Abstract
The mycelial biomass of basidiomycetes is a promising source of compounds and represents an alternative for industrial and biotechnological applications. Fungi use light as information and hold photoresponse mechanisms, in which sensors respond to light wavelengths and regulate various biological processes. Therefore, this study aimed to investigate the effects of blue, green, and red lights on the growth, chemical composition, and antioxidant and antimicrobial activity of Lentinus crinitus mycelial biomass. The chemical composition of the mycelial biomass was determined by chromatographic methods, antioxidant activity was analyzed by in vitro assays, and antimicrobial activity was investigated by the microdilution assay. The highest mycelial biomass yield was observed under blue-light cultivation. Many primordia arose under blue or green light, whereas the stroma was formed under red light. The presence of light altered the primary fungal metabolism, increasing the carbohydrate, tocopherol, fatty acid, and soluble sugar contents, mostly mannitol, and reducing the protein and organic acid concentrations. Cultivation under red light increased the phenol concentration. In contrast, cultivation under blue and green lights decreased phenol concentration. Benzoic and gallic acids were the main phenolic acids in the hydroalcoholic extracts, and the latter acids increased in all cultures under light, especially red light. Mycelial biomass cultivated under red light showed the highest antioxidant activity in the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay. The ferric reducing antioxidant power (FRAP) method showed that all light wavelengths increased the antioxidant activity of mycelial biomass, with the highest value under red light. Moreover, the β-carotene/linoleic acid co-oxidation (BCLA) assay demonstrated that the antioxidant activity was affected by light cultivation. Mycelial biomass grown under all conditions exhibited antibacterial and antifungal activities. Thus, mycelial biomass cultivation of L. crinitus under light conditions may be a promising strategy for controlling the mycelial chemical composition and biomass yield.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Basidiomycetes are known to possess various metabolites with biological activities, such as antioxidant [1], antimicrobial [2], antitumor [3], and anti-inflammatory [4]. Most of these compounds can be obtained from mycelial biomass [5], basidiocarps and their segments, such as stipe and pileus [6], cultivation medium [7], and the spent mushroom substrate [8]. Mushrooms are appreciated worldwide for their flavor and nutritional composition, including proteins, essential amino acids, carbohydrates, fibers, vitamins, and minerals [9]. However, mushroom bioactive compounds are becoming popular due to the growing demand for healthy living standards and better human health, cosmetics, and nutritional resources [10, 11].
Understanding the effects of light on the physiology and metabolism of basidiomycetes can positively impact research to develop bioprocesses that focus on bioactive compounds. Fungal growth and metabolism can change in response to different environmental conditions, such as pH, temperature, light, nutrient availability, and competition among species [12]. Fungi use light as information about environmental conditions [13,14,15,16]. Different fungal photoreceptors respond to various light signals of a wide range of wavelengths via blue light-sensitive flavin, green light-sensitive retinal, and red light-sensitive tetrapyrrole receptors [17]. Photoreceptor characteristics are highly conserved in fungi [18]. When chromophores absorb light energy, they trigger a light-dependent signaling pathway that affects the gene expression pattern of the organism [13]. Therefore, there is increasing interest in understanding how light affects fungal life and metabolism, encouraging research on light stimuli of mycelial biomass [19], with the formation of primordia and the development of fruiting bodies [20, 21].
Lentinus crinitus (L.) Fr. (Basidiomycota, Polyporaceae) is known for its antioxidant activity [6], microbial growth inhibition [2], antitumor polysaccharides [22], bioaccumulating iron [23] and lithium [24], and decolorizing dyes [25]. This white-rot fungus has a pantropical distribution [26] and is a protein source for Brazilian, Colombian, Peruvian, and Venezuelan Amazon indigenous [27]. Blue light affects carbohydrate-active enzyme production in L. crinitus mycelial biomass [28]. However, no reports on the effects of different wavelengths on the metabolism and chemical composition of L. crinitus mycelial biomass are available. Therefore, this study aimed to investigate the effects of blue, green, and red lights on the growth, chemical composition, and antioxidant and antimicrobial activities of L. crinitus mycelial biomass.
2 Materials and methods
2.1 Biological material
Lentinus crinitus (L.) Fr. U9-1 strain from the Universidade Paranaense collection was used in this study. The strain was registered in the National System of Genetic Patrimony Management and Associated Traditional Knowledge (SisGen) under the code AF1FC65. The U9-1 strain was cultivated in 20 g L−1 malt extract agar (MEA, Kasvi, São José dos Pinhais, PR, Brazil) at 28 ± 1 °C in the dark after the cryopreservation recovery [29]. We used six 6-mm-diameter MEA disks containing mycelia to inoculate the liquid cultivation medium for mycelial biomass growth.
2.2 Production of mycelial biomass
Lentinus crinitus mycelia were grown in conical flasks (250 mL) containing 100 mL of 20 g L−1 malt extract liquid medium autoclaved at 121 °C for 20 min [28]. After inoculation, the flasks were maintained for 21 days at 28 ± 1 °C in a biochemical oxygen demand incubator (SS Scientific, Londrina, PR, Brazil) without agitation, and under different light wavelengths or in the dark (control). The light in the incubator was a set of light-emitting diodes (LED) with wavelengths from 455 to 475 nm (blue), 515 to 530 nm (green), and 620 to 630 nm (red). The light-photon flux density inside the incubator was measured using a portable quantum photosynthetically active radiation meter (Hydrofarm, Pataluma, CA, USA) set at 20 μmol m−2 s−1 [19]. Mycelial biomass was harvested on the last day of cultivation by filtration using a Whatman® filter paper grade 1 and dried in an air-circulating oven at 60 °C until a constant mass was obtained. The total dried mycelial biomass was frozen in liquid nitrogen, ground, homogenized, and stored at − 20 °C.
2.3 Chemical characterization of mycelial biomass
2.3.1 Nutritional value
The macronutrient composition, such as protein, fat, ash, and carbohydrate content of the mycelial biomass, was determined according to the Association of Official Analytical Chemists (AOAC) [30]. The crude protein content (N × 4.38) was identified using the Macro–Kjeldahl method. The crude fat was determined by extracting a known mass of powdered sample with petroleum ether using a Soxhlet apparatus. Ash content was determined by the incineration of the mycelial biomass at 600 ± 15 °C. Total carbohydrates were calculated using the formula: (total carbohydrates = 100 − [gprotein + gfat + gash]), where the gprotein is grams of protein, gfat is grams of fat and gash is grams of ash. The results are expressed in grams per 100 g (dry weight). The energy was calculated according to the regulation (EU) number 1169/2011 of the European Parliament and the Council of 25 October 2011, on the provision of food information to consumers, as kcal per 100 g using the formula: (dry basis) = (4 × [gprotein + gcarbohydrates]) + (9 × gfat).
2.3.2 Free sugars
The soluble sugar content was determined using a high-performance liquid chromatography (HPLC) system with a refraction index detector (Knauer, Smartline System 1000) [31]. The peak was identified by comparing the relative retention time (Rt) with that of an authentic standard. The quantification was performed using melezitose as an internal standard (IS; Sigma-Aldrich, St. Louis, MO, USA), and calibration curves were constructed from authentic standards. The results were processed using Clarity software (Data Apex, Prague, Czech Republic) and expressed in grams per 100 g (dry basis).
2.3.3 Organic acids
Organic acids were determined by ultrafast liquid chromatography (UFLC; Shimadzu 20A series) coupled with a photodiode array detector [32]. Organic acids were quantified by comparing the peak area recorded at 215 nm with the calibration curves obtained from the commercial standards of each compound. The results are expressed in grams per 100 g (dry weight).
2.3.4 Fatty acids
The fatty acid methyl ester (FAME) profile was determined after the transesterification of the lipid fraction by gas–liquid chromatography with a flame ionization detector coupled to a capillary column using a YOUNG IN Chromass 6500 GC System instrument equipped with a split/splitless injector, flame ionization detector (FID), and Zebron-Fame column [33]. The different fatty acids were identified by comparing the relative retention times of the samples of interest with the commercial standards. The results were processed using Clarity DataApex software (version 4.0; Prague, Czech Republic) and expressed as the relative percentage of each fatty acid.
2.3.5 Tocopherols
The tocopherol content was determined following the protocol described by Barros et al. [32]. The analysis was performed using the same HPLC system as described for soluble sugars, coupled with a fluorescence detector (FP-2020; Jasco) programmed for excitation at 290 nm and emission at 330 nm. Tocopherols were identified by chromatographic comparisons with the standards, and the quantification was based on the fluorescence signal response of each standard using the IS method (IS solution in hexane and tocol, 50 µg mL−1). Tocol (Matreya, Pleasant Gap, State College, PA, USA) was used as an IS. The results are expressed in micrograms per 100 g (dry basis).
2.4 Phenolic and related compounds
The phenolic composition of the hydroalcoholic and methanolic extracts of the mycelial biomass was evaluated. For hydroalcoholic extract preparation, mycelial biomass (3 g) was mixed with 15 mL ethanol and water (4:1) and incubated at 50 °C for 45 min [34]. The mixture was then centrifuged at 4400×g at 10 °C for 20 min, and the supernatant was recovered and re-extracted as described previously. Next, the supernatants were combined, and the extract (100 mg mL−1) was diluted in ethanol and water (4:1) to 20, 40, 60, and 80 mg mL−1 and stored at − 20 °C for further analysis. Phenolic compounds were assessed using an HPLC system (Shimadzu, LC-20A Prominence) coupled with a UV–Vis detector (Shimadzu, SPD-20A) [35]. The extracts (40 mg mL−1) were filtered using a 0.22-µm pore membrane, and a 20 µL sample was injected into the equipment. The compounds were detected at 280 and 320 nm.
For methanolic extract preparation, the mycelial biomass (1.5 g) was stirred with methanol and water (4:1, 30 mL) at − 20 °C for 6 h, sonicated for 15 min, and filtered through a Whatman no. 4 filter paper. The residue was then extracted with two additional 30 mL portions of methanol and water solution. The combined extracts were evaporated at 40 °C under reduced pressure to remove methanol. The aqueous phase was subjected to liquid–liquid extraction with diethyl ether (3 × 30 mL) and ethyl acetate (3 × 30 mL). The organic phases were evaporated to dryness at 40 °C, dissolved in water and methanol (4:1, 1 mL), and filtered through a 0.22-μm disposable LC filter disk for HPLC analysis. The phenolic compounds were determined using the above-mentioned UFLC system. The diode-array detection (DAD) was performed using 280 and 370 nm wavelengths.
The phenolic compounds of the hydroalcoholic and methanolic extracts were characterized according to their UV spectra and retention times and compared with authentic standards. For quantitative analysis, calibration curves were prepared using different standard compounds [36]. The results were expressed in μg g−1 (dry basis).
2.5 Total phenolic content
The total phenol content in the mycelial biomass extracts was determined using the Folin–Ciocalteu method [37]. Samples (0.3 mL) of the extracts were mixed with 2.5 mL of 10% Folin–Ciocalteu reagent (volume basis) and 2.0 mL of 7.5 g L−1 sodium carbonate. The mixtures were incubated at 50 °C for 15 min, and the absorbance was determined at 760 nm (SpectraMax Plus 384, Molecular Devices, San Jose, CA, USA). A mixture of ethanol and water (4:1) was used as analytical control. The concentration of phenols was calculated against a gallic acid standard curve (10–700 µM). The results were expressed as μg of gallic acid equivalents (GAE) per 100 mg of mycelial biomass (dry basis).
2.6 Antioxidant activity of mycelial biomass
The antioxidant activity of the hydroalcoholic extracts of the mycelial biomass was determined by DPPH (2,2-diphenyl-1-picrylhydrazyl) free radical scavenging assay, ferric reducing antioxidant power (FRAP), and β-carotene/linoleic acid co-oxidation (BCLA). The extracts were diluted in ethanol and water (4:1) to 20, 40, 60, 80, and 100 mg mL−1. The antioxidant activity of the methanolic extract was determined using the thiobarbituric acid-reactive substance (TBARS) method.
2.6.1 DPPH radical-scavenging activity assay
On a 96-well plate, an aliquot of 0.01 mL of each extract dilution was mixed with 29 mL of 60 μM DPPH prepared in methanol. The mixture was incubated at 22 °C for 30 min in the dark, and the absorbance was measured at 515 nm (SpectraMax Plus 384, Molecular Devices, San Jose, CA, USA). The analytical control was prepared by replacing the extract with ethanol. The concentration necessary to reduce 50% of DPPH free radicals (IC50) in a sample was determined from the correlation between the absorbance and sample concentration [38]. Quercetin was used as a positive control.
2.6.2 Ferric reducing antioxidant power (FRAP) assay
The FRAP reagent was prepared by mixing 25 mL of acetate buffer (300 mM, pH 3.6), 2.5 mL of 10 mM 2,4,6-tri(2-pyridyl)-s-triazine (TPTZ), and 2.5 mL of 20 mM ferrous chloride solution. Next, on a 96-well plate, 0.01 mL of the diluted extracts was mixed with 0.29 mL of FRAP reagent. The reaction mixture was incubated at 35 °C for 30 min, and the absorbance was measured at 595 nm (SpectraMax Plus 384, Molecular Devices, San Jose, CA, USA). The percentage of antioxidant activity was calculated by comparison with a standard curve of ferrous sulfate (500–2000 μM). Trolox was used as a positive control [39].
2.6.3 β-Carotene/linoleic acid co-oxidation (BCLA) assay
On a 96-well plate, an aliquot of 0.02 mL of each extract dilution was mixed with 0.280 mL of BCLA and incubated at 35 °C. For each 10 min up to 120 min of reaction, the absorbance was determined at 470 nm (SpectraMax Plus 384, Molecular Devices, San Jose, CA, USA). Trolox was used as the positive control. The results are expressed as the percentage inhibition of β-carotene oxidation. The reduction in absorbance of the system without antioxidants was considered 100% oxidation [40].
2.6.4 Thiobarbituric acid reactive substances (TBARS) assay
TBARS was performed as described by Kostić et al. [41]. Porcine (Sus scrofa L.) brains were obtained from officially slaughtered animals, dissected, and homogenized in ice-cold Tris–HCl buffer (20 mM, pH 7.4) to yield a 1:2 (m/v) brain tissue homogenate. The brain homogenate was centrifuged for 10 min at 3000 g. An aliquot (100 µL) of the supernatant was incubated with different concentrations of the sample solutions (200 µL) in the presence of FeSO4 (10 mM; 100 µL) and 100 µL ascorbic acid (0.1 mM; 100 µL) at 37 °C for 1 h. The reaction was stopped by adding trichloroacetic acid (28% m/v, 500 µL), followed by thiobarbituric acid (TBA, 2% m/v, 380 µL). The mixture was incubated for 20 min at 80 °C. Finally, the samples were centrifuged at 3000×g for 10 min to remove the precipitated proteins, and the absorbance of the malondialdehyde–thiobarbituric acid complex in the supernatant was determined at 532 nm. The inhibition ratio was calculated using the following equation: inhibition ratio (%) = [(A − B)/A] × 100, where A and B are the absorbance of the control and sample solutions, respectively. The results were expressed as IC50 values (mg mL−1, sample concentration providing 50% antioxidant activity). Trolox (Sigma-Aldrich, St. Louis, MO, USA) was used as a positive control.
2.7 Antimicrobial activity of mycelial biomass
For the antibacterial assays, the methanolic extracts of L. crinitus mycelial biomass were used against the gram-positive bacteria Staphylococcus aureus subsp. aureus Rosenbach (ATCC 11632), Bacillus cereus Frankland and Frankland (clinical isolate), Listeria monocytogenes (Murray et al.) Pirie (ATCC 35152), and the gram-negative bacteria Escherichia coli (Migula) Castellani and Chalmers (ATCC 25922), Salmonella enterica subsp. enterica (ex Kauffmann and Edwards) Le Minor and Popoff serovar Typhimurium (ATCC 13311), Enterobacter cloacae subsp. cloacae (Jordan) Hormaeche, and Edwards, subsp. nov. (ATCC 35030).
For antifungal assays, the methanolic extracts of L. crinitus mycelial biomass were used against six micromycetes, including Aspergillus fumigatus Fresenius (human isolate), Aspergillus niger van Tieghem (ATCC 6275), Aspergillus versicolor (Vuillemin), Tiraboschi (ATCC 11730), Talaromyces funiculosus (Thom) Samson et al. (synonym = Penicillium funiculosum Thom) (ATCC 36839), Trichoderma virens (Miller et al.) von Arx (ATCC 9645), and Penicillium aurantiogriseum Dierckx (synonym = Penicillium verrucosum var. Cyclopium (Westling) Samson, Stolk, & Hadlok) (food isolate).
Microdilution was performed as described by Soković et al. [42]. The results were presented as the extract concentration that completely inhibited microbial growth (i.e., minimum inhibitory concentration, MIC), determined through the colorimetric microbial viability assay, and the minimum bactericidal and fungicidal concentration (MBC and MFC, respectively) values. The food preservatives sodium benzoate (E211) and potassium metabisulfite (E224) and commercial antimicrobials, such as streptomycin for bacteria and bifonazole for fungi, were used as positive controls.
2.8 Statistical analysis
For mycelial biomass yield, the assays were followed by a completely randomized design and consisted of four treatments with ninety replications. All other analytical tests were performed in triplicates. The results were evaluated using analysis of variance (ANOVA), and the differences among the means (p ≤ 0.05) were determined by the Scott–Knott test.
3 Results
3.1 Production of mycelial biomass
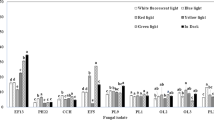
Fungal cultivation under different light wavelengths increased mycelial biomass compared to that under dark cultivation. The most significant increase in the mycelial biomass yield was observed under the blue light (7.2 ± 0.3 g L−1), with an increase of 118% compared to the dark (3.3 ± 0.3 g L−1) cultivation. Moreover, under the green (6.8 ± 0.3 g L−1) and red (4.6 ± 0.3 g L−1) light, cultivations of mycelial biomass increased by 106% and 39%, respectively.
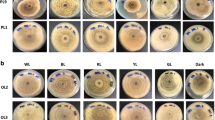
Similarly, mycelial morphology seemed to change under light cultivation compared to dark cultivation (Fig. 1). Here, the mycelial biomass remained in vegetative growth for 21 days. Under light cultivation, primordium or stroma formation was observed in regions of more compact and pigmented mycelia that normally precede the emergence of primordia (Fig. 1). The stroma arose under blue and green light on the fourth day of cultivation, followed by primordium emergence on the seventh day. On the tenth day, the flasks under blue light showed more (9.6 ± 6.0 units) and larger (around 2 cm large) primordia than flasks under green light (8.3 ± 6.0 units). In contrast, mycelial biomass under red light did not form primordia; however, compared to other groups, mycelial biomass under red light presented a greater stroma formation on the 15th day of cultivation with continued stromal growth.
3.2 Chemical characterization of mycelial biomass
Mycelial biomass cultivated under light conditions altered the macronutrient composition (Table 1). Specifically, the carbohydrate content increased under all light wavelengths, mainly under blue and green light. Additionally, mycelial biomass presented higher energy values under blue and green light than under dark cultivation, whereas mycelial biomass showed the lowest energy level under red light. In contrast, light did not affect fat content compared to dark cultivation. Compared to dark cultivation, mycelial biomass cultivation under light conditions decreased the protein content by 33%, 29%, and 15% for the blue, green, and red light, respectively.
Compared to that in dark cultivation, under blue and green light, the mycelial biomass increased soluble sugar contents by 1.1-fold and 1.4-fold, respectively (Table 1). For example, mannitol was 1.2-fold and 1.3-fold higher in mycelial biomass under blue and green light, respectively. However, only green light increased the trehalose content by 1.6-fold compared to that under dark cultivation.
Under dark cultivation, mycelial biomass had a higher organic acid concentration than under light conditions, with a high prevalence of citric and quinic acids (Table 2). Under cultivation in the dark, the mycelial biomass had 1.4-fold more organic acids than in blue and red light cultivation and 1.6-fold more organic acids than in green light cultivation. However, under light cultivation, mycelial biomass presented citric acid as the major organic acid, except under red light, where quinic acid was the major organic acid.
Concerning to the total tocopherol content (Table 3), the mycelial biomass cultivated under green or red light presented the highest concentration, 3.9-fold and 3-fold higher, respectively, than that under dark cultivation. Conversely, the mycelial biomass showed a 1.3-fold lower total tocopherol content under blue light than under dark cultivation. The major tocopherol isomer in mycelial biomass was γ-tocopherol, followed by α-tocopherol. Under green and red light cultivation, mycelial biomass was 4.6-fold and 3.8-fold higher for γ-tocopherol, respectively, than under dark cultivation.
Eighteen fatty acids, including saturated (SFA), monounsaturated (MUFA), and polyunsaturated fatty acids (PUFAs), were identified in mycelial biomass (Table 4). SFAs were predominant, and the highest percentage (66%) was detected in mycelial biomass cultivated under green and red light. However, PUFAs were the most prevalent (26%) under blue light compared to the control and other light cultivation conditions. The major compounds in all light cultivars were palmitic (C16:0), oleic (C18:1n9c), and linoleic (C18:2n6c) acids. However, under light conditions, the mycelial biomass increased, in general, the amount of each fatty acid compared to dark cultivation. For example, the amount of palmitic acid was greater in mycelial biomass cultivation under green and red light (51%) than in the dark (43%) cultivation. Likewise, the amount of linoleic acid was more significant in the mycelial biomass cultivated under blue light (26%) than under green, red light (14%), or dark (21%) cultivation.
3.3 Phenolic and related compounds
Five phenolic compounds were identified in the hydroalcoholic extracts of the mycelial biomass (Table 5). Under dark cultivation, benzoic acid was the predominant phenolic compound. In contrast, gallic acid was the major phenolic compound under light cultivation. The benzoic acid content under dark cultivation was 4-fold, 5.4-fold, and 4.3-fold higher than that under blue, green, and red light cultivation, respectively. Additionally, the gallic acid content of mycelial biomass cultivated under red light was fourfold and twofold higher than that under dark, and blue and green light conditions, respectively. The p-coumaric acid content was also higher under red light cultivation, approximately 1.8-fold higher than under dark cultivation, and 1.6-fold higher than in the other light cultivations. The syringic acid content was also slightly higher under blue light and dark conditions. However, vanillin concentrations did not differ between dark and light cultivation.
In the methanolic extract (Table 5), p-hydroxybenzoic acid was the major compound produced under light cultivation (Table 5). However, the p-hydroxybenzoic acid content in the mycelial biomass cultivated in the dark was almost 3-fold higher than that under blue light and 2.6-fold and 2-fold higher than that under green and red light cultivation, respectively. The mycelial biomass cultivated in the dark also had 1.4-fold higher protocatechuic acid than that under blue and green light and 2.4-fold higher than that under red light cultivation. Although cinnamic acid was present in smaller amounts in all light cultivations, the mycelial biomass cultivated under green light had 1.8-fold more cinnamic acid than that in the dark and more than 3-fold higher levels of cinnamic acid than that under blue and red light cultivation.
The total phenols quantified in the hydroalcoholic extracts of the mycelial biomass cultivated under light conditions ranged from 140 to 300 µg GAE 100 mg−1 (Table 6). The highest phenol content (p ≤ 0.05) was observed in the mycelial biomass cultivated under red light (300 µg GAE 100 mg−1), presenting a 33% increase compared to the control (220 µg GAE 100 mg−1). However, under blue (200 µg GAE 100 mg−1) or green (140 µg GAE 100 mg−1) light cultivation, the phenol content of the mycelial biomass was 13% and 67% lower, respectively, than under dark cultivation.
3.4 Antioxidant activity of mycelial biomass
Cultivation in red light provided the mycelial biomass extracts with the highest (p ≤ 0.05) capacity to scavenge the DPPH radical (76.7 mg mL−1), a 57% increase when compared to cultivation in the dark (120.4 mg mL−1). On the other hand, cultivation under blue and green light reduced the antioxidant activity of the extracts by DPPH method with an IC50 value of 136.1 mg mL−1 and 133.1 mg mL-1, respectively, a 10% reduction compared to under dark cultivation (Table 6).
Cultivation under light conditions also increased antioxidant activity by the FRAP method when compared to the positive control (Trolox) and dark cultivation (Table 6). The highest activity (23.0 µmol Fe+2 g−1) was observed under red light, which was 2.3- and 2.4-fold higher than Trolox (10.5 µmol Fe+2 g−1) and under dark (9.7 µmol Fe+2 g−1) cultivation, respectively. The antioxidant activity by the FRAP method was also higher than that of the control and under blue (11.0 µmol Fe+2 g−1) and green (11.5 µmol Fe+2 g−1) light cultivation, with an increase in the antioxidant activity under dark cultivation by 14% and 19%, respectively, although there were no significant differences (p > 0.05) between them. The mycelial biomass extracts cultivated under light protected 43% (red light) to 72% (blue light) of the β-carotene against oxidation (Table 6). However, the extracts under blue and green light cultivation did not differ (p > 0.05), and under dark cultivation, red light reduced the protective capacity of β-carotene. These results indicate that the antioxidant activity of this method is classified from intermediate to high, showing the great potential of mycelial biomass extracts to avoid lipid oxidation.
3.5 Antimicrobial activity of mycelial biomass
The mycelial biomass extracts showed bacteriostatic activity against all evaluated bacterial strains, with MIC values ranging from 0.5 to 4 mg mL−1 (Table 7). Regarding the commercial additives used, MIC values ranged from 0.5 to 4 mg mL−1 for E211 and E224. Mycelial biomass cultivation under light conditions did not affect the bacteriostatic activity of the extracts, except under red light, where the MIC value against S. aureus was higher than that under dark cultivation. Compared to E211, mycelial biomass under dark, blue, and green light cultivation had MIC values that were 50% lower against S. aureus. On the other hand, mycelial biomass grown in the dark and under all wavelengths had equal MIC values against B. cereus, E. coli, and E. cloacae, and higher values against L. monocytogenes and S. enterica. Compared with E224, mycelial biomass cultivated in the dark and under all light wavelengths had MIC values higher against all bacterial strains, except for B. cereus, where the MIC value was 75% lower. The mycelial biomass extracts showed bactericidal activity against all bacterial strains (Table 7). Mycelial biomass cultivation under all light conditions did not affect the bactericidal activity of the extracts, except under red light, where the MBC value was twofold higher than that under dark cultivation against S. aureus. Furthermore, the mycelial biomass extracts showed lower killing effects than streptomycin (MIC range from 0.04 to 0.2 mg mL − 1) on all bacterial strains (Table 7).
The mycelial biomass extracts were effective against all fungal strains, with MIC values ranging from 0.5 to 1 mg mL−1 (Table 7). The mycelial biomass extracts had lower MIC values for all fungal strains than did bifonazole (Table 7). However, the MIC value of mycelial biomass extracts produced under light wavelengths did not differ from that of dark cultivation. The same tendency was observed for MFC (Table 7). The mycelial biomass extracts were effective against all fungal strains with MFCs ranging from 1 to 2 mg mL−1, while the positive control, bifonazole, exhibited MIC ranging from 0.1 to 0.2 mg mL−1.
4 Discussion
Cultivation under different light conditions affects mycelial biomass growth, chemical composition, and antioxidant and antimicrobial activities. The response system to blue light, known as white-collar (WCC), is already known in fungi, whose proteins function as photoreceptors and transcription factors, affecting the expression of several genes [43]. Homologs of genes encoding WCC system proteins have been described in several basidiomycetes [18]. Red light response systems involving phytochrome receptors were initially described in Aspergillus nidulans. Their function is related to significant changes in gene expression, which culminate in development and secondary metabolism alterations [44]. Lavin et al. [45] evaluated the genomes of 47 basidiomycetes and verified at least one highly conserved phytochrome receptor gene in 41 species. Green light-sensitive receptors have also been described in ascomycetes, and are rhodopsin-like membrane receptors that contain a retinal-like chromophore linked to an opsin apoprotein [46]. However, rhodopsins have been described in a few basidiomycetes, and their function in fungi is still not fully understood [47]. Reports on the fungal response to light and the results of our study on the light response of mycelial biomass indicate that L. crinitus responds to light due to the presence of a photoresponse system that might trigger signal transduction pathways that alter gene expression, affect cellular metabolism, and mycelial biomass growth.
To the best of our knowledge, this study is the first to report the effects of light on mycelial biomass growth in L. crinitus. We verified primordium formation under blue and green light cultivation, indicating a possible trigger for the change in mycelial biomass growth from the vegetative to the reproductive phase. Our results are similar to those reported for P. ostreatus [48], in which blue light cultivation induced fruiting body formation in defined solid medium. In Lentinus tigrinus cultivated under blue light, there was a reduction in the time required for primordium formation and an increase in the number of fruiting bodies [49]. In our study, L. crinitus first primordia appeared on the seventh cultivation day under blue light, with an increase in primordium number after ten cultivation days. Although mycelial biomass was cultivated in liquid medium, the response to light was verified as a shift from vegetative to reproductive mycelia.
Many factors regulate the fungal life cycle, including the presence or absence of light [50]. The effect of light on the transition from vegetative to reproductive mycelial growth has already been described, with blue light reported as the most favorable for fruiting body formation in several species [51]. Blue light is essential for stimulating primordium formation in Cordyceps militaris, whereas red light has the opposite effect [52]. Moreover, blue light is responsible for the transition from primordia to fruiting bodies in Pleurotus eryngii cultivation, acting on signaling and regulating gene expression [53]. Cultivating P. eryngii under blue or red light is responsible for the differential expression of almost 4000 genes that might be involved in morphogenesis, increased respiratory activity, and signal transduction function, among others [21]. During L. crinitus mycelial biomass cultivation under all lights, but not in the dark, water droplets condensed on the flask walls, which may be related to the increase in respiratory activity [21]. Our results suggest that light cultivation, especially blue and green light, induces light-responsive gene action that stimulates L. crinitus primordium formation.
Our results showed an increase in mycelial biomass yield when cultivated under all light conditions, particularly blue light. Damaso et al. [49] evaluated the effect of different light intensities on mycelial biomass growth, fruiting body production, and antioxidant activity of L. tigrinus. They reported that mycelial biomass cultivated under blue light had a larger diameter (89 mm) than that cultivated under red light (81 mm). In contrast, P. eryngii mycelial biomass yield was lower under light cultivation, with a significant reduction under blue light cultivation [54]. According to the authors, the reduction in mycelial biomass may be related to higher blue light energy in the visible spectral range, which is harmful to mycelia [54]. Thus, mycelial biomass growth seems light-wavelength-dependent for each species.
Edible mushrooms are a good source of nutrients, primarily because of their high protein, carbohydrate, and low fat content [55]. In addition, basidiocarps and mycelial biomass have different bioactive compounds and, therefore, have potential as functional foods [56]. Our study is the first to report the effects of light on the chemical composition, secondary metabolism, and antioxidant activity of L. crinitus mycelial biomass.
Our results showed that the protein content of the mycelial biomass (7.9–11.8 g per 100 g) was similar to that found in previous studies with L. crinitus basidiocarps. In the literature, the protein content of L. crinitus basidiocarps varied from 9.8 g [57] to 13.0 g [6] per 100 g, being the pileus (14.4 g per 100 g) richer than the stipe (9.5 g per 100 g) [2]. However, mycelial biomass cultivated under light reduced the protein content of L. crinitus. The effect of light cultivation on protein and amino acid metabolism in basidiomycetes has been previously reported. RNA sequencing studies revealed that Cerrena unicolor under different light conditions had differential gene expression, especially the downregulation of multiple genes, including putative enzymes involved in protein and amino acid synthesis [58]. Transcriptome analysis of P. eryngii primordium differentiated into a fruiting body after blue light stimulation demonstrated that multiple genes related to amino acid biosynthesis are regulated [53].
The soluble sugar content in the mycelial biomass of L. crinitus differed from that of the basidiocarps. Cultivation under light increased the soluble sugar content of mycelial biomass. Previous studies showed that L. crinitus basidiocarps had a trehalose predominance, being 3.13 g per 100 g in the pileus and 3.2 g per 100 g in the stipe [2] and 3.5 g per 100 g in the whole basidiocarp [6]. However, mycelial biomass cultivated under light contained 3- to 4-times more sugars, mostly mannitol. Cultivation under green light resulted in the greatest increase in sugar content in the mycelial biomass, and the trehalose content was 1.5-times higher than that in the basidiocarps. In comparison, the mannitol content was 67-times higher than that of basidiocarps. It is worth noting that the mycelial biomass sample may contain some free sugars from the culture medium that could not be removed during extract preparation [59]. However, the increase in the sugar content after light cultivation indicated a significant difference in this parameter. Mannitol is found in many fungal spores, basidiocarps, and vegetative mycelia. This polyol plays a central role in stress resilience, involving osmoregulation and reactive oxygen species scavengers [60]. For Agaricus bisporus [61] and Volvariella volvacea [62], mannitol accumulation is related to resistance to saline stress and adaptation to low-temperature conditions, respectively. Furthermore, morphogenesis in A. bisporus seems to depend on mannitol metabolism since the enzyme activity involved in sugar metabolism increases during basidiocarp development [61]. Therefore, we can infer that mannitol plays a role in L. crinitus resistance to stress caused by prolonged exposure to light, but this requires further investigation.
These results suggest that light exposure of L. crinitus mycelial biomass affects fungal primary metabolism, reduces protein content, and increases carbohydrates and soluble sugars. Data on light-induced changes in C. unicolor gene expression demonstrated that enzymes involved in carbohydrate and amino acid metabolism were down-regulated [58], which could be associated with changes in primary metabolite production. Transcriptome studies of Pleurotus ostreatus revealed that basidiocarps under blue light cultivation had increased expression of enzymes involved in glycolysis and the pentose phosphate pathway, contributing to more significant growth. In contrast, under red-light cultivation, the activation of these metabolic pathways is weakened [20].
The mycelial biomass of L. crinitus was lower than that of total organic acids than the basidiocarps. Compared with the pileus [2] and the whole basidiocarp [6], the content of organic acids was 4.5- and 4-times lower in mycelial biomass. However, the stipe showed 1.5 times fewer organic acids. In the mycelial biomass, we observed a predominance of citric acid but did not detect malic acid. However, the malic acid content in the basidiocarp was more than 5-times higher, and no citric acid was reported. Despite the differences among the growth stages, the reduction in citric and oxalic acid content when the mycelial biomass was grown in light also suggests a light effect on the primary metabolism of L. crinitus. Less pyruvate is directed toward citric acid biosynthesis when some fungi are cultivated under light [63]. Pawlik et al. [58] observed a downregulation of enzyme expression involved in pyruvate metabolism and the tricarboxylic acid cycle when C. unicolor was cultivated under green light. For L. crinitus, the most significant reduction in organic acid content also occurred under green light cultivation, although blue and red lights also reduced the organic acid content.
Our study also detected the presence of quinic and shikimic acids in mycelial biomass, but these compounds were not found in L. crinitus basidiocarp [2, 6]. Quinic and shikimic acids are metabolically related to aromatic amino acid synthesis. In addition, they are chiral starting materials for the production of influenza virus neuraminidase inhibitors [64]. In the mycelial biomass of L. crinitus, quinic acid was the second most abundant, lower than that of citric acid. Previous studies have reported shikimic acid accumulation in the mycelial biomass of P. ostreatus cultivated under blue light, mainly because of the increased expression of rate-determining enzymes in the shikimate pathway and enzymes in the glycolysis and pentose phosphate pathways [65]. In addition to the many studies on the effect of light on amino acid biosynthesis enzyme expression, these findings may indicate a light effect on the shikimate pathway of L. crinitus.
Vitamin E content in the mycelial biomass of L. crinitus was different from that previously described for basidiocarps. While α- and γ-tocopherol isoforms were detected in the mycelial biomass, only β-tocopherol was detected in the basidiocarp. Because α- and β-tocopherol are produced separately in the tocopherol biosynthesis pathway [66], distinct pathways are active in the fruiting body and mycelial biomass of L. crinitus. Studies have indicated that the isoforms present in different species can vary. In the basidiocarp and mycelial biomass of Pleurotus ostreatoroseus, α- and β-tocopherol were detected [59]; in Laetiporus sulphureus, α-, β-, and γ-tocopherol isoforms were detected [67], whereas in Armillaria mellea, only δ-tocopherol was detected [41]. The tocopherol content in wild and cultivated mushrooms can vary between 50 and 300 µg per 100 g (dry basis) [68]. Mycelial biomass from Lepista nuda cultivated in different culture media was 20–200 µg per 100 g of γ-tocopherol [69]. In P. ostreatoroseus mycelial biomass, the α-tocopherol content was 9 µg per 100 g [59], twice that of L. crinitus mycelial biomass under dark and green light cultivation. The effect of green and red light on increasing tocopherol content and blue light on reducing tocopherols suggests that light affects tocopherol synthesis pathways. The hydroquinone ring of tocopherols is derived from the shikimate pathway [66], which is affected by light in the mycelial biomass of L. crinitus. However, further analysis is required to elucidate the mechanisms underlying the regulation of light.
The fatty acid profile of L. crinitus mycelial biomass differs from that of the basidiocarps. While SFAs prevailed in the mycelial biomass cultivated under dark or light conditions, PUFAs were the predominant class in basidiocarp [2, 6]. Blue light cultivation increased linoleic acid content in the mycelial biomass but reduced oleic acid content. Green and red light cultivation also decreased the MUFA content. Previous studies of ascomycetes have shown that light affects lipid metabolism [70]. In our study, the primary metabolism of L. crinitus was modified by light, and the metabolism of fatty acids was different when the mycelial biomass was cultivated under light. The reducing power required for the fatty acid synthesis pathway depends directly on the availability of acetyl-CoA, which in turn can be produced by citrate conversion. As previously discussed, the tricarboxylic acid pathway is altered by light in L. crinitus, which could be reflected in fatty acid metabolism [43].
Our results showed that light cultivation affected the total phenol content and antioxidant activity of the mycelial biomass. Few studies have evaluated the effects of light on the secondary metabolism of mycelial biomass of basidiomycetes [13]. Cultivation of L. crinitus under red light increased phenol accumulation in the mycelial biomass, whereas blue and green light cultivation had the opposite effect. When L. tigrinus was under light, there was a 3- and fourfold increase in the phenolic content of the mycelial biomass in red and blue light cultivation, respectively, compared to dark cultivation [49]. In Inonotus obliquus, blue and red light reduced the total phenols by 25% and 49%, respectively [71]. In Inonotus rheadae, blue light cultivation increased total phenols, but green and red light reduced phenol content [72]. Comparing the phenol content among fungi is difficult, as phenolic compounds may vary due to different aspects of cultivation, such as light, species or strain, and extraction forms.
Phenolic compounds exhibit extensive antioxidant activity and can act as reducing agents, hydrogen donors, singlet oxygen scavengers, and have metal-chelating potential [73]. Therefore, their accumulation in fungal tissues is of great interest. However, phenol levels may vary among different fungal species and growth stages. Our results demonstrate phenol accumulation in the mycelial biomass of L. crinitus cultivated in a liquid medium under light, a simple and affordable way to produce fungal biomass, which can be an advantageous alternative to mushroom production. However, most studies on fungal antioxidant compounds refer to the fruiting body and only a few of the mycelial biomass, making it difficult to compare the results.
Characterization of phenolic extracts by HPLC showed that light affected the composition of phenolic compounds. Benzoic acid was the most abundant compound in the dark. However, gallic acid was the most abundant under light cultivation conditions. There are no reports on the effect of light on the profile of phenolic compounds in the mycelial biomass. Nevertheless, these findings indicate that light can affect the shikimate pathway by altering the synthesis of secondary metabolites. However, our results are comparable to those of other basidiomycetes grown under dark conditions. Heleno et al. [74] reported the absence of p-coumaric acid in the mycelial biomass of Ganoderma lucidum. However, we identified p-coumaric acid in L. crinitus mycelial biomass under dark and light cultivation, and the highest content was 58.9 µg g−1 in the mycelial biomass under red light cultivation. Carvajal et al. [75] evaluated the antioxidant activity of Agaricus brasiliensis mycelial biomass and fruiting bodies and identified a higher amount of benzoic and syringic acid in the mycelial biomass (4-day cultivation) and gallic acid in the fruiting body and mycelial biomass (8-day cultivation). These compounds have also been identified in the mycelial biomass of L. crinitus.
Our results indicated higher concentrations of gallic acid (180%) and p-coumaric acid (195%) in the mycelial biomass of L. crinitus under red light cultivation than those described in comparative studies of fresh mushrooms and their mycelial biomass [76]. Although the compound concentrations varied, gallic and p-coumaric acids were not detected in Lentinula edodes. In contrast, protocatechuic acid was present in fresh mushroom and mycelial biomass (0.00036 and 0.00073 µg mg−1, respectively). The fresh mushroom and mycelial biomass of A. bisporus contained gallic acid (0.06276 and 0.03077 µg mg−1, respectively) and p-coumaric acid (0.00231 and 0.00366 µg mg−1, respectively).
The antioxidant activity of the mycelial biomass extracts showed that light affected their ability to scavenge DPPH radicals and reduce iron. Red light promoted the highest antioxidant activity among the analyzed methods. The greatest increase in antioxidant activity in the mycelial biomass of L. crinitus cultivated under light was correlated with the total phenol content, indicating that the greater the accumulation of phenols in the mycelial biomass, the lower the half-maximal effective concentration (EC50). DPPH radical reduction depends on the structural conformation of the antioxidant compound, as it interacts by reducing DPPH, and the number of reduced molecules is related to the number of hydroxyls available in the evaluated compound [77]. In gallic acid, the three hydroxyl groups can reduce the six DPPH radicals. Thus, the antioxidant activity of this molecule is associated with its structure [78].
There are very few reports on the effect of light on the antioxidant activity of mycelial biomass, making it difficult to compare results. Damaso et al. [49] evaluated the activity and scavenging potential of DPPH free radicals of L. tigrinus cultivated under LED light and reported that light reduced antioxidant activity. González-Palma et al. [79] evaluated the antioxidant capacity of P. ostreatus at various growth stages, such as in the vegetative mycelium, primordium, and fruiting body, using different aqueous and methanolic extracts. These authors reported that the methanolic extract of the fruiting body and primordium showed a greater ability to reduce DPPH when compared to the mycelial biomass; however, light conditions were only used to stimulate the fructification of P. ostreatus. Dundar et al. [80] evaluated the radical scavenging activity in the mycelial biomass of P. ostreatus in submerged culture, which showed the highest activity at 71.29 mg mL−1 when cultivated in the absence of light. Cultivation of L. crinitus under light increased the iron-reducing power of mycelial biomass, and the most significant increase was observed under red light cultivation. There are no reports on the effect of light in reducing the potential of mycelial biomass extracts from other fungi.
The mycelial biomass extracts of L. crinitus exhibited broad antimicrobial activities. However, cultivation under different light conditions did not affect the biological activity (p > 0.05). Previous studies have demonstrated the antimicrobial activity of extracts from the stipe, pileus [81], and whole basidiocarp of L. crinitus [2]. Both basidiocarp and their parts extracts showed bacteriostatic activity superior to that of mycelial biomass extracts against the same bacteria. The MIC values for the basidiocarp extract, for example, ranged from 0.12 to 0.499 mg mL−1 [2], while the MIC value for the mycelial biomass extract was between 0.5 and 4 mg mL−1. Similar results were observed for the pileus, and the stipe extracts, whose MIC values against the same bacteria ranged from 0.20 to 0.40 mg mL−1 and from 0.125 to 0.40 mg mL−1, respectively [81]. The same tendency was observed for bactericidal, fungistatic, and fungicidal activities of the basidiocarp and its parts compared with the mycelial biomass.
The antimicrobial activity of basidiomycetes may be related to several compounds from primary and secondary metabolism, such as organic acids, phenolic compounds, peptides, and steroids [82]. Although light affects the production of several compounds in the mycelial biomass of L. crinitus, its antimicrobial activity is not affected by light conditions. However, further studies are needed to evaluate the compounds that contribute to the antimicrobial activity of the mycelial biomass.
Our study demonstrated that different light cultivations could increase the number of compounds of interest in the mycelial biomass. This finding opens new perspectives for future studies and provides new alternatives to phenolic compound production from mycelial biomass.
5 Conclusions
The mycelial biomass of L. crinitus under blue or green light cultivation increases the mycelial biomass yield and stimulates primordium and stroma formation. However, blue or green light conditions reduce the protein content and total organic acid production. Under blue light cultivation, carbohydrates, PUFAs, p-coumaric acid, FRAP, and TBARS antioxidant activity increased. Under green light cultivation, energy, carbohydrates, and sugars, such as mannitol, trehalose, γ-tocopherol, SFAs, and cinnamic acid, were increased. Under red light cultivation, SFAs, gallic acid, total phenolic content, and DPPH and FRAP antioxidant activity increased. Under dark cultivation (control), ash, proteins, and total organic acids, such as oxalic, quinic, shikimic, citric acid, α-tocopherol, MUFAs, and phenolic acids, including benzoic, syringic, protocatechuic, p-hydroxybenzoic, and p-coumaric acids increased. The fat content, phenolic acids, such as vanillin, BCLA antioxidant activity, and antimicrobial activity of mycelial biomass were not affected by cultivation under dark or light conditions.
Although photoreceptors have not yet been identified in L. crinitus, light appears to act as a signaling agent that affects primary and secondary fungal metabolism. The effects of different light wavelengths on L. crinitus mycelial biomass production, primordium-inducing morphogenesis, chemical composition, metabolism, and antioxidant activities highlight the need for further studies. Nevertheless, cultivation under different light wavelengths can optimize the production of mycelial biomass and antioxidant compounds to meet the interests of the market and biotechnological, pharmaceutical, and food industries.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Shaffique, S., Kang, S. M., Kim, A. Y., Imran, M., Aaqil Khan, M., & Lee, I. J. (2021). Current knowledge of medicinal mushrooms related to antioxidant properties. Sustainability, 13(14), 7948. https://doi.org/10.3390/su13147948
Bertéli, M. B. D., Barros, L., Reis, F. S., Ferreira, I. C., Glamočlija, J., Soković, M., Valle, J. S., Linde, G. A., Ruiz, S. P., & Colauto, N. B. (2021). Antimicrobial activity, chemical composition and cytotoxicity of Lentinus crinitus basidiocarp. Food & Function, 12(15), 6780–6792. https://doi.org/10.1039/d1fo00656h
Bertéli, M. B. D., Lopes, A. D., Colla, I. M., Linde, G. A., & Colauto, N. B. (2016). Agaricus subrufescens: Substratum nitrogen concentration and mycelial extraction method on antitumor activity. Anais da Academia Brasileira de Ciências, 88, 2239–2246. https://doi.org/10.1590/0001-3765201620160161
Mourão, F., Umeo, S. H., Bertéli, M. B. D., Lourenço, E. L., Junior, A. G., Takemura, O. S., Linde, G. A., & Colauto, N. B. (2011). Anti-inflammatory activity of Agaricus blazei in different basidiocarp maturation phases. Food and Agricultural Immunology, 22(4), 325–3333. https://doi.org/10.1080/09540105.2011.581272
Song, X., Gaascht, F., Schmidt-Dannert, C., & Salomon, C. E. (2020). Discovery of antifungal and biofilm preventative compounds from mycelial cultures of a unique North American Hericium sp. fungus. Molecules, 25(4), 963. https://doi.org/10.3390/molecules25040963
Bertéli, M. B. D., Oliveira Filho, O., Freitas, J. D., Bortolucci, W. C., Silva, G. R., Gazim, Z. C., Lívero, F. A. R., Lovato, E. C. W., Valle, J. S., Linde, G. A., Barros, L., Reis, F. S., Ferreira, I. C. F. R., Paccola-Meirelles, L. D., & Colauto, N. B. (2021). Lentinus crinitus basidiocarp stipe and pileus: Chemical composition, cytotoxicity and antioxidant activity. European Food Research and Technology, 247(6), 1355–1366. https://doi.org/10.1007/s00217-021-03713-1
Ogidi, C. O., Ubaru, A. M., Ladi-Lawal, T., Thonda, O. A., Aladejana, O. M., & Malomo, O. (2020). Bioactivity assessment of exopolysaccharides produced by Pleurotus pulmonarius in submerged culture with different agro-waste residues. Heliyon, 6(12), e05685. https://doi.org/10.1016/j.heliyon.2020.e05685
Zhu, H., Tian, L., Zhang, L., Bi, J., Song, Q., Yang, H., & Qiao, J. (2018). Preparation, characterization and antioxidant activity of polysaccharide from spent Lentinus edodes substrate. International Journal of Biological Macromolecules, 112, 976–984. https://doi.org/10.1016/j.ijbiomac.2018.01.196
Lavelli, V., Proserpio, C., Gallotti, F., Laureati, M., & Pagliarini, E. (2018). Circular reuse of bio-resources: The role of Pleurotus spp. in the development of functional foods. Food & Function, 9(3), 1353–1372. https://doi.org/10.1039/c7fo01747b
Taofiq, O., González-Paramás, A. M., Martins, A., Barreiro, M. F., & Ferreira, I. C. (2016). Mushrooms extracts and compounds in cosmetics, cosmeceuticals and nutricosmetics—A review. Industrial Crops and Products, 90, 38–48. https://doi.org/10.1016/j.indcrop.2016.06.012
Martinez-Medina, G. A., Chávez-González, M. L., Verma, D. K., Prado-Barragán, L. A., Martínez-Hernández, J. L., Flores-Gallegos, A. C., Thakur, M., Srivastav, P. P., & Aguilara, C. N. (2021). Bio-functional components in mushrooms, a health opportunity: Ergothionine and huitlacohe as recent trends. Journal of Functional Foods, 77, 104326. https://doi.org/10.1016/j.jff.2020.104326
Wu, Y., Choi, M. H., Li, J., Yang, H., & Shin, H. J. (2016). Mushroom cosmetics: The present and future. Cosmetics, 3(3), 22. https://doi.org/10.3390/cosmetics3030022
Corrochano, L. M. (2019). Light in the fungal world: From photoreception to gene transcription and beyond. Annual Review of Genetics, 53, 149–170. https://doi.org/10.1146/annurev-genet-120417-031415
Dias, L. P., Pedrini, N., Braga, G. U. L., Ferreira, P. C., Pupin, B., Araújo, C. A. S., Corrochano, L. M., & Rangel, D. E. N. (2020). Outcome of blue, green, red, and white light on Metarhizium robertsii during mycelial growth on conidial stress tolerance and gene expression. Fungal Biology, 124, 263–272. https://doi.org/10.1016/j.funbio.2019.04.007
Dias, L. P., Pupin, B., Roberts, D. W., & Rangel, D. E. N. (2022). Low- or high-white light irradiance induces similar conidial stress tolerance in Metarhizium robertsii. Archives of Microbiology, 204, 83. https://doi.org/10.1007/s00203-021-02730-8
Dias, L. P., Souza, R. K. F., Pupin, B., & Rangel, D. E. N. (2021). Conidiation under illumination enhances conidial tolerance of insect-pathogenic fungi to environmental stresses. Fungal Biology, 125, 891–904. https://doi.org/10.1016/j.funbio.2021.06.003
Yu, Z., & Fischer, R. (2019). Light sensing and responses in fungi. Nature Reviews Microbiology, 17(1), 25–36. https://doi.org/10.1038/s41579-018-0109-x
Wu, B., Xu, Z., Knudson, A., Carlson, A., Chen, N., Kovaka, S., LaButti, K., Lipzen, A., Pennachio, C., Riley, R., Schakwitz, W., Umezawa, K., Ohm, R. A., Grigoriev, I. V., Nagy, L. G., Gibbons, J., & Hibbett, D. (2018). Genomics and development of Lentinus tigrinus: A white-rot wood-decaying mushroom with dimorphic fruiting bodies. Genome Biology and Evolution, 10(12), 3250–3261. https://doi.org/10.1093/gbe/evy246
Araújo, N. L., Avelino, K. V., Halabura, M. I. W., Marim, R. A., Kassem, A. S. S., Linde, G. A., Colauto, N. B., & Valle, J. S. (2021). Use of green light to improve the production of lignocellulose-decay enzymes by Pleurotus spp. in liquid cultivation. Enzyme and Microbial Technology, 149, 109860. https://doi.org/10.1016/j.enzmictec.2021.109860
Wang, H., Tong, X., Tian, F., Jia, C., Li, C., & Li, Y. (2020). Transcriptomic profiling sheds light on the blue-light and red-light response of oyster mushroom (Pleurotus ostreatus). AMB Express, 10(1), 1–10. https://doi.org/10.1186/s13568-020-0951-x
Du, F., Zou, Y., Hu, Q., Zhang, H., & Ye, D. (2020). Comparative transcriptomic analysis reveals molecular processes involved in pileus morphogenesis in Pleurotus eryngii under different light conditions. Genomics, 112(2), 1707–1715. https://doi.org/10.1016/j.ygeno.2019.09.014
López-Legarda, X., Arboleda-Echavarría, C., Parra-Saldivar, R., Rostro-Alanis, M., Alzate, J. F., Villa-Pulgarin, J. A., & Segura-Sánchez, F. (2020). Biotechnological production, characterization and in vitro antitumor activity of polysaccharides from a native strain of Lentinus crinitus. International Journal of Biological Macromolecules, 164, 3133–3144. https://doi.org/10.1016/j.ijbiomac.2020.08.191
Meniqueti, A. B., Ruiz, S. P., Faria, M. G. I., Valle, J. S., Gonçalves, A. C., Jr., Dragunski, D. C., Colauto, N. B., & Linde, G. A. (2021). Iron bioaccumulation in Lentinus crinitus mycelia cultivated in agroindustrial byproducts. Waste and Biomass Valorization, 12(9), 4965–4974. https://doi.org/10.1007/s12649-021-01353-w
Faria, M. G. I., Avelino, K. V., Valle, J. S., Silva, G. J., Gonçalves, A. C., Jr., Dragunski, D. C., Colauto, N. B., & Linde, G. A. (2019). Lithium bioaccumulation in Lentinus crinitus mycelial biomass as a potential functional food. Chemosphere, 235, 538–542. https://doi.org/10.1016/j.chemosphere.2019.06.218
Tavares, M. F., Avelino, K. V., Araújo, N. L., Marim, R. A., Linde, G. A., Colauto, N. B., & Valle, J. S. (2020). Decolorization of azo and anthraquinone dyes by crude laccase produced by Lentinus crinitus in solid state cultivation. Brazilian Journal of Microbiology, 51(1), 99–106. https://doi.org/10.1007/s42770-019-00189-w
Silva, G. T., & Gibertoni, T. B. (2006). Aphyllophorales (Basidiomycota) em áreas urbanas da região metropolitana do Recife, PE, Brasil. Hoehnea, 33(4), 533–543.
Vargas-Isla, R., Ishikawa, N. K., & Py-Daniel, V. (2013). Contribuições etnomicológicas dos povos indígenas da Amazônia. Biota Amazônia, 3(1), 58–65. https://doi.org/10.18561/2179-5746/biotaamazonia.v3n1p58-65
Marim, R. A., Avelino, K. V., Halabura, M. I. W., Araujo, N. L., Santana, T. T., Linde, G. A., Colauto, N. B., & Valle, J. S. (2020). Lentinus crinitus response to blue light on carbohydrate-active enzymes. Bioscience Journal, 36(3), 924–931. https://doi.org/10.14393/BJ-v36n3a2020-49986
Zaghi Junior, L. L., Bertéli, M. B. D., Freitas, J. D. S., Oliveira Filho, O. B. Q., Lopes, A. D., Ruiz, S. P., Valle, J. S., Linde, G. A., & Colauto, N. B. (2020). Five-year cryopreservation at −80 °C of edible and medicinal basidiomycetes by wheat grain technique. Journal of Microbiological Methods, 176, 106030. https://doi.org/10.1016/j.mimet.2020.106030
AOAC-Association of Official Analytical Chemists. (2016). Official methods of analysis of AOAC international. AOAC International.
Ćirić, A., Kruljević, I., Stojković, D., Fernandes, Â., Barros, L., Calhelha, R. C., Ferreira, I. C. F. R., Soković, M., & Glamočlija, J. (2019). Comparative investigation on edible mushrooms Macrolepiota mastoidea, M. rhacodes and M. procera: functional foods with diverse biological activities. Food & Function, 10(12), 7678–7686. https://doi.org/10.1039/c9fo01900f
Barros, L., Pereira, C., & Ferreira, I. C. F. R. (2013). Optimized analysis of organic acids in edible mushrooms from Portugal by ultra fast liquid chromatography and photodiode array detection. Food Analytical Methods, 6(1), 309–316. https://doi.org/10.1007/s12161-012-9443-1
Spréa, R. M., Fernandes, Â., Calhelha, R. C., Pereira, C., Pires, T. C. S. P., Alves, M. J., Canan, C., Barros, L., Amaral, J. S., & Ferreira, I. C. F. R. (2020). Chemical and bioactive characterization of the aromatic plant Levisticum officinale W.D.J. Koch: A comprehensive study. Food & Function, 11, 1292–1303. https://doi.org/10.1039/C9FO02841B
Saltarelli, R., Ceccaroli, P., Iotti, M., Zambonelli, A., Buffalini, M., Casadei, L., Vallorani, L., & Stocchi, V. (2009). Biochemical characterisation and antioxidant activity of mycelium of Ganoderma lucidum from Central Italy. Food Chemistry, 116(1), 143–151. https://doi.org/10.1016/j.foodchem.2009.02.023
Palacios, I., Lozano, M., Moro, C., D’arrigo, M., Rostagno, M. A., Martínez, J. A., & Villares, A. (2011). Antioxidant properties of phenolic compounds occurring in edible mushrooms. Food Chemistry, 128(3), 674–678. https://doi.org/10.1016/j.foodchem.2011.03.085
Fernandes, A., Barros, L., Antonio, A. L., Barreira, J. C. M., Oliveira, M. B. P. P., Martins, A., & Ferreira, I. C. F. R. (2014). Using gamma irradiation to attenuate the effects caused by drying or freezing in Macrolepiota procera organic acids and phenolic compounds. Food and Bioprocess Technology, 7, 3012–3021. https://doi.org/10.1007/s11947-013-1248-8
Singleton, V. L., Orthofer, R., & Lamuela-Raventós, R. M. (1999). Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. In L. Packer (Ed.), Methods in enzymology (1st ed., pp. 152–178). Academic Press. https://doi.org/10.1016/S0076-6879(99)99017-1
Rufino, M. D. S., Alves, R. E., Brito, E. S., Morais, S. M., Sampaio, C. D. G., Pérez-Jimenez, J., & Saura-Calixto, F. D. (2007). Metodologia científica: Determinação da atividade antioxidante total em frutas pela captura do radical livre DPPH. Comunicado Técnico, Embrapa, 127, 1–4.
Rufino, M. D. S., Alves, R. E., Brito, E. S., Morais, S. M., Sampaio, C. D. G., Pérez-Jimenez, J., & Saura-Calixto, F. D. (2006). Metodologia científica: Determinação da atividade antioxidante total em frutas pelo método de redução do ferro (FRAP). Comunicado Técnico, Embrapa, 125, 1–4.
Rufino, M. D. S., Alves, R. E., Brito, E. S., Mancini Filho, J., & Moreira, A. V. B. (2006). Metodologia científica: Determinação da atividade antioxidante total em frutas no sistema beta-caroteno/ácido linoleico. Comunicado Técnico, Embrapa, 126, 1–4.
Kostić, M., Smiljković, M., Petrović, J., Glamočlija, J., Barros, L., Ferreira, I. C. F. R., Ćirić, A., & Soković, M. (2017). Chemical, nutritive composition and a wide range of bioactive properties of honey mushroom Armillaria mellea (Vahl: Fr.) Kummer. Food & Function, 8(9), 3239–3249. https://doi.org/10.1039/c7fo00887b
Soković, M., Glamočlija, J., Marin, P. D., Brkić, D., & van Griensven, L. J. L. D. (2010). Antibacterial effects of the essential oils of commonly consumed medicinal herbs using an in vitro model. Molecules, 15, 7532–7546. https://doi.org/10.3390/molecules15117532
Tisch, D., & Schmoll, M. (2010). Light regulation of metabolic pathways in fungi. Applied Microbiology and Biotechnology, 85, 1259–1277. https://doi.org/10.1007/s00253-009-2320-1
Bayram, Ö., Feussner, K., Dumkow, M., Herrfurth, C., Feussner, I., & Braus, G. H. (2016). Changes of global gene expression and secondary metabolite accumulation during light-dependent Aspergillus nidulans development. Fungal Genetics and Biology, 87, 30–53. https://doi.org/10.1016/j.fgb.2016.01.004
Lavín, J. L., Ramírez, L., Pisabarro, A. G., & Oguiza, J. A. (2015). Genomewide analysis of phytochrome proteins in the phylum Basidiomycota. Journal of Basic Microbiology, 55(9), 1141–1147. https://doi.org/10.1002/jobm.201500078
Dasgupta, A., Fuller, K. K., Dunlap, J. C., & Loros, J. J. (2016). Seeing the world differently: Variability in the photosensory mechanisms of two model fungi. Environmental Microbiology, 18(1), 5–20. https://doi.org/10.1111/1462-2920.13055
Wang, Z., Wang, J., Li, N., Li, J., Trail, F., Dunlap, J. C., & Townsend, J. P. (2018). Light sensing by opsins and fungal ecology: NOP-1 modulates entry into sexual reproduction in response to environmental cues. Molecular Ecology, 27(1), 216–232. https://doi.org/10.1111/mec.14425
Arjona, D., Aragón, C., Aguilera, J. A., Ramírez, L., & Pisabarro, A. G. (2009). Reproducible and controllable light induction of in vitro fruiting of the white-rot basidiomycete Pleurotus ostreatus. Mycological Research, 113(5), 552–558. https://doi.org/10.1016/j.mycres.2008.12.006
Damaso, E. J., Jr., Dulay, R. M. R., Kalaw, S. P., & Reyes, R. G. (2018). Effects of color light emitting diode (led) on the mycelial growth, fruiting body production, and antioxidant activity of Lentinus tigrinus. International Journal of Science and Technology, 3(2), 9–16. https://doi.org/10.22137/ijst.2018.v3n2.02
Corrochano, L. M. (2007). Fungal photoreceptors: Sensory molecules for fungal development and behavior. Photochemical & Photobiological Sciences, 6(7), 725–736. https://doi.org/10.1039/B702155K
Kamada, T., Sano, H., Nakazawa, T., & Nakahori, K. (2010). Regulation of fruiting body photomorphogenesis in Coprinopsis cinerea. Fungal Genetics and Biology, 47(11), 917–921. https://doi.org/10.1016/j.fgb.2010.05.003
Yi, Z. L., Huang, W. F., Ren, Y., Onac, E., Zhou, G. F., Peng, S., Wang, X. J., & Li, H. H. (2014). LED lights increase bioactive substances at low energy costs in culturing fruiting bodies of Cordyceps militaris. Scientia Horticulturae, 175, 139–143. https://doi.org/10.1016/j.scienta.2014.06
Xie, C., Gong, W., Zhu, Z., Yan, L., Hu, Z., & Peng, Y. (2018). Comparative transcriptomics of Pleurotus eryngii reveals blue-light regulation of carbohydrate-active enzymes (CAZymes) expression at primordium differentiated into fruiting body stage. Genomics, 110(3), 201–209. https://doi.org/10.1016/j.ygeno.2017.09.012
Wu, J. Y., Chen, H. B., Chen, M. J., Kan, S. C., Shieh, C. J., & Liu, Y. C. (2013). Quantitative analysis of LED effects on edible mushroom Pleurotus eryngii in solid and submerged cultures. Journal of Chemical Technology & Biotechnology, 88(10), 1841–1846. https://doi.org/10.1002/jctb.4038
Barros, L., Cruz, T., Baptista, P., Estevinho, L. M., & Ferreira, I. C. F. R. (2008). Wild and commercial mushrooms as source of nutrients and nutraceuticals. Food and Chemical Toxicology, 46(8), 2742–2747. https://doi.org/10.1016/j.fct.2008.04.030
Câmara, J. S., Albuquerque, B. R., Aguiar, J., Corrêa, R. C., Gonçalves, J. L., Granato, D., Pereira, J. A. M., Barros, L., & Ferreira, I. C. F. R. (2020). Food bioactive compounds and emerging techniques for their extraction: Polyphenols as a case study. Foods, 10(1), 37. https://doi.org/10.3390/foods10010037
Dávila, G. L. R., Murillo, A. W., Zambrano, F. C. J., Suárez, M. H., & Méndez, A. J. J. (2020). Evaluation of nutritional values of wild mushrooms and spent substrate of Lentinus crinitus (L.) Fr. Heliyon, 6(3), e03502. https://doi.org/10.1016/j.heliyon.2020.e03502
Pawlik, A., Mazur, A., Wielbo, J., Koper, P., Zebracki, K., Kubik-Komar, A., & Janusz, G. (2019). RNA sequencing reveals differential gene expression of Cerrena unicolor in response to variable lighting conditions. International Journal of Molecular Sciences, 20(2), 290. https://doi.org/10.3390/ijms20020290
Corrêa, R. C. G., Souza, A. H. P., Calhelha, R. C., Barros, L., Glamočlija, J., Soković, M., Peralta, R. M., Bracht, A., & Ferreira, I. C. F. R. (2015). Bioactive formulations prepared from fruiting bodies and submerged culture mycelia of the Brazilian edible mushroom Pleurotus ostreatoroseus Singer. Food & Function, 6(7), 2155–2164. https://doi.org/10.1039/c5fo00465a
Meena, M., Prasad, V., Zehra, A., Gupta, V. K., & Upadhyay, R. S. (2015). Mannitol metabolism during pathogenic fungal–host interactions under stressed conditions. Frontiers in Microbiology, 6, 1019. https://doi.org/10.3389/fmicb.2015.01019
Stoop, J. M., & Mooibroek, H. (1998). Cloning and characterization of NADP-mannitol dehydrogenase cDNA from the button mushroom, Agaricus bisporus, and its expression in response to NaCl stress. Applied and Environmental Microbiology, 64(12), 4689–4696. https://doi.org/10.1128/AEM.64.12.4689-4696.1998
Zhao, X., Yu, C., Zhao, Y., Liu, S., Wang, H., Wang, C., Guo, L., & Chen, M. (2019). Changes in mannitol content, regulation of genes involved in mannitol metabolism, and the protective effect of mannitol on Volvariella volvacea at low temperature. BioMed Research International. https://doi.org/10.1155/2019/1493721
Casas-Flores, S., & Herrera-Estrella, A. (2016). The bright and dark sides of fungal life. In I. S. Druzhinina & C. P. Kubicek (Eds.), The mycota: Environmental and microbial relationships (3rd ed., pp. 41–77). Springer. https://doi.org/10.1007/978-3-319-29532-9_3
Liu, J. Y., Chang, M. C., Meng, J. L., Feng, C. P., Zhao, H., & Zhang, M. L. (2017). Comparative proteome reveals metabolic changes during the fruiting process in Flammulina velutipes. Journal of Agricultural and Food Chemistry, 65(24), 5091–5100. https://doi.org/10.1021/acs.jafc.7b01120
Kojima, M., Kimura, N., & Miura, R. (2015). Regulation of primary metabolic pathways in oyster mushroom mycelia induced by blue light stimulation: Accumulation of shikimic acid. Scientific Reports, 5(1), 1–7. https://doi.org/10.1155/2015/290161
Szarka, A., Tomasskovics, B., & Bánhegyi, G. (2012). The ascorbate-glutathione-α-tocopherol triad in abiotic stress response. International Journal of Molecular Sciences, 13(4), 4458–4483. https://doi.org/10.3390/ijms13044458
Petrović, J., Stojković, D., Reis, F. S., Barros, L., Glamočlija, J., Ćirić, A., Ferreira, I. C. F. R., & Soković, M. (2014). Study on chemical, bioactive and food preserving properties of Laetiporus sulphureus (Bull.: Fr.) Murr. Food & Function, 5(7), 1441–1451.
Kalač, P. (2013). A review of chemical composition and nutritional value of wild-growing and cultivated mushrooms. Journal of the Science of Food and Agriculture, 93(2), 209–218. https://doi.org/10.1002/jsfa.5960
Pinto, S., Barros, L., Sousa, M. J., & Ferreira, I. C. F. R. (2013). Chemical characterization and antioxidant properties of Lepista nuda fruiting bodies and mycelia obtained by in vitro culture: Effects of collection habitat and culture media. Food Research International, 51(2), 496–502. https://doi.org/10.1016/j.foodres.2013.01.009
Chen, C. H., Ringelberg, C. S., Gross, R. H., Dunlap, J. C., & Loros, J. J. (2009). Genome-wide analysis of light-inducible responses reveals hierarchical light signalling in Neurospora. EMBO Journal, 28(8), 1029–1042. https://doi.org/10.1038/emboj.2009.54
Zheng, W., Zhang, M., Zhao, Y., Miao, K., & Jiang, H. (2009). NMR-based metabolomic analysis on effect of light on production of antioxidant phenolic compounds in submerged cultures of Inonotus obliquus. Bioresource Technology, 100(19), 4481–4487. https://doi.org/10.1016/j.biortech.2009.04.027
Gornostai, T. Y. G., Borovskii, G. G., Kashchenko, N. I., & Olennikov, D. N. (2018). Phenolic compounds of Inonotus rheades (Agaricomycetes) mycelium: RP-UPLC-DAD-ESI/MS profile and effect of light wavelength on styrylpyrone content. International Journal of Medicinal Mushrooms, 20(7), 637–645. https://doi.org/10.1615/intjmedmushrooms.2018026595
Balasundram, N., Sundram, K., & Samman, S. (2006). Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chemistry, 99(1), 191–203. https://doi.org/10.1016/j.foodchem.2005.07.042
Heleno, S. A., Barros, L., Martins, A., Queiroz, M. J. R., Santos-Buelga, C., & Ferreira, I. C. F. R. (2012). Fruiting body, spores and in vitro produced mycelium of Ganoderma lucidum from Northeast Portugal: A comparative study of the antioxidant potential of phenolic and polysaccharidic extracts. Food Research International, 46(1), 135–140. https://doi.org/10.1016/j.foodres.2011.12.009
Carvajal, A. E. S., Koehnlein, E. A., Soares, A. A., Eler, G. J., Nakashima, A. T., Bracht, A., & Peralta, R. M. (2011). Bioactives of fruiting bodies and submerged culture mycelia of Agaricus brasiliensis (A. blazei) and their antioxidant properties. LWT-Food Science and Technology, 46(2), 493–499. https://doi.org/10.1016/j.lwt.2011.11.018
Reis, F. S., Barros, L., Martins, A., & Ferreira, I. C. F. R. (2012). Chemical composition and nutritional value of the most widely appreciated cultivated mushrooms: An inter-species comparative study. Food and Chemical Toxicology, 50(2), 191–197. https://doi.org/10.1016/j.fct.2011.10.056
Brand-Williams, W., Cuvelier, M. E., & Berset, C. L. W. T. (1995). Use of a free radical method to evaluate antioxidant activity. LWT-Food Science and Technology, 28(1), 25–30. https://doi.org/10.1016/S0023-6438(95)80008-5
Badhani, B., Sharma, N., & Kakkar, R. (2015). Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. RSC Advances, 5(35), 27540–27557. https://doi.org/10.1039/C5RA01911G
González-Palma, I., Escalona-Buendía, H. B., Ponce-Alquicira, E., Téllez-Téllez, M., Gupta, V. K., Díaz-Godínez, G., & Soriano-Santos, J. (2016). Evaluation of the antioxidant activity of aqueous and methanol extracts of Pleurotus ostreatus in different growth stages. Frontiers in Microbiology, 7, 1099. https://doi.org/10.3389/fmicb.2016.01099
Dundar, A., Okumus, V., Ozdemir, S., & Yildiz, A. (2013). Antioxidant properties of cultured mycelia from four Pleurotus species produced in submerged medium. International Journal of Food Properties, 16(5), 1105–1116. https://doi.org/10.1080/10942912.2011.576793
Bertéli, M. B. D., Souza, M. M. M., Barros, L., Ferreira, I. C. F. R., Glamočlija, J., Soković, M., Dragunski, D. C., Valle, J. S., Ferreira, E. S., Pinto, L. C., Souza, C. O., Ruiz, S. P., Linde, G. A., & Colauto, N. B. (2022). Basidiocarp structures of Lentinus crinitus: An antimicrobial source against foodborne pathogens and food spoilage microorganisms. World Journal of Microbiology and Biotechnology, 38(5), 74. https://doi.org/10.1007/s11274-022-03257-w
Heleno, S. A., Ferreira, I. C. F. R., Esteves, A. P., Ćirić, A., Glamočlija, J., Martins, A., Soković, M., & Queiroz, M. J. R. P. (2013). Antimicrobial and demelanizing activity of Ganoderma lucidum extract, p-hydroxybenzoic and cinnamic acids and their synthetic acetylated glucuronide methyl esters. Food and Chemical Toxicology, 58, 95–100. https://doi.org/10.1016/j.fct.2013.04.02
Acknowledgements
The authors thank the Universidade Paranaense (36900/2020), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brazil (CAPES), Fundação Araucária, and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, 307953/2017-3) for financial support and fellowships. The authors are also grateful to the Foundation for Science and Technology (FCT, Portugal) for financial support from the national funds FCT/MCTES to CIMO (UIDB/00690/2020) and P.I. for the institutional scientific employment program contract for L.B. and A.F. This research was also funded by the Serbian Ministry of Education, Science, and Technological Development (Contract No. 451-03-68/2022-14/200007).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Halabura, M.I.W., Avelino, K.V., Araújo, N.L. et al. Light conditions affect the growth, chemical composition, antioxidant and antimicrobial activities of the white-rot fungus Lentinus crinitus mycelial biomass. Photochem Photobiol Sci 22, 669–686 (2023). https://doi.org/10.1007/s43630-022-00344-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43630-022-00344-7