metabolites in the breathing [6, 88, 101]. Further, CRISPR-Cas method is another promising method for future diagnostic approach. Till now, this method is restricted to detect viruses. Genome editing procedures have been adapted in Mucor species by using CRISPR-Cas system. The whole system includes a specific single stranded nucleic acid and an enzyme with endonuclease activity [100]. Introduction of the above technique for diagnosis would be reliable, inexpensive, rapid with easy application procedure.
Current Treatment, Resistance pattern and new ways to come
Antifungals
There are few antifungals available to treat mucormycosis which have reported anti-Mucorales activity. Amphotericin B, triazoles such as Posaconazole and isavuconazoles are recommended for treatment while echinocandins are still under investigations. European conference on infection in Leukemia (ECIL) in 2017 and European Confederation of Medical Mycology (ECMM) in 2019 strongly recommends liposomal Amphotericin B (L-AMB) drug as the first line of treatment option for adult. Amphotericin B lipid complex (ABLC) is also a treatment option for mucormycosis without CNS involvement; recommended by ECIL. L-AMB and ABLC both are strongly recommended when pediatrics and neonates are associated [102, 103]. Recently, Isavuconazole (ISZ), a new drug approved in US and in Europe in 2015 for the treatment of Mucormycosis [104]. It is as effective as Amphotericin B in decreasing the fungal load and improving survival in infected murine model. A retrospective study also emphasized on the use of ISZ as effective therapeutics in the mucormycosis cases where CNS is involved due to is efficiency in crossing blood brain barrier [105]. Recent global guidelines recommends the use of ISZ as first line of therapy with moderate strength and highly recommended for salvage therapy in adults with renal complications and in children [5]. Posaconazole (PSZ) with broad spectrum antifungal activity against Mucorales and used as salvage therapy. Intravenous and Delayed release tablets of PSZ drug have been improved to have better bioavailability and recommended moderately by ECMM as first line of treatment procedure. They are usually taken as salvage and step-down therapy in mucormycosis [87, 106].
Surgery
Earlier attainment of aggressive removal of necrotic tissue through surgical procedure provides improve survival in rhino-cerebral and pulmonary mucormycosis. Surgical procedures prior to systemic dissemination of infection are helpful to improve the clinical outcomes. Surgery with high-dose of systemic antifungals is improving survival rate. Surgery in Rhino cerebral mucormycosis is often removal of all necrotic tissue resulting disfigurement such as removal of palate, nasal cartilage and sometimes orbit. However, with recent improvement in the surgical procedure with endoscopic debridement is associated with removal of only affected necrotic tissue. Patients with unifocal pulmonary infection can be benefitted with lobectomy or pneumonectomy. Surgical procedure is avoided in case of critically ill patients and in case of multifocal lesions or close to large vessels, or in case of thrombocytopenia. [102]
Adjuvants
In case of diabetes mellitus and ketoacidosis, reversal of immunosuppression, optimizing underlying medical conditions, reduction of neutropenia using hematopoietic growth factors, granulocyte stimulating factor, quick administration of antifungals and treatment with hyperbaric oxygen are recommended by ECMM and ECIL-6, while iron chelators are strongly discouraged.
New therapeutics
Few are under clinical trial procedures that include Rezafungin, SCY-078, Olorofim and encochleated amphotericin B.[102, 107, 108] VT1161 is a novel drug exhibiting in vitro antifungal activity against Mucorales such as R. oryzae, Cunninghamella and Lichtheimia by inhibiting fungal CYP51 [102]. A broad spectrum triazole SCH 42427 is showing antifungal activity against Mucorales in murine infection model. APX001A, otherwise known as fosmanogepix is proved to be an antifungal; targeting Gtw1 protein of fungus [109]. PC1244 is another novel antifungal displaying antifungal efficient activity against Mucorales while colistin is presenting modest performances. Nystatin-intralipid exhibited excellent antifungal activity against Mucorales in G. mellonella infection model [110]. As the mucormycosis is a disease complex involving multiple species, different clinical presentation and varied underlying conditions; studies are not always univocal about a certain drug. Certainly, few mechanisms are vital for the growth and invasion strategies of Mucorales and new therapeutics is underway. CotH targeting antibodies are efficient to prevent endothelial invasion as it is a role player in the above process. Antibodies against CotH also improve the survival of neutropenic and diabetic murine infection model. GPR-78 specific immune serum may protect the diabetic mice from mucormycosis [70]. Genetic modified cytotoxic T-cells are specifically targeting beta glucan of fungal cell wall. This strategy has not yet implicated in mucor infection model but tested in aspergillosis infection. [111]
Resistance Pattern
Mucorales exhibits intrinsic resistance towards all recommended antifungals in current therapy except Amphotericin B. This innate trait could be linked to wide spread duplication of genome region and whole genome in Mucorales [112]. Sequence alignment of lanosterol 14 alpha –demethylase CYP51-F5 proteins suggested substitution at the position Y29F results into resistance towards short tailed triazoles [93]. Upregulation of multidrug transporters could be another reason for innate resistance property. Drug resistance in M. circinelloides is well associated with pleiotropic drug resistance transporter (PDR), belonging to ABC transporters. Genes such as pdr1and pdr2 are involved in resistance towards isavuconazole, ravuconazole and posaconazole. Deletion of ABC transporter genes in M. lusitanicus increases susceptibility towards triazoles. Another study is suggestive towards epigenetic acquired modification in the specific gene for transient resistance shown in murine infection model; study also stated that murine infection is enhancing the process of epimutations. However, the resistance mechanism must be studied both in vivo and in vitro systems to confirm the resistance pattern and also get the idea for future targets. [100]
Conclusion
Mucormycosis is a neglected disease yet the fatal and invasive one. During the outbreak of COVID- 19, this superinfection gets much public attention. Mucor is ubiquitously available in the environment and usually harmless to immunocompetent individuals. But the patients with weakened immune system or with underlying disease conditions such as malignancies, diabetes, receiving steroids/ immunosuppressant are at higher risk to get infected with Mucorales to suffer with fatal consequences. India is getting higher attention because it gets 70 times higher than global disease toll. India is a tropical country; the warmer environment, dense and poorly controlled hospital set ups during COVID-19 infections, spurred diabetic populations, misuse and wide spread use of steroids/antibiotics, mucosal damage by COVID 19 virus itself cause rapid progress of the disease. But India’s statistics before COVID 19 was also high in compared to other parts of globe. Therefore, India needs a special attention for the therapeutics as well as in the prevention of the infection. But due to paucity of research in fungal disease in comparison to bacterial, parasitical and viral disease leave the clinicians with very few options for treatment and diagnosis of the disease. Although rate of infection is high in India, only few research groups are involved in the fungal research. Till date serological biomarker or any test are unavailable for rapid and easy diagnosis, leading to delayed treatment and high mortality. The management of Mucormycosis is another challenge to overcome; surgical debridement of infected and necrotic tissue is essential to survival but this can lead to visual loss and severe disfigurement. The only antifungal that is effective and available globally is Amphotericin B; but it has nephrotoxicity towards host. Improved liposomal formulation of Amphotericin B and few others such as Azaconazole and Posaconazole are also found to be useful in therapeutic purpose; but availability and affordability of these antifungals are raising concerns for people across the globe. The current situation presents both a challenge and an opportunity for the researcher to learn about the epidemiology, pathophysiology and pattern of resistance towards existing antifungals. It's also the right moment to develop a rapid and reliable diagnosis tool for mucormycosis. Research on new antifungals has to be accelerated in order to combat this deadly disease.
Data Availability
COVID-associated mucormycosis cases in different states and UTs of India till Nov 2021 are used in the manuscript, Data available at: Government of India Ministry of Health and Family Welfare.
References
World Health Organization. COVID-19 Weekly Epidemiological Update. World Heal Organ. 2022;(August):1–33. https://www.who.int/publications/m/item/covid-19-weekly-epidemiological-update
Bardi T, Pintado V, Gomez-Rojo M et al (2021) Nosocomial infections associated to COVID-19 in the intensive care unit: clinical characteristics and outcome. Eur J Clin Microbiol Infect Dis 40(3):495–502. https://doi.org/10.1007/s10096-020-04142-w
Kumar M, Sarma DK, Shubham S et al (2021) Mucormycosis in COVID-19 pandemic: Risk factors and linkages. Curr Res Microb Sci 2:100057. https://doi.org/10.1016/j.crmicr.2021.100057
Rocha ICN, Hasan MM, Goyal S et al (2021) COVID-19 and mucormycosis syndemic: double health threat to a collapsing healthcare system in India. Trop Med Int Heal 26(9):1016–1018. https://doi.org/10.1111/tmi.13641
Prakash H, Chakrabarti A (2019) Global epidemiology of mucormycosis. J Fungi. 5(1). https://doi.org/10.3390/jof5010026
Skiada A, Pavleas I, Drogari-Apiranthitou M (2020) Epidemiology and diagnosis of mucormycosis: An update. J Fungi 6(4):1–20. https://doi.org/10.3390/jof6040265
Prakash H, Ghosh AK, Rudramurthy SM et al (2019) A prospective multicenter study on mucormycosis in India: epidemiology, diagnosis, and treatment. Med Mycol 57(4):395–402. https://doi.org/10.1093/mmy/myy060
Jeong W, Keighley C, Wolfe R et al (2019) The epidemiology and clinical manifestations of mucormycosis: a systematic review and meta-analysis of case reports. Clin Microbiol Infect 25(1):26–34. https://doi.org/10.1016/j.cmi.2018.07.011
Lewis RE, Cahyame-Zuniga L, Leventakos K et al (2013) Epidemiology and sites of involvement of invasive fungal infections in patients with haematological malignancies: A 20-year autopsy study. Mycoses 56(6):638–645. https://doi.org/10.1111/myc.12081
Kontoyiannis DP, Yang H, Song J et al (2016) Prevalence, clinical and economic burden of mucormycosis-related hospitalizations in the United States: A retrospective study. BMC Infect Dis 16(1):1–6. https://doi.org/10.1186/s12879-016-2023-z
Zilberberg MD, Shorr AF, Huang H, Chaudhari P, Paly VF, Menzin J (2014) Hospital days, hospitalization costs, and inpatient mortality among patients with mucormycosis: A retrospective analysis of US hospital discharge data. BMC Infect Dis 14(1):1–10. https://doi.org/10.1186/1471-2334-14-310
Torres-Narbona M, Guinea J, Martínez-Alarcón J, Muñoz P, Gadea I, Bouza E (2007) Impact of zygomycosis on microbiology workload: A survey study in Spain. J Clin Microbiol 45(6):2051–2053. https://doi.org/10.1128/JCM.02473-06
Guinea J, Escribano P, Vena A et al (2017) Increasing incidence of mucormycosis in a large Spanish hospital from 2007 to 2015: Epidemiology and microbiological characterization of the isolates. PLoS ONE 12(6):1–10. https://doi.org/10.1371/journal.pone.0179136
Bitar D, Van Cauteren D, Lanternier F et al (2009) Increasing incidence of zygomycosis (mucormycosis), France, 1997–2006. Emerg Infect Dis 15(9):1395–1401. https://doi.org/10.3201/eid1509.090334
Ambrosioni J, Bouchuiguir-Wafa K, Garbino J (2010) Emerging invasive zygomycosis in a tertiary care center: Epidemiology and associated risk factors. Int J Infect Dis 14(SUPPL. 3):e100–e103. https://doi.org/10.1016/j.ijid.2009.11.024
Saegeman V, Maertens J, Meersseman W, Spriet I, Verbeken E, Lagrou K (2010) Increasing incidence of Mucormycosis in university hospital. Belgium Emerg Infect Dis 16(9):1456–1458. https://doi.org/10.3201/eid1609.100276
Dolatabadi S, Ahmadi B, Rezaei-Matehkolaei A et al (2018) Mucormycosis in Iran: A six-year retrospective experience. J Mycol Med 28(2):269–273. https://doi.org/10.1016/j.mycmed.2018.02.014
Ueno R, Nishimura S, Fujimoto G, Ainiwaer D (2021) The disease burden of mucormycosis in Japan: results from a systematic literature review and retrospective database study. Curr Med Res Opin 37(2):253–260. https://doi.org/10.1080/03007995.2020.1846510
Rammaert B, Lanternier F, Poirée S, Kania R, Lortholary O (2012) Diabetes and mucormycosis: A complex interplay. Diabetes Metab 38(3):193–204. https://doi.org/10.1016/j.diabet.2012.01.002
Chakrabarti A, Singh R (2014) Mucormycosis in India: Unique features. Mycoses 57(s3):85–90. https://doi.org/10.1111/myc.12243
Xess I, Mohapatra S, Shivaprakash MR et al (2012) Evidence implicating Thamnostylum lucknowense as an etiological agent of rhino-orbital mucormycosis. J Clin Microbiol 50(4):1491–1494. https://doi.org/10.1128/JCM.06611-11
Prakash H, Singh S, Rudramurthy SM et al (2020) An aero mycological analysis of Mucormycetes in indoor and outdoor environments of northern India. Med Mycol 58(1):118–123. https://doi.org/10.1093/mmy/myz031
Pamidimukkala U, Sudhaharan S, Kancharla A et al (2021) Mucormycosis due to Apophysomyces species complex- 25 years’ experience at a tertiary care hospital in southern India. Med Mycol 58(4):425–433. https://doi.org/10.1093/MMY/MYZ081
Sahu RK, Salem-Bekhit MM, Bhattacharjee B, et al. (2021) Mucormycosis in indian covid-19 patients: Insight into its patho-genesis, clinical manifestation, and management strategies. Antibiotics. 10(9). https://doi.org/10.3390/antibiotics10091079
Ghazi BK, Rackimuthu S, Wara UU et al (2021) Rampant increase in cases of mucormycosis in India and Pakistan: A serious cause for concern during the ongoing COVID-19 pandemic. Am J Trop Med Hyg 105(5):1144–1147. https://doi.org/10.4269/ajtmh.21-0608
Stone N, Gupta N, Schwartz I (2021) Mucormycosis: time to address this deadly fungal infection. The Lancet Microbe 2(8):e343–e344. https://doi.org/10.1016/S2666-5247(21)00148-8
Yasmin F, Najeeb H, Siddiqui SA, Ochani RK (2022) Mucormycosis and COVID-19: Double trouble for India? J Glob Health 12:3–7. https://doi.org/10.7189/jogh.12-03001
Kwon-Chung KJ (2012) Taxonomy of fungi causing mucormycosis and entomophthoramycosis (zygomycosis) and nomenclature of the disease: Molecular mycologic perspectives. Clin Infect Dis. 54(SUPPL. 1). https://doi.org/10.1093/cid/cir864
Borkar SG (2021) Mucormycosis: A Surge in Mucorales Fungal Infection in Post – Covid Patients in Indian States and Insight into Known and Unknown Factors. Int J Glob Heal 1(3):26–60. https://doi.org/10.14302/issn.2693-1176.ijgh-21-3907
Walther G, Wagner L, Kurzai O. (2019) Updates on the taxonomy of mucorales with an emphasis on clinically important taxa. J Fungi. 5(4). https://doi.org/10.3390/jof5040106
Ibrahim AS, Spellberg B, Walsh TJ, Kontoyiannis DP (2012) Pathogenesis of mucormycosis. Clin Infect Dis 54(SUPPL. 1):1–7. https://doi.org/10.1093/cid/cir865
Petraitis V, Petraitiene R, Antachopoulos C et al (2013) Increased virulence of Cunninghamella bertholletiae in experimental pulmonary mucormycosis: Correlation with circulating molecular biomarkers, sporangiospore germination and hyphal metabolism. Med Mycol 51(1):72–82. https://doi.org/10.3109/13693786.2012.690107
Nicolás FE, Murcia L, Navarro E, Navarro-Mendoza MI, Pérez-Arques C, Garre V (2020) Mucorales species and macrophages. J Fungi 6(2):1–12. https://doi.org/10.3390/jof6020094
Kaerger K, Schwartze VU, Dolatabadi S et al (2015) Adaptation to thermotolerance in Rhizopus coincides with virulence as revealed by avian and invertebrate infection models, phylogeny, physiological and metabolic flexibility. Virulence 6(4):395–403. https://doi.org/10.1080/21505594.2015.1029219
Soare AY, Watkins TN, Bruno VM (2020) Understanding Mucormycoses in the Age of “omics.” Front Genet 11(June):1–11. https://doi.org/10.3389/fgene.2020.00699
Ibrahim AS, Gebremariam T, Lin L et al (2010) The high affinity iron permease is a key virulence factor required for Rhizopus oryzae pathogenesis. Mol Microbiol 77(3):587–604. https://doi.org/10.1111/j.1365-2958.2010.07234.x
Laaberki MH, Dworkin J (2008) Role of spore coat proteins in the resistance of Bacillus subtilis spores to Caenorhabditis elegans predation. J Bacteriol 190(18):6197–6203. https://doi.org/10.1128/JB.00623-08
Chibucos MC, Soliman S, Gebremariam T et al (2016) An integrated genomic and transcriptomic survey of mucormycosis-causing fungi. Nat Commun 7:1–11. https://doi.org/10.1038/ncomms12218
Lax C, Pérez‐arques C, Navarro‐mendoza MI, et al. (2020) Genes, pathways, and mechanisms involved in the virulence of mucorales. Genes (Basel). 11(3). https://doi.org/10.3390/genes11030317
Gebremariam T, Alkhazraji S, Soliman SSM et al (2019) Anti-CotH3 antibodies protect mice from mucormycosis by prevention of invasion and augmenting opsonophagocytosis. Sci Adv 5(6):1–15. https://doi.org/10.1126/sciadv.aaw1327
Liu H, Lee MJ, Solis NV et al (2016) Aspergillus fumigatus CalA binds to integrin α5β1 and mediates host cell invasion. Nat Microbiol 2(November):1–10. https://doi.org/10.1038/nmicrobiol.2016.211
Baldin C, Ibrahim AS (2017) Molecular mechanisms of mucormycosis—The bitter and the sweet. PLoS Pathog 13(8):1–9. https://doi.org/10.1371/journal.ppat.1006408
Binder U, Maurer E, Lass-Flörl C (2014) Mucormycosis - from the pathogens to the disease. Clin Microbiol Infect 20(6):60–66. https://doi.org/10.1111/1469-0691.12566
Prakash H, Rudramurthy SM, Gandham PS et al (2017) Apophysomyces variabilis: Draft genome sequence and comparison of predictive virulence determinants with other medically important Mucorales. BMC Genomics 18(1):1–13. https://doi.org/10.1186/s12864-017-4136-1
Hassan MIA, Voigt K (2019) Pathogenicity patterns of mucormycosis: Epidemiology, interaction with immune cells and virulence factors. Med Mycol 57:S245–S256. https://doi.org/10.1093/mmy/myz011
Sugui JA, Christensen JA, Bennett JE, Zelazny AM, Kwon-Chung KJ (2011) Hematogenously disseminated skin disease caused by Mucor velutinosus in a patient with acute myeloid leukemia. J Clin Microbiol 49(7):2728–2732. https://doi.org/10.1128/JCM.00387-11
Shelburne SA, Ajami NJ, Chibucos MC et al (2015) Implementation of a pan-genomic approach to investigate holobiont-infecting microbe interaction: A case report of a leukemic patient with invasive mucormycosis. PLoS ONE 10(11):1–17. https://doi.org/10.1371/journal.pone.0139851
Lee SC, Li A, Calo S et al (2015) Calcineurin orchestrates dimorphic transitions, antifungal drug responses and host-pathogen interactions of the pathogenic mucoralean fungus Mucor circinelloides. Mol Microbiol 97(5):844–865. https://doi.org/10.1111/mmi.13071
Vellanki S, Billmyre RB, Lorenzen A, et al. (2020) A novel resistance pathway for calcineurin inhibitors in the human-pathogenic mucorales mucor circinelloides. MBio. 11(1). https://doi.org/10.1128/mBio.02949-19
Lee SC, Li A, Calo S, Heitman J. (2013) Calcineurin Plays Key Roles in the Dimorphic Transition and Virulence of the Human Pathogenic Zygomycete Mucor circinelloides. PLoS Pathog. 9(9). https://doi.org/10.1371/journal.ppat.1003625
Trieu TA, Navarro-Mendoza MI, Pérez-Arques C et al (2017) RNAi-Based Functional Genomics Identifies New Virulence Determinants in Mucormycosis. PLoS Pathog 13(1):1–26. https://doi.org/10.1371/journal.ppat.1006150
Papon N, Naglik JR, Hube B, Goldman GH (2021) Fungal pathogenesis: A new venom. Curr Biol 31(8):R391–R394. https://doi.org/10.1016/j.cub.2021.03.015
Soliman SSM, Baldin C, Gu Y et al (2021) Mucoricin is a ricin-like toxin that is critical for the pathogenesis of mucormycosis. Nat Microbiol 6(3):313–326. https://doi.org/10.1038/s41564-020-00837-0
Pérez-Arques C, Navarro-Mendoza MI, Murcia L, et al. (2019) Mucor circinelloides thrives inside the phagosome through an atf-mediated germination pathway. MBio. 10(1). https://doi.org/10.1128/mBio.02765-18
Navarro-Mendoza MI, Pérez-Arques C, Murcia L et al (2018) Components of a new gene family of ferroxidases involved in virulence are functionally specialized in fungal dimorphism. Sci Rep 8(1):1–13. https://doi.org/10.1038/s41598-018-26051-x
Gebremariam T, Liu M, Luo G et al (2014) CotH3 mediates fungal invasion of host cells during mucormycosis. J Clin Invest 124(1):237–250. https://doi.org/10.1172/JCI71349
Patiño-Medina JA, Maldonado-Herrera G, Pérez-Arques C et al (2018) Control of morphology and virulence by ADP-ribosylation factors (Arf) in Mucor circinelloides. Curr Genet 64(4):853–869. https://doi.org/10.1007/s00294-017-0798-0
Ribes JA, Vanover-Sams CL, Baker DJ (2000) Zygomycetes in human disease. Clin Microbiol Rev 13(2):236–301. https://doi.org/10.1128/CMR.13.2.236-301.2000
Li Z, Lu G, Meng G. (2019) Pathogenic fungal infection in the lung. Front Immunol. 10(JUL):1–20. https://doi.org/10.3389/fimmu.2019.01524
Radotra B, Challa S (2022) Pathogenesis and Pathology of COVID-Associated Mucormycosis: What Is New and Why. Curr Fungal Infect Rep 16(4):206–220. https://doi.org/10.1007/s12281-022-00443-z
Challa S (2019) Mucormycosis: Pathogenesis and Pathology. Curr Fungal Infect Rep 13(1):11–20. https://doi.org/10.1007/s12281-019-0337-1
Joseph A. Lemire; Joe J. Harrison & Raymond J. Turner. (2013) Antimicrobial activity of metals_ mechanisms, molecular targets and applications _ Nature Reviews Microbiology _ Nature Publishing Group. 11(June). https://doi.org/10.1038/nrmicro3028
Petrikkos G, Tsioutis C (2018) Recent Advances in the Pathogenesis of Mucormycoses. Clin Ther 40(6):894–902. https://doi.org/10.1016/j.clinthera.2018.03.009
Jorens PG, Boelaert JR, Halloy V, Zamora R, Schneider YJ, Herman AG (1995) Human and rat macrophages mediate fungistatic activity against Rhizopus species differently: In vitro and ex vivo studies. Infect Immun 63(11):4489–4494. https://doi.org/10.1128/iai.63.11.4489-4494.1995
Chamilos G, Lewis RE, Lamaris G, Walsh TJ, Kontoyiannis DP (2008) Zygomycetes hyphae trigger an early, robust proinflammatory response in human polymorphonuclear neutrophils through toll-like receptor 2 induction but display relative resistance to oxidative damage. Antimicrob Agents Chemother 52(2):722–724. https://doi.org/10.1128/AAC.01136-07
Posch W, Wilflingseder D, Lass-Flörl C (2020) Immunotherapy as an Antifungal Strategy in Immune Compromised Hosts. Curr Clin Microbiol Reports 7(3):57–66. https://doi.org/10.1007/s40588-020-00141-9
Pushparaj K, Kuchi Bhotla H, Arumugam VA et al (2022) Mucormycosis (black fungus) ensuing COVID-19 and comorbidity meets - Magnifying global pandemic grieve and catastrophe begins. Sci Total Environ 805:150355. https://doi.org/10.1016/j.scitotenv.2021.150355
Kovalenko M, Rönnstrand L, Heldin CH et al (1997) Phosphorylation site-specific inhibition of platelet-derived growth factor β-receptor autophosphorylation by the receptor blocking tyrphostin AG1296. Biochemistry 36(21):6260–6269. https://doi.org/10.1021/bi962553l
Watkins TN, Gebremariam T, Swidergall M et al (2018) Inhibition of EGFR signaling protects from mucormycosis. MBio 9(4):1–13. https://doi.org/10.1128/mBio.01384-18
Liu M, Spellberg B, Phan QT et al (2010) The endothelial cell receptor GRP78 is required for mucormycosis pathogenesis in diabetic mice. J Clin Invest 120(6):1914–1924. https://doi.org/10.1172/JCI42164
Lugito NPH, Cucunawangsih C (2021) How Does Mucorales Benefit from the Dysregulated Iron Homeostasis During SARS-CoV-2 Infection? Mycopathologia 186(6):877–882. https://doi.org/10.1007/s11046-021-00594-6
Alqarihi A, Gebremariam T, Gu Y et al (2020) GRP78 and integrins play different roles in host cell invasion during mucormycosis. MBio 11(3):1–18. https://doi.org/10.1128/mBio.01087-20
Lin L, Gebremariam T, Ibrahim AS. (2015) Passive immunization with CotH3 antibodies protects neutropenic mice from lethal mucormycosis through enhancing macrophage killing (MPF6P.660). J Immunol. 194(1_Supplement):202.18–202.18. https://doi.org/10.4049/jimmunol.194.supp.202.18
Galaris D, Barbouti A, Pantopoulos K (2019) Iron homeostasis and oxidative stress: An intimate relationship. Biochim Biophys Acta - Mol Cell Res 1866(12):118535. https://doi.org/10.1016/j.bbamcr.2019.118535
Ibrahim AS, Spellberg B, Edwards J (2008) Iron acquisition: A novel perspective on mucormycosis pathogenesis and treatment. Curr Opin Infect Dis 21(6):620–625. https://doi.org/10.1097/QCO.0b013e3283165fd1
Symeonidis AS (2009) The role of iron and iron chelators in zygomycosis. Clin Microbiol Infect 15(SUPPL. 5):26–32. https://doi.org/10.1111/j.1469-0691.2009.02976.x
Shirazi F, Kontoyiannis DP, Ibrahim AS (2015) Iron starvation induces apoptosis in rhizopus oryzae in vitro. Virulence 6(2):121–126. https://doi.org/10.1080/21505594.2015.1009732
Howard DH (1999) Acquisition, transport, and storage of iron by pathogenic fungi. Clin Microbiol Rev 12(3):394–404. https://doi.org/10.1128/cmr.12.3.394
Tabassum T, Araf Y, Moin AT, Rahaman TI, Hosen MJ (2022) COVID-19-associated-mucormycosis: possible role of free iron uptake and immunosuppression. Mol Biol Rep 49(1):747–754. https://doi.org/10.1007/s11033-021-06862-4
Larcher G, Dias M, Razafimandimby B, Bomal D, Bouchara JP (2013) Siderophore Production by Pathogenic Mucorales and Uptake of Deferoxamine B. Mycopathologia 176(5–6):319–328. https://doi.org/10.1007/s11046-013-9693-5
Alejandre-Castañeda V, Patiño-Medina JA, Valle-Maldonado MI et al (2022) Secretion of the siderophore rhizoferrin is regulated by the cAMP-PKA pathway and is involved in the virulence of Mucor lusitanicus. Sci Rep 12(1):1–16. https://doi.org/10.1038/s41598-022-14515-0
Khanna M, Challa S, Kabeil AS, et al. (2021) Risk of Mucormycosis in Diabetes Mellitus: A Systematic Review. Cureus. 13(10). https://doi.org/10.7759/cureus.18827
Ibrahim AS, Gebermariam T, Fu Y et al (2007) The iron chelator deferasirox protects mice from mucormycosis through iron starvation. J Clin Invest 117(9):2649–2657. https://doi.org/10.1172/JCI32338
Ponnaiah M, Ganesan S, Bhatnagar T, et al. (2022) Hyperglycemia and steroid use increase the risk of rhino-orbito-cerebral mucormycosis regardless of COVID-19 hospitalization: Case-control study, India. PLoS One. 17(8 August):1–17. https://doi.org/10.1371/journal.pone.0272042
Fouad YA, Abdelaziz TT, Askoura A et al (2021) Spike in Rhino-Orbital-Cerebral Mucormycosis Cases Presenting to a Tertiary Care Center During the COVID-19 Pandemic. Front Med 8(May):1–6. https://doi.org/10.3389/fmed.2021.645270
Pai V, Sansi R, Kharche R, Bandili SC, Pai B. (2021) Rhino-orbito-cerebral Mucormycosis: Pictorial Review. Insights Imaging. 12(1). https://doi.org/10.1186/s13244-021-01109-z
Spellberg B, Edwards J, Ibrahim A (2005) Novel perspectives on mucormycosis: Pathophysiology, presentation, and management. Clin Microbiol Rev 18(3):556–569. https://doi.org/10.1128/CMR.18.3.556-569.2005
Luo Z, Zhang L (2017) Diagnosis and treatment of pulmonary mucormycosis: A case report. Exp Ther Med 14(4):3788–3791. https://doi.org/10.3892/etm.2017.4986
Petrikkos G, Skiada A, Lortholary O, Roilides E, Walsh TJ, Kontoyiannis DP (2012) Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis 54(SUPPL. 1):23–34. https://doi.org/10.1093/cid/cir866
Castrejón-Pérez AD, Miranda I, Welsh O, Welsh EC, Ocampo-Candiani J (2017) Cutaneous mucormycosis. An Bras Dermatol 92(3):304–311. https://doi.org/10.1590/abd1806-4841.20176614
Naqvi H, Yousaf MN, Mills L. (2020) S3012 Gastric Mucormycosis; An Infection of Fungal Invasion Into the Gastric Mucosa in Immunocompromised Patients. Am J Gastroenterol. 115(1):S1587-S1587. https://doi.org/10.14309/01.ajg.0000714096.55092.bc
Garrido-Molina JM, Márquez-Hernández VV, Alcayde-García A et al (2021) 乳鼠心肌提取 HHS Public Access. J Hosp Infect 45(1):617–621. https://doi.org/10.1016/S1473-3099(19)30312-3.Global
Ponnaiyan D, Anitha C m., Prakash P s. g., et al. (2022) Mucormycosis diagnosis revisited: Current and emerging diagnostic methodologies for the invasive fungal infection (Review). Exp Ther Med. 25(1):1–6. https://doi.org/10.3892/etm.2022.11746
Sharma A, Goel A (2022) Mucormycosis: risk factors, diagnosis, treatments, and challenges during COVID-19 pandemic. Folia Microbiol (Praha) 67(3):363–387. https://doi.org/10.1007/s12223-021-00934-5
Afshar P, Larijani LV, Rouhanizadeh H (2018) A comparison of conventional rapid methods in diagnosis of superficial and cutaneous mycoses based on KOH, chicago sky blue 6b and calcofluor white stains. Iran J Microbiol 10(6):433–440
Lass-Flörl C (2009) Zygomycosis: Conventional laboratory diagnosis. Clin Microbiol Infect 15(SUPPL. 5):60–65. https://doi.org/10.1111/j.1469-0691.2009.02999.x
Frater JL, Hall GS, Procop GW (2001) Histologic Features of Zygomycosis. Arch Pathol Lab Med 125(3):375–378. https://doi.org/10.5858/2001-125-0375-hfoz
Mansoor S, Ahmed TI, Happa K, Sultan M, Manhas S, Shamas S (2022) Spectrum of Mucormycosis Before and During COVID-19: Epidemiology, Diagnosis, and Current Therapeutic Interventions. Curr Fungal Infect Rep 16(4):131–142. https://doi.org/10.1007/s12281-022-00438-w
Dadwal SS, Kontoyiannis DP (2018) Recent advances in the molecular diagnosis of mucormycosis. Expert Rev Mol Diagn 18(10):845–854. https://doi.org/10.1080/14737159.2018.1522250
Garre V (2022) Recent Advances and Future Directions in the Understanding of Mucormycosis. Front Cell Infect Microbiol 12(February):1–7. https://doi.org/10.3389/fcimb.2022.850581
Acosta-España JD, Voigt K. (2022) Mini Review: Risk Assessment, Clinical Manifestation, Prediction, and Prognosis of Mucormycosis: Implications for Pathogen- and Human-Derived Biomarkers. Front Microbiol. 13(June). https://doi.org/10.3389/fmicb.2022.895989
Brunet K, Rammaert B (2020) Mucormycosis treatment: Recommendations, latest advances, and perspectives. J Mycol Med 30(3):1–6. https://doi.org/10.1016/j.mycmed.2020.101007
Reed C, Bryant R, Ibrahim AS et al (2008) Combination polyene-caspofungin treatment of rhino-orbital-cerebral mucormycosis. Clin Infect Dis 47(3):364–371. https://doi.org/10.1086/589857
Natesan SK, Chandrasekar PH (2016) Isavuconazole for the treatment of invasive aspergillosis and mucormycosis: Current evidence, safety, efficacy, and clinical recommendations. Infect Drug Resist 9:291–300. https://doi.org/10.2147/IDR.S102207
Schwartz S, Cornely OA, Hamed K et al (2021) Isavuconazole for the treatment of patients with invasive fungal diseases involving the central nervous system. Med Mycol 58(4):417–424. https://doi.org/10.1093/MMY/MYZ103
Sun QN, Fothergill AW, McCarthy DI, Rinaldi MG, Graybill JR (2002) In vitro activities of posaconazole, itraconazole, voriconazole, amphotericin B, and fluconazole against 37 clinical isolates of zygomycetes. Antimicrob Agents Chemother 46(5):1581–1582. https://doi.org/10.1128/AAC.46.5.1581-1582.2002
Zhao Y, Perlin DS (2020) Review of the novel echinocandin antifungal rezafungin: Animal studies and clinical data. J Fungi 6(4):1–9. https://doi.org/10.3390/jof6040192
Rathod M, Patel JJ, Universiy P, et al. (2022) A Comprehensive Review on Mucormycosis A Comprehensive Review on Mucormycosis Dr Mrudangsinh Rathod a , Mr Jaydev Patel * a , Mr Manthan Prajapati a , Mr Madhav oza a. https://doi.org/10.35629/7781-0705883890
Shaw KJ, Ibrahim AS (2020) Fosmanogepix: A review of the first-in-class broad spectrum agent for the treatment of invasive fungal infections. J Fungi 6(4):1–21. https://doi.org/10.3390/jof6040239
Maurer E, Hörtnagl C, Lackner M et al (2019) Galleria mellonella as a model system to study virulence potential of mucormycetes and evaluation of antifungal treatment. Med Mycol 57(3):351–362. https://doi.org/10.1093/mmy/myy042
Seif M, Kakoschke TK, Ebel F et al (2022) CAR T cells targeting Aspergillus fumigatus are effective at treating invasive pulmonary aspergillosis in preclinical models. Sci Transl Med 14(664):1–9. https://doi.org/10.1126/scitranslmed.abh1209
Ma LJ, Ibrahim AS, Skory C, et al. (2009) Genomic analysis of the basal lineage fungus Rhizopus oryzae reveals a whole-genome duplication. PLoS Genet. 5(7). https://doi.org/10.1371/journal.pgen.1000549
Funding
Author S.P has received research support from ICMR-Postdoctoral fellowship (No. 3/1/3/PDF(25)/2021-HRD), ICMR, Govt. Of India.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design of the study. The first draft was prepared by Dr. Sunita Panda. It was reviewed by all the authors. The final draft was approved by all the authors.
Corresponding authors
Ethics declarations
Ethics Approval
Not Applicable
Consent to Participate
Not Applicable
Consent to Publish
Not Applicable
Competing Interest
The authors have no relevant financial or non-financial interest to disclose.
Additional information
Responsible Editor: Roxane M Piazza
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Panda, S., Sahu, M.C., Turuk, J. et al. Mucormycosis: A Rare disease to Notifiable Disease. Braz J Microbiol 55, 1065–1081 (2024). https://doi.org/10.1007/s42770-024-01315-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-024-01315-z