Abstract
Alzheimer’s disease (AD) is the leading cause of dementia in the aging population, but despite extensive research, there is no consensus on the biological cause of AD. While AD research is dominated by protein/peptide-centric research based on the amyloid hypothesis, a theory that designates dysfunction in beta-amyloid production, accumulation, or disposal as the primary cause of AD, many studies focus on metabolomics as a means of understanding the biological processes behind AD progression. In this review, we discuss mass spectrometry (MS)-based AD metabolomics studies, including sample type and preparation, mass spectrometry data acquisition strategies, and data analysis, as well as biological insights gleaned from these studies, with the hope of informing future AD metabolomic studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease (AD) is the most common cause of dementia in the aging population and the sixth-leading cause of death in the United States. Health care and hospice expenses related to AD cost the US nearly $305 billion in 2020 alone. Today, more than 5 million Americans suffer from AD; by 2050, this number is projected to grow to 13.8 million (Gaugler et al. 2019). Despite extensive research, there are currently no treatments that slow or stop AD development. As the burden of AD grows larger worldwide, there is an increasing need to diagnose AD early in its progression. Currently, AD diagnosis requires the patient to show significant behavioral and cognitive decline; studies have shown that by the time clinical symptoms are showing, cellular decline could already have been occurring for the past 20 years (Wilkins and Trushina 2018).
The biggest challenge within the world of AD is diagnosing individuals in the preclinical stage before the appearance of more severe, irreversible symptoms. Biomarker discovery is imperative to accurately diagnose AD patients; a reliable biomarker molecule must show a measurable change that can help distinguish healthy and ill patients and would ideally distinguish healthy control and AD during the mild cognitive impairment (MCI) phase of disease progression. Traditional biomarker discovery is often done through study of proteins (proteomics) or genes (genomics), but studying the metabolome reveals the patient phenotype, which includes both genotypic factors and environmental effects. Because metabolomics measures metabolites present at any given time, it can reveal the time-sensitive physiological state of a patient and dynamic responses to stimulus (Yeung 2018).
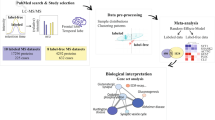
Many early AD biomarker studies used postmortem brain tissue as a source of biomarker molecules, but as brain tissue is not a viable sample source for biomarker screening in living patients, attention has turned to circulating biofluids. Mass spectrometry (MS) is a powerful, label-free technique that can be used for both metabolite identification and quantitation. In this review, we explore the past 5 years of MS-based AD biomarker research to reveal overlap between different biofluids and pathways that warrant additional research. It is worth mentioning that MS is not the only means to study the metabolome; nuclear magnetic resonance (NMR) spectroscopy is also commonly employed in metabolomic studies. In the interest of focusing on MS technique, NMR spectroscopy is outside the scope of this review article, but a detailed comparison of the two techniques can be found elsewhere (Emwas 2015).
Study Design
Patient Selection
Patient recruitment and selection is crucial to any disease research, but as AD is not definitively diagnosable until after death, choosing patients for an AD study comes with uncertainty (National Institute on Aging 2017). Definitive AD diagnosis requires a postmortem brain examination to confirm presence of amyloid plaques and tau tangles. Current antemortem diagnostic tools are fairly sensitive and specific, but one 2016 study of the National Alzheimer's Coordinating Center database showed about 11% of AD-diagnosed patients did not have AD pathology (e.g., amyloid plaques) confirmed by autopsy. Most misdiagnoses were either vascular dementia or Lewy body dementia, both of which have similar symptom presentation to AD. There were also false positive cases, where AD pathology was present postmortem but the patient had been misdiagnosed with another form of dementia (Qian et al. 2016).
In the studies included in this review, there is variation n AD diagnostic criteria. Two studies diagnosed their patients following the National Institute of Aging-Alzheimer’s Association (NIA-AA) criteria, while four chose to use the criteria issued by the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA). The NIA-AA criteria (Jack Jr et al. 2018) are the most recently updated criteria, as they were released in 2011 and updated in 2018; these criteria are an update to the NINCDS-ADRDA criteria (McKhann et al. 1984), which were issued in 1984. Yet another study utilized different criteria, the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) (Frances et al. 1995).
AD progresses on a spectrum, spanning from asymptomatic preclinical AD to mild cognitive impairment, and finally to AD dementia, and the NIA-AA criteria recognize this spectrum. The NINCDS-ADRDA criteria focuses on memory loss as the main symptom of AD dementia, but the NIA-AA criteria recognize that other symptoms, like loss of word-finding ability, are often impaired before memory loss occurs. Thus, NIA-AA can distinguish between AD dementia and other non-AD dementias more accurately.
Additionally, while it is unnecessary for diagnosis, the NIA-AA criteria recognize the potential of using biomarkers as a means of diagnosis. The NIA-AA accepted biomarkers for AD diagnosis consist of both imaging and biochemical biomarkers and can be sorted into three categories in what is called the AT(N) biomarker grouping: aggregated β-amyloid (Aβ), aggregated tau (neurofibrillary tangles), and neurodegeneration/neuronal injury (Table 1). An additional category, cognitive symptoms, is added for staging severity (Jack Jr et al. 2018). Biomarker technologies included in these criteria are limited but are flexible to allow for additions if new biomarkers are validated.
The aforementioned sets of criteria are similar in many ways, but their differences are not insignificant; a study comparing DSM-IV plus NINCDS-ADRDA to NIA-AA found that the two means of diagnosis agreed only 70% of the time on the same patient cohort (Dolci et al. 2017). There is a clear need for a more consistent definition of AD to ensure AD patients, and only AD patients, are included in AD-related studies.
Sample Type
Cerebrospinal Fluid (CSF)
Because AD is a neurological condition, CSF is one of the most widely utilized biofluids for AD research, as it has direct contact with the brain. Among many metabolite subgroups detected across several studies (Bergau et al. 2019; van der Velpen et al. 2019; Hajjar et al. 2020), carbohydrates and amino acids are the most highly represented in CSF (Table S1).
While it is a useful biofluid in understanding neurological conditions, collecting CSF is an invasive procedure and must be performed by a doctor, making it less feasible for large-scale screening (Wang et al. 2015). Using CSF in tandem with another biofluid, like plasma (Fig. 1) may help establish reliable biomarkers through translation of CSF findings to a more accessible biofluid (van der Velpen et al. 2019).
Workflow depicting concurrent analysis of plasma and CSF from the same patients. In this workflow followed by van der Velpen et al. (2019), plasma and CSF from the same patients were analyzed concomitantly to allow for comparison across more than one system. This provides valuable information about how the CSF metabolome compares to the plasma metabolome of the same patient; because CSF is more well-characterized in the AD domain, this could lead to more reliable plasma biomarker results. Reproduced from van der Velpen et al. (2019) Systemic and central nervous system metabolic alterations in Alzheimer’s disease. Alzheimer's research and therapy 11: 93 under a Creative Commons CC-BY license (http://creativecommons.org/licenses/by/4.0/)
Blood Plasma
Unlike CSF collection, plasma can be accessed through a simple blood draw, an innocuous procedure that can be performed by a nurse and does not require a lengthy appointment. Blood circulates throughout the body and comes into contact with all organs, making it a potentially accurate representation of the entire body system; the blood-brain barrier does keep molecules from passing between the brain and the rest of the circulatory system, however, meaning that the blood metabolic profile may not reflect the brain metabolic profile.
Additionally, some studies have shown the metabolic profile of blood plasma can vary by sex, age, race, and other factors like diet, lifestyle, and medication causing significant variation as well (Lawton et al. 2008; Almanza-Aguilera et al. 2018). This disadvantage must be considered in experiments using plasma, as lifestyle differences across patients could confound the AD-related metabolic profile.
Many studies have utilized plasma as a sample source for MS-based AD metabolomic studies (Graham et al. 2015; de Leeuw et al. 2017; Habartová et al. 2019; Kim et al. 2019; Peña-Bautista et al. 2019; van der Velpen et al. 2019; Niedzwiecki et al. 2020), with representation across dozens of different metabolite families (Table S2).
Blood Serum
Serum and plasma are closely related; plasma is obtained from unclotted blood, while serum is the fluid present after clotting. Serum is not technically a physiological fluid, as it is only naturally present when wound healing is taking place. Investigation of the metabolite profiles of both serum and plasma found that while serum has higher overall metabolite concentrations, quantitative reproducibility between samples is better in plasma (Yu et al. 2011). Additionally, some metabolites, like arginine and glucose, are affected by coagulation; arginine, for example, is released by platelets during coagulation and may appear artificially increased in serum (Teerlink et al. 2002). Among other groups represented in serum studies (González‐Domínguez et al. 2014; Ciavardelli et al. 2016; Sun et al. 2020), amino acids and fatty acids and their esters were commonly detected (Table S3).
Urine
Urine is a useful biofluid in that it is noninvasive, sterile, and easy to collect. While it is an attractive biomarker source for these reasons, urine will likely contain mostly hydrophilic metabolites, with a low concentration of lipids and other nonpolar metabolites (Trillo et al. 2013); this supposition is in agreement with what was found across two studies included in this review (Zhang et al. 2019; Yilmaz et al. 2020), with a higher representation of acidic metabolites (Table S4). Given that dysregulated lipid metabolism is of potential interest in AD research, the biomolecular composition of urine could be a disadvantage.
Saliva
Saliva has a unique distinction as one of the most easily accessible biofluids. It requires no needles and takes little collection time. It is important to note, however, that AD often causes decreased unstimulated salivary flow and xerostomia, or dry mouth, which could lead to difficulty in collecting enough sample for analysis (Delwel et al. 2018; Gao et al. 2020). Compared to the vast number of studies using other biofluids like blood, few studies have attempted metabolomic analysis with saliva as a sample source, let alone studies focused solely on AD; the study cited in this review (Huan et al. 2018) found metabolites related to amino acids, fatty acyl glycosides, purine nucleosides, and pterins in their analysis (Table S5).
Sample Collection and Preparation
Fasting
Of the 15 studies included in this review, nine required subjects to fast before sample collection, two did not require fasting, and four did not specify. The two that did not require fasting were both blood-based experiments utilizing plasma (de Leeuw et al. 2017; Niedzwiecki et al. 2020). Fasting before blood testing is standard for several types of blood tests, as digestive processes could raise the level of different metabolites and nutrients like glucose and triglycerides. Studies are in disagreement on the importance of fasting before blood tests, with some indicating that fasting concentrations of some metabolites are not significantly different from non-fasting concentrations (Sidhu and Naugler 2012).
Extraction
Solvent choice is important and determines what metabolites are extracted for analysis. An ideal solvent will extract all metabolites with minimal degradation, but this is certainly easier said than done. Extraction solvents can generally be sorted into two categories: monophasic and biphasic extractions. Monophasic extraction methods use a single solvent to extract metabolites into one phase. The simplicity of monophasic extractions is attractive, as evidenced by the nine studies included in this review utilizing a monophasic extraction, seven of which used acetonitrile (Kim et al. 2002; González‐Domínguez et al. 2014; Graham et al. 2015; de Leeuw et al. 2017; Habartová et al. 2019; Peña-Bautista et al. 2019; Hajjar et al. 2020; Niedzwiecki et al. 2020; Sun et al. 2020). Methanol and acetonitrile are both commonly used monophasic extraction solvents, as they both have a moderate polarity that can extract a range of both polar and nonpolar metabolites; methanol is both hydrophilic and lipophilic, making it an attractive solvent in cases where lipids are of interest (Mushtaq et al. 2014). It is important to note that the solvent used should ideally be MS-compatible; reconstitution in an MS-compatible solvent adds time and uncertainty into the analysis, as taking extra time to dry and reconstitute samples outside of low-temperature storage increases risk of metabolite degradation.
Biphasic extractions utilize a solvent combination to extract metabolites into polar and nonpolar phases; typically, water and an organic solvent are used. While some monophasic solvents are capable of simultaneously extracting moderate amounts of polar and nonpolar metabolites, biphasic extractions are necessary for deeper metabolome coverage. Biphasic solvent combinations are the most useful method in global metabolomic studies planning to utilize complementary separation methods, for example, reversed phase (RP) chromatography for nonpolar compounds and hydrophilic liquid chromatography (HILIC) for polar compounds. Separating metabolites by polarity also increases resolution during analysis by removing interference from metabolites of the opposite polarity (Chetwynd et al. 2017). There are some disadvantages to biphasic extractions, namely the time-consuming nature and technical difficulty of working with many biphasic solvent combinations. Generally, the layer of cell and protein debris is located at the junction between the polar and nonpolar solvents, making it difficult to isolate the bottom solvent without accidentally pipetting some cell debris as well; this debris can contaminate or clog the instrument and should be avoided. It is worth noting that some methods, including methyl tert-butyl ether (MTBE) extraction (Matyash et al. 2008) have experimented with different combinations of solvents that allow for the layer of interest to be above the debris.
Notably, the greatly simplified sample preparation or minimal sample preparation needed for urine samples is an advantage that this biofluid has over others. Urine samples do not often require extraction for metabolomic analysis; in this study of urinary metabolites, for example, samples were filtered via molecular weight cut-off ultracentrifugation filters and then diluted so that all samples had the same osmolality (Hao et al. 2016). Simplified sample preparation also extends to NMR spectroscopy, the other common method of metabolomic profiling; compared to the other sample preparation described in this 2007 study, urine preparation is incredibly simplistic, only requiring the addition of sodium azide for stability (Beckonert et al. 2007).
Derivatization
Chemical derivatization of analytes can significantly aid in measurement. Unlike proteins, which can always be expected to contain certain functional groups like amines, metabolites are chemically diverse and cannot all be derivatized with a single method. Despite this hurdle, several methods exist to aid in analysis. A brief discussion of the advantages of derivatization follows; description of individual derivatization procedures is outside the scope of this review, but more detailed descriptions of several procedures can be found elsewhere (Zhao and Li 2020).
Separation methods will be discussed in more depth later in the review, but it is important to note here that derivatization can be a boon in cases where one separation mode is desired; while using more than one separation mode increases metabolome coverage through including a range of molecule polarity, using multiple separations increases time and cost of analysis. By changing metabolite structure through derivatization, molecular polarity and size can be altered. One example is decreasing the polarity of polar molecules to enable separation using a nonpolar stationary phase.
Derivatization can also be used to stabilize analyte molecules and is generally required for gas chromatography (GC) to increase thermal/chemical stability and volatility. Derivatization in GC–MS can also improve separation by changing the properties of analyte molecules (e.g., polarity) to enhance separation. Commonly used derivatizations include silylation, oximation, alkylation, and acylation; both studies that utilized GC–MS separation chose a two-step process of oximation followed by silylation (de Leeuw et al. 2017; Sun et al. 2020).
Derivatizations are sometimes performed to improve fragmentation in tandem MS (MS/MS). While most studies included in this review used a label-free approach, one chose to dansylate metabolites before analysis (Huan et al. 2018). Dansylated metabolites produce a distinct fragment ion in MS/MS experiments, making it a useful tool for metabolites that are otherwise in low abundance and difficult to detect.
Use of isobaric labels for metabolite quantitation is recent, and with label development still being an active research area, isobaric labeling will likely continue to expand into many metabolite classes. MS/MS reporter-based tags like DiLeu (Xiang et al. 2010) and iTRAQ (Nabetani et al. 2011) can be used to label amine-containing metabolites; these tags rely on heavy isotopes incorporated into an amine-specific reacting molecule, and have been successfully used for metabolomics studies (Hao et al. 2017). While other chemical tag varieties also utilize heavy isotopes, the term “isobaric” refers to the fact that each isotopologue of this tag has the same nominal mass, reducing the MS1 spectrum complexity. An isobaric tag comprises three components: the reporter group, the balance group, and the reactive group. The reporter group is designed to be broken off during collision-induced dissociation (CID) and each reporter will have a different mass due to the different isotope combinations; these are balanced by isotopes in the balance group. The reactive group reacts with a selected functional group on the analyte of interest.
As previously mentioned, no single method can derivatize all metabolites due to the heterogeneity of the human metabolome. Other tags have been developed for other functional groups, like aminoxyTMT or SUGAR for carbonyl-containing molecules (Hahne et al. 2012; Feng et al. 2019). There are several advantages to using isobaric labelling for quantitation. In addition to improving accuracy and sensitivity, isobaric tags also enable multiplexing, allowing researchers to pool multiple samples at once. With the ability to pool samples, less material per sample is needed, and sample preparation and analysis time are reduced. Because samples are analyzed simultaneously, liquid chromatography (LC)-MS data overlap will also increase.
Analysis Methods
Separation of Molecules
The most commonly used stationary phase across all reviewed studies was C18-bonded silica, a nonpolar stationary phase used in reversed phase liquid chromatography (RPLC). C18 is a popular choice in LC–MS experiments, as it is simple and highly reproducible. C18 is ideal for separating nonpolar metabolites, as it utilizes a nonpolar stationary phase. While RPLC methods are capable of covering the nonpolar and semipolar portion of the human metabolome (Naser et al. 2018), polar metabolites are poorly retained on C18 columns and will elute too quickly for separation and detection. Many metabolites that are involved in many biochemical pathways and are therefore of great interest, including organic acids, sugars, and many amino acids, are polar and cannot be analyzed well using RPLC without derivatization.
Hydrophilic-interaction chromatography (HILIC) is another commonly used separation mode (Graham et al. 2015; van der Velpen et al. 2019; Hajjar et al. 2020). HILIC separates molecules based on their hydrophilicity, with hydrophobic metabolites eluting earlier and hydrophilic, typically polar metabolites being well-retained. HILIC is complementary to RPLC and is often used orthogonally to ensure complete coverage of the AD metabolome in untargeted experiments (Fig. 2). HILIC also provides higher sensitivity due to the more volatile mobile phase required for analyses (often acetonitrile). However, reliance on a large amount of acetonitrile presents disadvantages for HILIC users; aside from the hazardous nature of the solvent, it also has gone through phases of supply shortage and high price (dos Santos Pereira et al. 2009).
Workflow describing orthogonal methods for complete coverage of AD metabolome. This workflow utilized by Hajjar et al. employs orthoganol methods to get the most complete coverage of the AD metabolome. Using C18 to separate nonpolar compounds and HILIC to separate polar compounds ensures that metabolites are not innadvertently omitted due to poor retention. Reproduced from Hajjar et al. (2020) Untargeted metabolomics reveal dysregulations in sugar, methionine, and tyrosine pathways in the prodromal state of AD. Alzheimers Dement (Amst) 12: e12064. under a Creative Commons CC-BY license (https://creativecommons.org/licenses/by-nc-nd/3.0/)
While LC–MS is frequently utilized for metabolomic experiments, GC–MS is also a valuable tool. A two-step derivatization reaction is usually required for volatilization, with carbonyl groups converted into oximes in step one and polar functional groups silylated in step two. GC–MS generates reproducible fragmentation patterns that can be compared to a commercial library for identification, making it an attractive method for complex samples. Not all metabolites can be volatilized or made thermally stable enough for GC–MS, however, meaning that this method alone cannot provide a fully comprehensive picture of the AD metabolome.
A more recent addition to AD metabolomic studies, capillary electrophoresis (CE) is a cost-effective method of separation, as it requires small sample volume. While CE is capable of separating a range of compounds, it is best used for polar and charged metabolites, making it a complementary method to RPLC (Soga and Imaizumi 2001). The nanoliter-sized injection volume, compared to microliters for LC, is an advantage in cases where samples are precious and hard to come by, but this small sample volume also leads to low concentration sensitivity. Despite some disadvantages, CE is gaining popularity, and the outlook on the use of CE in metabolomics is positive, with more recent advancements like CE microchips and multi-dimensional separation providing shortened analysis times and improved separation, respectively (Ramautar et al. 2009; Toraño et al. 2019).
A unique method to separate and identify metabolites is found in ion-mobility spectrometry-MS (IMS-MS). While all of the aforementioned separation techniques separate metabolites prior to ionization, IMS-MS utilizes a buffer gas to separate molecules post-ionization. In particular, IMS-MS is proficient at separating isomeric molecules, something that may be of particular interest to AD lipidomic studies; recently, IMS-MS was employed to separate and identify bile acids, a class of molecules that share structural similarities that make LC separation difficult (Zheng et al. 2019). While there is still much to be learned regarding the role of lipids in AD, it is commonly accepted that various lipids play a significant role in AD progression (Kao et al. 2020). Conceivably, the same technique that was applied to bile acids could be applied to lipids of interest in AD studies to allow for greater separation and identification.
Ionization
There are several different ionization techniques, but the ionization technique of choice for global metabolomics is electrospray ionization (ESI), as it gives coverage across a wide range of molecules (Kamleh et al. 2009). ESI can be operated in both positive (ESI+) and negative (ESI−) polarity mode; ESI+ charges molecules through the addition of a cation, like hydrogen or sodium, while ESI− adds an anion to analyte molecules. Based on this mechanism, easily protonated molecules (bases) will be more easily detected in ESI+, while molecules that can be deprotonated (acids) will be more detectable in ESI− (Steckel and Schlosser 2019). More compounds tend to ionize easily in ESI+ than ESI−, but this can be a double-edged sword; with more ionizable analyte molecules come more ionizable background molecules, leading to a higher background noise in ESI+ compared to ESI−. ESI− benefits from lower background noise, but is commonly assumed to ionize fewer compounds than ESI+, leading to spotty metabolome coverage (Cech and Enke 2001).
Including both methods can lead to better metabolome coverage in untargeted metabolomics experiments. Studies have shown that many metabolites will only ionize in one ionization mode, with one study on human serum showing a 90% increase in detected ions by utilizing both ionization modes, rather than positive only (Nordström et al. 2008).
Mass Analyzers
Metabolomic experiments can be categorized into two groups: untargeted and targeted. Targeted metabolomic studies aim to quantify specific metabolites already identified as analytes of interest and benefit greatly from high sensitivity to detect these analytes at even lower concentrations. Conversely, untargeted metabolomic studies seek a more global metabolome coverage and aim for high resolution to distinguish between features with similar masses.
To increase confidence in metabolite identification in cases with lower mass resolution, MS/MS can be used and ost studies covered in this review employed MS/MS for their experiments. MS can only measure mass-to-charge ratio (m/z) of molecules, making it difficult to separate and identify metabolites that have nearly identical masses. MS/MS often utilizes two mass analyzers: one to separate parent ions based on their m/z and another to separate product ions based on that same ratio, following fragmentation between the first and second mass analyzers. It is worth noting that in trap-based instruments, MS/MS occurs in a single space, by altering the pulse sequence for isolation, then fragmentation. The MS/MS fragmentation step is key in identifying metabolites, as metabolites with similar parent masses often have different fragment ions that can set them apart from one another. As a note, isobaric tags require MS/MS instrumentation to fragment into the reporter ions used for quantitation.
MS1 is less frequently employed in metabolomics but can be seen in experiments using Fourier transform ion cyclotron resonance (FTICR), time-of-flight (TOF), and Orbitrap mass analyzers. These analyzers offer high mass resolution and high mass measurement accuracy, making it easier to distinguish between similar metabolite masses without the need for MS/MS fragmentation.
Data analysis and Bioinformatics
Quantitation
Metabolites vary greatly in ionization efficiency, making absolute metabolite quantitation in a global metabolomic study extremely difficult. Stable isotope-labelled internal standards can be employed for small, targeted studies with known metabolites, but it is simply not feasible to include a standard for each metabolite in an untargeted study, leaving untargeted studies to rely mostly on relative quantitation.
Targeted metabolomic studies often have a smaller analyte range, making inclusion of internal standards possible for absolute quantitation. Some studies included in this review utilized stable-isotope standards for absolute quantitation, but most used relative quantitation and fold change calculations, with standards included for quality control.
Quantifying large numbers of metabolites in a targeted study is a tantalizing prospect, and techniques such as multiple reaction monitoring (MRM) and more recently, parallel reaction monitoring (PRM) are providing ways to achieve large-scale targeted metabolomics quantification. MRM and PRM rely on specific mass spectrometric parameters to isolate precursor ions of interest. In MRM, which is traditionally done using triple quadrupole (QQQ) instrumentation, the precursor ion is selected in the first quadrupole, fragmented in the second, and a pre-selected product ion is isolated in the third quadrupole. Because only one product ion is isolated in the third quadrupole, MS/MS data is often insufficient for proper metabolite identification. PRM is an improvement upon MRM; while the precursor ion selection step is the same, the ion is then fragmented via HCD or CID, with all product ions being simultaneously analyzed in an Orbitrap or similarly high-resolution mass analyzer. A more detailed description of large-scale targeted metabolomics can be found elsewhere (Zhou and Yin 2016).
Of note, as previously mentioned, isobaric tags can also be used to enable quantitation as well but are limited in the functional groups that can be labeled.
Raw Data Processing
With the human metabolome comprising of over 100,000 molecules (Wishart et al. 2018), metabolomics datasets are often large and complex, requiring powerful software tools to extract as much useful information as possible. Preprocessing is a vital step in a metabolomics study, comprising of steps like deconvolution and feature detection, as well as including quality control steps like blank corrections and normalization. While there are commercial products such as Compound Discoverer™ and MetaboScape®, these are vendor specific. It is increasingly becoming popular to convert raw data files into open formats that are compatible with open-source software packages like MS-DIAL (Tsugawa et al. 2020) and XCMS (Tautenhahn et al. 2008). There is a push to create better preprocessing software that is increasingly user-friendly and coherent across platforms, with many new programs appearing in the recent years; these programs are discussed fully in a recent, detailed review (Misra 2021).
Statistical Analysis
The studies included in this review could be divided into two categories regarding statistical analysis: those that associated metabolite concentration with accepted biomarkers (e.g., Aβ) and those that associated metabolite concentrations with presence of AD dementia. The former category may be a valuable means of detecting significant biomarkers, as changes in metabolite concentration could be correlated more easily with increase in tauopathy or amyloid aggregates, rather than perceived cognitive changes (Fig. 3).
Schematic workflow showing methods and data analysis of AD plasma. Kim et al. followed this workflow, choosing to correlate plasma metabolite concentrations with accepted CSF biomarkers in addition to other AD clinical variables. This particular method of analysis removes the uncertainty that comes with antemortem AD diagnosis, potentially providing more confidence in the significance of the results. Reproduced with permission from Kim et al. (2019) Primary fatty amides in plasma associated with brain amyloid burden, hippocampal volume, and memory in the European Medical Information Framework for Alzheimer's Disease biomarker discovery cohort Alzheimer’s Dement 15: 817–827, copyright © 2019 John Wiley and Sons
In metabolomics experiments, datasets frequently present far more features than there are number of samples. Multivariate analysis (MA) can be used to manage noise, missing data, and massive number of features. Principal component analysis (PCA) and partial least squares discriminant analysis (PLS-DA) are both common choices for comparing between different sample classes (e.g., AD vs. CTRL); these tools take complicated datasets and condense them into 2- or 3-dimensional plots.
While MA is an attractive choice when confronted with a large dataset, univariate analysis (UA) is different, but complementary, to MA. When conducting a global metabolomics study, it is often recommended to include both statistical analysis types. Hypothesis testing, using student’s t–test or analysis of variance (ANOVA), can be used to determine whether a single metabolite is significantly different between a diseased state and control. Fold change (FC) is calculated to determine whether a metabolite is up- or down-regulated in disease state versus control. Detailed discussion of the intricacies of statistics for metabolomics is outside the scope of this review, but detailed reviews on both UA (Vinaixa et al. 2012) and MA (Liland 2011) can be found elsewhere.
Biological Insights
The 15 studies included in this paper represent a diverse cohort of AD metabolomics studies, all focused on different biofluids. Ideally, metabolomics studies of the same disease should reveal similar findings, in order to focus research efforts on particular pathways of interest that could be further studied. The purpose of choosing these studies was to compare findings derived from a variety of separation and MS techniques and determine how coherent the results are. As mentioned previously, these studies also cover a variety of sample types: CSF, plasma, serum, saliva, and urine. A comparison across multiple biofluids not only shows what similarities can be seen across multiple systems, but also could elucidate relationships between different biofluids; a metabolite that is increased in one biofluid and decreased in another, for example, could indicate an exchange or relationship between the two associated bodily systems. While brain tissue is arguably one of the most informative sample types for a disease affecting the brain, it was excluded from this review as it is not accessible in living patients, and therefore would not be practical for widespread biomarker detection in living patient cohorts.
The following section is a detailed analysis of several important metabolic pathways that emerged as significant after consolidating the data from these studies. While AD is not technically classified as a metabolic disease, metabolic change is one of the first symptoms of AD (Calsolaro and Edison 2016). Furthermore, AD has been connected to other metabolic conditions, sharing similar demographic profiles and risk factors with type 2 diabetes and obesity (Moreira 2012). Of note, while the AD research community is focused on the amyloid hypothesis, a theory that designates dysfunction in beta-amyloid production, accumulation, or disposal as the primary cause of AD (Selkoe and Hardy 2016), few significant steps have been made towards finding a treatment to stop or slow AD, indicating that there may be vital information missing in our understanding of AD pathology; connections between Aβ aggregation and metabolic pathways like the urea cycle, insulin metabolism, and fatty acid metabolism as described below could give nuance to the amyloid hypothesis, ultimately leading to new discoveries and effective therapeutic strategies.
Energy Metabolism
Energy-related metabolites were detected in several biofluids. Glucose is the primary energy source in the human brain, with the brain using 25% of whole-body glucose under normal conditions; however, AD brains classically show a marked decrease in glucose metabolism (Fig. S1) (Calsolaro and Edison 2016). This change in metabolism is a reliable hallmark of AD and occurs before the appearance of clinical symptoms. Several glycolysis intermediates were detected as significantly different in AD/MCI vs control groups: phosphoenolpyruvate, 2-phosphoglycerate, 3-phosphoglycerate, pyruvic acid, and dihydroxyacetone phosphate were all found to be decreased in AD CSF as compared to control (Bergau et al. 2019). This corroborates other evidence of dysregulated glucose metabolism; reduced glucose metabolism has previously been reported in imaging using fluorodeoxyglucose-positron emission tomography (FDG-PET) and magnetic resonance imaging (MRI), and there is evidence that glucose-metabolism-regulated proteins are increased in the AD brain (Musunuri et al. 2014; Verclytte et al. 2016).
The dysregulation of energy metabolism in AD is also demonstrated by insulin resistance and low insulin expression in the brain (Steen et al. 2005). Insulin plays a vital role in the human brain, helping regulate processes like neuronal survival, plasticity, and signaling pathways associated with learning and memory, in addition to energy metabolism (Bingham et al. 2002). Insulin is also an important growth factor for neurons, indicating that insulin resistance could cause neural degeneration (Li and Hölscher 2007). Insulin also plays a role in the physiological processing of Aβ, a hallmark peptide in AD that aggregates in the brain (de la Monte 2009).
The significance of insulin in AD is underscored by the ties between AD and diabetes, a condition also characterized by insulin dysregulation. The 1999 Rotterdam study linked higher risk of AD development to diabetes, with diabetes increasing the risk by twofold (Ott et al. 1999). The common insulin resistance in AD and diabetes led to the coining of the term “type 3 diabetes” for AD in 2005 (Steen et al. 2005). Treatment with insulin has improved cognition in AD patients, further strengthening the connection between the two conditions (Park et al. 2000; Craft et al. 2003; Reger et al. 2008).
The glucose-sorbitol pathway (Fig. S2) is also implicated across several studies; the increase in sorbitol concentration indicates a potential activation of this pathway in AD patients. Sorbitol and inositol, molecules detected in AD urine (Yilmaz et al. 2020), are both osmolytes, which protect cells from hypertonicity and help stabilize protein structure (Yancey et al. 1982; Arakawa and Timasheff 1985). While they have protective effects in physiological concentrations, increased sorbitol concentration is implicated in long-term diabetic complications, such as neuropathy and atherosclerosis. Evidence shows the glucose-sorbitol pathway is activated in the brain, where increased glucose concentration not used in glycolysis is instead metabolized into sorbitol (Rosales-Corral et al. 2015).
In the absence of glucose metabolism, ketone bodies can be utilized as an alternative energy source. 3-hydroxybutyric acid, detected as decreased in MCI urine compared to control (Yilmaz et al. 2020), is one of the three ketone bodies that can be metabolized for energy production. Also known as beta-hydroxybutyrate (BHB), this ketone body is consistently decreased in AD patients (Shippy et al. 2020). Higher BHB concentrations correlate with greater cognitive function, and diets that encourage ketosis have been shown to improve cognitive function in adults with mild cognitive impairment (Reger et al. 2004; Krikorian et al. 2012). While ketosis is associated with decreased inflammation and increased energy metabolism, ketone bodies are thought to provide the same metabolic effects as insulin (Kashiwaya et al. 1997).
Amino Acid Metabolism
Arginine Metabolism
Metabolites associated with arginine metabolism were detected as significantly changed in multiple studies. Across all 15 studies, 10 metabolites associated with arginine/proline metabolism were found to be significantly altered, with three metabolites appearing in multiple biofluids. The arginine biosynthesis pathway was also impacted, with five metabolites being significantly altered. Two of these metabolites, arginine and ornithine, were detected in multiple biofluids.
Dysregulation of arginine, citrulline, and ornithine point to a possible defect in the urea cycle (Fig. S3). Arginine is decreased in AD in both CSF and plasma (Graham et al. 2015; van der Velpen et al. 2019; Zhang et al. 2019). This suggests a potential increase in arginase expression, perhaps due to the interplay between arginase and nitric oxide (NO) synthase, both of which utilize arginine as a substrate. NO is key in maintaining brain function, acting as a neurotransmitter and regulating cerebral blood flow (Clemente et al. 2020). With an increase in arginase expression, NO is no longer produced in acceptable quantities, which could lead to oxidative stress.
This is partially supported by what little is known about arginase expression in AD patients. Arginase has two isoforms, arginase I (ARG1) and arginase II (ARG2). While both arginase isoforms are expressed in low levels in the central nervous system, a 2010 study showed ARG2 gene expression is higher in the AD brain than that in control brain. Interestingly, that same study showed a potential link between AD risk and a rare ARG2 allele that leads to decreased ARG2 activity (Hansmannel et al. 2010). This could indicate the urea cycle is protective, although it does not negate the theory of increased arginase expression, as there is no consensus on which arginase isoform increases in expression in the AD brain (Liu et al. 2014). If ARG2 is indeed decreased in AD, the increase of arginine in urine (Zhang et al. 2019) would make sense; ARG2 is expressed highest in the kidneys, and a decrease in ARG2 function would lead to an excess of arginine that could be excreted through the urine.
Barring a change in arginase expression, decrease in arginine could also be attributed to arginine metabolism into guanidino compounds; if arginase is inefficient, this could lead to an excess of arginine converted into those compounds. Many guanidino compounds are neurotoxic and are known to increase oxidative stress, a common characteristic of AD brains (de Souza Wyse et al. 2001; Delwing-de Lima et al. 2010). These compounds build up in cases of hyperargininemia, a condition in which ARG1 activity is decreased. This condition is characterized in part by neurological symptoms, including epilepsy and cognitive deficits (Iyer et al. 1998). Some uremic toxins were detected across the experiments included in this review, including guanidinoacetate, argininate, and asymmetric dimethylarginine (ADMA) (González‐Domínguez et al. 2014; Kim et al. 2019; Yilmaz et al. 2020).
This hypothesis is supported by the potential linkage between chronic kidney disease (CKD) and cognitive impairment. The risk of developing AD is increased in patients with CKD (Etgen et al. 2012). Interestingly, non-AD cognitive impairment in CKD patients can be decreased, and even reversed completely, following a kidney transplant (Radić et al. 2011). Vascular injury, a common characteristic between CKD and AD, is attributed to uremic toxin accumulation due to kidney dysfunction (Shi et al. 2018). ADMA in particular is implicated in vascular stiffness and is important in cerebral blood vessel regulation (Aldámiz-Echevarría and Andrade 2012; Asif et al. 2013). The increase in vascular stiffness, as well as the aforementioned neurotoxic effects of uremic toxins, could be linked to AD pathology.
Current biomarkers associated with both AD and CKD also show some overlap. Creatinine, which is upregulated in CKD patient serum, is shown to be increased in AD serum as well (González‐Domínguez et al. 2014; Shi et al. 2018). Creatinine level in CKD patients is known to be closely related to cognitive decline (McAdams-DeMarco et al. 2017). Aβ is also increased in CKD patient serum (Kanemaru et al. 2016). Connections between the two conditions lend themselves to a potential kidney-brain axis, with arginine metabolism possibly holding a key role in both.
Aside from a change in the urea cycle, a decrease of ornithine in AD could also point to a possible change in polyamine metabolism (Fig. S4). Putrescine, spermidine, and spermine were all detected as significantly increased in AD plasma vs control, as well as several related compounds (Graham et al. 2015). Ornithine is decreased in several studies, while all downstream metabolites are increased, suggesting a potential change in ornithine decarboxylase (ODC) expression (Graham et al. 2015; de Leeuw et al. 2017; Zhang et al. 2019). While putrescine does not appear neurotoxic, both spermine and spermidine can activate processes implicated in neuronal degeneration, including N-methyl-d-aspartate (NMDA)-preferring glutamate receptor function and apoptosis (Williams et al. 1989; Poulin et al. 1995). ODC expression has been detected in the AD brain, although it is region-specific (Poulin et al. 1995). Polyamines have been pursued as a potential AD therapeutic target for several clinical trials, with a pilot trial demonstrating a positive effect on memory performance in older adults (Wirth et al. 2019).
Isoleucine, Leucine, and Valine Metabolism
Isoleucine, leucine, and valine comprise a group of three essential amino acids called branched chain amino acids (BCAA). Across the studies included in this review, all three BCAAs were detected as decreased in AD compared to control (de Leeuw et al. 2017; van der Velpen et al. 2019; Yilmaz et al. 2020).
One hypothesis regarding the importance of BCAAs to the brain is related to brain glutamate metabolism. BCAAs pass through the blood-brain barrier with ease, and there is ample branched-chain amino acid transaminase (BCAT) that could use BCAAs to produce glutamate (Fig. S5) (Yudkoff 2017). Glutamate is a vital neurotransmitter that plays a key role in learning and memory; a decrease in BCAA concentration in the body could lead to a decrease in glutamate production and glutamatergic neuron loss in the brain (Francis 2003).
Valine in particular is associated with increased apoptosis at high concentrations in maple syrup urine disease (Vilela et al. 2017). Decreased valine concentration could be the body’s response to increased apoptosis in AD (Tynkkynen et al. 2018). Further investigation into the importance of BCAAs to the brain is warranted, although it should be noted BCAAs have been demonstrated to be associated with general dementia, rather than AD specifically (Tynkkynen et al. 2018).
Methionine and Cysteine Metabolism
Cysteine/methionine metabolic dysregulation was detected in urine (Zhang et al. 2019), saliva (Huan et al. 2018), serum (González‐Domínguez et al. 2014), and CSF (Hajjar et al. 2020). Abnormal cysteine/methionine metabolism has been previously implicated in AD; abnormal homocysteine accumulation in blood has been linked to higher risk of AD development (Seshadri et al. 2002; Shea and Rogers 2002). High homocysteine levels are associated with regional brain atrophy and vascular lesions, and homocysteine may affect both tau-protein phosphorylation and cholinesterase degradation (Clarke et al. 1998; Williams et al. 2002; Darvesh et al. 2007; Zhang et al. 2008).
S-adenosylhomocysteine (SAH) was detected in CSF as increased in MCI, while S-adenosylmethionine (SAM) was detected as downregulated (Hajjar et al. 2020). This is congruent with similar findings in AD CSF, and could indicate a dysregulation in SAM methylation to SAH (Trillo et al. 2013). Across the other studies, cysteine and methionine were also found to be dysregulated, with methionine decreased in AD and MCI in serum, and cysteine increased in AD urine (Zhang et al. 2019). Methionine synthase facilitates transformation of homocysteine back to methionine; the increase in SAH could also be related to the decrease in methionine through methionine synthase dysregulation.
Choline Metabolism
A large body of evidence suggests that the central cholinergic system is dysregulated in AD; one of the earliest pathological events of AD progression is thought to be cholinergic neuron loss in the basal forebrain (Davies and Maloney 1976; Coyle et al. 1983). Acetylcholine is an essential neurotransmitter utilized by cholinergic neuronal systems, including motor neurons and neurons involved with memory. Acetylcholine was first associated with human memory in the 1970, when it was shown that competitive agonists of acetylcholine impact memory storage/recall and other cognitive tasks (Drachman and Leavitt 1974).
Acetylcholine is produced by choline acetyltransferase (ChAT), an enzyme whose activity is significantly decreased in AD brains, with the degree of reduction in activity correlating with cognitive impairment severity. ChAT takes choline and adds an acetyl group, yielding acetylcholine, an important neurotransmitter (Auld et al. 2002). Multiple studies in this review detected an increase in choline concentration in AD patients, which could be due to decreased ChAT activity (González‐Domínguez et al. 2014; Peña-Bautista et al. 2019).
While there are currently no pharmaceutical treatments that can reverse AD progression, cholinesterase inhibitors are commonly prescribed for AD patients to temporarily improve some cognitive symptoms. Acetylcholinesterases break down acetylcholine into choline, removing acetylcholine from the system. With decreased ChAT activity, the AD brain is unable to replenish what is broken down, which leads to cholinergic synapse loss. Cholinesterase inhibitors can increase acetylcholine concentrations temporarily to stave off cognitive decline.
Fatty Acid Metabolism
Several carnitine-related metabolites were detected as showing significant alterations across several studies, indicating a disturbance of fatty acid metabolism. Medium-chain acyl-carnitines were significantly decreased in AD plasma, and correlated well with mini-mental state examination (MMSE) scores that measure cognitive performance (Ciavardelli et al. 2016). Some acyl-carnitines have been reported as being predictive of cognitive decline, along with several phosphatidylcholines; these were seen to be decreased in patients who later developed AD or MCI symptoms (Mapstone et al. 2014). Two carnitine species, trans-hexadec-2-enoyl carnitine and palmitoyl-L-carnitine, were detected as increased in AD in another study of plasma (Habartová et al. 2019). Carnitines were also reported as significantly different in serum and saliva (González‐Domínguez et al. 2014; Huan et al. 2018). Carnitine metabolism is complex, which could account for disagreement in the directionality of dysregulation. The repeated emergence of carnitines in AD metabolomic studies is worth further investigation.
Arachidonic acid was detected as decreased in AD plasma with its precursor linoleic acid being increased (Fig. S6) (Habartová et al. 2019). This suggests a potential disturbance in the metabolic pathway. Arachidonic acid is the second most concentrated polyunsaturated fatty acid (PUFA) in the brain, with the first being docosahexaenoic acid (also detected as changed in this study). These three PUFAs have been detected as dysregulated in the AD brain as well, although there is disagreement over whether they are increased or decreased in AD (Nasaruddin et al. 2016; Snowden et al. 2017). Linoleic and arachidonic acid both have been shown to induce tau and Aβ polymerization, implying that they could be related to, or even induce, some AD pathology (Amtul et al. 2012).
Concluding Remarks and Outlook
AD is an extremely complex disease, and it is highly unlikely that only one metabolic dysfunction is responsible for disease pathology. Additionally, it is unclear which of the metabolic changes in AD are directly contributing to AD pathology and which are compensatory mechanisms to correct dysfunction of another pathway. The studies highlighted here all present strong candidates for AD-related metabolic dysfunction, but more consistency across AD metabolomics studies will allow for much more certainty regarding affected pathways.
The AD research community would greatly benefit from more uniform criteria for building patient cohorts. The range of criteria used currently does not always agree on probable AD diagnoses, and studies may be inadvertently including non-AD dementia. Because AD has a unique pathology compared to other dementias, inclusion of non-AD dementia patients must be avoided to prevent clouding of results with the metabolism of other dementias. Emphasis should also be placed on increasing the diversity of patient cohorts; while AD disproportionately affects African American/Black and Hispanic adults, these communities are vastly underrepresented in AD studies. A 2020 study comparing protein expression of African American/Black brain tissue to non-Hispanic White brain tissue showed that many proteins have significant race/diagnosis interactions, suggesting that diversifying patient cohorts would substantially deepen current understanding of AD (Stepler et al. 2020).
Consistency in AD study design should extend to experimental methods as well. Variation in technique across the AD research community is to be expected, but it is difficult to compare results across studies that do not use comparable methods. Tailoring experimental methods to analytes in targeted analyses enables absolute quantitation of metabolites, but an untargeted experiment with the goal of comprehensive metabolomic investigations should employ a variety of separation and ionization techniques to ensure comprehensive coverage of all dysregulated metabolites. In this review, many metabolites reported as significantly changed in AD compared to control were only detected in one study, but this does not mean that they are not present in patients in other studies as well.
For a biomarker to be pursued as a potential AD diagnostic, it needs to be consistently dysregulated and continually appear in AD metabolism studies. Untargeted analyses that do not employ orthogonal methods may miss key metabolites, slowing AD biomarker discovery progress; if an untargeted study that only uses a HILIC column for separation finds that certain sugars are dysregulated, for example, another study using an RP column may not replicate these results. Ensuring that untargeted studies are truly global will help reveal patterns in AD metabolism that can be further investigated. A major barrier to using multiple methods for a single study is the money, time, and sample volume required to use complementary LC–MS methods; incorporating derivatization into the experimental methods may provide a means to shrink that barrier. Derivatization can alter metabolite properties; some studies have used derivatization to alter polar metabolites for better separation on an RP column (Li et al. 2016).
While each sample type presented here has several advantages and disadvantages, a shift towards less invasive sample collection through urine or saliva would be prudent for eventual biomarker-based AD diagnosis. Patients with AD often present neuropsychiatric and psychotic symptoms, which can make them hostile, belligerent, and agitated (Zvěřová 2019). In 2019, it was reported 83% of help provided to elderly adults in the United States came from family or friends, and nearly half of those caregivers provided care specifically for adults with AD or other dementias; these caregivers more frequently report emotional, financial, and physical difficulties than those caregiving for adults without dementia (Gaugler et al. 2019). For future diagnostic testing, any sample that can be collected from the comfort of an AD patient’s home will greatly reduce stress on both patient and caregiver. With more research, many of these potential metabolic biomarkers could be used in tandem with currently accepted protein biomarkers to diagnose AD more accurately in early stages of the disease.
References
Aldámiz-Echevarría L, Andrade F (2012) Asymmetric dimethylarginine, endothelial dysfunction and renal disease. Int J Mol Sci 13:11288–11311. https://doi.org/10.3390/ijms130911288
Almanza-Aguilera E, Brunius C, Bernal-Lopez MR et al (2018) Impact in plasma metabolome as effect of lifestyle intervention for weight-loss reveals metabolic benefits in metabolically healthy obese women. J Proteome Res 17:2600–2610. https://doi.org/10.1021/acs.jproteome.8b00042
Amtul Z, Uhrig M, Wang L, Rozmahel RF, Beyreuther K (2012) Detrimental effects of arachidonic acid and its metabolites in cellular and mouse models of Alzheimer’s disease: structural insight. Neurobiol Aging 33(831):e821–831. https://doi.org/10.1016/j.neurobiolaging.2011.07.014
Arakawa T, Timasheff S (1985) The stabilization of proteins by osmolytes. Biophys J 47:411–414. https://doi.org/10.1016/S0006-3495(85)83932-1
Asif M, Louis Soiza R, McEvoy M, Mangoni A (2013) Asymmetric dimethylarginine: a possible link between vascular disease and dementia. Curr Alzheimer Res 10:347–356. https://doi.org/10.2174/1567205011310040001
Auld DS, Kornecook TJ, Bastianetto S, Quirion R (2002) Alzheimer’s disease and the basal forebrain cholinergic system: relations to β-amyloid peptides, cognition, and treatment strategies. Prog Neurobiol 68:209–245. https://doi.org/10.1016/s0301-0082(02)00079-5
Beckonert O, Keun HC, Ebbels TM et al (2007) Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nat Protoc 2:2692. https://doi.org/10.1038/nprot.2007.376
Bergau N, Maul S, Rujescu D, Simm A, Navarrete Santos A (2019) Reduction of glycolysis intermediate concentrations in the cerebrospinal fluid of Alzheimers Disease patients. Front Neurosci 13:871. https://doi.org/10.3389/fnins.2019.00871
Bingham EM, Hopkins D, Smith D et al (2002) The role of insulin in human brain glucose metabolism: an 18fluoro-deoxyglucose positron emission tomography study. Diabetes 51:3384–3390. https://doi.org/10.2337/diabetes.51.12.3384
Calsolaro V, Edison P (2016) Alterations in glucose metabolism in Alzheimer’s disease. Recent Pat Endocr Metab Immune Drug Discov 10:31–39. https://doi.org/10.2174/1872214810666160615102809
Cech NB, Enke CG (2001) Practical implications of some recent studies in electrospray ionization fundamentals. Mass Spectrom Rev 20:362–387. https://doi.org/10.1002/mas.10008
Chetwynd AJ, Dunn WB, Rodriguez-Blanco G (2017) Collection and preparation of clinical samples for metabolomics. Metabolomics: from fundamentals to clinical applications. Springer, Berlin, pp 19–44. https://doi.org/10.1007/978-3-319-47656-8_2
Ciavardelli D, Piras F, Consalvo A et al (2016) Medium-chain plasma acylcarnitines, ketone levels, cognition, and gray matter volumes in healthy elderly, mildly cognitively impaired, or Alzheimer’s disease subjects. Neurobiol Aging 43:1–12. https://doi.org/10.1016/j.neurobiolaging.2016.03.005
Clarke R, Smith AD, Jobst KA, Refsum H, Sutton L, Ueland PM (1998) Folate, vitamin B12, and serum total homocysteine levels in confirmed Alzheimer disease. Arch Neurol 55:1449–1455. https://doi.org/10.1001/archneur.55.11.1449
Clemente G, van Waarde A, Antunes I, Dömling A, Elsinga P (2020) Arginase as a potential biomarker of disease progression: a molecular imaging perspective. Int J Mol Sci 21:5291. https://doi.org/10.3390/ijms21155291
Coyle JT, Price DL, Delong MR (1983) Alzheimer’s disease: a disorder of cortical cholinergic innervation. Science 219:1184–1190. https://doi.org/10.1126/science.6338589
Craft S, Asthana S, Cook DG et al (2003) Insulin dose–response effects on memory and plasma amyloid precursor protein in Alzheimer’s disease: interactions with apolipoprotein E genotype. Psychoneuroendocrinology 28:809–822. https://doi.org/10.1016/s0306-4530(02)00087-2
Darvesh S, Walsh R, Martin E (2007) Homocysteine thiolactone and human cholinesterases. Cell Mol Neurobiol 27:33–48. https://doi.org/10.1007/s10571-006-9114-0
Davies P, Maloney A (1976) Selective loss of central cholinergic neurons in Alzheimer’s disease. Lancet 308:1403. https://doi.org/10.1016/s0140-6736(76)91936-x
de la Monte SM (2009) Insulin resistance and Alzheimer’s disease. BMB Rep 42:475. https://doi.org/10.5483/bmbrep.2009.42.8.475
de Leeuw FA, Peeters CF, Kester MI et al (2017) Blood-based metabolic signatures in Alzheimer’s disease. Alzheimers Dement (amst) 8:196–207. https://doi.org/10.1016/j.dadm.2017.07.006
de Souza Wyse AT, Bavaresco CS, Hagen MEK et al (2001) In vitro stimulation of oxidative stress in cerebral cortex of rats by the guanidino compounds accumulating in hyperargininemia. Brain Res 923:50–57. https://doi.org/10.1016/s0006-8993(01)03197-3
Delwel S, Binnekade TT, Perez RS, Hertogh CM, Scherder EJ, Lobbezoo F (2018) Oral hygiene and oral health in older people with dementia: a comprehensive review with focus on oral soft tissues. Clin Oral Investig 22:93–108. https://doi.org/10.1007/s00784-017-2264-2
Delwing-de Lima D, Wollinger LF, Casagrande ACM et al (2010) Guanidino compounds inhibit acetylcholinesterase and butyrylcholinesterase activities: effect neuroprotector of vitamins E plus C. Int J Dev Neurosci 28:465–473. https://doi.org/10.1016/j.ijdevneu.2010.06.008
Dolci GA, Damanti S, Scortichini V et al (2017) Alzheimer’s disease diagnosis: discrepancy between clinical, neuroimaging, and cerebrospinal fluid biomarkers criteria in an Italian cohort of geriatric outpatients: a retrospective cross-sectional study. Front Med 4:203. https://doi.org/10.3389/fmed.2017.00203
dos Santos PA, David F, Vanhoenacker G, Sandra P (2009) The acetonitrile shortage: is reversed HILIC with water an alternative for the analysis of highly polar ionizable solutes? J Sep Sci 32:2001–2007. https://doi.org/10.1002/jssc.200900272
Drachman DA, Leavitt J (1974) Human memory and the cholinergic system: a relationship to aging? Arch Neurol 30:113–121. https://doi.org/10.1001/archneur.1974.00490320001001
Emwas A-HM (2015) The strengths and weaknesses of NMR spectroscopy and mass spectrometry with particular focus on metabolomics research. Springer, In Metabonomics, pp 161–193. https://doi.org/10.1007/978-1-4939-2377-9_13
Etgen T, Chonchol M, Förstl H, Sander D (2012) Chronic kidney disease and cognitive impairment: a systematic review and meta-analysis. Am J Nephrol 35:474–482. https://doi.org/10.1159/000338135
Feng Y, Chen B, Yu Q et al (2019) Isobaric multiplex labeling reagents for carbonyl-containing compound (SUGAR) tags: a probe for quantitative glycomic analysis. Anal Chem 91:3141–3146. https://doi.org/10.1021/acs.analchem.8b05757
Frances A, First MB, Pincus HA (1995) DSM-IV guidebook. American Psychiatric Association
Francis PT (2003) Glutamatergic systems in Alzheimer’s disease. Int J Geriatr Psychiatry 18:S15–S21. https://doi.org/10.1002/gps.934
Gao SS, Chu CH, Young FYF (2020) Oral health and care for elderly people with Alzheimer’s disease. Int J Environ Res Public Health 17:5713. https://doi.org/10.3390/ijerph17165713
Gaugler J, James B, Johnson T, Marin A, Weuve J (2019) 2019 Alzheimer’s disease facts and figures. Alzheimers Dement 15:321–387. https://doi.org/10.1016/j.jalz.2019.01.010
González-Domínguez R, García A, García-Barrera T, Barbas C, Gómez-Ariza JL (2014) Metabolomic profiling of serum in the progression of Alzheimer’s disease by capillary electrophoresis–mass spectrometry. Electrophoresis 35:3321–3330. https://doi.org/10.1002/elps.201400196
Graham SF, Chevallier OP, Elliott CT et al (2015) Untargeted metabolomic analysis of human plasma indicates differentially affected polyamine and L-arginine metabolism in mild cognitive impairment subjects converting to Alzheimer’s disease. PLoS ONE 10:e0119452. https://doi.org/10.1371/journal.pone.0119452
Habartová L, Hrubešová K, Syslová K et al (2019) Blood-based molecular signature of Alzheimer’s disease via spectroscopy and metabolomics. Clin Biochem 72:58–63. https://doi.org/10.1016/j.clinbiochem.2019.04.004
Hahne H, Neubert P, Kuhn K et al (2012) Carbonyl-reactive tandem mass tags for the proteome-wide quantification of N-linked glycans. Anal Chem 84:3716–3724. https://doi.org/10.1021/ac300197c
Hajjar I, Liu C, Jones DP, Uppal K (2020) Untargeted metabolomics reveal dysregulations in sugar, methionine, and tyrosine pathways in the prodromal state of AD. Alzheimers Dement (amst) 12:12064. https://doi.org/10.1002/dad2.12064
Hansmannel F, Sillaire A, Kamboh MI et al (2010) Is the urea cycle involved in Alzheimer’s disease? J Alzheimers Dis 21:1013–1021. https://doi.org/10.3233/JAD-2010-100630
Hao L, Greer T, Page D et al (2016) In-depth characterization and validation of human urine metabolomes reveal novel metabolic signatures of lower urinary tract symptoms. Sci Rep 6:1–11. https://doi.org/10.1038/srep30869
Hao L, Johnson J, Lietz CB et al (2017) Mass defect-based N, N-dimethyl leucine labels for quantitative proteomics and amine metabolomics of pancreatic cancer cells. Anal Chem 89:1138–1146. https://doi.org/10.1021/acs.analchem.6b03482
Huan T, Tran T, Zheng J et al (2018) Metabolomics analyses of saliva detect novel biomarkers of Alzheimer’s disease. J Alzheimers Dis 65:1401–1416. https://doi.org/10.3233/JAD-180711
Iyer R, Jenkinson C, Vockley J, Kern R, Grody W, Cederbaum S (1998) The human arginases and arginase deficiency. J Inherit Metab Dis 21:86–100. https://doi.org/10.1023/a:1005313809037
Jack CR Jr, Bennett DA, Blennow K et al (2018) NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement 14:535–562. https://doi.org/10.1016/j.jalz.2018.02.018
Kamleh MA, Dow JA, Watson DG (2009) Applications of mass spectrometry in metabolomic studies of animal model and invertebrate systems. Brief Funct Genomic Proteomic 8:28–48. https://doi.org/10.1093/bfgp/eln052
Kanemaru K, Kanemaru A, Murayama S (2016) Association between renal functions and csf biomarkers in alzheimer’s disease. Alzheimers Dement 7:P665. https://doi.org/10.1016/j.jalz.2016.06.1508
Kao Y-C, Ho P-C, Tu Y-K, Jou I, Tsai K-J (2020) Lipids and Alzheimer’s disease. Int J Mol Sci 21:1505. https://doi.org/10.3390/ijms21041505
Kashiwaya Y, King MT, Veech RL (1997) Substrate signaling by insulin: a ketone bodies ratio mimics insulin action in heart. Am J Cardiol 80:50A–64A. https://doi.org/10.1016/s0002-9149(97)00458-x
Kim PS, Iyer RK, Lu KV et al (2002) Expression of the liver form of arginase in erythrocytes. Mol Genet Metab 76:100–110. https://doi.org/10.1016/s1096-7192(02)00034-3
Kim M, Snowden S, Suvitaival T et al (2019) Primary fatty amides in plasma associated with brain amyloid burden, hippocampal volume, and memory in the European Medical Information Framework for Alzheimer’s Disease biomarker discovery cohort. Alzheimers Dement 15:817–827. https://doi.org/10.1016/j.jalz.2019.03.004
Krikorian R, Shidler MD, Dangelo K, Couch SC, Benoit SC, Clegg DJ (2012) Dietary ketosis enhances memory in mild cognitive impairment. Neurobiol Aging 33(425):e419–425. https://doi.org/10.1016/j.neurobiolaging.2010.10.006
Lawton KA, Berger A, Mitchell M, et al (2008) Analysis of the adult human plasma metabolome. Pharmacogenomics 9(4):383–397. https://doi.org/10.2217/14622416.9.4.383
Li L, Hölscher C (2007) Common pathological processes in Alzheimer disease and type 2 diabetes: a review. Brain Res Rev 56:384–402. https://doi.org/10.1016/j.brainresrev.2007.09.001
Li S, Jin Y, Wang J et al (2016) Urinary profiling of cis-diol-containing metabolites in rats with bisphenol A exposure by liquid chromatography-mass spectrometry and isotope labeling. Analyst 141:1144–1153. https://doi.org/10.1039/c5an02195b
Liland KH (2011) Multivariate methods in metabolomics–from pre-processing to dimension reduction and statistical analysis. Trends Analyt Chem 30:827–841. https://doi.org/10.1016/j.trac.2011.02.007
Liu P, Fleete MS, Jing Y et al (2014) Altered arginine metabolism in Alzheimer’s disease brains. Neurobiol Aging 35:1992–2003. https://doi.org/10.1016/j.neurobiolaging.2014.03.013
Mapstone M, Cheema AK, Fiandaca MS et al (2014) Plasma phospholipids identify antecedent memory impairment in older adults. Nat Med 20:415–418. https://doi.org/10.1038/nm.3466
Matyash V, Liebisch G, Kurzchalia TV, Shevchenko A, Schwudke D (2008) Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics*. J Lipid Res 49:1137–1146. https://doi.org/10.1194/jlr.D700041-JLR200
McAdams-DeMarco MA, Bae S, Chu N et al (2017) Dementia and Alzheimer’s disease among older kidney transplant recipients. J Am Soc Nephrol 28:1575–1583. https://doi.org/10.1681/ASN.2016080816
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (1984) Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group* under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34:939–939. https://doi.org/10.1212/wnl.34.7.939
Misra BB (2021) New software tools, databases, and resources in metabolomics: updates from 2020. Metabolomics 17:1–24. https://doi.org/10.1007/s11306-021-01796-1
Moreira PI (2012) Alzheimer’s disease and diabetes: an integrative view of the role of mitochondria, oxidative stress, and insulin. J Alzheimers Dis 30:S199–S215. https://doi.org/10.3233/JAD-2011-111127
Mushtaq MY, Choi YH, Verpoorte R, Wilson EG (2014) Extraction for metabolomics: access to the metabolome. Phytochem Anal 25:291–306. https://doi.org/10.1002/pca.2505
Musunuri S, Wetterhall M, Ingelsson M et al (2014) Quantification of the brain proteome in Alzheimer’s disease using multiplexed mass spectrometry. J Proteome Res 13:2056–2068. https://doi.org/10.1021/pr401202d
Nabetani T, Makino A, Hullin-Matsuda F et al (2011) Multiplex analysis of sphingolipids using amine-reactive tags (iTRAQ). J Lipid Res 52:1294–1302. https://doi.org/10.1194/jlr.D014621
Nasaruddin ML, Hölscher C, Kehoe P, Graham SF, Green BD (2016) Wide-ranging alterations in the brain fatty acid complement of subjects with late Alzheimer’s disease as detected by GC–MS. Am J Transl Res 8:154
Naser FJ, Mahieu NG, Wang L, Spalding JL, Johnson SL, Patti GJ (2018) Two complementary reversed-phase separations for comprehensive coverage of the semipolar and nonpolar metabolome. Anal Bioanal Chem 410:1287–1297. https://doi.org/10.1007/s00216-017-0768-x
National Institute on Aging (2017) How is Alzheimer's disease diagnosed?
Niedzwiecki MM, Walker DI, Howell JC et al (2020) High-resolution metabolomic profiling of Alzheimer’s disease in plasma. Ann Clin Transl Neurol 7:36–45. https://doi.org/10.1002/acn3.50956
Nordström A, Want E, Northen T, Lehtiö J, Siuzdak G (2008) Multiple ionization mass spectrometry strategy used to reveal the complexity of metabolomics. Anal Chem 80:421–429. https://doi.org/10.1021/ac701982e
Ott A, Stolk R, Van Harskamp F, Pols H, Hofman A, Breteler M (1999) Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology 53:1937–1937. https://doi.org/10.1212/wnl.53.9.1937
Park CR, Seeley RJ, Craft S, Woods SC (2000) Intracerebroventricular insulin enhances memory in a passive-avoidance task. Physiol Behav 68:509–514. https://doi.org/10.1016/s0031-9384(99)00220-6
Peña-Bautista C, Roca M, Hervás D et al (2019) Plasma metabolomics in early Alzheimer’s disease patients diagnosed with amyloid biomarker. J Proteomics 200:144–152. https://doi.org/10.1016/j.jprot.2019.04.008
Poulin R, Pelletier G, Pegg A (1995) Induction of apoptosis by excessive polyamine accumulation in ornithine decarboxylase-overproducing L1210 cells. Biochem J 311:723–727. https://doi.org/10.1042/bj3110723
Qian W, Schweizer T, Munoz D, Fischer CE (2016) O3–04-06: Misdiagnosis of Alzheimer’s disease: inconsistencies between clinical diagnosis and neuropathological confirmation. Alzheimers Dement 12:293–293. https://doi.org/10.1016/j.jalz.2016.06.529
Radić J, Ljutić D, Radić M, Kovačić V, Dodig-Ćurković K, Šain M (2011) Kidney transplantation improves cognitive and psychomotor functions in adult hemodialysis patients. Am J Nephrol 34:399–406. https://doi.org/10.1159/000330849
Ramautar R, Somsen GW, de Jong GJ (2009) CE-MS in metabolomics. Electrophoresis 30:276–291. https://doi.org/10.1002/elps.200800512
Reger MA, Henderson ST, Hale C et al (2004) Effects of β-hydroxybutyrate on cognition in memory-impaired adults. Neurobiol Aging 25:311–314. https://doi.org/10.1016/S0197-4580(03)00087-3
Reger M, Watson G, Green P et al (2008) Intranasal insulin improves cognition and modulates β-amyloid in early AD. Neurology 70:440–448. https://doi.org/10.1212/01.WNL.0000265401.62434.36
Rosales-Corral S, Tan D-X, Manchester L, Reiter RJ (2015) Diabetes and Alzheimer disease, two overlapping pathologies with the same background: oxidative stress. Oxid Med Cell Longev 2015:1–14. https://doi.org/10.1155/2015/985845
Selkoe DJ, Hardy J (2016) The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med 8:595–608. https://doi.org/10.15252/emmm.201606210
Seshadri S, Beiser A, Selhub J et al (2002) Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N Engl J Med 346:476–483. https://doi.org/10.1056/NEJMoa011613
Shi Y, Liu Z, Shen Y, Zhu H (2018) A novel perspective linkage between kidney function and Alzheimer’s disease. Front Cell Neurosci 12:384. https://doi.org/10.3389/fncel.2018.00384
Shippy DC, Wilhelm C, Viharkumar PA, Raife TJ, Ulland TK (2020) β-Hydroxybutyrate inhibits inflammasome activation to attenuate Alzheimer’s disease pathology. J Neuroinflammation 17:1–12. https://doi.org/10.1186/s12974-020-01948-5
Sidhu D, Naugler C (2012) Fasting time and lipid levels in a community-based population: a cross-sectional study. Arch Intern Med 172:1707–1710. https://doi.org/10.1001/archinternmed.2012.3708
Snowden SG, Ebshiana AA, Hye A et al (2017) Association between fatty acid metabolism in the brain and Alzheimer disease neuropathology and cognitive performance: a nontargeted metabolomic study. PLoS Med 14:1002266. https://doi.org/10.1371/journal.pmed.1002266
Soga T, Imaizumi M (2001) Capillary electrophoresis method for the analysis of inorganic anions, organic acids, amino acids, nucleotides, carbohydrates and other anionic compounds. Electrophoresis 22:3418–3425. https://doi.org/10.1002/1522-2683(200109)22:16
Steckel A, Schlosser G (2019) An organic chemist’s guide to electrospray mass spectrometric structure elucidation. Molecules 24:611. https://doi.org/10.3390/molecules24030611
Steen E, Terry BM, Rivera J et al (2005) Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease–is this type 3 diabetes? J Alzheimers Dis 7:63–80. https://doi.org/10.3233/jad-2005-7107
Stepler KE, Mahoney ER, Kofler J, Hohman TJ, Lopez OL, Robinson RA (2020) Inclusion of African American/Black adults in a pilot brain proteomics study of Alzheimer’s disease. Neurobiol Dis 146:105129. https://doi.org/10.1016/j.nbd.2020.105129
Sun C, Gao M, Wang F et al (2020) Serum metabolomic profiling in patients with Alzheimer disease and amnestic mild cognitive impairment by GC/MS. Biomed Chromatogr 34:4875. https://doi.org/10.1002/bmc.4875
Tautenhahn R, Böttcher C, Neumann S (2008) Highly sensitive feature detection for high resolution LC/MS. BMC Bioinformatics 9:1–16. https://doi.org/10.1186/1471-2105-9-504
Teerlink T, Nijveldt RJ, De Jong S, Van Leeuwen PA (2002) Determination of arginine, asymmetric dimethylarginine, and symmetric dimethylarginine in human plasma and other biological samples by high-performance liquid chromatography. Anal Biochem 303:131–137. https://doi.org/10.1006/abio.2001.5575
Toraño JS, Ramautar R, de Jong G (2019) Advances in capillary electrophoresis for the life sciences. J Chromatogr B Biomed Sci Appl 1118:116–136. https://doi.org/10.1016/j.jchromb.2019.04.020
Trillo L, Das D, Hsieh W et al (2013) Ascending monoaminergic systems alterations in Alzheimer’s disease. Translating basic science into clinical care. Neurosci Biobehav Rev 37:1363–1379. https://doi.org/10.1016/j.neubiorev.2013.05.008
Tsugawa H, Ikeda K, Takahashi M et al (2020) A lipidome atlas in MS-DIAL 4. Nat Biotechnol 38:1159–1163. https://doi.org/10.1038/s41587-020-0531-2
Tynkkynen J, Chouraki V, van der Lee SJ et al (2018) Association of branched-chain amino acids and other circulating metabolites with risk of incident dementia and Alzheimer’s disease: a prospective study in eight cohorts. Alzheimers Dement 14:723–733. https://doi.org/10.1016/j.jalz.2018.01.003
van der Velpen V, Teav T, Gallart-Ayala H et al (2019) Systemic and central nervous system metabolic alterations in Alzheimer’s disease. Alzheimers Res Ther 11:93. https://doi.org/10.1186/s13195-019-0551-7
Verclytte S, Lopes R, Lenfant P et al (2016) Cerebral hypoperfusion and hypometabolism detected by arterial spin labeling MRI and FDG-PET in early-onset Alzheimer’s disease. J Neuroimaging 26:207–212. https://doi.org/10.1111/jon.12264
Vilela TC, Scaini G, Furlanetto CB et al (2017) Apoptotic signaling pathways induced by acute administration of branched-chain amino acids in an animal model of maple syrup urine disease. Metab Brain Dis 32:115–122. https://doi.org/10.1007/s11011-016-9892-0
Vinaixa M, Samino S, Saez I, Duran J, Guinovart JJ, Yanes O (2012) A guideline to univariate statistical analysis for LC/MS-based untargeted metabolomics-derived data. Metabolites 2:775–795. https://doi.org/10.3390/metabo2040775
Wang Y-F, Fuh J-L, Lirng J-F et al (2015) Cerebrospinal fluid leakage and headache after lumbar puncture: a prospective non-invasive imaging study. Brain 138:1492–1498. https://doi.org/10.1093/brain/awv016
Wilkins JM, Trushina E (2018) Application of metabolomics in Alzheimer’s disease. Front Neurol 8:719. https://doi.org/10.3389/fneur.2017.00719
Williams K, Romano C, Molinoff PB (1989) Effects of polyamines on the binding of [3H] MK-801 to the N-methyl-d-aspartate receptor: pharmacological evidence for the existence of a polyamine recognition site. Mol Pharmacol 36:575–581
Williams JH, Pereira EA, Budge MM, Bradley KM (2002) Minimal hippocampal width relates to plasma homocysteine in community-dwelling older people. Age Ageing 31:440–444. https://doi.org/10.1093/ageing/31.6.440
Wirth M, Schwarz C, Benson G et al (2019) Effects of spermidine supplementation on cognition and biomarkers in older adults with subjective cognitive decline (SmartAge)—study protocol for a randomized controlled trial. Alzheimers Res Ther 11:36. https://doi.org/10.1186/s13195-019-0484-1
Wishart DS, Feunang YD, Marcu A et al (2018) HMDB 4.0: the human metabolome database for 2018. Nucleic Acids Res 46:D608–D617. https://doi.org/10.1093/nar/gkx1089
Xiang F, Ye H, Chen R, Fu Q, Li L (2010) N, N-dimethyl leucines as novel isobaric tandem mass tags for quantitative proteomics and peptidomics. Anal Chem 82:2817–2825. https://doi.org/10.1021/ac902778d
Yancey PH, Clark ME, Hand SC, Bowlus RD, Somero GN (1982) Living with water stress: evolution of osmolyte systems. Science 217:1214–1222. https://doi.org/10.1126/science.7112124
Yeung PK (2018) Metabolomics and biomarkers for drug discovery. Metabolites 8(1):11. https://doi.org/10.3390/metabo8010011
Yilmaz A, Ugur Z, Bisgin H et al (2020) Targeted metabolic profiling of urine highlights a potential biomarker panel for the diagnosis of Alzheimer’s disease and mild cognitive impairment: a pilot study. Metabolites 10:357. https://doi.org/10.3390/metabo10090357
Yu Z, Kastenmüller G, He Y et al (2011) Differences between human plasma and serum metabolite profiles. PLoS ONE 6:21230. https://doi.org/10.1371/journal.pone.0021230
Yudkoff M (2017) Interactions in the metabolism of glutamate and the branched-chain amino acids and ketoacids in the CNS. Neurochem Res 42:10–18. https://doi.org/10.1007/s11064-016-2057-z
Zhang C-E, Tian Q, Wei W et al (2008) Homocysteine induces tau phosphorylation by inactivating protein phosphatase 2A in rat hippocampus. Neurobiol Aging 29:1654–1665. https://doi.org/10.1016/j.neurobiolaging.2007.04.015
Zhang Y-q, Tang Y-b, Dammer E et al (2019) Dysregulated urinary arginine metabolism in older adults with amnestic mild cognitive impairment. Front Aging Neurosci 11:90. https://doi.org/10.3389/fnagi.2019.00090
Zhao S, Li L (2020) Chemical derivatization in LC–MS based metabolomics study. TrenDs Analyt Chem 131:115988. https://doi.org/10.1016/j.trac.2020.115988
Zheng X, Smith FB, Aly NA et al (2019) Evaluating the structural complexity of isomeric bile acids with ion mobility spectrometry. Anal Bioanal Chem 411:4673–4682. https://doi.org/10.1007/s00216-019-01869-0
Zhou J, Yin Y (2016) Strategies for large-scale targeted metabolomics quantification by liquid chromatography-mass spectrometry. Analyst 141:6362–6373. https://doi.org/10.1039/c6an01753c
Zvěřová M (2019) Clinical aspects of Alzheimer’s disease. Clin Biochem 72:3–6. https://doi.org/10.1016/j.clinbiochem.2019.04.015
Acknowledgements
JM would like to thank her colleague Dylan Tabang for his critical evaluation of the manuscript and helpful suggestions.
Funding
The preparation of this manuscript is partially supported by the National Institutes of Health (NIH) grants RF1AG052324 and R21AG065728. J.M. acknowledges a predoctoral traineeship supported by the NIH T32 training grant on Biology of Aging and Age-related Diseases (T32AG000213). L.L. acknowledges a Vilas Distinguished Achievement Professorship and Charles Melbourne Johnson Distinguished Chair Professorship with funding provided by the Wisconsin Alumni Research Foundation and University of Wisconsin-Madison School of Pharmacy.
Author information
Authors and Affiliations
Contributions
JM performed the literature survey and wrote the manuscript. LL provided the theme and guided the manuscript writing, reviewed, revised, and edited the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors claim no conflict of interest.
Availability of data and materials
Not applicable.
Code availability
Not applicable.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mill, J., Li, L. Recent Advances in Understanding of Alzheimer’s Disease Progression Through Mass Spectrometry-Based Metabolomics. Phenomics 2, 1–17 (2022). https://doi.org/10.1007/s43657-021-00036-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43657-021-00036-9