Abstract
TiO2 particles of high photocatalytic activity immobilised on various substrates usually suffer from low mechanical stability. This can be overcome by the utilisation of an inorganic binder and/or incorporation in a robust hydrophobic matrix based on rare-earth metal oxides (REOs). Furthermore, intrinsic hydrophobicity of REOs may result in an increased affinity of TiO2-REOs composites to non-polar aqueous pollutants. Therefore, in the present work, three methods were used for the fabrication of composite TiO2/CeO2 films for photocatalytic removal of dye Acid Orange 7 and the herbicide monuron, as representing polar and non-polar pollutants, respectively. In the first method, the composition of a paste containing photoactive TiO2 particles and CeCl3 or Ce(NO3)3 as CeO2 precursors was optimised. This paste was deposited on glass by doctor blading. The second method consisted of the deposition of thin layers of CeO2 by spray coating over a particulate TiO2 photocatalyst layer (prepared by drop casting or electrophoresis). Both approaches lead to composite films of similar photoactivity that of the pure TiO2 layer, nevertheless films made by the first approach revealed better mechanical stability. The third method comprised of modifying a particulate TiO2 film by an overlayer based on colloidal SiO2 and tetraethoxysilane serving as binders, TiO2 particles and cerium oxide precursors at varying concentrations. It was found that such an overlayer significantly improved the mechanical properties of the resulting coating. The use of cerium acetylacetonate as a CeO2 precursor showed only a small increase in photocatalytic activity. On the other hand, deposition of SiO2/TiO2 dispersions containing CeO2 nanoparticles resulted in significant improvement in the rate of photocatalytic removal of the herbicide monuron.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Titanium dioxide (TiO2), a non-toxic and inexpensive material, has been systematically utilised as a photocatalyst due to its chemical stability and high photocatalytic efficiency. Among various commercially available TiO2 materials, the powder P25 (Evonik) [1] represents one of the most active photocatalysts thus serves as a benchmark, although there are other photocatalysts developed for more specific applications. Despite its superior properties, layers consisting of TiO2 particles immobilised on various substrates usually suffer from low mechanical stability. This can be overcome by the utilisation of an inorganic binder, made from an appropriate oxide or hydroxide. Due to the intrinsic hydrophobicity of REOs [2,3,4,5], the resulting composite TiO2-REOs layers may exhibit increased affinity to sparingly soluble non-polar aqueous pollutants. Thus, we aimed to develop efficient photocatalytic materials based on TiO2 incorporated in a robust hydrophobic matrix based on rare-earth metal oxides (CeO2, La2O3, Yb2O3) with expected high affinity to non-polar species resulting in the possible application in wastewater treatment. Among various REOs, cerium (IV) oxide (CeO2) has been attracting our interest at first.
Rare-earth oxides are promising candidates for the fabrication of hydrophobic surfaces. Their robustness in applications under various harsh conditions has been demonstrated in several experimental studies [2, 3, 6, 7]. However, doubts remain as to the wettability of REOs. Reports demonstrating their intrinsic hydrophobicity [2,3,4,5] have been countered with claims that they are inherently hydrophilic [8, 9]. Their hydrophobic nature (resulting in water-contact angles exceeding 90°) can be attributed to their unique electronic structure [5] or to the adsorption of volatile organic compounds [8, 9]. Previous studies indicated that the deposition of thin CeO2 films of desired wettability was a challenging task. Moreover, the wettability and other properties of thin ceramic CeO2 films depends on the deposition technique and on associated process parameters.
Besides influencing surface hydrophobicity, CeO2 has been also reported to improve photocatalytic properties when combined with TiO2 [10, 11]. For example, a CeO2/TiO2 composite with CeO2 content 1–10 mol % increased the photocatalytic conversion of toluene up to 3 times when compared to the bare TiO2 [10]. Another study showed that the coating of TiO2 nanoparticles by atomic layer deposition (ALD) of CeO2 resulted in photoactivity enhancement over uncoated TiO2 for the degradation of MB, that was attributed to formation of e−/h+ pair trap centres and reduction of e−/h+ recombination rate [11].
TiO2 thin films consisting of TiO2 particles can be conventionally prepared using a simple doctor blading where a paste of TiO2 particles is blended with additives (e.g. thickeners, binders, surfactants, plasticisers), and such paste is coated on various substrates following by calcination. Another deposition technique is drop casting from aqueous TiO2 particle suspensions [12] and electrophoretic deposition from a suspension of TiO2 particles in methanol [13]. Such fabricated particulate films have high photocatalytic activity but on the other hand might suffer from low mechanical stability.
The present approach of fabrication of composite TiO2/CeO2 layers is thus based on the incorporation of CeO2 precursors (chlorides, nitrates) to the TiO2 paste to be then deposited by doctor blading. Another approach is the deposition of a CeO2 precursor over a TiO2 particulate layer; this method may result also in a significant improvement of mechanical properties. Such overlayer can be deposited by spray pyrolysis of metal salt solutions, a comparatively simple and versatile technique for the preparation of homogeneous thin films of CeO2 [14,15,16,17,18,19]. Alternatively, a CeO2 overlayer can be also deposited by a spray or dip coating of a solution of CeO2 precursor or suspension of CeO2 particles with optional subsequent calcination.
The aim of this work was the fabrication of mechanically stable photoactive composite TiO2/CeO2 layers by modifying particulate TiO2 layers with various CeO2 precursors, or, alternatively, by partial overcoating of such layers with cerium oxides. Photoactivity was evaluated using two model substances, the herbicide monuron as a representative of a non-polar pollutant and the anionic dye Acid Orange 7 (AO7) as a representative of a polar pollutant.
2 Experimental
2.1 Materials/chemicals
Microscope slides (Thermo Scientific, Menzel–Gläser, size 76 × 26 mm2, 1 mm thick) were used as the supporting substrates. An aqueous suspension of TiO2 particles (P25, Aeroxide, Evonik industries, Lot. No.: 616031498) was used for the preparation of TiO2 layers. CeO2 precursors (CeCl3 (Sigma Aldrich, 99.9%), Ce(NO3)3 (Sigma Aldrich, 99%) and Ce(AcAc)3 (Sigma Aldrich, 381403)) and two types of CeO2 particles (“CeO2 nano” (Sigma Aldrich, particle size 25 nm (BET), 544841 and CeO2 (Sigma Aldrich, 99.0%, 22390-F)) were used for the preparation of CeO2 layers.
The chemicals used were absolute ethanol (Penta, > 99.8%), TEOS (Tetraethoxysilan, Sigma Aldrich, 98%, R.G.), colloidal silica (Levasil CS30-125 MS, Vodní Sklo a.s.), HCl (Penta, 35%, A.G.), isopropyl alcohol (Lach-Ner, s.r.o., > 99.99%, G.R.), hydroxyethylcellulose (Fluka Analytical). Monuron (Sigma Aldrich, 99%, Lot. No.: 06805EN) and Acid Orange 7 (Sigma Aldrich, Lot. No:10903HC-485) were used as model pollutants.
2.2 Films preparation
Three methods were used for the fabrication of composite TiO2/CeO2 films. Figure 1 shows an illustrative scheme of these different types of samples.
An illustrative scheme of three types of composite TiO2/CeO2 layers. A paste containing TiO2 and CeO2 particles deposited by doctor blading and calcination at 500 °C (A), CeO2 layer deposited over a particulate TiO2 layer (B) and a composite SiO2/TiO2/CeO2 layer deposited over a particulate TiO2 layer (C)
2.2.1 Doctor blading
A paste was prepared by mixing the following solutions/suspensions. Hydroxyethylcellulose (HEC) (0.5 g in 12.5 ml of distilled water (DW)) was mixed for 5 min. Then the following was added: (i) TiO2 suspension (1 g in 5 ml of DW mixed for 20 min, following by sonication for 5 min.) or (ii) aqueous solutions of CeCl3 at predefined concentrations or (iii) TiO2 suspension (1 g in 5 ml of CeCl3 or Ce(NO3)3 solution (at predefined concentrations) mixed for 20 min, then sonicated for 5 min). Pastes of different molar ratios of CeO2 precursor/TiO2 were deposited on the soda-lime glass by doctor blading. The deposited films were dried at 60 °C and then calcined at 500 °C for 1 h to remove the thickener (HEC) and transform the precursor to CeO2. The thickness of the film was measured by profilometry. Layer thickness was at least 2 μm, which makes it an optically-thick film, in terms of light absorption as shown previously for the case of P25 layer where layer with thickness 2 μm (mass 0.2 mg/cm2) absorbs more than 95% of incident light [20].
2.2.2 Deposition of a CeO2 overlayer over a particulate TiO2 layer
Underlying TiO2 P25 layers were prepared using drop casting of aqueous TiO2 suspension (2.5 g · dm−3) and electrophoretic deposition from methanolic TiO2 suspension (10 g · dm−3) at 10 V [13]. In the case of drop casting, coverage of TiO2 was controlled by the volume of deposited suspension, in the case of electrophoretic deposition coverage of TiO2 was controlled by the electrophoresis time.
CeO2 overlayers were prepared by spray pyrolysis of an aqueous solution of cerium chloride (c = 0.05 mol · dm−3) using a homemade automatic spraying system (air pressure 4 bar, deposition temperature 450 °C). The thickness of the CeO2 layer was 500 nm as measured by profilometry. CeO2 overlayers were also prepared utilising spray and dip coating of cerium acetylacetonate (0.01 mol · dm−3) in ethanol with subsequent calcination at 500 °C for 1 h. Here, thickness of the CeO2 layer was 100 and 200 nm, respectively.
2.2.3 Deposition of a SiO2/TiO2/CeO2 overlayer over a particulate TiO2 layer
Underlying TiO2 P25 layers were prepared using drop casting of TiO2 suspension (2.5 g · dm−3), the coverage amount being 0.5 mg · cm−2. Such prepared underlayers were modified by overlayers comprising of SiO2/TiO2 and cerium acetylacetonate or CeO2 nanoparticles. 3.2 ml of TiO2 P25 aqueous suspension (c = 240 g · dm−3) was added under vigorous stirring to the SiO2 binder consisting of 1.4 ml tetraethoxysilane (TEOS), 2.2 ml colloidal SiO2 (Ludox) and 6.4 ml isopropanol. The mixture was diluted with n-propanol (1:1.35). Nanoparticles of CeO2 were mixed under vigorous stirring in the suspension of TiO2 to get a molar ratio Ce:Ti = 1:40, 1:20 and 1:10. Such suspensions were deposited on the glass substrate by (i) spray coating (airbrush) and by (ii) dip coating (dipping and withdrawal speed was 30 mm min−1 with a 60 s delay) and subsequent annealing at 500 °C for 1 h.
2.3 Film characterisation
The prepared composite films were characterised by XRD, SEM and contact angle measurement. The mechanical stability (adhesion to the substrate) was demonstrated using the Scotch Tape Test (Scotch Magic™ Tape, width 19 mm), UV–VIS spectroscopy and via long term exposure in an aqueous environment.
The photocatalytic activity of the composite films was evaluated using two model substances, the herbicide monuron as a representative of a non-polar pollutant and the anionic dye Acid Orange 7 (AO7) as a representative of a polar pollutant. A magnetically-stirred water-cooled rectangular glass reactor was used [21]. Samples with catalyst layers were irradiated by Sylvania Lynx CFS 11 W BL350 fluorescent UV light tubes. These tubes emit irradiation in the wavelength range from 320 to 390 nm with a maximum at 355 nm. The light intensity was 1.9 mW · cm−2.The initial concentration of AO7 and monuron was 1·10–4 mol dm−3. The initial pH of both model pollutants was 5.7. The irradiated area and the reactor volume were (2.5 × 4) cm2 and 25 ml, respectively. The concentration of AO7 was measured using UV–VIS spectroscopy [21], the concentration of monuron was followed by HPLC analysis [22], employing a Shimadzu modular system Nexera lite with photo diode array detector SPD-M40. A mobile phase methanol/water (60:40, v/v) was applied, with a flow rate of 1 ml min−1 and a LiChrospher 100 RP-18 column (type LiChroCART 125–4, Merck, Germany). The concentration of each pollutant was followed as a function of irradiation time. From the concentration decay the initial degradation rate was calculated as described elsewhere [21].
3 Results
3.1 TiO2/CeO2 films prepared by doctor blading
Table 1 summarizes the surface mass and water-contact angles of composite films of various molar ratios of Ce:Ti. Surprisingly, pure CeO2 layer exhibited a low contact angle of about 25°. In the case of a Ce:Ti ratio 1:1 and 1:2, the contact angle was higher (in the range of 30–60°). When nitrate was used as a precursor, the contact angle was even lower (about 20°). We studied in detail the influence of post-calcination at 500 °C for 1 h in air and following exposure to the air (see Figure S1 in SI). It was observed that immediately after calcination, the contact angle became lower than 5°, nevertheless, within 50 min it increased to almost 50°, which is much higher that the contact angle of as prepared CeO2 layer. These findings support previous claims that the hydrophobicity of REOs is primarily determined by the environment to which the REO surface is exposed [7]. Thus, the measured values in Table 1 cannot be regarded as reflecting the true property of the material.
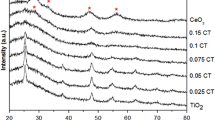
The TiO2/CeO2 films having Ce:Ti ratios of 1:2 and 1:1 showed very good mechanical stability (see Figure S6 in SI). SEM morphology and XRD difractogram of films with Ce:Ti ratio of 1:2 are shown in Figure S2 and Figure S3, respectively. The film contained crystalline CeO2 having a fluorite-type cubic structure, together with anatase and rutile lines corresponding to P25 TiO2 particles. The photocatalytic activity of the prepared films was evaluated based on the degradation of the anionic dye Acid Orange 7 (AO7) in an aqueous solution under UV light irradiation. The time dependence of AO7 concentration during irradiation is shown in Figure S4 (in SI). Films modified with CeO2 exhibited a significant decrease in photocatalytic activity (app. 5 times) compared with films containing only TiO2. This decrease cannot be explained by the lower content of TiO2 in the CeO2 modified film (66% vs. 100% in unmodified film). Anyway, it seems that the amount of CeO2 has to be decreased.
In the next step instead of CeO2 precursors CeO2 particles (BET surface area 47 m2/g) were added to the paste and the Ce:Ti ratio was decreased to 1:10, 1:20 and 1:40.
Table 2 summarises the surface mass of TiO2/CeO2 films of various molar ratios of Ce:Ti. Profilometry of compositeTiO2 layer (see Figure S5 in SI) shows rather smooth character of the layer and thickness around 6–7 μm.
The photocatalytic activity was evaluated using Acid Orange 7 (AO7) as a representative of a polar pollutant and the herbicide monuron as a representative of a non-polar pollutant. Figure 2a and Fig. 2b show the time dependence of AO7 and monuron concentration during irradiation, respectively. Adsorption of monuron on the composite TiO2-CeO2 paste layers (Fig. 2b, open rectangles) was negligible, on the other side adsorption of AO7 (Fig. 2a, open rectangles) was not negligible (decrease of concentration was about 2% after 30 min and then almost constant up to 4 h). Therefore, the measured concentrations of AO7 were corrected for adsorption in the dark and resulting AO7 concentrations are shown as a function of irradiation in Fig. 2a. The photocatalytic activity can be compared via apparent first order rate constant (k, min−1). Another approach for the comparison of photocatalytic activity is via initial degradation rate (ri, nmol · cm−2 · min−1), calculated based on the concentration decay during first 60 min.
a Photocatalytic degradation of AO7 on composite TiO2/CeO2 films of various Ce:Ti ratio prepared by doctor blading. Photocatalyst mass 0.9 – 1.3 mg · cm−2 (see Table 2). b Photocatalytic degradation of monuron on composite TiO2/CeO2 films of various Ce:Ti ratio prepared by doctor blading. Photocatalyst mass 0.9 – 1.3 mg · cm−2 (see Table 2)
The initial degradation rates of monuron and Acid Orange 7 with composite TiO2/CeO2 layers of various Ce:Ti ratios, prepared by doctor blading are shown in Fig. 3. Error bars (calculated from three identical experiments) were included to evaluate the significance of observed differences. For AO7 there is a decrease about 40% for Ce:Ti ratio 1:40 and about 10–20% for Ce:Ti ratio of 1:20 and 1:10. For monuron there is a decrease about 15% for Ce:Ti ratio 1:40, but the difference between various Ce:Ti ratios is within an experimental error.
Initial degradation rate of monuron and Acid Orange 7 (left axis) and the ratio of initial degradation rates of both pollutants (right axis) for composite TiO2/CeO2 layers of various Ce:Ti ratio prepared by doctor blading with CeO2 nano. The irradiated area was 10 cm2. Photocatalyst mass 0.9 – 1.3 mg · cm−2 (see Table 2)
This decrease is not as strong as in the case of pastes with much higher Ce:Ti as shown in Figure S4 (in SI). Figure 3 also shows the ratio of initial degradation rates of both pollutants, it can be seen that this parameter is around 2 and does not depend on the Ce:Ti ratio. All in all, for this type of films, the addition of cerium oxide did not improve the photocatalytic activity for both AO7 and monuron.
3.2 TiO2/CeO2 films - CeO2 overlayers on particulate TiO2 layers
Composite layers consisting of TiO2 particles and CeO2 particles, prepared by doctor blading (3.1) were mechanically stable but exhibited lower photocatalytic activity than pure TiO2 layers. The next approach for the preparation of composite film consisted of two steps. The first step was the deposition of particulate TiO2 layer of known mass and thickness, the second step was the coverage of particulate TiO2 layer by an CeO2 overlayer.
Particulate TiO2 layers were prepared by drop casting and electrophoretic deposition from suspension of TiO2 particles (P25). To observe the adhesion and cohesion of particles, a fixed area of samples (5 cm2) was covered with a scotch tape, then a weight was put for a fixed time on the tape and then the tape was removed. In general, the adhesion of TiO2 layers to the substrate decreased with the increasing deposited amount of TiO2. It was found previously that the deposited mass of TiO2 higher than 0.5 mg · cm−2 does not improve photocatalytic activity [20]. Therefore, layer of such mass was investigated in detail. The results of the Scotch tape test (the surface images of TiO2 layer and corresponding tape after the scotch tape test) are shown for both nanoparticulate layers of the same deposit mass (0.5 mg · cm−2) and thickness (4.5 μm) in Figure S7 (in SI). In both cases there were particles that remained on the scotch tape after its removal from the layer. But the images of the films after the Scotch tape test show that the nanoparticulate TiO2 layers prepared by drop casting of TiO2 particles had much better adhesion to the substrate in comparison with TiO2 layer prepared by electrophoretic deposition.
At first, particulate TiO2 films (0.5 mg · cm−2) were covered by an optimised CeO2 coating (thickness 500 nm) utilising spray pyrolysis of CeCl3. The observed photocatalytic activity of composite films with CeO2 coating fabricated by spray pyrolysis (shown in Figure S8 in SI) was significantly lower (5 times) than the original particulate TiO2 layer prepared by drop casting. Very poor photocatalytic activity can be explained by the fact that particulate TiO2 films were overcoated by CeO2 films and thus shielded TiO2 from incident light. As CeO2 is also a semiconductor and may contribute to the overall activity of a composite layer, photocatalytic activity of pristine CeO2 layer was also evaluated and found negligible (see Figure S8 in SI).
As a next step, particulate TiO2 films (0.5 mg · cm−2) were covered by a thinner coating (100 and 200 nm) of CeO2 using cerium acetylacetonate as a precursor to make the TiO2 particles less shielded and more accessible to the aqueous media. Spray and dip coating were used for the deposition of this overlayer. Photocatalytic degradation of the herbicide monuron in aqueous solution on such fabricated composite films is shown in Fig. 4. Although the thickness of the CeO2 overlayer was only 100 nm, the resulting composite layer had significantly lower photoactivity than the original particulate TiO2 layer. With increasing thickness of CeO2 overlayer to 200 nm we observed even stronger decrease in photocatalytic activity of the composite layer (method of overlayer deposition (spray or dip coating) does not play any role). Explanation for very poor photocatalytic activity is similar to the case of composite films with an overlayer prepared by spray pyrolysis. Particulate TiO2 films were overcoated by CeO2 films and thus shielded TiO2 from incident light. It should be noted that previous studies showed that a few (less than 12) atomic layers of silica [23] or alumina [24] are sufficient to prevent any photocatalytic activity. Hence, it is quite interesting that some activity is still found with an overcoated layer of 100 nm. This activity may indicate that the overcoating layer is porous and does not fully cover the underlying titania.
The kinetics of photocatalytic degradation of monuron, by particulate TiO2 layers (TNP),overcoated with a CeO2 overlayer of different thickness., Here, the thickness of the TNP was 4.5 μm, and the ceria precursor was 0,01 mol · dm−3 Ce(AcAc)3) in ethanol. The deposition was performed by dip coating or spray coating, following by calcination at 500 °C. Photocatalyst mass 0.5 mg · cm−2
3.3 SiO2/TiO2/CeO2 films - SiO2/TiO2/CeO2 overlayers on a particulate TiO2 layers
The third approach to the preparation of ceria-containing photocatalysts was based on the improvement of the cerium oxide-based overlayer and consisted of two steps: (i) deposition of particulate TiO2 underlayer by drop casting, (ii) deposition of an overlayer consisting of TiO2 particles, a binder based on a mixture of colloidal SiO2 and tetraethoxysilane (TEOS) and cerium acetylacetonate as cerium oxide precursor or CeO2 nanoparticles in various proportions. The ratio of Si:Ti in all overlayers was 2:1 as determined by EDX analysis.
3.3.1 Particulate TiO2 layers (drop casting) modified by an overlayer consisting of TiO2 particles and cerium acetylacetonate in SiO2 binder
A particulate TiO2 layer of known mass (0.5 mg · cm−2) was deposited by drop casting from an aqueous suspension. Then, an overlayer containing cerium oxide precursor was deposited by spray coating (cerium acetylacetonate in ethanol mixed with TiO2 particles and SiO2 binder (ratio Ce:Ti = 1:40)) at room temperature, following by annealing at 500 °C. The thickness of such overlayers was 200 nm as measured by profilometry.
The kinetics of the photocatalytic oxidation of monuron are shown in Fig. 5. A thin layer comprising of nothing but the overlayer deposited on glass showed very small photocatalytic activity (see Fig. 5, black triangles). But when such an overlayer was deposited on the TiO2 layer (prepared by drop casting) (see Fig. 5, open spheres), the photocatalytic activity was found to be better than the original layer (Fig. 5, black diamonds). Another advantage was a significant increase in the mechanical stability of the coating (see Fig. 9).
The concentration of monuron as a function of UV irradiation time on TiO2 photocatalyst coatings modified by an overlayer containing cerium oxide. ○ P25 TiO2 layer (drop casting), ▼ spray-coated overlayer prepared from TiO2 particles, SiO2 binder and Ce(AcAc)3 (200 nm), ♦ P25 TiO2 layer + spray-coated overlayer containing TiO2 particles, SiO2 binder and cerium oxide prepared from Ce(AcAc)3, ∆ P25 TiO2 layer + spray-coated overlayer containing TiO2 particles and SiO2 binder. Photocatalyst mass 0.5 mg · cm−2
The improvement in photoactivity could be due to the presence of CeO2 as well as due to the presence of the SiO2 binder. To clarify this, particulate TiO2 layers were also covered by an overlayer consisting of TiO2 particles, SiO2 binder but without CeO2 precursor. The results clearly showed that the main factor here was the presence of the silica binder such that the addition of Ce(AcAc)3 as CeO2 precursor had hardly any positive effect.
3.3.2 Particulate TiO2 layers (drop casting) modified by an overlayer consisting of TiO2 and CeO2 particles in SiO2 binder
In the next phase, attention was focussed on the integrating CeO2 nanoparticles (instead of CeO2 precursors) with TiO2 particles and SiO2 binder. The resulting TiO2/SiO2/CeO2 suspension was deposited on the glass substrate using spray coating (thickness 200 nm) and dip coating (thickness 250 nm). These two overlayers (200 and 250 nm) were also used for the coverage of TiO2 particulate layers prepared by drop casting (0.5 mg · cm−2). The kinetics of photocatalytic oxidation of monuron on such coatings are shown in Fig. 6.
Concentration of monuron as a function of UV irradiation time of TiO2 photocatalyst coatings modified by overlayer SiO2/TiO2/CeO2 consisting of CeO2 and TiO2 nanoparticles and SiO2 binder. ○ P25 TiO2 layer (drop casting), ◊ spray-coated SiO2/TiO2/CeO2 layer (200 nm), x dip-coated SiO2/TiO2/CeO2 layer (250 nm), ∆ P25 TiO2 layer + spray-coated overlayer containing TiO2 particles and SiO2 binder (250 nm), ■ P25 TiO2 layer + spray-coated SiO2/TiO2/CeO2 overlayer (200 nm), ▲ P25 TiO2 layer + dip-coated SiO2/TiO2/CeO2 overlayer (250 nm). Photocatalyst mass 0.5 mg · cm−2
TiO2/SiO2/CeO2 layers prepared by dip and spray coating on glass exhibit photocatalytic activity (even very small) for monuron degradation, but a more interesting effect was obtained after the deposition of such TiO2/SiO2/CeO2 overlayers on the TiO2 P25 particulate layer. Both composite layers showed significant improvement in photocatalytic degradation of monuron compared to the particulate TiO2 P25 layer. However, in the case of spray coating, the composite film exhibited a similar performance as the TiO2 P25 layer covered by TiO2-SiO2 overlayer without CeO2. No further improvement was indicated with CeO2 addition. But on the other hand, the overlayer deposited by dip coating exhibited much better performance even in comparison with TiO2 P25 layer covered by TiO2-SiO2 overlayer. The possible explanation could be in better penetration of the dip-coated overlayer into the porous TiO2 underlayer. However, the thickness of both overlayer coatings (measured on glass substrate by profilometry) was similar (200 and 250 nm).
In the next step TiO2/SiO2/CeO2 overlayers of various Ce:Ti ratios were deposited by dip coating on the TiO2 P25 particulate layer and used for photocatalytic degradation of monuron and Acid Orange 7. The kinetics of photocatalytic oxidation of monuron on such coatings are shown in Fig. 7. The corresponding first order constants and initial degradation rates are shown in Table S1 (in SI). The comparison of initial degradation rates of both model pollutants on various coatings, namely particulate TiO2 layer, TiO2 layer + SiO2/TiO2 overlayer and TiO2 layer + SiO2/TiO2/CeO2 overalyers containing CeO2 nano and CeO2 particles of different Ce:Ti molar ratio is shown in Fig. 8. Coverage of the TiO2 P25 layer by TiO2-SiO2 overlayer without CeO2 results in the similar increase of initial degradation rates for both AO7 and monuron. But the presence of CeO2 particles in TiO2-SiO2 overlayer exhibits improvement only in the case of monuron which means that the ratio of initial degradation rates of both pollutants significantly increased. The positive effect of the incorporation of CeO2 particles in TiO2-SiO2 overlayer can be explained by increasing the adsorption of hydrophobic compound or by inducing charge separation. It was proved in our recent work that particles of rare-earth oxides (oxides of Er, La, Gd and Ce) incorporated in TiO2-SiO2 coatings assist in photocatalytic degradation of hydrophobic pollutant ciprofloxacin by serving as electron sinks [25]. The later explanation thus seems to be more probable.
Concentration of monuron as a function of UV irradiation time of TiO2 photocatalyst coatings modified by overlayer SiO2/TiO2/CeO2 consisting of CeO2 and TiO2 nanoparticles and SiO2 binder. ○ P25 TiO2 layer (drop casting), ∆ P25 TiO2 layer + SiO2/TiO2 overlayer, ▲ P25 TiO2 layer + SiO2/TiO2/CeO2 overlayer (Ce:Ti = 1:40), ▲ P25 TiO2 layer + SiO2/TiO2/CeO2 overlayer (Ce:Ti = 1:20), ▲ P25 TiO2 layer + SiO2/TiO2/CeO2 overlayer (Ce:Ti = 1:10). Photocatalyst mass 0.5 mg · cm−2
Initial degradation rate of monuron and Acid Orange 7 (left axis) and the ratio of initial degradation rates of both polutants (right axis) for the drop-casted TiO2 particulate layer and that covered by various (dip coated) composite overlayers containing CeO2 nano or CeO2 particles in different Ce:Ti molar ratio in SiO2 binder. The irradiated area was 10 cm2. Photocatalyst mass 0.5 mg · cm−2. (TS means TiO2/SiO2, TSCe-nano means TiO2/SiO2/CeO2 nano and TSCe means TiO2/SiO2/CeO2)
The beneficial effect of silica on the degradation kinetics of the non-polar pollutant monuron could be explained via the “Adsorb & Shuttle” phenomenon in which molecules are being adsorbed in the vicinity of photocatalytic domains and then diffuse to the photocatalytic domains [26]. This phenomenon would assists in adsorbing monuron in close proximity to the photocatalyst. It can be supported by our recent work where we studied the effect of modifying TiO2 with REOs of the lanthanide family (Er, La, Gd, Ce) on the photocatalytic activity towards degrading ciprofloxacin, another model non-polar compound, and show that the hydrophobicity of the silica binder plays an important role in promoting ciprofloxacin degradation [25]. The fact that the presence of silica had a positive effect also on the degradation kinetics of Acid Orange 7 suggests that other mechanisms could be involved. In that context, it is noteworthy that a composite of silica-titania was found to be very efficient in the photocatalytic degradation of the water-soluble dye rhodamine 6G, which tends to adsorb on silica but not on titanium dioxide [27], so apparently the effect of silicon dioxide is not limited to hydrophobic compounds. In addition, we cannot negate the possibility that the effect of silica was due to increasing the surface area of the films. This explanation is in line with the smaller diameter of the silicon dioxide particles.
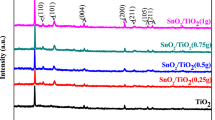
For Ce:Ti ratio 1:20, the influence of two types of CeO2 was investigated. XRD patterns are shown in Figure S9 (in SI), crystal size (calculated form XRD) and BET surface area are given in Table S2 (in SI). Calculated crystal size is similar, 20 nm for “CeO2 nano” particles and 31 nm for CeO2 particles, while BET surface area is for CeO2 particles 8 m2 · g−1, for “CeO2 nano” particles it is 6 times higher (47 m2 · g−1). In the case where CeO2–nano particles were replaced by CeO2 particles, degradation rates of both pollutants are similar. This suggests that neither the particle size of CeO2 nor its surface area plays a significant role.
The stability of the TiO2/SiO2/CeO2 composite layers in photocatalytic applications was verified by repeated photocatalytic degradations of monuron. 5 cycles of repeated use were performed and then the effect of regeneration under UV light irradiation was tested (see Figure S10 in SI). The mechanical stability of composite layers was sufficient (no particle loss was detected during repeated photocatalytic degradation). After 5 cycles of repeated photocatalytic degradation, XRD analysis did not show any change in the phase composition, also SEM morphology of the composite layers did not change (see Figs. S11 and S12 in SI).
3.3.3 Film morphology and mechanical stability
To determine the mechanical stability of the composite TiO2 film, a scotch tape test was performed on the TiO2 particulate layer covered by dip-coated SiO2/TiO2 overlayer containing CeO2 nanoparticles (see Fig. 9b). As shown in Fig. 9, the amount of material transferred to the scotch tape was larger in the case of the particulate layer (Fig. 9a) than that of the composite TiO2 layer (Fig. 9b). This means that there was a significant increase in the mechanical stability of the TiO2 layer following modification with the TiO2-SiO2-CeO2 overlayer.
Scotch tape test. The comparison of mechanical stability of the TiO2 layer (coverage 0.5 mg · cm−2) (A) and composite layer – TiO2 layer partly covered by the TiO2-SiO2-CeO2 overlayer (B), molar ratio of Ce:Ti = 1:20. Numbers in the picture B means: 1 – unmodified part of the layer and 2 – modified part of the layer, i.e. TiO2 layer covered by the TiO2-SiO2-CeO2 overlayer
To quantify the difference in the mechanical stability of the TiO2 layers the transmittance of the films was measured in the range of 300–800 nm, prior to and after performing the scotch tape test. The UV–VIS spectra of the underlying TiO2 particulate layers prepared by drop casting and that of the composite TiO2 particulate layers covered byTiO2-SiO2-CeO2 overlayer of molar ratio 1:20 (Ce:Ti) were corrected for the transmittance of the glass substrate and are shown in Figure S13 (in SI). In the case of the underlying drop-casted TiO2 nanoparticulate layer, there were considerable differences in transmittance, before and after the scotch tape test (Figure S13a). On the other side, in the case of the composite layer (drop casted underlying TiO2 nanoparticulate layer + TiO2-SiO2-CeO2 overlayer), the values of transmittance were almost the same, before and after the scotch tape test (Figure S13b). The modification with an TiO2-SiO2-CeO2 overlayer thus resulted in better adhesion to the substrate and therefore in higher mechanical stability.
The morphology of a drop-casted TiO2 particulate layer and the morphology of the composite TiO2/CeO2 layer consisting of a drop-casted particulate TiO2 layer with a dip-coated SiO2/TiO2/CeO2 overlayer is shown in Fig. 10 and Fig. 11, respectively.
In the case of the particulate TiO2 layer (Fig. 10), one can clearly see a typically open structure consisting of small particles of TiO2 (size around 50 nm). In the case of films containing a SiO2/TiO2/CeO2 overlayer (Fig. 11), one sees only a slight difference in morphology. The size of the TiO2 particles in both films is around 50 nm, but due to the presence of a binder, agglomerates/clusters of particles are observed. This explains the better adhesion to the substrate. Film with different Ce:Ti ratio (1:20 and 1:40) did not show any significant difference in their surface morphology (see Figure S14 in SI).
Particle size around 50 nm is in agreement with the calculated crystalline size of TiO2 (Scherrer equation) determined as 40 ± 20 nm. CeO2 particles present in the overlayer had crystalline size 20 ± 5 nm, but due to the small Ce content (C:/Te ratio = 1:20) and similar size as TiO2, both oxides could not be distinguished on the SEM images. The surface chemical composition of a composite TiO2/CeO2 layer (TiO2 particulate layer modified by SiO2/TiO2/CeO2 overlayer) done by EDX is shown in Table 3. The calculated Ti:Ce ratios were 43, 21 and 11, respectively, which are very close to the Ti:Ce ratios in the suspensions deposited by spray or dip coating. This confirms that the SiO2/TiO2/CeO2 suspension was deposited homogeneously.
From cross-section SEM micrographs it follows that the thickness of the drop-casted TiO2 layer was around 4.5 μm, and the thickness of the composite SiO2/TiO2/CeO2 overlayer was around 300 nm. Profilometry was used as another method for the determination of layer thickness. The profile of drop-casted TiO2 particulate layer (mass of deposit 0.5 mg · cm−2) is shown in Figure S15a (in SI). The profile of a SiO2/TiO2/CeO2 overlayer is shown in Figure S15b. The surface roughness is higher than in the case of layers prepared by doctor blading (see Figure S5 in SI), the measured thickness was lower, 4.5 ± 0.5 μm. Comparison of layer thicknesses (TiO2 particulate layer and SiO2/TiO2/CeO2 overlayer) obtained from SEM and profilometry is shown in Table S3 (in SI). Thicknesses of both layers obtained from SEM analysis and profilometry are in very good agreement.
The film prepared by drop casting with dip or spray-coated overlayer has higher roughness and thus higher surface area than the layer prepared by doctor blading. The difference between photocatalytic performance could be thus primary related to the film architecture which comes from a deposition method.
4 Conclusion
Thin composite films of TiO2 particles embedded in a CeO2 matrix were produced using three methods. In the first approach, paste deposition by doctor blading, it was necessary to optimise the composition of the paste containing photoactive TiO2 and CeCl3, Ce(NO3)3 as precursors of CeO2 or CeO2 particles as well as the process conditions for the preparation of the composite layer. The resulting composite films were mechanically stable; however, the photoactivity was similar to that of pristine TiO2.
In the second approach, thin layers of CeO2 by spray or dip coating were deposited on particulate TiO2 layers (prepared by drop casting and electrophoresis). The mechanical stability of such fabricated composite films in terms of adhesion to the substrate as well as stability in an aqueous environment was found to be satisfactory. But the photoactivity was lower than that of original pure TiO2 films.
In the third approach, a TiO2 layer prepared by drop casting was modified by an overlayer of various molar ratio Ti:Ce (40:1, 20:1 and 10:1) consisting of a binder (colloidal SiO2 and TEOS as a film-forming substance), TiO2 particles and various cerium oxide precursors. The dispersion of TiO2 particles (P25) in the binder system deposited by spray or dip coating on the pre-prepared TiO2 particle layers significantly improved the mechanical properties of the coating. The addition of acetylacetonate to the prepared binder system and its deposition resulted in only small increase in photocatalytic activity. Modification with an overlayer containing a SiO2 binder without CeO2 nanoparticles resulted in significant improvement in the mechanical properties as well as in a 30% increase (in comparison with the original pure TiO2 layer) of initial degradation rates for both AO7 and monuron. Modification with an overlayer containing CeO2 nanoparticles in a SiO2 binder resulted in another 50% increase (in comparison with the TiO2 layer covered by a TiO2/SiO2 overlayer) but only in the case of the herbicide monuron, used as a model compound of a non-polar pollutant.
References
Ohtani, B., Prieto-Mahaney, O. O., Li, D., & Abe, R. (2010). What is Degussa (Evonik) P25? Crystalline composition analysis, reconstruction from isolated pure particles and photocatalytic activity test. Journal of Photochemistry and Photobiology A: Chemistry, 216, 179–182.
Azimi, G., Kwon, H.-M., & Varanasi, K. K. (2014). Superhydrophobic surfaces by laser ablation of rare-earth oxide ceramics. MRS Communications, 4, 95–99.
Bai, M., Kazi, H., Zhang, X., Liu, J., & Hussain, T. (2018). Robust hydrophobic surfaces from suspension HVOF thermal sprayed rare-earth oxide ceramics coatings. Science and Reports, 8, 1–8.
Fronzi, M., Assadi, M. H. N., & Hanaor, D. A. (2019). Theoretical insights into the hydrophobicity of low index CeO2 surfaces. Applied Surface Science, 478, 68–74.
Azimi, G., Dhiman, R., Kwon, H.-M., Paxson, A. T., & Varanasi, K. K. (2013). Hydrophobicity of rare-earth oxide ceramics. Nature materials, 12, 315–320.
Liang, J., Hu, Y., Fan, Y., & Chen, H. (2013). Formation of superhydrophobic cerium oxide surfaces on aluminum substrate and its corrosion resistance properties. Surface and interface analysis, 45, 1211–1216.
P. Singh, K. Srivatsa, On the wettability and optical properties of nanocrystalline CeO 2 thin films, (2016).
Lundy, R., Byrne, C., Bogan, J., Nolan, K., Collins, M. N., Dalton, E., & Enright, R. (2017). Exploring the role of adsorption and surface state on the hydrophobicity of rare earth oxides. ACS applied materials & interfaces, 9, 13751–13760.
Fu, S.-P., Rossero, J., Chen, C., Li, D., Takoudis, C. G., & Abiade, J. T. (2017). On the wetting behavior of ceria thin films grown by pulsed laser deposition. Applied Physics Letters, 110, 081601.
Muñoz-Batista, M. J., Gómez-Cerezo, M. N., Kubacka, A., Tudela, D., & Fernández-García, M. (2014). Role of interface contact in CeO2–TiO2 photocatalytic composite materials. ACS Catalysis, 4, 63–72.
Wang, X., Jin, Y., & Liang, X. (2017). Significant photocatalytic performance enhancement of TiO2 by CeO2 atomic layer deposition. Nanotechnology, 28, 505709.
Paušová, Š, Riva, M., Baudys, M., Krýsa, J., Barbieriková, Z., & Brezová, V. (2019). Composite materials based on active carbon/TiO2 for photocatalytic water purification. Catalysis Today, 328, 178–182.
Waldner, G., & Krysa, J. (2005). Photocurrents and degradation rates on particulate TiO2 layers effect of layer thickness, concentration of oxidizable substance and illumination direction. Electrochimica Acta, 50, 4498–4504.
Souza, J., Silva, A., & Paes, H. (2007). Synthesis and characterization of CeO 2 thin films deposited by spray pyrolysis. Journal of Materials Science-materials in Electronics - J MATER SCI-MATER ELECTRON, 18, 951–956.
Konstantinov, K., Stambolova, I., Peshev, P., Darriet, B., & Vassilev, S. (2000). Preparation of ceria films by spray pyrolysis method. International Journal of Inorganic Materials, 2, 277–280.
Silva, T. G., Ferreira, A., Ribeiro, E., Silveira, E., & Mattoso, N. (2015). Low-defect CeO2 films synthesis by combined spray pyrolysis using different precursors. Applied Physics A, 118, 1489–1494.
Wang, S., Wang, W., Liu, Q., Zhang, M., & Qian, Y. (2000). Preparation and characterization of cerium (IV) oxide thin films by spray prolysis method. Solid State Ionics, 133, 211–215.
Elidrissi, B., Addou, M., Regragui, M., Monty, C., Bougrine, A., & Kachouane, A. (2000). Structural and optical properties of CeO2 thin films prepared by spray pyrolysis. Thin Solid Films, 379, 23–27.
Liu, B., Zhao, X., Zhang, N., Zhao, Q., He, X., & Feng, J. (2005). Photocatalytic mechanism of TiO2–CeO2 films prepared by magnetron sputtering under UV and visible light. Surface Science, 595, 203–211.
Zlamal, M., Krysa, J., & Jirkovsky, J. (2009). Photocatalytic degradation of acid orange 7 on TiO2 films prepared from various powder catalysts. Catalysis Letters, 133, 160–166.
Krýsa, J., Paušová, Š, Zlámal, M., & Mills, A. (2012). Photoactivity assessment of TiO2 thin films using acid orange 7 and 4-chlorophenol as model compounds. Part I: key dependencies. Journal of Photochemistry and Photobiology A: Chemistry, 250, 66–71.
Krysa, J., Waldner, G., Mest’ankova, H., Jirkovsky, J., & Grabner, G. (2006). Photocatalytic degradation of model organic pollutants on an immobilized particulate TiO2 layer - roles of adsorption processes and mechanistic complexity. Applied Catalysis B-Environmental, 64, 290–301.
Nussbaum, M., Shaham-Waldmann, N., & Paz, Y. (2014). Synergistic photocatalytic effect in Fe Nb-doped BiOCl. J Photoch Photobio A, 290, 11–21.
Arbell, N., Bauer, K., & Paz, Y. (2021). Kinetic resolution of racemic mixtures via enantioselective photocatalysis. Acs Applied Materials & Interfaces, 13, 39781–39790.
O. Toker, J. Krysa, Y. Paz, The Effect of Modifying TiO2 with Lanthanides on the Photocatalytic Degradation of Ciprofloxacin, a Hydrophobic Compound, Journal of Photocatalysis, (2022) accepted.
Haick, H., & Paz, Y. (2001). Remote photocatalytic activity as probed by measuring the degradation of self-assembled monolayers anchored near microdomains of titanium dioxide. The Journal of Physical Chemistry B, 105, 3045–3051.
Anderson, C., & Bard, A. J. (1995). An Improved photocatalyst of TiO2/SiO2 prepared by a Sol-Gel synthesis. The Journal of Physical Chemistry, 99, 9882–9885.
Acknowledgements
This work was supported by the Ministry of Education, Youth and Sport of the Czech Republic (project LTAIZ19011) and by the Israeli Ministry of Science (grant No. 3-16075).
Funding
Ministerstvo Školství, Mládeže a Tělovýchovy, LTAIZ19011, Josef Krysa, Israeli Ministry of Science, No. 3-16075, Yaron Paz
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
There are no conflicts to declare.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rusek, J., Baudys, M., Toker, O. et al. Composite TiO2 films modified by CeO2 and SiO2 for the photocatalytic removal of water pollutants. Photochem Photobiol Sci 21, 2127–2138 (2022). https://doi.org/10.1007/s43630-022-00283-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43630-022-00283-3