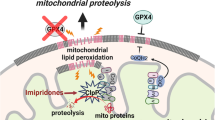
Abstract
Acute myelocytic leukemia seriously impairs the health and lifespan of patients; thus, effective treatment methods for acute myelocytic leukemia need to be urgently determined. This study aimed to investigate the role of calycosin in acute myelocytic leukemia and elucidate its mechanism of action. Cells were treated with calycosin and then subjected to cell counting Kit-8, flow cytometry, and western blot assays to detect cell viability, cells apoptosis, and apoptosis-related proteins, respectively. To demonstrate that calycosin induces ferroptosis in acute myelocytic leukemia cells, the levels of iron ion, lipid- reactive oxygen species (ROS), cysteine, glutathione, and glutathione peroxidase 4 were measured using corresponding kits. The ferroptosis-related genes Ptgs2 and Chac1 were detected using reverse transcription quantitative polymerase chain reaction (RT-qPCR). The solute carrier family 7a member 11 (SLC7A11) and phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)/protein kinase B signaling pathways were analyzed by western blotting. We found that calycosin inhibited cell viability and increased the level of apoptosis in acute myelocytic leuke cells. It also increased the iron ion levels, accompanied by an increase in lipid ROS levels, and increased the expression of ferroptosis-related genes. In contrast, cysteine, glutathione, and glutathione peroxidase 4 expressions, as well as SLC7A11 expression, were decreased by calycosin. Calycosin inhibited PI36/AKT signaling in a dose-dependent manner. However, these effects were reversed by SLC7A11 overexpression. Thus, calycosin can alleviate acute myelocytic leukemia and may be a novel treatment strategy for patients with acute myelocytic leukemia.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute myeloid leukemia (AML) is one of the most common types of leukemia and has a 5-year survival rate of less than 33%. The treatment of AML is challenging. It was estimated that AML affected 1 million people and caused 147,000 deaths worldwide in 2015 (Kouchkovsky and Abdul-Hay 2016). Acute myeloid leukemia is most commonly observed in older adults, and men are more affected than women. Moreover, the survival rate of patients with AML is significantly different for different age groups—approximately 35% for patients under 60 years and 10% for patients over 60 years (Vago and Gojo 2020). Acute myeloid leukemia is characterized by rapidly growing abnormal cells that accumulate in the bone marrow and blood and interfere with normal blood cells. Symptoms include fatigue, shortness of breath, and easy bruising and bleeding, and AML is associated with an increased risk of infection (Rubnitz et al. 2010). Acute myeloid leukemia progresses rapidly and becomes fatal within weeks or months usually, if left untreated. Therefore, it is crucial to identify effective drugs for treating AML.
Calycosin (1) is a natural isoflavone found in Astragalus membranaceus Fisch. ex Bunge (Syn. of A. mongholicus Bunge, Fabaceae), and has anti-oxidative, anti-radiation, anti-cancer, anti-viral, and anti-lipid effects (Deng et al. 2021a, b). Calycosin has been reported to treat myocardial fibrosis via transforming growth factor-beta receptor 1 (TGFBR1) (Chen et al. 2022) and to inhibit the invasion and migration of breast cancer cells (Zhang et al. 2021). Yan et al. (2019) reported that calycosin protects against cerebral ischemia–reperfusion injury through the SIRT1 signaling pathway. However, whether calycosin has a therapeutic effect on AML and its mechanism of action remain unclear. Therefore, this study aimed to determine the effects of calycosin on AML.

Ferroptosis was first identified by Dr. Brent R. Sockwell at the Columbia University in 2012. It is a newly discovered iron-dependent programmed cell death that differs from apoptosis, necrosis, and autophagy (Jiang et al. 2021). Ferroptosis is implicated in the progression of several diseases, and its potential role in Parkinson’s disease has been demonstrated previously (Tian et al. 2020). Ferroptosis has been reported to be a novel therapeutic direction for treating cardiovascular diseases (Fang et al. 2023). Furthermore, it is involved in the development of many cancers (Mou et al. 2019; Zhang et al. 2022). Although the relationship between ferroptosis and AML has been previously studied, it remains unclear whether calycosin causes ferroptosis in AML.
The phosphatidylinositol 3-kinase (PI3K) and protein kinase B (AKT) signaling pathway, which is a classical and important signaling pathway axis (Wang et al. 2022a, b), has been reported to be involved in many physiological processes of diseases, such as metastasis (Chen et al. 2016), cell proliferation (Xie et al. 2019), and glucose metabolism (Hoxhaj and Manning 2020). In addition, the PI3K/AKT pathway has been confirmed to be associated with several conditions in humans, such as fracture healing (Wang et al. 2017), Alzheimer’s disease (Long et al. 2021), and ischemia–reperfusion injury (Feng et al. 2020). PI3K/AKT is activated in various tumors, including AML (Nepstad et al. 2020; He et al. 2021; Wang et al. 2022a, b). The PI3K/AKT signaling pathway is closely related to cell growth, apoptosis, and ferroptosis (Hao et al. 2022; Xu et al. 2023). Research has shown that inhibiting the PI3K/AKT signaling pathway is a key strategy for treating AML (Sandhöfer et al. 2015). In addition, research has shown that calycosin can inhibit the PI3K/AKT signaling pathway in various situations (Xue et al. 2017; Zhu et al. 2022). However, whether calycosin attenuates AML through the PI3K/AKT pathway has not been investigated yet.
Solute carrier family 7 member 11 (SLC7A11) plays a role in regulating amino acid transport (Sato et al. 1999). High levels of SLC7A11 are associated with the malignant behavior of cancer cells (Yan et al. 2023). Meanwhile, studies have shown that inhibition of SLC7A11, a key factor in ferroptosis (Koppula et al. 2021), can inactivate the PI3K/AKT signaling pathway (Zhu et al. 2020; Jiang et al. 2023). Therefore, calycosin may affect AML cell ferroptosis by SLC7A11 through the PI3K/AKT pathway.
Thus, the present study aimed to determine the effect of calycosin on AML and elucidate its underlying mechanisms.
Materials and Methods
Cell Culture and Stimulation
U-937, a human histiocytic lymphoma cell line, was purchased from Procell (Wuhan, China). The cells were cultured in RPMI-1640 medium (Biological Industries, Israel) supplemented with 1% penicillin and streptomycin (P/S) (BI, Israel) and 15% fetal bovine serum (FBS) (Sigma, USA) in an atmosphere of 5% CO2 at 37 °C. Next, U937 cells were treated with 0, 20, 40, and 80 μM (Chang et al. 2023) of calycosin (99.93% purity, Lote S903801, Selleck, China) for 24 h until samples were collected for subsequent assays.
Cell Counting
Cell growth was evaluated using CCK-8 kit (Fcmacs, Nanjing, China). After calycosin treatment, U937 cells were seeded in 96-well plates at 3 × 103 cells and cultured in 10 μl solution for 2 h at 37 °C and 5% CO2 in dark. The OD values were calculated at 450 nm using an ultraviolet spectrophotometer (Infinite Pro; Tecan).
Cell Apoptosis
Calycosin-induced cells (1 × 106) were harvested in a 500 μl solution containing 5 μl Annexin V-FITC and 5 μl propidium Iodide (Beyotime, Shanghai, China) at room temperature in dark for 30 min. Then, cell apoptotic rate was analyzed using flow cytometry (FCM) (C6; Thermo Fisher, USA) with Kaluza analysis software (v.2.1.1.20653; Beckman Coulter, Inc.).
Cell Transfection
To overexpress SLC7A11 in U937 cells, SLC7A11 overexpression plasmid (SLC7A11-plasmid) and control-plasmid were obtained from Santa Cruz Biotechnology (USA) and transfected into cells grown to 60% confluence using jetPRIME (Polyplus, France). Subsequently, cells were collected after culturing for 24 h at 37 °C and 5% CO2.
Iron Assay
Iron levels in U937 cells were measured using an iron assay kit (Novus, USA). Briefly, the culture medium and cells were first digested with trypsin (Thermo Fisher, NY, USA), and then, the samples were harvested and centrifuged at 1000 g for 5 min. Iron assay buffer was co-cultured with the samples for 30 min, and thereafter, the samples were collected after centrifugation at 15,000 g for 5 min. Values were measured using a microplate reader (Wix, China).
Lipid ROS Detection
Lipid ROS levels were measured using BODIPY C11 probe (D3861; Thermo Fisher). Briefly, cells were pretreated with BODIPY C11 for 12 h after calycosin treatment for 24 h or before transfection. Then, lipid ROS in the cells were detected using FCM (C6; Thermo Fisher, USA).
Cysteine, Glutathione, and Glutathione Peroxidase 4 Measurements
The cysteine (ELK9092; ELK Biotechnology), glutathione (GSH, A006-2; Nanjing Jiancheng Biotechnology), and glutathione peroxidase 4 (GPX4, ELK4775; ELK Biotechnology) levels in the cells were measured using corresponding biochemical assay kits after co-culture with calycosin, according to the manufacturer’s instructions. The OD value of each well was determined at 525 nm.
RT-qPCR Assay
Following the manufacturer’s protocol, total RNA was isolated from the cells using TRIzol reagent (Multi Sciences, Hangzhou, China), and cDNA was obtained by reverse transcribing the isolated RNA using an RT-PCR kit (Yeasen, China) and RT-qPCR analysis was conducted using PerfectStart® SYBR qPCR Mix (Vazyme, Nanjing, China). The expression levels were calculated using the 2 − ΔΔCt assay (Livak and Schmittgen 2001). The relevant primer sequences are listed in Table 1.
Western Blot Assay
Calycosin-treated U937 cells were lysed using RIPA buffer (CST, USA). Then, a 10% gel was used to detach the proteins, which were then transferred to PVDF membranes (Whatman, USA). Next, 1 × PBST (Univ, China) and 5% non-fat milk powder (CST, USA) were used to block the PVDF membranes. The PVDF membranes were then cultured for 12 h with primary antibodies against Bcl-2 (ab196495, 1: 1000, Abcam), Bax (#2772, 1: 2000, CST), SLC7A11 (ab175186, 1: 2000, Abcam), PI3K (ab191606, 1: 1000, Abcam), p-PI3K (ab182651, 1: 500, Abcam), AKT (#4691, 1: 3000, CST), p-AKT (#4060 1: 1000, CST), and GAPDH (ab181602, 1: 10,000, Abcam). The membranes were blocked with secondary antibodies (Arigo, Taiwan, China) the following day. The immunoblot pattern was photographed using an image capture system (Wix, USA), and the grayscale value of the target was counted using ImageJ.
Statistical Analysis
The results were analyzed via SPSS v.20.0 (IBM Corp., Armonk, NY, USA) and expressed as mean ± standard deviation (SD) of three independent measurements. Student’s t-test was used to analyze two cohorts, whereas Tukey’s multiple comparison test was used to compare multiple groups. Statistical significance was set at p < 0.05.
Results and Discussion
Apoptosis of Tested Cells
To elucidate the effect of calycosin on AML, U937 cells were treated with different concentrations (0, 20, 40, and 80 μM) of calycosin for 24 h, followed by subsequent assays. The CCK-8 results showed that calycosin inhibited cell viability in a dose-dependent manner, and this inhibitory effect increased with time (Fig. 1A). Further, we found that the apoptotic rate of U937 cells was enhanced by calycosin (Fig. 1B and C). Apoptosis-related proteins were detected using a western blot assay and qPCR. We observed that the protein and mRNA levels of Bcl-2 were decreased in the calycosin-treated group, whereas those of Bax were increased (Fig. 1D–F), which indicated that calycosin inhibited the proliferation of AML cells.
Ferroptosis Induction
Calycosin inhibited the cell viability and increased the level of apoptosis in AML cells. To investigate the mechanism underlying this phenomenon, ferroptosis-related assays were performed. U937 cells were again treated with various concentrations of calycosin for 24 h, and samples were collected for detection. As shown in Fig. 2A and B, the concentrations of iron and Fe2+ increased in the calycosin-treated group in a dose-dependent manner. The FCM assay indicated that the level of lipid ROS was enhanced in the calycosin-treated group, and the ROS accumulation level increased with increasing calycosin concentration (Fig. 2C). The mRNA levels of ferroptosis-related genes (Ptgs2 and Chac1) were increased following calycosin treatment (Fig. 2D and E). At the same time, the levels of Cys, GSH, and GPX4 decreased in the calycosin-treated group (Fig. 2F–H). These results indicated that calycosin induced ferroptosis in AML cells.
Effect of calycosin (1) on ferroptosis in U937 cells. A. The concentrations of iron ions in calycosin-induced U937 cells. B. The level of Fe2+. C. The release of lipid ROS. D and E. The mRNA level of ferroptosis-related gene Chac1 and Ptgs2. F–H. The levels of Cys, GSH, and GPX4. *p < 0.05, **p < 0.01, ***p < 0.001. Data are presented as mean ± SD of three independent experiments
Suppression of SLC7A11
SLC7A11 is a key protein that regulates ferroptosis (Koppula et al. 2021). As shown in Fig. 2, we confirmed that calycosin caused ferroptosis in AML cells. Thus, we attempted to examine the effect of calycosin on the expression level of SLC7A11. Western blotting and RT-qPCR were used to calculate the SLC7A11 levels. We found that calycosin downregulated SLC7A11 expression in a dose-dependent manner (Fig. 3A and B).
PI3K/AKT Pathway Inhibition
To identify the pathway through which calycosin regulates ferroptosis in AML cells, the PI3K/AKT pathway, a classical signaling pathway reported to be associated with ferroptosis, was analyzed using western blotting. U937 cells were treated with 0, 20, 40, and 80 μM of calycosin for 24 h. We observed that calycosin reduced the protein levels of p-PI3K and p-AKT in a dose-dependent manner and decreased the ratios of p-PI3K/PI3K and p-AKT/AKT (Fig. 4A–C). These results suggested that the PI3K/AKT signaling pathway plays a key role in calycosin-stimulated AML cells.
SLC7A11 in Ferroptosis
To demonstrate the role of SLC7A11 in calycosin-induced ferroptosis, we constructed an SLC7A11-plasmid and performed in vitro transfection experiments to overexpress SLC7A11. U937 cells were transfected with control-plasmid and SLC7A11-plasmid for 24 h, and the efficiency of transfection was calculated by western blot assay and RT-qPCR. The results showed that the SLC7A11-plasmid notably increased SLC7A11 expression in U937 cells compared with the control-plasmid (Fig. 5A and B). The SLC7A11 level was measured after U937 cells were treated with 80 μM calycosin and simultaneously transfected with SLC7A11-plasmid. The protein and mRNA levels of SLC7A11 after calycosin treatment was substantially reduced; however, these effects were clearly reversed by the SLC7A11-plasmid (Fig. 5C and D).
SLC7A11-plasmid reversed the effects of calycosin (1) on SLC7A11 expression in U937 cells. A and B. The efficiency of SLC7A11-plasmid transfection. C and D. The protein and mRNA level of SLC7A11 in calycosin-induced U937 cells with or without SLC7A11-plasmid co-transfection. ***p < 0.001 vs. control-plasmid; ###p < 0.001 vs. control; &&&p < 0.001 vs. calycosin + control-plasmid. Data are presented as mean ± SD of three independent experiments
Repression of the PI3K/AKT Pathway
To explore whether calycosin induced ferroptosis in AML cells by affecting the PI3K/AKT pathway through SLC7A11, U937 cells were treated with 80 μM calycosin for 24 h and simultaneously transfected with SLC7A11-plasmid. The concentrations of iron and Fe2+ were increased in the calycosin group compared with the control group; however, the SLC7A11-plasmid disrupted this effect (Fig. 6A and B). The accumulation of lipid ROS was increased after calycosin treatment, but this phenomenon was also reversed by the SLC7A11-plasmid co-transfection (Fig. 6C). The mRNA levels of Chac1 and Ptgs2 were enhanced in the calycosin group, but co-transfection with the SLC7A11-plasmid reduced their expression levels (Fig. 6D and E). The levels of Cys, GSH, and GPX4 were lower in the calycosin group than in the control group, whereas this phenomenon was reversed by SLC7A11-plasmid co-transfection (Fig. 6F–H).
Effects of SLC7A11 on calycosin-induced ferroptosis in U937 cells. A. The level of iron. B. The level of Fe2+ was calculated by co-responding kit. C. The lipid ROS was detected by FCM. D and E. qPCR was used to detect Ptgs2 and Chac1 gene expression. F–H. The level of Cys, GSH, and GPX4 was calculated by corresponding kit. ***p < 0.001 vs. control; ##p < 0.01 vs. calycosin + control-plasmid. Data are presented as mean ± SD of three independent experiments
In comparison with controls, calycosin notably decreased the protein levels of p-PI3K and p-AKT and decreased the ratios of p-PI3K/PI3K and p-AKT/AKT; however, these alterations were significantly reversed after SLC7A11-plasmid co-transfection (Fig. 7A–C). Taken together, these results suggested that calycosin induced ferroptosis in AML cells by affecting the activation of the PI3K/AKT signaling pathway via SLC7A11.
Effect of SLC7A11 on calycosin-induced inhibition of PI3K/AKT in U937 cells. A. The protein level of PI3K, p-PI3K, AKT, and p-AKT. B and C. The ratio of p-PI3K/PI3K and p-AKT/AKT. ***p < 0.001 vs. control; ##p < 0.01, ###p < 0.001 vs. calycosin + control-plasmid. Data are presented as mean ± SD of three independent experiments
The American Cancer Society estimates for leukemia in the US in 2021 were as follows: among 61,090 new cases and 23,660 deaths from all types of leukemia, AML accounted for 20,240 cases, mostly comprising adult patients. Approximately 11,400 deaths were attributed to AML, and almost all these patients were adults (Kayser and Levis 2019). Currently, AML is one of the most common types of leukemia in adults. The goal of AML treatment is to achieve complete remission (CR; bone marrow and blood cell counts return to normal), preferably a complete molecular remission and maintenance (Chopra and Bohlander 2019). In most AML cases, remission occurs in approximately two-thirds of the patients treated with standard induction chemotherapy. If remission is achieved, patients typically undergo additional chemotherapy (consolidation) to remove any remaining leukemic cells (Wojcicki et al. 2020). At present, most treatment methods for AML involve chemotherapy; however, chemotherapy is harmful to patients. Thus, it is crucial to explore new and safe therapies for treating AML.
Our study demonstrated that calycosin inhibited the viability of U937 cells and promoted cell apoptosis. Additionally, calycosin induced ferroptosis in U937 cells by repressing the PI3K/AKT pathway through SLC7A11. Recent studies have shown that calycosin is biologically active in various human diseases. Ma et al. (2022) showed that calycosin ameliorates atherosclerosis by regulating autophagy. Meanwhile, Jin et al. (2022) demonstrated that calycosin optimizes bone loss in a rat model. In addition, previous studies have indicated that calycosin can be a potential treatment for human papillary thyroid carcinoma (Qu et al. 2022). Recently, Huang et al. (2022) reported that calycosin alleviates diabetic nephropathy by regulating ferroptosis. However, to our knowledge, no studies have investigated the effects of calycosin on AML. Therefore, in this study, we aimed to explore the effects of calycosin on AML.
Ferroptosis is a regulated form of cell death characterized by iron-dependent accumulation of lipid peroxides to lethal levels (Liang et al. 2022). Sensitivity to ferroptosis is tightly linked to many biological processes (Li et al. 2020), including amino acid, iron, and polyunsaturated fatty acid metabolism, as well as GSH, phospholipid, NADPH, and coenzyme Q10 biosynthesis. Moreover, ferroptosis is closely related to conditions, such as Alzheimer’s disease (Lei et al. 2021), cancer (Zhao et al. 2022), stroke (Li et al. 2021), and traumatic brain injury (Geng et al. 2021). Ferroptosis is mainly regulated by System Xc- and GSH metabolism, GPX4 activity, and ROS production (Yan et al. 2021). System Xc- is composed of SLC3A2 and SLC7A11 dimers embedded on the surface of the cell membrane. SLC7A11 is the main functional subunit that transports cystine into cells for GSH synthesis (Tang et al. 2021). Therefore, inhibition of SLC7A11 expression induces ferroptosis.
The PI3K/AKT signaling is a critical cytoprotective mechanism, and growing evidence suggested that the PI3K/AKT signaling pathway plays a critical role in human diseases. Yang et al. (2021a) found that betulin target human ovarian cancer cells and inhibit cell migration and invasion via the PI3K/AKT signaling pathway. Wang and Chen (2021) suggested that EMT in oral squamous cell carcinoma cells is inhibited by the inactivation of PI3K/AKT. Deng et al. (2021a, b) demonstrated that the PI3K/AKT pathway is associated with hepatocellular carcinoma, while Yang et al. (2021b) highlighted the key role of the PI3K/AKT signaling pathway in esophageal squamous cell carcinoma. In the present study, we found that calycosin induces ferroptosis via the PI3K/AKT pathway.
Conclusion
We demonstrated that calycosin inhibits the growth of AML cells, increases cell apoptosis, induces ferroptosis, and suppresses PI3K/AKT pathway in AML cells. Therefore, calycosin may serve as a novel therapeutic strategy for AML treatment.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Chang L, Zhang A, Liu W, Cao P, Dong L, Gao X (2023) Calycosin inhibits hepatocyte apoptosis in acute liver failure by suppressing the TLR4/NF-κB pathway: an in vitro study. Immun Inflamm Dis 11:e935. https://doi.org/10.1002/iid3.935
Chen H, Zhou L, Wu X, Li R, Wen J, Sha J, Wen X (2016) The PI3K/AKT pathway in the pathogenesis of prostate cancer. Front Biosci 21:1084–1091. https://doi.org/10.2741/4443
Chen G, Xu H, Xu T, Ding W, Zhang G, Hua Y, Wu Y, Han X, Xie L, Liu B, Zhou Y (2022) Calycosin reduces myocardial fibrosis and improves cardiac function in post-myocardial infarction mice by suppressing TGFBR1 signaling pathways. Phytomedicine 104:154277. https://doi.org/10.1016/j.phymed.2022.154277
Chopra M, Bohlander SK (2019) The cell of origin and the leukemia stem cell in acute myeloid leukemia. Genes Chromosomes Cancer 58:850–858. https://doi.org/10.1002/gcc.22805
De Kouchkovsky I, Abdul-Hay M (2016) Acute myeloid leukemia: a comprehensive review and 2016 update. Blood Cancer J 6:e441. https://doi.org/10.1038/bcj.2016.50
Deng M, Chen H, Long J, Song J, Xie L, Li X (2021a) Calycosin: a review of its pharmacological effects and application prospects. Expert Rev Anti Infect Ther 19:911–925. https://doi.org/10.1080/14787210.2021.1863145
Deng X, Luo T, Li Z, Wang Z (2021b) Design, synthesis and anti-hepatocellular carcinoma activity of 3-arylisoquinoline alkaloids. Eur J Med Chem 228:113985. https://doi.org/10.1016/j.ejmech.2021.113985
Fang X, Ardehali H, Min J, Wang F (2023) The molecular and metabolic landscape of iron and ferroptosis in cardiovascular disease. Nat Rev Cardiol 20:7–23. https://doi.org/10.1038/s41569-022-00735-4
Feng C, Wan H, Zhang Y, Yu L, Shao C, He Y, Wan H, Jin W (2020) Neuroprotective effect of danhong injection on cerebral ischemia-reperfusion injury in rats by activation of the PI3K-Akt pathway. Front Pharmacol 11:298. https://doi.org/10.3389/fphar.2020.00298
Geng Z, Guo Z, Guo R, Ye R, Zhu W, Yan B (2021) Ferroptosis and traumatic brain injury. Brain Res Bull 172:212–219. https://doi.org/10.1016/j.brainresbull.2021.04.023
Hao J, Zhang W, Huang Z (2022) Bupivacaine modulates the apoptosis and ferroptosis in bladder cancer via phosphatidylinositol 3-kinase (PI3K)/AKT pathway. Bioengineered 13:6794–6806. https://doi.org/10.1080/21655979.2022.2036909
He Y, Sun MM, Zhang GG, Yang J, Chen KS, Xu WW, Li B (2021) Targeting PI3K/Akt signal transduction for cancer therapy. Signal Transduct Target Ther 6:425. https://doi.org/10.1038/s41392-021-00828-5
Hoxhaj G, Manning BD (2020) The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat Rev Cancer 20:74–88. https://doi.org/10.1038/s41568-019-0216-7
Huang D, Shen P, Wang C, Gao J, Ye C, Wu F (2022) Calycosin plays a protective role in diabetic kidney disease through the regulation of ferroptosis. Pharm Biol 60:990–996. https://doi.org/10.1080/13880209.2022.2067572
Jiang X, Stockwell BR, Conrad M (2021) Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol 22:266–282. https://doi.org/10.1038/s41580-020-00324-8
Jiang Y, Cui J, Cui M, Jing R (2023) SLC7A11 promotes the progression of gastric cancer and regulates ferroptosis through PI3K/AKT pathway. Pathol Res Pract 248:154646. https://doi.org/10.1016/j.prp.2023.154646
Jin X, Wang H, Liang X, Ru K, Deng X, Gao S, Qiu W, Huai Y, Zhang J, Lai L, Li F, Miao Z, Zhang W, Qian A (2022) Calycosin prevents bone loss induced by hindlimb unloading. NPJ Microgravity 8:23. https://doi.org/10.1038/s41526-022-00210-x
Kayser S, Levis MJ (2019) Clinical implications of molecular markers in acute myeloid leukemia. Eur J Haematol 102:20–35. https://doi.org/10.1111/ejh.13172
Koppula P, Zhuang L, Gan B (2021) Cystine transporter SLC7A11/xCT in cancer: ferroptosis, nutrient dependency, and cancer therapy. Protein Cell 12:599–620. https://doi.org/10.1007/s13238-020-00789-5
Lei P, Ayton S, Bush AI (2021) The essential elements of Alzheimer’s disease. J Biol Chem 296:100105. https://doi.org/10.1074/jbc.REV120.008207
Li J, Cao F, Yin HL, Huang ZJ, Lin ZT, Mao N, Sun B, Wang G (2020) Ferroptosis: past, present and future. Cell Death Dis 11:88. https://doi.org/10.1038/s41419-020-2298-2
Li C, Sun G, Chen B, Xu L, Ye Y, He J, Bao Z, Zhao P, Miao Z, Zhao L, Hu J, You Y, Liu N, Chao H, Ji J (2021) Nuclear receptor coactivator 4-mediated ferritinophagy contributes to cerebral ischemia-induced ferroptosis in ischemic stroke. Pharmacol Res 174:105933. https://doi.org/10.1016/j.phrs.2021.105933
Liang D, Minikes AM, Jiang X (2022) Ferroptosis at the intersection of lipid metabolism and cellular signaling. Mol Cell 82:2215–2227. https://doi.org/10.1016/j.molcel.2022.03.022
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Long HZ, Cheng Y, Zhou ZW, Luo HY, Wen DD, Gao LC (2021) PI3K/AKT signal pathway: a target of natural products in the prevention and treatment of Alzheimer’s disease and Parkinson’s disease. Front Pharmacol 12:648636. https://doi.org/10.3389/fphar.2021.648636
Ma C, Wu H, Yang G, Xiang J, Feng K, Zhang J, Hua Y, Kang L, Fan G, Yang S (2022) Calycosin ameliorates atherosclerosis by enhancing autophagy via regulating the interaction between KLF2 and MLKL in apolipoprotein E gene-deleted mice. Br J Pharmacol 179:252–269. https://doi.org/10.1111/bph.15720
Mou Y, Wang J, Wu J, He D, Zhang C, Duan C, Li B (2019) Ferroptosis, a new form of cell death: opportunities and challenges in cancer. J Hematol Oncol 12:34. https://doi.org/10.1186/s13045-019-0720-y
Nepstad I, Hatfield KJ, Grønningsæter IS, Reikvam H (2020) The PI3K-Akt-mTOR signaling pathway in human acute myeloid leukemia (AML) cells. Int J Mol Sci 21:2907. https://doi.org/10.3390/ijms21082907
Qu N, Qu J, Huang N, Zhang K, Ye T, Shi J, Chen B, Kan C, Zhang J, Han F, Hou N, Sun X, Pan R (2022) Calycosin induces autophagy and apoptosis via Sestrin2/AMPK/mTOR in human papillary thyroid cancer cells. Front Pharmacol 13:1056687. https://doi.org/10.3389/fphar.2022.1056687
Rubnitz JE, Gibson B, Smith FO (2010) Acute myeloid leukemia. Hematol Oncol Clin North Am 24:35–63. https://doi.org/10.1016/j.hoc.2009.11.008
Sandhöfer N, Metzeler KH, Rothenberg M, Herold T, Tiedt S, Groiß V, Carlet M, Walter G, Hinrichsen T, Wachter O, Grunert M, Schneider S, Subklewe M, Dufour A, Fröhling S, Klein HG, Hiddemann W, Jeremias I, Spiekermann K (2015) Dual PI3K/mTOR inhibition shows antileukemic activity in MLL-rearranged acute myeloid leukemia. Leukemia 29:828–838. https://doi.org/10.1038/leu.2014.305
Sato H, Tamba M, Ishii T, Bannai S (1999) Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. J Biol Chem 274:11455–11458. https://doi.org/10.1074/jbc.274.17.11455
Tang D, Chen X, Kang R, Kroemer G (2021) Ferroptosis: molecular mechanisms and health implications. Cell Res 31:107–125. https://doi.org/10.1038/s41422-020-00441-1
Tian Y, Lu J, Hao X, Li H, Zhang G, Liu X, Li X, Zhao C, Kuang W, Chen D, Zhu M (2020) FTH1 inhibits ferroptosis through ferritinophagy in the 6-OHDA model of Parkinson’s disease. Neurotherapeutics 17:1796–1812. https://doi.org/10.1007/s13311-020-00929-z
Vago L, Gojo I (2020) Immune escape and immunotherapy of acute myeloid leukemia. J Clin Invest 130:1552–1564. https://doi.org/10.1172/JCI129204
Wang X, Chen Q (2021) FERMT1 knockdown inhibits oral squamous cell carcinoma cell epithelial-mesenchymal transition by inactivating the PI3K/AKT signaling pathway. BMC Oral Health 21:598. https://doi.org/10.1186/s12903-021-01955-9
Wang T, Zhang X, Bikle DD (2017) Osteogenic differentiation of periosteal cells during fracture healing. J Cell Physiol 232:913–921. https://doi.org/10.1002/jcp.25641
Wang J, Hu K, Cai X, Yang B, He Q, Wang J, Weng Q (2022a) Targeting PI3K/AKT signaling for treatment of idiopathic pulmonary fibrosis. Acta Pharm Sin B 12:18–32. https://doi.org/10.1016/j.apsb.2021.07.023
Wang F, Yang L, Xiao M, Zhang Z, Shen J, Anuchapreeda S, Tima S, Chiampanichayakul S, Xiao Z (2022b) PD-L1 regulates cell proliferation and apoptosis in acute myeloid leukemia by activating PI3K-AKT signaling pathway. Sci Rep 12:11444. https://doi.org/10.1038/s41598-022-15020-0
Wojcicki AV, Kasowski MM, Sakamoto KM, Lacayo N (2020) Metabolomics in acute myeloid leukemia. Mol Genet Metab 130:230–238. https://doi.org/10.1016/j.ymgme.2020.05.005
Xie Y, Shi X, Sheng K, Han G, Li W, Zhao Q, Jiang B, Feng J, Li J, Gu Y (2019) PI3K/Akt signaling transduction pathway, erythropoiesis and glycolysis in hypoxia (Review). Mol Med Rep 19:783–791. https://doi.org/10.3892/mmr.2018.9713
Xu J, Li Y, Kang M, Chang C, Wei H, Zhang C, Chen Y (2023) Multiple forms of cell death: a focus on the PI3K/AKT pathway. J Cell Physiol 238:2026–2038. https://doi.org/10.1002/jcp.31087
Xue JF, Shi ZM, Zou J, Li XL (2017) Inhibition of PI3K/AKT/mTOR signaling pathway promotes autophagy of articular chondrocytes and attenuates inflammatory response in rats with osteoarthritis. Biomed Pharmacother 89:1252–1261. https://doi.org/10.1016/j.biopha.2017.01.130
Yan X, Yu A, Zheng H, Wang S, He Y, Wang L (2019) Calycosin-7-O-β-D-glucoside attenuates OGD/R-induced damage by preventing oxidative stress and neuronal apoptosis via the SIRT1/FOXO1/PGC-1α pathway in HT22 cells. Neural Plast 2019:8798069. https://doi.org/10.1155/2019/8798069
Yan HF, Zou T, Tuo QZ, Xu S, Li H, Belaidi AA, Lei P (2021) Ferroptosis: mechanisms and links with diseases. Signal Transduct Target Ther 6:49. https://doi.org/10.1038/s41392-020-00428-9
Yan Y, Teng H, Hang Q, Kondiparthi L, Lei G, Horbath A, Liu X, Mao C, Wu S, Zhuang L, James You M, Poyurovsky MV, Ma L, Olszewski K, Gan B (2023) SLC7A11 expression level dictates differential responses to oxidative stress in cancer cells. Nat Commun 14:3673. https://doi.org/10.1038/s41467-023-39401-9
Yang Q, Fei Z, Huang C (2021a) Betulin terpenoid targets OVCAR-3 human ovarian carcinoma cells by inducing mitochondrial mediated apoptosis G2/M phase cell cycle arrest, inhibition of cell migration and invasion and modulating mTOR/PI3K/AKT signalling pathway. Cell Mol Biol Noisy-Le-Grand 67:14–19. https://doi.org/10.14715/cmb/2021.67.2.3
Yang C, Chen C, Xiao Q, Wang X, Shou Y, Li H, Sun M (2021b) Relationship between PTEN and angiogenesis of esophageal squamous cell carcinoma and the underlying mechanism. Front Oncol 11:739297. https://doi.org/10.3389/fonc.2021.739297
Zhang Z, Lin M, Wang J, Yang F, Yang P, Liu Y, Chen Z, Zheng Y (2021) Calycosin inhibits breast cancer cell migration and invasion by suppressing EMT via BATF/TGF-β1. Aging 13:16009–16023. https://doi.org/10.18632/aging.203093
Zhang C, Liu X, Jin S, Chen Y, Guo R (2022) Ferroptosis in cancer therapy: a novel approach to reversing drug resistance. Mol Cancer 21:47. https://doi.org/10.1186/s12943-022-01530-y
Zhao L, Zhou X, Xie F, Zhang L, Yan H, Huang J, Zhang C, Zhou F, Chen J, Zhang L (2022) Ferroptosis in cancer and cancer immunotherapy. Cancer Commun 42:88–116. https://doi.org/10.1002/cac2.12250
Zhu JH, De Mello RA, Yan QL, Wang JW, Chen Y, Ye QH, Wang ZJ, Tang HJ, Huang T (2020) MiR-139-5p/SLC7A11 inhibits the proliferation, invasion and metastasis of pancreatic carcinoma via PI3K/Akt signaling pathway. Biochim Biophys Acta Mol Basis Dis 1866:165747. https://doi.org/10.1016/j.bbadis.2020.165747
Zhu L, Liu S, Liao YF, Sheng YM, He JC, Cai ZX, Man Q, Wu YY (2022) Calycosin suppresses colorectal cancer progression by targeting ERβ, upregulating PTEN, and inhibiting PI3K/Akt signal pathway. Cell Biol Int 46:1367–1377. https://doi.org/10.1002/cbin.11840
Funding
This study was supported by Wuhan Municipal Health and Family Planning Commission Research Program Funding Project (grant no. WX18Z35).
Author information
Authors and Affiliations
Contributions
CX contributed to the study design, data collection, statistical analysis, data interpretation and manuscript preparation. WC, HJ, XL, SL, and DW contributed to the data collection and statistical analysis. YX contributed to data collection, statistical analysis and manuscript preparation. All authors read and approved the final manuscript.
Corresponding author
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, C., Chang, W., Jiang, H. et al. Calycosin Induces Ferroptosis by SLC7A11 Through the PI3K/Akt Pathway in Acute Myelocytic Leukemia. Rev. Bras. Farmacogn. 34, 776–784 (2024). https://doi.org/10.1007/s43450-023-00502-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43450-023-00502-7