Abstract
Background
Bone marrow oedema (BMO), seen on magnetic resonance imaging, can be associated with various injuries to the knee but may also occur in asymptomatic athletes. The prevalence and causal factors for these observations are not well understood. The aim of this study was to determine the prevalence of BMO in asymptomatic knees of athletes, competing at a high level, and to investigate the associated factors.
Materials and Methods
Twenty-five asymptomatic university athletes, competing at regional to international level, were recruited. Bilateral knee magnetic resonance imaging was performed in each athlete (total 50 knee scans) at the end of their competitive season. Imaging studies were reported independently by two experienced consultant musculoskeletal radiologists.
Results
There was almost perfect agreement between reporters for diagnosis of BMO (κ = 0.896). Seven participants (28%) were found to have BMO (six in one knee and one bilaterally). The amount of time spent training, during the season, was significantly associated with the appearance of BMO (p < 0.05).
Conclusion
The occurrence of BMO in asymptomatic knees of athletes is common (occurring in over one-quarter of knees) and may be associated with training intensity. This should be considered when treating athletes and deciding upon appropriate treatment plans.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The significance of bone marrow oedema (BMO), in an asymptomatic individual, is controversial [1,2,3,4,5,6]. Although specific oedema patterns have been shown to be indicative of particular injuries in the knee, many studies have shown that BMO is common in athletes and may not be associated with symptoms [1,2,3,4,5,6]. Therefore, the relevance of such findings, when identified in symptomatic patients, remains uncertain.
Some patterns of BMO have been shown to correlate with specific injuries (pivot shift of anterior cruciate ligament rupture = midpoint of lateral femoral condyle and postero-lateral tibial plateau on sagittal images; lateral patellar dislocation = lateral femoral condyle and medial patella facet on axial images; medial collateral ligament injury [valgus stress] = lateral femoral condyle and lateral tibial plateau on coronal images) [7, 8]. However, less specific patterns have also been observed across multiple contacts and non-contact sports [9,10,11].
The predictors for the appearance of BMO in asymptomatic knees of athletes have not been studied. The aim of this prospective study was to determine the prevalence of BMO in the asymptomatic knee joint of athletes playing sports at county, national and international level and to assess associated factors.
Materials and Methods
Review board approval was obtained from the Institutional Ethics Committee (Reference Number: 1/7/08#8). All procedures were performed in accordance with guidelines set-out by the Declaration of Helsinki.
The study was performed in collaboration between the University Sports and Health Science Department and the University Hospital (both located in the South-West of England). 25 asymptomatic athletes were recruited, from a University’s student population, who were currently in full-time training and competition and were asymptomatic of illness and pre-existing knee symptoms. Participants with recent knee trauma, previous knee injury, knee surgery or bleeding disorders were excluded. All participants in this investigation competed at an international, national or county level during their most recent season.
Magnetic resonance imaging (MRI) scans were conducted at the end of each participant’s competitive season so that the effect of training and match play, on the prevalence of BMO, could be measured. Each participant had an MRI scan of both knees (total = 50 scans). Details of the sport played and the amount (and type) of training were collected from each athlete. Rather than attempting to quantify training level and intensity, we chose to group athletes by sport as training is likely to mirror game-play (contact vs. non-contact) and intensity is likely to vary throughout training cycles in the year. Further granulation of the data would also make interpretation more problematic.
A 1.5-Tesla MRI Scanner (Philips, The Netherlands) was used to image all knees. Standard sagittal T1 and proton-density fat-saturated images in three orthogonal planes were acquired. This is the normal knee protocol, at our institution, with the proton-density fat-saturated sequences being highly sensitive for bone marrow oedema. All MRIs were reported, independently, by two experienced consultant musculoskeletal radiologists with particular attention directed to determining the presence of BMO. Any discrepancy in the interpretation of BMO was noted and those scans were reviewed again by two further radiologists. Abnormalities other than BMO were also noted and reported.
As the topic of investigation was novel, no power calculation was performed to determine the necessary sample size.
Statistical analysis was performed using the SPSS Statistics programme (IBM, New York, USA). Inter-observer correlation [Cohen’s kappa (κ)] was used to assess agreement for MRI diagnosis of BMO with the strength of agreement assessed according to the work of Landis and Koch [12]. A multiple logistic regression model was used to identify the factors associated. Subgroup analysis was performed to examine the relationship between cumulative training within sport (years) and time within season training (weeks) with the presence of BMO. In each case, athletes were grouped into bands to aid interpretation. These bands were compared to the variables using Pearson’s correlation co-efficient.
Results
A total of 25 athletes were recruited in the study. All 25 athletes had a MRI scan of both knees. There were 12 males and 13 females. The median age was 21 years (range 19–23 years). The details of all 25 participants and their MRI findings are summarised in Table 1. The majority of recruited athletes were playing rugby (52%). 13 athletes were competing at national or international level with the remainder competing at county level (Table 2). Participants had been competing in their respective sport for a median of 10 years (range 3–16 years). The median number of weeks spent in training, for this particular season, was 34 weeks (range 14–41 weeks).
Level of Agreement on Diagnosis of BMO on MRI Scan
There was agreement on the absence of BMO in 18 patients and the presence of BMO in six patients. In one patient, there was initial disagreement but further review, by two additional radiologists, confirmed BMO. Therefore, a total of seven patients were found to have BMO. Statistical analysis revealed that there was almost perfect agreement on the diagnosis of BMO on MRI scan by the two independent reporters (κ = 0.896). With such a high level of agreement between observers, it is unlikely that the single case examined by all four radiologists had any impact on the results found.
Analysis of Participants’ Sports
BMO was identified in seven out of 25 participants (28%). The male: female ratio was 5:2. BMO was unilateral in six subjects and bilateral in one individual. Thus, the total number of knees with BMO was eight out of 50 knees (16%). Of the seven participants with positive BMO, three were playing at the international level, two at the national level and two at the county level. Five participants were rugby players, one was a netball player and one was a hockey player. Findings of BMO on MRI scan of athletes playing different sports are summarised in Table 3.
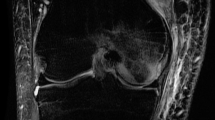
Of the eight knees with BMO, lesions were identified in the anterior of the medial femoral condyle (AMFC) in five knees (62.5%—all in rugby players and all male), in the lateral tibial plateau (LTP) in two knees (25%—one in a female rugby player and one in a male hockey player) and in the trochlea in one patient (12.5%—one female netball player). Six out of the eight knees with BMO (75%) were isolated lesions and, in two knees (25%), there were associated high signals in medial meniscus but no tear. Figure 1 demonstrates an example of BMO in the AMFC of a rugby player.
Associated Variables (Multiple Logistic Regression and Correlation)
The distribution of continuous variables was explored using box plots. Multiple logistic regression was used to explore the influence of variables on the observed outcome of BMO on MRI scan. Our sample size limited the analyses to the three variables with sufficient numbers—age, length of the training period, and total time participating in the sport. Analysis was not possible for the type of sport and individual level of completion due to small sub-group size.
BMO was associated with a longer period spent in training (median 35 weeks; range 34–38 weeks) compared with those with no BMO (median 31 weeks; range 14–41 weeks). This was the most significant associated factor in the regression model (p = 0.048). However, no correlation was found between length of time in sports (p = 0.646) or length of training period within the season (p = 0.404). No difference was found in comparing either age of the athlete or number of years participating in the sport.
The mean data according to age of athlete, years of sports participation and training weeks are summarised in Table 4 with Figs. 2, 3 and 4 graphically showing the tested relationships (box and whisker plots).
Discussion
The main findings of this study were that BMO was found in over one-quarter of athletes, following a season competing at regional to international level, and that the prevalence was associated with an increased training duration in the regression model. BMO was reliably detected with almost perfect inter-observer agreement. No association was found with other tested factors. These findings add to previous published reports suggesting caution in the interpretation of imaging in these athletes.
The increased use of MRI (particularly in high-level athletes) has heralded the discovery of BMO in both symptomatic and asymptomatic individuals. Characterised by increased signal on T2-weighted images and low-signal on T1-weighted images, BMO represents free water in the form of oedema, haemorrhage or an inflammatory response [8]. In the general population, BMO has been shown to be associated with arthritis, inflammatory conditions and tumours and is frequently associated with symptoms [7, 8, 13]. The presence of asymptomatic BMO lesions in non-athletes is not well understood and would require a large longitudinal study to ensure early-stage disease is not the cause. The significance of BMO in athletes is more controversial with studies showing a high prevalence, in asymptomatic individuals, and the mechanisms less well understood [1,2,3,4,5,6, 9, 10]. The alternative term of “bone bruising”, implies that an injury has occurred and thus, perhaps, training should be adjusted. The specific BMO patterns, shown to exist with injuries such as anterior cruciate ligament and patella dislocation [4, 8, 10], may cause anxiety in those treating these athletes and prompt adaptations in training that may be unnecessary and counter-productive. Rather, it is proposed that, in athletes, this may represent normal remodelling, in response to loads regularly exceeding physiological thresholds [4, 10, 11, 14, 15]. Therefore, the interpretation of BMO in athletes remains difficult as the significance of changes may differ from the non-athletic population.
Previous studies, in athletes, have examined both the prevalence of asymptomatic changes and association of loading patterns (both individual events and duration of exposure). Our study found the prevalence of BMO was 28% in 25 participants or 16% in 50 knees. The first description of BMO in asymptomatic athletes, by Brunner et al., found MRI evidence of BMO in 50% (10/20) of professional basketball and collegiate football players [1]. Major et al. found a prevalence of 41%, in collegiate basketball players (before training started) [4], and during the course of training, the prevalence of BMO, in long-distance runners, has been shown to range from 10 to 62.5% [11, 16, 17]. Using modern 3.0-T MRI scanners, the prevalence is seen to be even higher with a recent study showing 75% of NCAA basketball players had BMO changes preseason and 86% post-season [18]. Kornaat and Van de Velde investigated the progression of lesions over the season, by following 16 professional athletes with repeated MRI examination, finding a high prevalence (88%) of asymptomatic BMO as well as change in over a half (58%) of the lesions over the course of the season (with new lesions appearing and old lesions disappearing) [9]. This suggests a dynamic process of injury and repair at a subclinical level.
The effect of training on BMO around the knee joint has been examined. Studies, investigating the effect of completing a marathon, have concluded that a single run does not appear to lead to significant BMO [15, 19]. Krampla et al. found that only one of seven (non-professional) runners, with knee BMO following a marathon, still had changes 6 weeks following the race (and reduction in training) [17]. In a follow-up to this study, 10 years after initial involvement in the study, no permanent damage to the internal structure of the knee joint was found suggesting that transient BMO leads to no lasting changes [20]. However, the continuation of training (as seen with the high-level athletes in the present study) is less well understood. Some previous authors have proposed that structured training may, in fact, be protective against the development of BMO lesions [21]. In a study of Israeli special forces recruits, Hadid et al. found BMO lesions in 26 of 55 recruits (47.3%) at entry and only nine (16.4%) following basic training [21]. The authors concluded that a more balanced training regime may be beneficial compared to the self-directed training that the recruits were undertaking prior to enrolment. The results of the present study show no correlation with the duration of time within the sport or training duration (throughout the season). However, examining median values within a multiple regression model did suggest duration to be a significant factor. These conflicting results are likely resultant from the small sample numbers involved. Similar to a study by Pappas et al. (who showed that asymptomatic BMO prevalence increased over the course of a season) [18], it may be that there is a difference between the BMO seen following unaccustomed exercise in the recruits (showing resolution) and chronic accustomed exposure in athletes (with increasing changes). Further work is needed to examine this relationship.
The level of participation was the next factor we examined. In our study, all of the international-level athletes (despite participating in different sports) had BMO on their MRI scans. Although the small numbers in the present study prevented including the level of participation in our statistical analyses, this may be worth further study to examine whether this is a true risk factor or the effect of additional training associated with competing at this level.
It is difficult to interpret whether it is the severity of impact, or duration of repetitive impacts, that has the greater influence of the appearance of BMO on MRI scan. In our study, five (71.4%) out of seven participants with BMO on MRI scan were rugby players. The prevalence of 38.5% (5 out of 13 rugby players) in this group was almost twice the average in our study (with no BMO seen in the two swimmers in our study). These observations suggest that high impact weight-bearing sports (like rugby) may also be predictors for the appearance of BMO on MRI scan. However, due to the small sample size in our study, it is not possible, to establish whether this is truly a risk factor. Higher body mass would also contribute to the forces involved. This data was not collected for athletes so cannot be commented on directly. However, it is also likely that some adaptation occurs within heavier athletes that may reduce the consequences of accustomed impacts. Other groups, studying sports involving repetitive contact or jumping, show a higher incidence but this direct relationship has not been formally investigated [1, 4, 18].
The distribution of lesions is another area of interest. In our subgroup of rugby players, the BMO lesions were predominantly in the tibiofemoral joint (medial femoral condyle or the lateral tibial plateau). In contrast, 50% of the lesions found in the knees of runners, in the study by Kornaat and Van de Velde, were in the patellofemoral joint [9]. The distribution of this is likely explained by the nature of the activity performed (cutting and change of direction in rugby vs. linear movement in a runner) and links well with previous work focussing on the biomechanics associated with the BMO patterns observed with specific soft-tissue injuries [10]. Furthermore, a recent study of NCAA basketball players showed a prevalence of BMO of 75% pre-season and 86% following a completed season [18]. These changes were found in both the tibiofemoral and patellofemoral joints reflecting the loading patterns seen in jumping sports [18]. Although our group only included two netball players (BMO found in one), it may be that sports involving repetitive jumping on hard surfaces may be a particular risk for BMO. Again, this may be a subject for further research.
One of the strengths of the present study is the variety of sports included. However, this has led to some limitations in the interpretation due to the small sample size in subgroup analyses. In particular, the interpretation of time within the sport and training duration (during the season) is difficult due to the relative small numbers within each banding. Many other factors could have been examined including the athletes’ body mass, training surface and footwear. Each may have had an impact on the results presented. These may be further topics for future research. Finally, the results of this paper do not reflect the incidence of new lesions within the season. The intention was to examine asymptomatic lesions across multiple sports and more specific questions about the incidence of lesions following specific training programmes or within specific athletic groups would be better answered by an alternative study design.
In conclusion, BMO seems to be common in asymptomatic athletes and it should be recognised that these observations might not be related to the clinical complaints of these patients. The results of the present study show that there may be a training effect associated with BMO but further research is required to establish causal relationships. When interpreting MRI scans, it is, therefore, vital to consider the level of competition and the sport participated in. Competitive athletes are likely to have a higher prevalence of BMO, but this should be correlated with both symptoms and typical loading patterns (associated with the individual’s sport) before attributing symptoms to this potentially normal finding. Restricting play and initiating inappropriate therapy have significant effects that could be avoided. This issue is likely to become even more significant as MRI scanners become more powerful and the clinical threshold for imaging decreases with greater portability and accessibility.
References
Brunner, M. C., Flower, S. P., Evancho, A. M., Allman, F. L., Apple, D. F., & Fajman, W. A. (1989). MRI of the athletic knee: Findings in asymptomatic professional basketball and collegiate football players. Investigative Radiology,24, 72–75.
Lazzarini, K. M., Troiano, R. N., & Smith, R. C. (1997). Can running cause the appearance of marrow edema on MR images of the foot and ankle? Radiology,202, 540–542.
Schweitzer, M. E., & White, L. M. (1996). Does altered biomechanics cause marrow edema? Radiology,198, 851–853.
Major, N. M., & Helms, C. A. (2002). MR imaging of the knee: Findings in asymptomatic collegiate basketball players. American Journal of Roentgenology,179, 641–644.
Lovell, G., Galloway, H., Hopkins, W., & Harvey, A. (2006). Osteitis pubis and assessment of bone marrow edema at the pubic symphysis with MRI in an elite junior male soccer squad. Clinical Journal of Sport Medicine,16, 117–122.
Connor, P. M., Banks, D. M., Tyson, A. B., Coumas, J. S., & D’Alessandro, D. F. (2003). Magnetic resonance imaging of the asymptomatic shoulder of overhead athletes: A 5-year follow-up study. American Journal of Sports Medicine,31, 724–727.
Mandalia, V., & Henson, J. H. L. (2008). Traumatic bone bruising—A review article. European Journal of Radiology,67, 54–61.
Baranyay, F. J., Wang, Y., Wluka, A. E., English, D. R., Giles, G. G., Sullivan, R. O., et al. (2007). Association of bone marrow lesions with knee structures and risk factors for bone marrow lesions in the knees of clinically healthy, community-based adults. Seminars in Arthritis and Rheumatism,37, 112–118.
Kornaat, P. R., & Van de Velde, S. K. (2014). Bone marrow edema lesions in the professional runner. American Journal of Sports Medicine,42, 1242–1246.
Patel, S. A., Hageman, J., Quatman, C. E., Wordeman, S. C., & Hewett, T. E. (2014). Prevalence and location of bone bruises associated with anterior cruciate ligament injury and the implications for mechanism of injury: A systematic review. Sports Medicine (Auckland, N. Z.),44, 281–293.
Schueller-Weidekamm, C., Schueller, G., Uffmann, M., & Bader, T. (2006). Incidence of chronic knee lesions in long-distance runners based on training level: Findings at MRI. European Journal of Radiology,58, 286–293.
Landis, J. R., & Koch, G. G. (1977). The measurement of observer agreement for categorical data. Biometrics,33, 159–174.
Guymer, E., Baranyay, F., Wluka, A. E., Hanna, F., Bell, R. J., Davis, S. R., et al. (2007). A study of the prevalence and associations of subchondral bone marrow lesions in the knees of healthy, middle-aged women. Osteoarthritis Cartilage,15, 1437–1442.
Kornaat, P. R., de Jonge, M. C., & Maas, M. (2008). Bone marrow edema-like signal in the athlete. European Journal of Radiology,67, 49–53.
Shellock, F. G., & Mink, J. H. (1991). Knees of trained long-distance runners: MR imaging before and after competition. Radiology,179, 635–637.
Stahl, R., Luke, A., Ma, C. B., Krug, R., Steinbach, L., Majumdar, S., et al. (2008). Prevalence of pathologic findings in asymptomatic knees of marathon runners before and after a competition in comparison with physically active subjects-a 3.0 T magnetic resonance imaging study. Skeletal Radiology,37, 627–638.
Krampla, W., Mayrhofer, R., Malcher, J., Kristen, K. H., Urban, M., & Hruby, W. (2001). MR imaging of the knee in marathon runners before and after competition. Skeletal Radiology,30, 72–76.
Pappas, G. P., Vogelsong, M. A., Staroswiecki, E., Gold, G. E., & Safran, M. R. (2016). Magnetic resonance imaging of asymptomatic knees in collegiate basketball players: The effect of one season of play. Clin J Sports Med,26, 483–489.
Hohmann, E., Wortler, K., & Imhoff, A. B. (2004). MR imaging of the hip and knee before and after marathon running. American Journal of Sports Medicine,32, 55–59.
Krampla, W. W., Newrkla, S. P., Kroener, A. H., & Hruby, W. F. (2008). Changes on magnetic resonance tomography in the knee joints of marathon runners: A 10-year longitudinal study. Skeletal Radiology,37, 619–626.
Hadid, A., Moran, D. S., Evans, R. K., Fuks, Y., Schweitzer, M. E., & Shabshin, N. (2014). Tibial stress changes in new combat recruits for special forces: Patterns and timing at MR imaging. Radiology,273, 483–490.
Funding
No outside funding or financial support was provided for the work performed.
Author information
Authors and Affiliations
Contributions
This submission represents honest and significant work from all authors. KB, VM, CW and PS conceived the idea for the study. VM, KB and RP designed the protocol. AR and DS performed the radiological assessment. JK, VM and RP were involved in the analysis and interpretation of the results. VM and JK wrote the first draft. All authors were involved in the editing of the manuscript and gave approval to the final draft.
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have a conflict of interest to declare.
Ethical standard statement
This article does not contain any studies with human or animal subjects performed by the any of the authors.
Informed consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Rights and permissions
About this article
Cite this article
Mandalia, V., Williams, C., Kosy, J. et al. Bone Marrow Oedema in the Knees of Asymptomatic High-Level Athletes: Prevalence and Associated Factors. JOIO 54, 324–331 (2020). https://doi.org/10.1007/s43465-020-00052-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43465-020-00052-3