Abstract
Marrubium astracanicum Jacq., Lamiaceae, is an herbaceous plant distributed in Iran, Turkey, and southern Caucasia. In the present study, α-glucosidase inhibitory principles of M. astracanicum aerial parts were investigated by a bioassay-guided approach. The extraction procedure from the plant aerial parts was carried out successively using petroleum ether, ethyl acetate, and methanol. The ethyl acetate extract, with the highest in vitro α-glucosidase inhibitory activity, was subjected to further chemical investigation. The structures of the isolated compounds were elucidated by 1H-NMR and 13C-NMR spectral analyses as apigenin-7-O-(3′′-(E)-p-coumaroyl)-β-d-glucoside, apigenin-7-O-(6′′-(E)-p-coumaroyl)-β-d-glucoside, martynoside, apigenin-7-O-β-d-glucoside, and verbascoside. In addition, the antidiabetic potential of the isolated compounds was evaluated in an in vitro α-glucosidase inhibitory assay, and kinetic and molecular docking studies were done for the most active compound. Apigenin-7-O-(3′′-(E)-p-coumaroyl)-β-d-glucoside and apigenin-7-O-(6′′-(E)-p-coumaroyl)-β-d-glucoside showed the most potent in vitro α-glucosidase inhibitory activity with IC50 values of 57.4 ± 1.2 and 186.4 ± 2.5 μM, respectively, which was about 4 to 13 times lower than the IC50 value of the reference compound acarbose (751.2 ± 0.4 μM).
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus type II which is also called adult type or non-insulin-dependent diabetes mellitus is a metabolic disease characterized by chronic hyperglycemia resulting from reduced response of the organs to insulin. It has been predicted that the number of patients with diabetes increases from 415 million in 2015 to approximately 642 million by 2040 (Carracher et al. 2018). This global growth in prevalence of diabetes mellitus type II has made it as one of the world’s greatest health problems (Khan et al. 2020). α-Glucosidase inhibitors such as acarbose, voglibose, and miglitol are one of the drug classes used in the management of diabetes mellitus type II (Derosa and Maffioli 2012). The enzyme α-glycosidase is located in the brush border of the small intestine mucosa that acts to hydrolyze carbohydrates containing α-(1→4) glucose linkage (Hossain et al. 2020). α-Glucosidase inhibitor drugs such as acarbose are efficient in suppressing postprandial blood glucose levels by the inhibition of the carbohydrate degradation to free absorbable glucose and help to prevent oxidative stress–induced damages caused by increase in blood glucose levels (Wright Jr et al. 2006). Different classes of natural products especially phenolics have been reported to possess α-glucosidase inhibitory effects (Kumar et al. 2011; Proença et al. 2020). Therefore, plants are considered as a rich source of biologically natural product for development of new α-glucosidase inhibitor drugs with diminished side effects.
The genus Marrubium L. from the family Lamiaceae consists of 49 species mainly distributed in Mediterranean and Irano-Turanian phytogeographic regions (Talebi et al. 2019). Among the Marrubium taxa, M. vulgare L., M. cuneatum Banks & Sol., Ballota deserti (Noë) Jury, Rejdali & A.J.K.Griffiths, syn. M. deserti (Noë) Coss., and M. globosum ssp. libanoticum (Boiss.) P.H.Davis have been mentioned for their medicinal use as antidiabetic agents in folk medicine of some countries (Rigano et al. 2007; Loizzo et al. 2008; Laouer et al. 2009; Hamza et al. 2019). Previous studies have demonstrated the in vitro α-glucosidase inhibitory activity of the methanolic extract of M. vulgare aerial parts (IC50 12.66 μg/ml), as well as significant dose-dependent blood glucose–lowering effects of its aqueous extract in alloxan-induced diabetic rats (Boudjelal et al. 2012). In 2008, Loizzo et al. reported the methanolic extract of M. radiatum aerial parts as one of the most potent extracts with α-glucosidase and α-amylase inhibitory activities (IC50 68.8 and 61.1 μg/ml, respectively), between different extracts obtained from some plants used in Lebanon traditional medicine against diabetes. In another study on M. alysson L., a diterpenoid lactone, called marrubiin, was isolated from its ethyl acetate extract with a strong inhibitory effect on α-glucosidase enzyme with IC50 value of 16.62 μg/ml compared with acarbose as positive control with IC50 value of 64.14 μg/ml (El-Mohsen et al. 2014).
Marrubium astracanicum Jacq. is one of eleven Marrubium species represented in the flora of Iran (Jamzad, 2012). The infusion of the aerial parts is used by indigenous people in Iran and Turkey for alleviation of stomachache and joint pains, as antipyretic and for the treatment of colds (Mosaddegh et al. 2012; Altundag and Ozturk 2011). Sesquiterpenes such as β-caryophyllene, germacrene D, and bicyclogermacrene have also been identified as main compounds of essential oil extracted from this plant aerial part (Javidnia et al. 2007; Teimori et al. 2008). Regarding traditional uses and α-glucosidase inhibitory potentials of Marrubium species, the present study was designed to isolate the α-glucosidase inhibitory principles of M. astracanicum in a bioassay-guided isolation approach.
Materials and Methods
Plant Material
The flowering aerial parts of Marrubium astracanicum Jacq., Lamiaceae, were collected from the Pol-e Zanguleh region (Chalus County, Mazandaran Province, Iran) in June 2018. The plant was authenticated by botanist Dr. Yousef Ajani (Research Institute of Forests and Rangelands, Karaj, Iran) and a voucher specimen was deposited at the Herbarium of the Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran (7085-TEH).
Extraction and Isolation
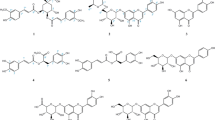
The air dried and grinded plant aerial parts (0.6 kg) were macerated, successively, with petroleum ether, ethyl acetate, and methanol (3× 4 l each) at room temperature. The extracts were concentrated using a rotary evaporator at 40 °C and dried in a vacuum oven (40 °C). The ethyl acetate extract (15 g) was subjected to CC on a silica gel (650 g, 230–400 mesh, Merck, Germany) and eluted with CH2Cl2-EtOAc (100:0 to 0:100%), and then EtOAc-MeOH (80:20) to get twenty fractions (E1-20). Compounds 1 (23 mg) and 2 (18 mg) were obtained as yellow precipitates from fractions E2 and E5, respectively. Fraction E10 (200 mg) was chromatographed on a Sephadex LH-20 column (20 g, Fluka, Switzerland) with MeOH to get compounds 3 (22 mg) and 4 (18 mg). Compound 5 (9 mg) was purified from fraction E20 via Sephadex LH20 column chromatography (10 g) using MeOH as the eluent. In all steps, column chromatography was monitored by TLC under UV at 254 and 366 nm, following ammonia vapor exposure. The structures of the isolated compounds were elucidated using 1H-NMR and 13C-NMR spectral analyses (Varian- INOVA, 500 MHz for 1H-NMR and 125 MHz for 13C-NMR), as well as comparing with published data (see Supporting Information).
α-Glucosidase Inhibition Assay
α-Glucosidase inhibitory activity of the extracts and isolated compounds were evaluated in an in vitro colorimetric assay (Asgari et al. 2019). The α-glucosidase enzyme (EC3.2.1.20, Saccharomyces cerevisiae, 20 U/mg) solution (1 U/ml, 20 μl), potassium phosphate buffer (50 mM, pH 6.8, 135 μl), and sample solution (500 μg/ml for the extracts and 750 μM for the pure compounds, 20 μl) were added to a 96-well plate and incubated at 37 °C for 10 min. After that, p-nitrophenyl-α-d-glucoside (PNPG) solution (4 mM, 25 μl) was added to the mixture and the plate was incubated for 20 min at 37 °C. Finally, the absorbances were recorded by a spectrophotometer (Gen5, PowerWave XS2, BioTek) at 405 nm and the percentage of enzyme inhibition was determined in comparison with the negative control (10% dimethyl sulfoxide). Acarbose is the antidiabetic drug used as positive control. The IC50 value was determined for the tested compound with inhibition percentage higher than 50% in preliminary assay. Each sample was tested in triplicate and results were analyzed using Sigma Plot 11.0 software and expressed as mean ± SD.
Enzyme Kinetic Study
The inhibition mode of the two most potent compounds against α-glucosidase enzyme activity was investigated (Asgari et al. 2019). For this purpose, α-glucosidase inhibitory activity was measured in the absence and presence of different concentrations of the tested compounds in combination with different concentrations of PNPG (1–10 mM) as the substrate. A Lineweaver-Burk plot was generated to identify the type of inhibition and the Michaelis-Menten constant (Km) value was determined from plot between reciprocal of the substrate concentration (1/[S]) and reciprocal of enzyme rate (1/V) over various inhibitor concentrations. Experimental inhibitor constant (Ki) value was constructed by secondary plots of the inhibitor concentrations [I] versus Km.
Molecular Docking Study
The interaction of α-glucosidase protein and compound 2 was simulated by Auto Dock 4 software and Auto Dock Tools (ADT 1.5.6) using the hybrid Lamarckian genetic algorithm (LGA). In human’s intestine, maltase-glucoamylase (MGAM) catalyzes the hydrolysis of disaccharides. Therefore, the C-terminal subunit of maltase-glucoamylase enzyme (GH31) was used for docking to better understand the isolated compounds-enzyme interactions. The three-dimensional (3D) crystal structure of human intestinal α-glucosidase (PDB code 3TOP) with a resolution of 2.88 °A was taken from the RCSB Protein Data Bank (Sakayanathan et al. 2018). The Hyperchem.8.0.10 software was used to develop and optimize 3D structures of selected compounds for molecular docking. The cubic grid box was built by 50 °A size (x, y, z) in the center with coordination of x = −29.9365, y = 34.8669, z = 25.8692 which was determined according to the position of the co-crystalized ligand (acarbose). All other parameters were left to default settings of Auto Dock 4. For validation of results, root mean-square deviation (RMSD) less than 2.0 °A was used as threshold. The RMSD was calculated in comparison to re-docking of original inhibitor, acarbose, that co-crystallized with 3TOP enzyme. The most favorable conformation by lowest free energy of binding was chosen and their target residues were determined.
Results and Discussion
Isolation and Structural Elucidation
Chemical investigation of ethyl acetate extract of M. astracanicum aerial parts resulted in the isolation of three flavone glycosides, apigenin-7-O-(3′′-(E)-p-coumaroyl)-β-d-glucoside (1), apigenin-7-O-(6′′-(E)-p-coumaroyl)-β-d-glucoside (2), and apigenin-7-O-β-d-glucoside (4), together with two phenylethanoid glycosides, martynoside (3) and verbascoside (5). Molecular structures of the isolated compounds were elucidated through 1H-NMR and 13C-NMR spectral analyses. This is the first report on isolation of these phenolic derivatives from the aerial parts of M. astracanicum.

α-Glucosidase Inhibition Assay
The inhibitory potentials of the extracts and isolated compounds 1–5 were evaluated in vitro against the α-glucosidase enzyme in comparison with acarbose, as a standard drug. As shown in Table 1, among the tested extracts, ethyl acetate extract demonstrated the most α-glucosidase inhibitory activity with the 43.5 ± 0.7% inhibition in concentration of 500 μg/ml. The α-glucosidase inhibition assay of the compounds 1–5 isolated from ethyl acetate extract revealed that except compound 4, all the compounds were more potent than the standard drug against α-glucosidase enzyme (Table 1). Compounds 1 and 2 with IC50 values of 57.4 ± 1.2 and 186.4 ± 2.5 μM, respectively, were the most potent α-glucosidase inhibitors in comparison with acarbose (IC50 751.2 ± 0.4 μM). Compound 1 showed a potent in vitro α-glucosidase inhibitory effect which was about thirteen times stronger the reference compound acarbose.
Enzyme Kinetic Study
The most potent compounds, apigenin-7-O-(3′′-(E)-p-coumaroyl)-β-d-glucoside (1) and apigenin-7-O-(6′′-(E)-p-coumaroyl)-β-d-glucoside (2), were subjected to a kinetic study to identify their mechanism of actions. As can be seen in Fig. 1, the Km and Vmax values increased by increasing the concentrations of compound 1 (0-60 μM), which indicated a non-competitive mode of inhibition. The enzyme kinetic study of apigenin-7-O-(6′′-(E)-p-coumaroyl)-β-d-glucoside (2), however, showed that the Km value increased, and Vmax was not affected by increasing the inhibitor’s concentrations (0–185 μM) (Fig. 2a). This result showed that compound 2 binds to the enzyme active site in a competitive mode of inhibition. Furthermore, the Ki value of compound 2 was determined as 185 μM (Fig. 2b).
Molecular Docking Study
For analyzing the interactions between the isolated compounds and α-glucosidase enzyme, molecular docking study was performed by Auto Dock Tools (version 1.5.6). Previous studies demonstrated a significant homology between S. cerevisiae α-glucosidase (G13) and human maltase-glucoamylase enzyme (GH31). They are similar in the catalytic domain (Rigden 2002). Therefore, the crystal structure of C-terminal domain of human intestinal α-glucosidase (PDB code: 3TOP) was used for docking simulations (Liu et al. 2019). In Fig. 3, the structures of acarbose and compound 2 (apigenin-7-O-(6′′-(E)-p-coumaroyl)-β-d-glucoside), which was one of the most potent inhibitors among the isolated compounds with competitive mode of action, were superimposed in the active site of enzyme. The docking poses of the acarbose and the isolated compounds in the active site of enzyme are shown in Fig. 4. Results for docking studies of the isolated compounds, as well as acarbose in the studied protein, including free binding energy (FBE), inhibition constant (Ki), and interacted residues of active site are also summarized in Table 2 and according to the results, the lowest binding energy for compound 2 was calculated as −7.61 kcal/mol.
As shown in Figs. 3 and 4, compound 2 fitted to the active site pocket of enzyme in a slightly different manner in comparison to acarbose. The OH in 4′ position of the B ring in compound 2 interacts with GLN1533 (2.77 Å) and PRO1160 (1.76 Å) residues of the active site. As previous reports claimed, the hydroxyl groups in 3′ and 4′ positions (B ring) of flavonoids can bond to the active site residues of α-glucosidase. In addition, the type of flavonoid structure (aromatic rings and specific distributing of electron clouds) facilitates the proton donation of the C-3′ or C-4′ OH groups. Also, the present results showed that the hydrogen of the C-4′ OH group in compound 2 donated the proton to form a hydrogen bond with PRO1160 and the O of the same position made additional hydrogen bond with GLN1533 residue of the α-glucosidase enzyme. On the other hand, compound 2 interacts with ASP1157 and ASP 1279 via C-4′′ OH (in the sugar moiety) and 4′′′ OH (hydroxyl of p-coumaroyl), respectively. These interactions and amino acid residues are in agreement with previous reports (Liu et al. 2019; Singh et al. 2018). Finally, a review on IC50 values of isolated apigenin derivatives 1, 2, and 4 (Table 1) shows that a significant increase in α-glucosidase inhibitory activity is observed by additon of a p-coumaroyl group on the glucosyl moiety of apigenin-7-O-β-d-glucoside, among which p-coumaroylation of the OH at C-3′′ position was found to be more effective to enhance enzyme inhibition.
A literature review revealed that there are limited reports on antidiabetic potentials of coumaroylated flavonoid glycosides (Ma et al. 2015; Ikechukwu and Ifeanyi 2016; Li et al. 2019). An investigation on red onion bulb (Allium cepa var. cepa L., Alliaceae) resulted in isolation of kaempferol-3-O-(6′′-(E)-p-coumaroyl)-β-d-glucoside as the bioactive compound of the aqueous extract with considerable hypoglycemic activity at the dose of 25 mg/kg in alloxan-induced diabetic rats (Ikechukwu and Ifeanyi 2016). Furthermoe, Ma et al. (2015) reported the α-glucosidase inhibitory activity of some phenolics including seven flavonoid derivatives from the flowers of Edgeworthia gardneri (Wall.) Meisn., Thymelaeaceae, which is used by indigenus people in Tibet as herbal tea for diabetes and hyperlipidemia (Ma et al. 2015). They showed that oral administration of kaempferol-3-O-(6′′-(E)-p-coumaroyl)-β-d-glucoside (tiliroside), the main compound isolated from E. gardneri flowers with IC50 value of 202 ± 12 μg/ml in α-glucosidase inhibitory assay, significantly decreased the postprandial blood glucose levels of normal and streptozotocin-induced diabetic mice at the dose of 300 mg/kg. In the mentioned study, quercetin (3,3′,4′5,7-pentahydroxyflavone) was identified as the most potent α-glucosidase inhibitor principle of E. gardneri flowers with IC50 value of 5.1 ± 0.3 μg/ml (IC50 value of acarbose 465 ± 37 μg/ml). The addition of second coumaroyl moiety can enhance the affinity of flavonoid glycoside toward α-glucosidase enzyme. In 2019, some dicoumaroylated flavonol rhamnosides were reported as α-glucosidase inhibitor principles of the ethyl acetate extract of Machilus litseifolia S.K. Lee., Lauraceae, of which 4′-O-methyl-2″,4″-di-(Z)-p-coumaroyl afzelin with IC50 value of 5.9 μM was found 91 times more potent than standard drug acarbos (IC50 266 μM) (Li et al. 2019).
Beside the mechanisms including regeneration of pancreatic B cells, regulation of insulin secretion, and delaying glucose absorption, the antioxidant activity of plant extracts and natural products is also contributed to antidiabetic effect by alleviating the oxidative stress damages induced by diabetes (Kang et al. 2020). In a study on the antioxidant activity of 24 Lamiaceae species growing in Iran, the methanol extract of M. astracanicum was found to have considerable antioxidant activity in DPPH free radical scavenging (IC50 618.3 ± 54.7 μg/ml) and ferric-reducing antioxidant power (FRAP value 16.4 ± 1.5 quercetin equivalent/g extract) assays (Firuzi et al. 2010). Moreover, apigenin-7-O-(6′′-(E)-p-coumaroyl)-β-d-glucoside (1), the most potent α-glucosidase inhibitor isolated from M. astracanicum in the present study, has also been reported to possess a potent antioxidant activity with IC50 value of 7.69×10−4 mg/ml in DPPH free radical scavenging assay (Delazar et al. 2005).
Conclusions
The bioassay-guided isolation of the constituents of M. astracanicum aerial parts resulted in isolation of five phenolic glycosides (1–5) from its ethyl acetate extract, among which apigenin-7-O-(3′′-(E)-p-coumaroyl)-β-d-glucoside (1) and apigenin-7-O-(6′′-(E)-p-coumaroyl)-β-d-glucoside (2) demonstrated potent inhibition toward α-glucosidase. These coumaroyl flavone glycosides could be considered appropriate natural candidates in antidiabetic drug development research due to their potential as α-glucosidase inhibitors in controlling postprandial blood glucose levels in diabetic patients. The present study also highlights the beneficial potentials of Marrubium astracanicum as a helpful herbal supplement for diabetic patients. However, further pharmacological and toxicological studies are needed to establish its efficacy and safety in management of diabetes mellitus type II.
References
Altundag E, Ozturk M (2011) Ethnomedicinal studies on the plant resources of east Anatolia, Turkey. Procedia Soc Behav Sci 19:756–777. https://doi.org/10.1016/j.sbspro.2011.05.195
Asgari MS, Mohammadi-Khanaposhtani M, Kiani M, Ranjbar PR, Zabihi E, Pourbagher R, Rahimi R, Faramarzi MA, Biglar M, Larijani B, Mahdavi M (2019) Biscoumarin-1,2,3-triazole hybrids as novel anti-diabetic agents: design, synthesis, in vitro α-glucosidase inhibition, kinetic, and docking studies. Bioorg Chem 92:103206. https://doi.org/10.1016/j.bioorg.2019.103206
Boudjelal A, Henchiri C, Siracusa L, Sari M, Ruberto G (2012) Compositional analysis and in vivo anti-diabetic activity of wild Algerian Marrubium vulgare L. infusion. Fitoterapia 83:286–292. https://doi.org/10.1016/j.fitote.2011.11.005
Carracher AM, Marathe PH, Close KL (2018) International Diabetes Federation 2017. Wiley Online Library.
Delazar A, Celik S, Göktürk R, Unal O, Nahar L, Sarker S (2005) Two acylated flavonoid glycosides from Stachys bombycina, and their free radical scavenging activity. Die Pharmazie 60:878–880. https://doi.org/10.1002/chin.200610208
Derosa G, Maffioli P (2012) α-Glucosidase inhibitors and their use in clinical practice. Arch Med Sci 8:899–906. https://doi.org/10.5114/aoms.2012.31621
El-Mohsen A, Rabeh M, Abou-Setta L, El-Rashedy A, Hussein A (2014) Marrubiin: a potent α-glucosidase inhibitor from Marrubium alysson. Int J Appl Res Nat Prod 7:21–27
Firuzi O, Javidnia K, Gholami M, Soltani M, Miri R (2010) Antioxidant activity and total phenolic content of 24 Lamiaceae species growing in Iran. Nat Prod Commun 5:261–264. https://doi.org/10.1177/1934578x1000500219
Hamza N, Berke B, Umar A, Cheze C, Gin H, Moore N (2019) A review of Algerian medicinal plants used in the treatment of diabetes. J Ethnopharmacol 238:111841. https://doi.org/10.1016/j.jep.2019.111841
Hossain U, Das AK, Ghosh S, Sil PC (2020) An overview on the role of bioactive α-glucosidase inhibitors in ameliorating diabetic complications. Food Chem Toxicol 145:111738. https://doi.org/10.1016/j.fct.2020.111738
Ikechukwu OJ, Ifeanyi OS (2016) The antidiabetic effects of the bioactive flavonoid (kaempferol-3-O-β-D-6 {p-coumaroyl} glucopyranoside) isolated from Allium cepa. Recent Pat Antiinfect Drug Discov 11:44–52. https://doi.org/10.2174/1574891x11666151105130233
Jamzad Z (2012) Flora of Iran: Lamiaceae. Research Institute of Forests and Rangelands, Tehran
Javidnia K, Miri R, Soltani M, Khosravi A (2007) Constituents of the essential oil of Marrubium astracanicum Jacq. from Iran. J Essent Oil Res 19:559–561. https://doi.org/10.1080/0972-060x.2004.10643399
Kang GG, Francis N, Hill R, Waters D, Blanchard C, Santhakumar AB (2020) Dietary polyphenols and gene expression in molecular pathways associated with type 2 diabetes mellitus: a review. Int J Mol Sci 21:140. https://doi.org/10.3390/ijms21010140
Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H, Al Kaabi J (2020) Epidemiology of type 2 diabetes–global burden of disease and forecasted trends. J Epidemiol Glob Health 10:107–111. https://doi.org/10.2991/jegh.k.191028.001
Kumar S, Narwal S, Kumar V, Prakash O (2011) α-Glucosidase inhibitors from plants: a natural approach to treat diabetes. Pharmacog Rev 5:19–29. https://doi.org/10.4103/0973-7847.79096
Laouer H, Yabrir B, Djeridane A, Yousfi M, Beldovini N, Lamamra M (2009) Composition, antioxidant and antimicrobial activities of the essential oil of Marrubium deserti. Nat Prod Commun 4:1133–1138. https://doi.org/10.1177/1934578x0900400824
Li T, Kongstad KT, Staerk D (2019) Identification of α-glucosidase inhibitors in Machilus litseifolia by combined use of high-resolution α-glucosidase inhibition profiling and HPLC-PDA-HRMS-SPE-NMR. J Nat Prod 82:249–258. https://doi.org/10.1021/acs.jnatprod.8b00609.s001
Liu JL, Kong YC, Miao JY, Mei XY, Wu SY, Yan YC, Cao XY (2019) Spectroscopy and molecular docking analysis reveal structural specificity of flavonoids in the inhibition of α-glucosidase activity. Int J Biol Macromol. 152:981–989. https://doi.org/10.1016/j.ijbiomac.2019.10.184
Loizzo MR, Saab AM, Tundis R, Menichini F, Bonesi M, Piccolo V, Statti GA, de Cindio B, Houghton PJ, Menichini F (2008) In vitro inhibitory activities of plants used in Lebanon traditional medicine against angiotensin converting enzyme (ACE) and digestive enzymes related to diabetes. J Ethnopharmacol 119:109–116. https://doi.org/10.1016/j.jep.2008.06.003
Ma YY, Zhao DG, Zhou AY, Zhang Y, Du Z, Zhang K (2015) α-Glucosidase inhibition and antihyperglycemic activity of phenolics from the flowers of Edgeworthia gardneri. J Agric Food Chem 63:8162–8169. https://doi.org/10.1021/acs.jafc.5b03081
Mosaddegh M, Naghibi F, Moazzeni H, Pirani A, Esmaeili S (2012) Ethnobotanical survey of herbal remedies traditionally used in Kohghiluyeh va Boyer Ahmad province of Iran. J Ethnopharmacol 141:80–95. https://doi.org/10.1016/j.jep.2012.02.004
Proença C, Ribeiro D, Freitas M, Fernandes E (2020) Flavonoids as potential agents in the management of type 2 diabetes through the modulation of α-amylase and α-glucosidase activity: a review. Crit Rev Food Sci Nutr 62:3137–3207. https://doi.org/10.1080/10408398.2020.1862755
Rigano D, Formisano C, Basile A, Lavitola A, Senatore F, Rosselli S, Bruno M (2007) Antibacterial activity of flavonoids and phenylpropanoids from Marrubium globosum ssp. libanoticum. Phytother Res 21:395–397. https://doi.org/10.1002/ptr.2061
Rigden DJ (2002) Iterative database searches demonstrate that glycoside hydrolase families 27, 31, 36 and 66 share a common evolutionary origin with family 13. FEBS Lett. 523:17–22. https://doi.org/10.1016/s0014-5793(02)02879-x
Sakayanathan P, Loganathan C, Iruthayaraj A, Periyasamy P, Poomani K, Periasamy V, Thayumanavan P (2018) Biological interaction of newly synthesized astaxanthin-s-allyl cysteine biconjugate with Saccharomyces cerevisiae and mammalian α-glucosidase: in vitro kinetics and in silico docking analysis. Int J Biol Macromol 118:252–262. https://doi.org/10.1016/j.ijbiomac.2018.06.027
Singh G, Singh A, Verma RK, Mall R, Azeem U (2018) Synthesis, biological evaluation and molecular docking studies of novel benzimidazole derivatives. Comput Biol Chem. 72:45–52. https://doi.org/10.1016/j.compbiolchem.2017.12.010
Talebi SM, Sheidai M, Ariyanejad F (2019) Stem anatomical study of some Iranian Marrubium L. species. Biodiversitas 20:2589–2595. https://doi.org/10.13057/biodiv/d200922
Teimori M, Khavari-Nejad R, Yassa N, Nejadsatari T (2008) Analysis of the essential oil of Marrubium crassidens Bioos. and M. astracanicum Jacq. J Appl Sci 8:1793–1795. https://doi.org/10.3923/jas.2008.1793.1795
Wright E Jr, Scism-Bacon JL, Glass LC (2006) Oxidative stress in type 2 diabetes: the role of fasting and postprandial glycaemia. Int J Clin Pract 60:308–314. https://doi.org/10.1111/j.1368-5031.2006.00825.x
Funding
This research was supported by Tehran University of Medical Sciences and Health Services Grants (No. 99-1-104-48267).
Author information
Authors and Affiliations
Contributions
RK and AH carried out the phytochemical analysis. MRD and MK supervised this study and suggested the original idea. MRD also has participated in interpreting related spectra and writing the manuscript. MAF and SM have contributed to enzyme inhibition assay. HPK conducted the molecular docking study. The current manuscript was critically read and approved by all authors.
Corresponding authors
Supplementary Information
ESM 1
(PDF 1281 kb)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kazemi, R., Delnavazi, MR., Parsa-Khankandi, H. et al. α-Glucosidase Inhibitors from Marrubium astracanicum: Isolation and Molecular Docking. Rev. Bras. Farmacogn. 32, 618–626 (2022). https://doi.org/10.1007/s43450-022-00287-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43450-022-00287-1