Abstract
Thymus fedtschenkoi Ronniger from Lamiaceae family is an endemic Iranian plant and used as traditional remedy by local people. The present study, evaluated ɑ-glucosidase inhibitory potentials of T. fedtschenkoi fractions and its compounds. The aerial part of T. fedtschenkoi was extracted with methanol/water and fractioned by n-hexane, chloroform, and n-butanol solvents, successively. The n-butanol fraction with the highest α-glucosidase inhibitory effect was further investigated with open column chromatography. The structures of isolated compounds were elucidated by 1H-NMR and 13C-NMR spectral analyses. In addition, antidiabetic potentials of the fractions and the isolated compounds were assessed through an in vitro ɑ-glucosidase inhibitory test and kinetic and molecular docking studies were done for the isolated compounds. Eight phenolic compounds including 3, 4-di-O-feruloyl quinic acid (1), luteolin-7-O-rutinoside (2), luteolin (3), rosmarinic acid methyl ester (4), rosmarinic acid (5), apigenin-7-O-glucoside (6), luteolin-7-O-glucuronide (7), and luteolin-7-O-glucoside (8) were isolated. According to the results, compound 5 was the most potent α-glucosidase inhibitor with IC50 value of 43.38 ± 0.05 μM which was about 17 times lower than the IC50 value of the acarbose as reference compound (750.0 ± 1.0 μM). The present study showed a good potency for the n-butanol fraction of T. fedtschenkoi and its compounds to inhibit α-glucosidase enzyme.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes as a chronic metabolic disorder seriously affected the well-being throughout the world with economic and social consequences; such that according to the International Diabetes Federation (IDF) data, diabetes is responsible for one death every eight seconds in 2019. It is estimated that the number of affected persons with diabetes is going to reach 700 million by 2045. In addition, based on various epidemiological studies diabetes is related to other pathological conditions such as renal failure, blindness, neurodegeneration and major depressive disorder (Chatterjee et al. 2017; Duarte-Silva et al. 2021; Groenewegen et al. 2021; Huang et al. 2020; IDF Diabetes Atlas 2019; Saeedi et al. 2019; Zhu et al. 2021).
Diabetes types 1 and 2 along with gestational diabetes mellitus are the main subcategories of diabetes in which the type 2 diabetes mellitus (T2D) responsible for the 90% of the total cases. T2D is generally known for abnormal insulin secretion and postprandial hyperglycemia. One of the promising ways for diabetes treatment is using inhibitors of the enzymes like α-glucosidase and α-amylase which resulting in reduction in postprandial hyperglycemia by delaying the digestion of carbohydrates. On the other hand, these carbohydrase inhibitors can be useful in the management of the weight gain and obesity. Considering this application and the adverse effects of available α-glycosidase inhibitors (acarbose, miglitol, etc.) in the market, searching plant extracts and natural compounds targeting α-glucosidase is a mainstream way in drug discovery for manage of diabetes and related disorders (Lankatillake et al. 2021; Rathod and Yadav 2021; Sharma et al. 2021; Zhu et al. 2021).
The genus Thymus L. from the Lamiaceae family has about 215 species all over the world. This genus originated from the Mediterranean zone and spreads throughout Europe, Greenland, Northern America, and Asia. Many plants of this genus have aromatic nature and widely used in food, cosmetic and medicinal purposes. Some of Thymus plants are important medicines in various traditional medicines and their infusions and decoctions were applied as carminative, digestive, antispasmodic, anti-inflammatory, emmenagogue, and tonic agents. Many studies focused on essential oils in Thymus plants which generally contained two well-known compounds named thymol and carvacrol. However nonvolatile compounds like flavonoids, simple phenylpropanoids, lignans, tannins, etc. could be the responsible substances for biological activities of these medicinal herbs. Thymus species showed a variety of activities in either in vivo or in vitro pharmacological researches e.g., as antimicrobial, antioxidant, antitumor, anti-inflammatory, anti-hypertensive, antidiabetic effects (Li et al. 2019; Salehi et al. 2019; Tohidi et al. 2019).
As mentioned above, Thymus species have a place in diabetes treatments. T. spicata var. spicata, T. fallax and T. kotschyanus have been used for treatment of diabetes in Iran and Turkey folk medicines (Li et al. 2019; Ozturk et al. 2018; Salteh and Amani 2020). In addition, some modern researches reveal the antidiabetic properties of Thymus species. Various studies reported good antidiabetic effect for T. quinquecostatus, T. praecox, and T. argaeus (Li et al. 2019). Taleb et al. (2017) ran a clinical trial for aqueous extract of T. kotschyanus in patients with type II diabetes. As a result, intake of T. kotschyanus infusion prepared from 10 g dried herb twice a day for three months along with drugs led to the better control of glucose levels and improved function of pancreatic beta cells.
Thymus fedtschenkoi Ronniger is a perennial subshrub wildly grown in the rocky slopes of the Irano-Turanian floristic region in Iran and Turkey. This plant which is closely related to T. kotschyanus, is used by indigenous people as antitussive, expectorant and antiseptic in treatment of common cold and sore throat (Hosseini et al. 2021). There are only a few studies conducted on this plant and the antimicrobial and antifungal activity is the only reported biological effect in the literature (Alinezhad et al. 2011; Aminkhani et al. 2019; Delazar et al. 2011). The essential oil of T. fedtschenkoi was analyzed in several studies. In many of these studies, thymol or carvacrol was the main compound of the essential oil (Abousaber et al. 2002; Aminkhani et al. 2019; Choi et al. 2002; Delazar et al. 2011; Tohidi et al. 2017). Nevertheless, other reports showed that linalool could be the main part of the essential oil of T. fedtschenkoi depending on the different phenological stages and environmental factors (Khorshidi et al. 2014; Rustaiee et al. 2011).
Therefore, on the one hand because of the importance of finding active natural products for diabetes management and on the other hand as a result of previous studies in Thymus species, this study was conducted to evaluate the potentials of T. fedtschenkoi fractions and its compounds in inhibiting α-glucosidase, in order to use in diabetes treatment.
Experimental
Materials
Plant materials
The aerial part of Thymus fedtschenkoi Ronniger was collected from Mishu-dagh Mountains (Shanjan, Shabestar, East-Azerbaijan, Iran) before its flowering stage in May 2017. The collected plant was authenticated by taxonomist Dr. Yousef Ajani and a voucher specimen was deposited in the herbarium of Research Institute of Forests and Rangelands (TARI), Tehran, Iran (Code Number: 107145).
Methods
Extraction and fractionation
The collected plant (1 kg) was macerated at room temperature with 70% methanol in water (5 L) for 72 h after shade drying and grounding. The process was repeated four times with fresh solvent. After maceration, the obtained dried hydroalcoholic extract was dispersed in minimum volume of water and fractionated successively with n-hexane, chloroform and n-butanol by liquid–liquid fractionation method. All of the fractions were concentrated and dried by rotary evaporator and vacuum oven (40 °C).
Isolation of compounds
The n-butanol part (10 g) was fractionated on a sephadex LH-20 column eluting with methanol to obtain 16 fractions (B1–B16). Open column chromatography of B3 with silica gel (230–400 mesh, Merck, Germany) using CHCl3–MeOH–H2O (8.5:1:0.5 to 6.5:3:0.5) as gradient solvent resulted in isolation of compounds 1 (15.2 mg) and 2 (9.1 mg). Fraction B7 (230 mg) was moved on silica gel column and eluted with CHCl3–MeOH (9.5:0.5) to get compounds 2 (13.1 mg) and 3 (21.3 mg). Compounds 5 (16 mg) and 6 (15 mg) from fraction B9 (80 mg), compound 7 (23.6 mg) from fraction B11 (160 mg), and compound 8 (15.1 mg) from fraction B13 (230 mg) isolated by the same method used for fraction B3. All collected fractions were checked by TLC under UV at 254 and 366 nm following ammonia vapor exposure in each step. The structures of the isolated compounds were elucidated using 1H-NMR and 13C-NMR spectral analyses, as well as comparing with published data.
ɑ-Glucosidase inhibition assay
The inhibitory activity of the fractions and isolated compounds against α‐Glucosidase were assayed with the previously published method (Adib et al. 2019; Peytam et al. 2020; Akocak et al. 2021; Markus et al. 2022; Taslimi et al. 2021). The α‐glucosidase enzyme (EC3.2.1.20, Saccharomyces cerevisiae, 20 U/mg) was purchased from Sigma-Aldrich and sample solutions from the fractions and pure compounds were prepared at 500 μg/mL and 750 µM concentrations, respectively. The studied enzyme solution (1 U/mL, 20 µL), potassium phosphate buffer (50 mM, pH 6.8, 135 µL) and sample solution (20 µL) were added to a 96‐well plate and incubated at 37 °C for 10 min. In next step after adding 25 µL p‐nitrophenyl‐α‐D‐glucoside (PNPG, purchased from Sigma-Aldrich) at 4 mM concentration, the plate was incubated for 20 min at 37 °C. At last, the absorbance of each sample was measured with a spectrophotometer (Gen5, PowerWave XS2, BioTek) at 405 nm and the percentage of enzyme inhibition was calculated in comparison with the negative control. The 10% Dimethyl sulfoxide solution and Acarbose were used as negative and positive control, respectively. All experiments were performed in triplicate, and results were analyzed using Sigma plot 11.0 software and expressed as mean ± SD.
Enzyme kinetic assay
For revealing the inhibition type of the most potent compound, ɑ-glucosidase enzyme activity was computed in the absence and presence of different concentrations of the tested compound (0, 55, 120 and 185 µM) along with substrate (PNPG) at concentrations 1 to 10 mM (Adib et al. 2019; Peytam et al. 2020). The type of inhibition determined through applying a line weaver Burk plot. In addition, the Michaelis–Menten constant (Km) value was calculated from plot between reciprocal of the substrate concentration (1/[S]) and reciprocal of enzyme rate (1/V) at different inhibitor concentrations, and the experimental inhibitor constant (Ki) value was resulted from secondary plots of the inhibitor concentrations [I] versus Km.
Molecular docking study
Molecular docking of the isolated compounds in the active site of the α-glucosidase protein was conducted with Auto Dock 4 software and Auto Dock Tools (ADT 1.5.6) using the hybrid Lamarckian Genetic Algorithm (LGA). In human’s intestine, Maltase-glucoamylase (MGAM) catalyzes the hydrolysis of the disaccharides. Therefore, the N-terminal subunit of maltase-glucoamylase enzyme was used for docking to better understanding of the isolated compounds-enzyme interactions. The three-dimensional (3D) crystal structure of human intestinal α-glucosidase (PDB code 2QMJ) with a resolution of 1.90 ˚A was taken from the RCSB Protein Data Bank (https://www.rcsb.org). The 3D structures of selected compounds prepared by Hyperchem.8.0.10 software. The cubic grid box was built by 50 ˚A size in dimensions (x = − 20.808, y = − 6.586, z = − 5.074) in accordance with the position of the co-crystalized ligand (acarbose). All other parameters were left to default settings of AutoDock4. For validation of results, the root mean-square deviation (RMSD) was determined in comparison to re-docking of original inhibitor, acarbose, that co-crystallized with enzyme (2QMJ) and the RMSD less than 2.0 ˚A was used as threshold. The most favorable conformation by lowest free energy of binding was chosen and their target residues were determined. The result was pictured using Discovery Studio Visualizer (Biovia, D.S. Discovery Studio Modeling Environment 2015) (Askin et al. 2021; Güleç et al. 2022; Osmaniye et al. 2022; Setiawansyah et al. 2022).
Results
Isolation and structural elucidation
In consequence of chromatographical analyzing of the n-butanol fraction obtained from T. fedtschenkoi aerial part, eight phenolic compounds were isolated which were identified as: 3, 4-di-O-feruloyl quinic acid (1), luteolin-7-O-rutinoside (2), luteolin (3), rosmarinic acid methyl ester (4), rosmarinic acid (5), apigenin-7-O-glucoside (6), luteolin-7-O-glucuronide (7), and luteolin-7-O-glucoside (8) (Fig. 1). Molecular structures of the isolated compounds were determined with 1H-NMR and 13C-NMR spectral data. The elucidated structures were confirmed through comparison with published data (Chirikova et al. 2019; He et al. 2014; Hyun et al. 2015; Orhan et al. 2012; Lu and Yeap Foo 2000; Sevindik et al. 2015; Shu et al. 2013).
Spectroscopic data of isolated compounds
3, 4-Di-O-feruloyl quinic acid (C27H28O12) (1); 1H-NMR (DMSO-d6, 400 MHz): δ 7.55 (2H, d, J = 15.8 Hz, H-7′ and H-7″), 7.32 (1H, br s, H-2′), 7.30 (1H, br s, H-2"), 7.11 (1H, br d, J = 7.8 Hz, H-6′), 7.09 (1H, br d, J = 7.8 Hz, H-6″), 6.81 (1H, d, J = 7.8 Hz, H-5′), 6.80 (1H, d, J = 8.1 Hz, H-5″), 6.48 (1H, d, J = 15.8 Hz, H-8′), 6.44 (1H, d, J = 15.8 Hz, H-8″), 5.31 (1H, br d, J = 11.1 Hz, H-4), 4.80 (1H, m, H-3), 4.16 (1H, m, H-5), 1.5–2.0 (4H, m, H-2 and H-6). 13C-NMR (DMSO-d6, 100 MHz): δ 179.62 (C-7), 166.90 (C-9′), 166.48 (C-9″), 149.74 (C-4′), 149.65 (C-4″), 148.42 (C-7′), 148.39 (C-7″), 145.13 (C-3′), 144.96 (C-3″), 126.16 (C-1′), 126.09 (C-1″), 123.65 (C-6′), 123.46 (C-6″), 115.95 (C-5′), 115.95 (C-5″), 115.75 (C-8′), 115.64 (C-8″), 111.36 (C-2′), 111.31 (C-2″), 73.46 (C-1), 70.85 (C-3), 70.66 (C-5), 69.63 (C-4), 56.12 (OCH3), 56.8 (OCH3), 36.88 (C-2), 36.55 (C-6) (Chirikova et al. 2019).
Luteolin-7-O-rutinoside (C27H30O16) (2); 1H-NMR (DMSO-d6, 400 MHz): δ 7.37 (1H, br d, J = 8.0 Hz, H-6′), 7.37 (1H, br s, H-2′), 6.81 (1H, d, J = 8.0 Hz, H-5′), 6.72 (1H, s, H-3), 6.67 (1H, br s, H-8), 6.42 (1H, br s, H-6), 5.05 (1H, d, J = 7.3 Hz, H-1″), 4.55 (1H, br s, H-1″′), 3.0–4.0 (11H, overlapped signals, H2″ to H6″ and H2‴ to H5‴), 1.08 (3H, d, J = 6.0 Hz, H-6‴). 13C-NMR (DMSO-d6, 100 MHz): δ 181.01 (C-4), 164.77 (C-2), 162.46 (C-7), 161.25 (C-5), 158.24 (C-9), 148.60 (C-4′), 147.48 (C-3′), 125.72 (C-1′), 118.35 (C-6′), 116.45 (C-5′), 113.83 (C-2′), 105.21 (C-1′), 103.87 (C-3), 101.59 (C-1″), 101.01 (C-1‴), 101.1 (C-6), 95.63 (C-8), 76.85 (C-5″), 76.11 (C-3″), 73.49 (4‴), 72.29 (C-2″), 70.94 (C-2‴), 70.74 (C-3‴), 70.36 (C-4″), 68.89 (C-5‴), 65.14 (C-6″), 18.23 (C-6‴) (Shu et al. 2013).
Luteolin (C15H10O6) (3); 1H-NMR (DMSO-d6, 400 MHz): δ 12.95 (1H, br s, OH-5), 7.40 (1H, br d, J = 8.1 Hz, H-6'), 7.39 (1H, br s, H-2'), 6.88 (1H, d, J = 8.1 Hz, H-5'), 6.65 (1H, s, H-3), 6.43 (1H, br s, H-8), 6.17 (1H, br s, H-6). 13C-NMR (DMSO-d6, 100 MHz): δ 182.11 (C-4), 164.65 (C-7), 164.33 (C-2), 161.93 (C-5), 157.74 (C-9), 150.19 (C-4'), 146.20 (C-3'), 121.91 (C-1'), 119.45 (C-6'), 116.46 (C-5'), 113.79 (C-2'), 104.12 (C-10), 103.30 (C-3), 99.29 (C-6), 94.30 (C-8) (Hyun et al. 2015).
Rosmarinic acid methyl ester (C19H18O8) (4); 1H-NMR (DMSO-d6, 400 MHz): δ 7.49 (1H, d, J = 15.8 Hz, H-7), 7.07 (1H, br s, H-2), 7.02 (1H, br d, J = 8.1 Hz, H-6), 6.77 (1H, d, J = 8.1 Hz, H-5), 6.65 (1H, br s, H-2′), 6.64 (1H, d, J = 8.0 Hz, H-5′), 6.50 (1H, br d, J = 8.0 Hz, H-6′), 6.26 (1H, d, J = 15.8 Hz, H-8), 5.11 (1H, dd, J = 6.5 and 5.5 Hz, H-8′), 3.64 (3H, s, COOCH3), 2.95 (2H, m, H-7′). 13C-NMR (DMSO-d6, 100 MHz): δ 170.37 (C-9′), 166.32 (C-9), 149.29 (C-4), 146.80 (C-7), 146.09 (C-3), 145.43 (C-3′), 144.57 (C-4′), 127.06 (C-1′), 125.64 (C-1), 122.15 (C-6), 120.49 (C-6′), 117.11 (C-2′), 116.20 (C-5), 115.35 (C-5′), 115.36 (C-2), 113.21 (C-8), 73.23 (C-8′), 52.43 (COOCH3), 36.62 (C-7′) (Sevindik et al. 2015).
Rosmarinic acid (C18H16O8) (5); 1H-NMR (DMSO-d6, 400 MHz): δ 7.42 (1H, d, J = 15.8 Hz, H-7), 7.06 (1H, br s, H-2), 6.98 (1H, br d, J = 8.0 Hz, H-6), 6.76 (1H, d, J = 8.0 Hz, H-5), 6.68 (1H, br s, H-2′), 6.62 (1H, d, J = 8.0 Hz, H-5′), 6.51 (1H, br d, J = 8.0 Hz, H-6′), 6.22 (1H, d, J = 15.8 Hz, H-8), 4.96 (1H, dd, J = 9.2 and 1.3 Hz, H-8′), 3.01 (1H, br d, J = 13.9 Hz, H-7′b), 2.84 (1H, dd, J = 13.5 and 9.2 Hz, H-7′a). 13C-NMR (DMSO-d6, 100 MHz): δ 172.13 (C-9′), 166.51 (C-9), 149.09 (C-4), 146.18 (C-7), 145.76 (C-3), 145.39 (C-3′), 144.31 (C-4′), 128.78 (C-1′), 125.86 (C-1), 121.79 (C-6), 120.36 (C-6′), 117.13 (C-2′), 116.35 (C-5), 115.87 (C-5′), 115.42 (C-2), 114.31 (C-8), 74.50 (C-8′), 37.00 (C-7′) (Sevindik et al. 2015).
Apigenin-7-O-glucoside (C21H20O10) (6); 1H-NMR (DMSO-d6, 400 MHz): δ 7.88 (2H, d, J = 8.1 Hz, H-2′ and H-6′), 6.89 (2H, d, J = 8.1 Hz, H-3′ and H-5′), 6.81 (1H, s, H-3), 6.79 (1H, br s, H-8), 6.40 (1H, br s, H-6), 5.06 (1H, d, J = 7.0 Hz, H-1″), 3.1–3.9 (6H, H-2″ to H-6″). 13C-NMR (DMSO-d6, 100 MHz): δ 182.36 (C-4), 164.72 (C-2), 163.31 (C-7), 161.63 (C-5), 161.50 (C-4′), 157.39 (C-9), 129.06 (C-6′ and C-2′), 121.45 (C-1′), 116.42 (C-3′ and C-5′), 105.76 (C-10), 103.54 (C-3), 100.30 (C-1″), 99.95 (C-6), 95.38 (C-8), 77.48 (C-5″), 76.59 (C-3″), 73.39 (C-2″), 69.91 (C-4″), 60.95 (C-6″) (He et al. 2014).
Luteolin-7-O-glucorunide (C21H18O12) (7); 1H-NMR (DMSO-d6, 400 MHz): δ 7.41 (1H, br s, H-2′), 7.39 (1H, br d, J = 8.0 Hz, H-6′), 6.86 (1H, d, J = 8.0 Hz, H-5′), 6.79 (1H, br s, H-8), 6.71 (1H, s, H-3), 6.42 (1H, br s, H-6), 5.09 (1H, d, J = 7.3 Hz, H- 1″), 3.65 (1H, d, J = 9.7 Hz, H-5″), 3.2–3.6 (3H, H-2″ to H-4″). 13C-NMR (DMSO-d6, 100 MHz): δ 182.25 (C-4), 173.01 (C-6"), 166.88 (C-2), 163.42 (C-7), 161.51 (C-5), 157.37 (C-9), 152.09 (C-4′), 146.88 (C-3′), 120.55 (C-1′), 119.53 (C-6′), 116.63 (C-5′), 113.75 (C-2′), 105.68 (C-10), 102.98 (C-3), 100.03 (C-1″ and C-6), 95.86 (C-8), 76.87 (C-3″), 74.21 (C-5″), 73.38 (C-2″), 72.38 (C-4″) (Lu and Yeap Foo 2000).
Luteolin-7-O-glucoside (cynaroside) (C21H20O11) (8); 1H-NMR (DMSO-d6, 400 MHz): δ 7.73 (1H, br s, H-2′), 7.58 (1H, br d, J = 8.5 Hz, H-6′), 6.81 (1H, d, J = 8.5 Hz, H-5′), 6.63 (1H, s, H-3), 6.45 (1H, br s, H-8), 6.14 (1H, br s, H-6), 4.75 (1H, d, J = 7.5 Hz, H-1″), 3.1–3.8 (6H, H-2″ to H-6″). 13C-NMR (DMSO-d6, 100 MHz): δ 181.76 (C-4), 164.52 (C-2), 162.89 (C-7), 161.09 (C-5), 156.88 (C-9), 150.36 (C-4′), 145.87 (C-3′), 120.98 (C-1′), 119.09 (C-6′), 116.00 (C-5′), 113.31 (C-2′), 105.30 (C-10), 103.00 (C-3), 99.97 (C-1″), 99.55 (C-6), 95.38 (C-8), 77.12 (C-5″), 76.37 (C-3″), 73.08 (C-2″), 69.58 (C-4″), 60.63 (C-6″) (Shu et al. 2013).
ɑ-Glucosidase inhibition assay
The inhibitory activity of the fractions and isolated compounds (1–8) were measured against α-glucosidase enzyme in contrast to standard inhibitor (acarbose). As shown in Table 1, n-butanol fraction demonstrated the most α-glucosidase inhibitory activity among the tested fractions with the 67.4 ± 2.1% inhibition in concentration of 500 μg/ml. Among the tested compounds, rosmarinic acid (5) showed the strongest ɑ-glucosidase inhibition (IC50 = 43.38 ± 0.05 μM) and the compounds 4, 6, 8, 3 and 7 were in the next order. In fact, except of 1 and 2, other isolated compounds demonstrated more potency than acarbose as a standard drug with IC50 value of 750.0 ± 1.0 μM (Table 1).
Enzyme kinetic study
The kinetic of rosmarinic acid (5) as the most potent among the isolated compounds was studied. The Lineweaver–Burk and related plots showed that the Km value increased, while Vmax remained constant through the presence of rosmarinic acid in various concentrations. In addition, the Ki value of rosmarinic acid was calculated as 43 μM (Fig. 2).
Molecular docking study
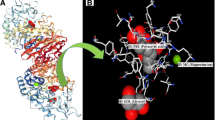
The interactions between the isolated compounds and α-glucosidase enzyme were examined using ADT (version 1.5.6). As formerly demonstrated, there is a significant homology between catalytic domains of S. cerevisiae α-glucosidase (G13) and human maltase-glucoamylase enzyme (GH31). In result, the crystal structure of N-terminal domain of human intestinal α-glucosidase (PDB code: 2QMJ) was used for docking simulations (Rigden 2002; Setiawansyah et al. 2022). The structures of standard drug (acarbose) and compound 5 (rosmarinic acid) as the most potent inhibitor of the isolated compounds were superimposed in the active site of enzyme (Fig. 3). In addition, the docking poses of acarbose and the isolated compounds were shown in Fig. 4.
The ˗log of free binding energy and related data for the isolated compounds were briefed in Table 2. According to the resulted poses, acarbose interacted with the active site of enzyme through hydrogen bonds with residues ASP203, THR205, TYR299, MET444, ASP542, and GLN603. There are also van der waals interactions for acarbose with residues ARG202, THR204, TYR214, TRP406, ASP443, PHE450, LYS480, ARG526, PHE575, ALA576, and TYR605. The docked pose of rosmarinic acid (5) showed a slightly different alignment in the interaction site of enzyme, whereas the hydrogen bond interactions with residues ASP203, THR205, and GLN603 are common interacted residues between rosmarinic acid (5) and acarbose. The TYR299, SER448, PHE450, PHE575, GLY602, and TYR605 are residues which linked with rosmarinic acid via van der waals interactions. Rosmarinic acid (5) also has pi–pi interaction via its aromatic rings with residues TRP406, ASP542, and ALA576.
Discussion
Diabetes is one of the major health concerns worldwide and characterized by hyperglycemia resulted from inefficient metabolism of carbohydrates and insulin secretion. One of the strategies to control diabetes is reducing postprandial hyperglycemia via inhibiting enzymes like α-glucosidase (Ruiz-Vargas et al. 2019; Uysal et al. 2019). In the current survey, the potential α-glucosidase inhibitory of T. fedtschenkoi various fractions assessed. Bioassay guided isolation of the n-butanol fraction resulted in five flavone derivatives (2, 3 and 6–8) in addition to three caffeic acid derivatives (1, 4 and 5). This is the first report of all of these compounds from the aerial part of T. fedtschenkoi. This study also reports the isolation of 3, 4-di-O-feruloylquinic acid (1) from Lamiaceae family for the first time. This compound has just been detected in some limited natural sources such as some Coffea species (Rubiaceae), Dacus carota (Apiaceae), Panax vietnamensis (Araliaceae), Artemisia annua and Lychnophora ericoides (Asteraceae) (Chirikova et al. 2019; Gobbo-Neto and Lopes 2008; Perrone et al. 2008; Viacava et al. 2020; Zhao et al. 2015).
The most effective α-glucosidase inhibitory activity was observed for rosmarinic acid. It was not a surprising result as for this compound, the antidiabetic properties were well established in numerous previous reports (Istifli 2021; Ruiz-Vargas et al. 2019; Swain and Puttaswamy 2020; Uysal et al. 2019; Zhu et al. 2019). In fact, rosmarinic acid is one of the effective natural compounds not only in α-glucosidase inhibition but also in other pathways which could be effective in diabetes treatment. Some studies found rosmarinic acid in diabetes management as effective as standard drugs like acarbose (Singh et al. 2012; Zhu et al. 2021). Considering these reports, rosmarinic acid is possibly one of the main active substances in the n-butanol fraction and it could make T. fedtschenkoi preparations as effective phytomedicine for diabetes treatment.
Luteolin, as a flavone, showed beneficial effects in diabetes, and its α-glucosidase inhibitory activity was established by copious reports (Cheng et al. 2014; Dao et al. 2021; Jia et al. 2019; Kim et al. 2000; Park et al. 2016; Yan et al. 2014). In fact luteolin and its glycosides like daidzein are well known as strong α-glucosidase inhibitors (Lodhi and Kori 2021). In the present study, among the isolated luteolin derivatives, luteolin-7-O-glucoside (cynaroside) had lowest IC50 against α-glucosidase. Kim et al. (Kim et al. 2000) and Asghari et al. (Asghari et al. 2015) studied the α-glucosidase inhibition of cynaroside. In correlation with current study, they reported good inhibition for cynaroside too. The compounds 2 (luteolin-7-O-rutinoside) and 7 (luteolin-7-O-glucuronide) showed weak inhibitory effects with IC50 values higher than 750 μM. However, this is the first report of α-glucosidase inhibitory activity of compound 2, but the glucuronide derivative of luteolin was assessed by Asghari et al. (2015) and controversially, the compound 7 showed good inhibitory effect even better than cynaroside.
As shown in Fig. 4, the residues ASP203, THR205, and GLN603 are common interacted sites between all docked compounds and probably they are important residues for interaction of substrate with N-terminal domain of human intestinal α-glucosidase. The flavonoid glycosides such as compounds 2, 7, and 8 interacted with ASP203 and THR205 via their glycoside hydroxyls (OH in positions 3′′, 4′′ or even 6′′). On the other hand, luteolin (3), as a non-glycoside flavonoid, bond to ASP203 via OH in position 5 of A-ring instead of glycoside hydroxyls and there is no reaction between luteolin and THR205. On the other hand, all of the isolated glycoside flavonoids except to compound 6, linked to GLN603 by OH of B-ring. Compound 6 and 3 (luteolin) have van der Waals reaction via B-ring with residue GLN603. This is in accordance with previous reports which claimed that flavonoids can bond to the active site residues of α-glucosidase through the hydroxyl groups in B-ring. Aromatic rings and specific distributing of electron clouds of flavonoid facilitate the donating of hydrogens in 3′ or 4′ OH groups (Liu et al. 2020; Singh et al. 2018; Xu 2010). Rosmarinic acid (5) reacted with residues ASP203 and THR205 by OH groups in phenyl lactic and p-coumaryl parts (3, 4, 3′, and 4′ positions) as an alternative of glycosidic hydroxyls in flavonoids, and it linked to residue GLN603 with carboxyl group of phenyl lactic part (9′ position).
The compound 6, as an apigenin glycoside showed the second lowest IC50 (96.33 ± 0.11 μM) in α-glucosidase inhibition. Furthermore, the results of other studies demonstrated that apigenin-7-O-glucoside (6) is a strong α-glucosidase inhibitor (Jia et al. 2019; Villa-Rodriguez et al. 2018). Considering that apigenin-7-O-glucoside could be effective in diabetes treatment through improving insulin resistance, and increasing glucose uptake by HepG2 cells, this plant metabolite is a potential lead compound for antidiabetic drug development surveys (Jia et al. 2019).
Conclusions
Analyzing the n-butanol fraction of T. fedtschenkoi resulted in the isolation and identification of five flavonoids, alongside with 3, 4-di-O-feruloylquinic acid, rosmarinic acid and rosmarinic acid methyl ester as caffeic acid derivatives. In in vitro enzyme inhibition assay, rosmarinic acid (5) demonstrated a potent competitive inhibition toward α-glucosidase enzyme with the lowest IC50 value (43.38 ± 0.05 μM). Furthermore, apigenin-7-O-glucoside (6) and cynaroside (7) were showed strong inhibition of α-glucosidase. The present study suggests these phenolic compounds as appropriate natural candidates in antidiabetic drug development research and also highlights the beneficial potentials of T. fedtschenkoi as a helpful herbal supplement for diabetic patients. However, further pharmacological and toxicological studies followed by clinical trial are needed to establish the efficacy and safety of this medicinal plant in management of diabetes mellitus type II.
References
Abousaber M, Hadjakhoondi A, Shafiee A (2002) Composition of the essential oil of Thymus pubescens boiss. Et kotschy ex celak and Thymus fedtschenkoi ronniger from Iran. J Essent Oil Res 14:154–155. https://doi.org/10.1080/10412905.2002.9699808
Adib M, Peytam F, Shourgeshty R, Mohammadi-Khanaposhtani M, Jahani M, Imanparast S, Faramarzi MA, Larijani B, Moghadamnia AA, Esfahani EN, Bandarian F (2019) Design and synthesis of new fused carbazole-imidazole derivatives as anti-diabetic agents: in vitro α-glucosidase inhibition, kinetic, and in silico studies. Bioorg Med Chem Lett 29:713–718. https://doi.org/10.1016/j.bmcl.2019.01.012
Akocak S, Taslimi P, Lolak N, Işık M, Durgun M, Budak Y, Türkeş C, Gülçin İ, Beydemir Ş (2021) Synthesis, characterization, and inhibition study of novel substituted phenylureido sulfaguanidine derivatives as α-glycosidase and cholinesterase inhibitors. Chem Biodivers 18:e2000958. https://doi.org/10.1002/cbdv.202000958
Alinezhad S, Kamalzadeh A, Shams-Ghahfarokhi M, Rezaee MB, Jaimand K, Kawachi M, Zamani Z, Tolouei R, Razzaghi-Abyaneh M (2011) Search for novel antifungals from 49 indigenous medicinal plants: Foeniculum vulgare and Platycladus orientalis as strong inhibitors of aflatoxin production by Aspergillus parasiticus. Ann Microbiol 61:673–681. https://doi.org/10.1007/s13213-010-0194-1
Aminkhani A, Sharifi R, Ranjbar E, Chalabian F, Katouzian F (2019) Antimicrobial activities and chemical constituents of essential oil extracted from stem, leaf, and flower of Thymus fedtschenkoi from Khoy, Iran. J Food Process Preserv 43(10):e14149. https://doi.org/10.1111/jfpp.14149
Asghari B, Salehi P, Sonboli A, Ebrahimi SN (2015) Flavonoids from Salvia chloroleuca with α-amylsae and α-glucosidase inhibitory effect. Iran J Pharm Res 14:609–615
Askin S, Tahtaci H, Türkeş C, Demir Y, Ece A, Akalın Çiftçi G, Beydemir Ş (2021) Design, synthesis, characterization, in vitro and in silico evaluation of novel imidazo[2,1-b][1,3,4]thiadiazoles as highly potent acetylcholinesterase and non-classical carbonic anhydrase inhibitors. Bioorg Chem 113:105009. https://doi.org/10.1016/j.bioorg.2021.105009
Chatterjee S, Khunti K, Davies MJ (2017) Type 2 diabetes. Lancet 389:2239–2251. https://doi.org/10.1016/S0140-6736(17)30058-2
Cheng N, Yi WB, Wang QQ, Peng SM, Zou XQ (2014) Synthesis and α-glucosidase inhibitory activity of chrysin, diosmetin, apigenin, and luteolin derivatives. Chin Chem Lett 25:1094–1098. https://doi.org/10.1016/j.cclet.2014.05.021
Chirikova N, Olennikov D, Grigor’ev R, Klyushin A, Nosov A (2019) Acylquinic acids, flavonoids, and maltol O-glucoside from Panax vietnamensis. Chem Nat Compd 55(6):1161–1163. https://doi.org/10.1007/s10600-019-02922-1
Choi HS, Kim MSL, Sawamura M (2002) The essential oils of Thymus migricus and T. fedtschenkoi var. handelii from Turkey. Flavour Fragrance J 17:41–45. https://doi.org/10.1002/ffj.1036
Dao TB, Nguyen TM, Nguyen VQ, Tran TM, Tran NM, Nguyen CH, Nguyen TH, Nguyen HH, Sichaem J, Tran CL, Duong TH (2021) Flavones from combretum quadrangulare growing in Vietnam and their alpha-glucosidase inhibitory activity. Molecules 26(9):2531. https://doi.org/10.3390/molecules26092531
Delazar A, Bahmani M, Shoar HH, Tabatabaei-Raisi A, Asnaashari S, Nahar L, Sarker SD (2011) Effect of altitude, temperature and soil on essential oil production in Thymus fedtschenkoi flowers in Osko and surrounding areas in Iran. J Essent Oil Bear Plant 14:23–29. https://doi.org/10.1080/0972060X.2011.10643897
Duarte-Silva E, de Melo MG, Maes M, Filho AJMC, Macedo D, Peixoto CA (2021) Shared metabolic and neuroimmune mechanisms underlying type 2 diabetes mellitus and major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry 111:110351. https://doi.org/10.1016/j.pnpbp.2021.110351
Gobbo-Neto L, Lopes NP (2008) Online identification of chlorogenic acids, sesquiterpene lactones, and flavonoids in the Brazilian Arnica Lychnophora ericoides Mart. (Asteraceae) leaves by HPLC-DAD-MS and HPLC-DAD-MS/MS and a validated HPLC-DAD method for their simultaneous analysis. J Agric Food Chem 56:1193–1204. https://doi.org/10.1021/jf072812l
Groenewegen A, Zwartkruis VW, Cekic B, de Boer R, Rienstra M, Hoes AW, Rutten FH, Hollander M (2021) Incidence of atrial fibrillation, ischaemic heart disease and heart failure in patients with diabetes. Cardiovasc Diabetol 20(1):1–2. https://doi.org/10.1186/s12933-021-01313-7
Güleç Ö, Türkeş C, Arslan M, Demir Y, Yeni Y, Hacımüftüoğlu A, Ereminsoy E, Küfrevioğlu Öİ, Beydemir Ş (2022) Cytotoxic effect, enzyme inhibition, and in silico studies of some novel N-substituted sulfonyl amides incorporating 1,3,4-oxadiazol structural motif. Mol Divers 9:1–21. https://doi.org/10.1007/s11030-022-10422-8
He F, Wang M, Gao M, Zhao M, Bai Y, Zhao C (2014) Chemical composition and biological activities of Gerbera anandria. Molecules 19(4):4046–4057. https://doi.org/10.3390/molecules19044046
Hosseini SH, Bibak H, Ghara AR, Sahebkar A, Shakeri A (2021) Ethnobotany of the medicinal plants used by the ethnic communities of Kerman province, Southeast Iran. J Ethnobiol Ethnomed 17(1):1–35. https://doi.org/10.1186/s13002-021-00438-z
Huang I, Lim MA, Pranata R (2020) Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia—a systematic review, meta-analysis, and meta-regression: diabetes and COVID-19. Diabetes Metab Syndr Clin Res Rev 14:395–403. https://doi.org/10.1016/j.dsx.2020.04.018
Hyun HB, Shrestha S, Boo KH, Cho SK (2015) Evaluation of antioxidant potential of ethyl acetate fraction of Rosmarinus officinalis L. and its major components. J Korean Soc Appl Biol Chem 58:715–722. https://doi.org/10.1007/s13765-015-0097-8
IDF Diabetes Atlas (2019) 9th edn. International Diabetes Federation, Brussels, Belgium
Istifli ES (2021) Chemical composition, antioxidant and enzyme inhibitory activities of onosma bourgaei and onosma trachytricha and in silico molecular docking analysis of dominant compounds. Molecules 26(10):2981. https://doi.org/10.3390/molecules26102981
Jia Y, Ma Y, Cheng G, Zhang Y, Cai S (2019) Comparative study of dietary flavonoids with different structures as α-glucosidase inhibitors and insulin sensitizers. J Agric Food Chem 67:10521–10533. https://doi.org/10.1021/acs.jafc.9b04943
Khorshidi J, Rasouli M, Rustaiee AR, Mohamadparast B (2014) Chemical composition of the essential oil of Thymus fedtschenkoi growing wild in Iran. J Essent Oil Bear Plant 17:173–175. https://doi.org/10.1080/0972060X.2013.831574
Kim JS, Kwon CS, Son KH (2000) Inhibition of alpha-glucosidase and amylase by luteolin, a flavonoid. Biosci Biotechnol Biochem 64:2458–2461. https://doi.org/10.1271/bbb.64.2458
Lankatillake C, Luo S, Flavel M, Lenon GB, Gill H, Huynh T, Dias DA (2021) Screening natural product extracts for potential enzyme inhibitors: protocols, and the standardisation of the usage of blanks in α-amylase, α-glucosidase and lipase assays. Plant Methods 17(1):1–19. https://doi.org/10.1186/s13007-020-00702-5
Li X, He T, Wang X, Shen M, Yan X, Fan S, Wang L, Wang X, Xu X, Sui H, She G (2019) Traditional uses, chemical constituents and biological activities of plants from the genus Thymus. Chem Biodiversity 16(9):e1900254. https://doi.org/10.1002/cbdv.201900254
Liu JL, Yc K, Miao JY, Mei XY, Wu SY, Yan YC, Cao XY (2020) Spectroscopy and molecular docking analysis reveal structural specificity of flavonoids in the inhibition of α-glucosidase activity. Int J Biol Macromol 152:981–989. https://doi.org/10.1016/j.ijbiomac.2019.10.184
Lodhi S, Kori ML (2021) Structure-activity relationship and therapeutic benefits of flavonoids in the management of diabetes and associated disorders. Pharm Chem J 54:1106–1125. https://doi.org/10.1007/s11094-021-02329-9
Lu Y, Yeap Foo L (2000) Flavonoid and phenolic glycosides from Salvia officinalis. Phytochemistry 55:263–267. https://doi.org/10.1016/S0031-9422(00)00309-5
Markus A, Schepmann D, Wünsch B (2022) Synthesis of oxazolo-annulated 3-benzazepines designed by merging two negative allosteric NMDA receptor modulators. Arch Pharm 355:2200020. https://doi.org/10.1002/ardp.202200020
Orhan F, Barış Ö, Yanmış D, Bal T, Güvenalp Z, Güllüce M (2012) Isolation of some luteolin derivatives from Mentha longifolia (L.) Hudson subsp. longifolia and determination of their genotoxic potencies. Food Chem 135(2):764–769. https://doi.org/10.1016/j.foodchem.2012.04.137
Osmaniye D, Türkeş C, Demir Y, Özkay Y, Beydemir Ş, Kaplancıklı ZA (2022) Design, synthesis, and biological activity of novel dithiocarbamate-methylsulfonyl hybrids as carbonic anhydrase inhibitors. Arch Pharm 355:2200132. https://doi.org/10.1002/ardp.202200132
Ozturk M, Altay V, Altundağ E, Ibadullayeva SJ, Aslanipour B, Gönenç TM (2018) Herbals in Iğdır (Turkey), Nakhchivan (Azerbaijan), and Tabriz (Iran). In: Plant and human health, vol 1. Springer, Cham, pp 197–266
Park MS, Zhu YX, Pae HO, Park SH (2016) In vitro and in vivo α-glucosidase and α-amylase inhibitory effects of the water extract of leaves of pepper (Capcicum annuum L. Cultivar Dangjo) and the active constituent luteolin 7-O-glucoside. J Food Biochem 40:696–703. https://doi.org/10.1111/jfbc.12252
Perrone D, Farah A, Donangelo C, Paulis T, Martin P (2008) Comprehensive analysis of major and minor chlorogenic acids and lactones in economically relevant Brazilian coffee cultivars. Food Chem 106:859–867. https://doi.org/10.1016/j.foodchem.2007.06.053
Peytam F, Adib M, Shourgeshty R, Mohammadi-Khanaposhtani M, Jahani M, Imanparast S, Faramarzi MA, Mahdavi M, Moghadamnia AA, Rastegar H, Larijani B (2020) Design and synthesis of new imidazo[1,2-b]pyrazole derivatives, in vitro α-glucosidase inhibition, kinetic and docking studies. Mol Divers 24(1):69–80. https://doi.org/10.1007/s11030-019-09925-8
Rathod P, Yadav RP (2021) Anti-diabesity potential of various multifunctional natural molecules. J Herbal Med 27:100430. https://doi.org/10.1016/j.hermed.2021.100430
Rigden DJ (2002) Iterative database searches demonstrate that glycoside hydrolase families 27, 31, 36 and 66 share a common evolutionary origin with family 13. FEBS Lett 523:17–22. https://doi.org/10.1016/s0014-5793(02)02879-x
Ruiz-Vargas JA, Morales-Ferra DL, Ramírez-Ávila G, Zamilpa A, Negrete-León E, Acevedo-Fernández JJ, Peña-Rodríguez LM (2019) α-Glucosidase inhibitory activity and in vivo antihyperglycemic effect of secondary metabolites from the leaf infusion of Ocimum campechianum mill. J Ethnopharmacol 243:112081. https://doi.org/10.1016/j.jep.2019.112081
Rustaiee AR, Mirahmadi SF, Sefidkon F, Tabatabaei MF, Omidbaigi R (2011) Essential oil content and composition of Thymus fedtschenkoi ronniger at different phenological stages. J Essent Oil Bear Plant 14:625–629. https://doi.org/10.1080/0972060X.2011.10643981
Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, Shaw JE (2019) Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas. Diabetes Res Clin Pract 157:107843. https://doi.org/10.1016/j.diabres.2019.107843
Salehi B, Abu-Darwish MS, Tarawneh AH, Cabral C, Gadetskaya AV, Salgueiro L, Hosseinabadi T, Rajabi S, Chanda W, Sharifi-Rad M, Mulaudzi RB (2019) Thymus spp. plants- food applications and phytopharmacy properties. Trends Food Sci Technol 85:287–306. https://doi.org/10.1016/j.tifs.2019.01.020
Salteh SA, Amani M (2020) Ethnobotanical study of medicinal plants from West Azerbaijan, Northwestern Iran. https://doi.org/10.21203/rs.3.rs-55496/v1
Setiawansyah A, Reynaldi MA, Tjahjono DH (2022) Molecular docking-based virtual screening of antidiabetic agents from Songga (Strychnos lucida R. Br.): an Indonesian native plant. Curr Res Biosci Biotech 3:208–214. https://doi.org/10.5614/crbb.2022.3.2/82kytcpw
Sevindik HG, Ozgen U, Atila A, Ozturk Er H, Kazaz C, Duman H (2015) Phtytochemical studies and quantitative HPLC analysis of rosmarinic acid and luteolin 5-O-β-D-glucopyranoside on Thymus praecox subsp. grossheimii var. grossheimii. Chem Pharm Bull (tokyo) 63:720–725. https://doi.org/10.1248/cpb.c14-00877
Sharma P, Singh S, Thakur V, Sharma N, Grewal AS (2021) Novel and emerging therapeutic drug targets for management of type 2 diabetes mellitus. Obes Med 23:100329. https://doi.org/10.1016/j.obmed.2021.100329
Shu X, Wang M, Liu D, Wang D, Lin X, Liu J, Wang X, Huang L (2013) Preparative separation of polyphenols from artichoke by polyamide column chromatography and high-speed counter-current chromatography. Quim Nova 36:836–839. https://doi.org/10.1590/s0100-40422013000600017
Singh P, Jha S, Irchhaiya R (2012) Antidiabetic and antioxidant activity of hydroxycinnamic acids from Calamintha officinalis Moench. Med Chem Res 21:1717–1721. https://doi.org/10.1007/s00044-011-9690-5
Singh G, Singh A, Verma RK, Mall R, Azeem U (2018) Synthesis, biological evaluation and molecular docking studies of novel benzimidazole derivatives. Comput Biol Chem 72:45–52. https://doi.org/10.1016/j.compbiolchem.2017.12.010
Swain A, Puttaswamy H (2020) α-glucosidase inhibition kinetics and molecular docking studies with the bioactive constituents from Canna indica L. rhizome extract. Asian J Chem 32:1986–1990. https://doi.org/10.14233/ajchem.2020.22727
Taleb A, Qannadi F, Changizi-Ashtiyani S, Zarei A, Rezvanfar M, Akbari A, Hekmatpou D (2017) The effect of aqueous extract Thymus kotschyanus Boiss. et Hohen on glycemic control and dyslipidemia associated with type II diabetes: a randomized controlled trial. Iran J Endocrinol Metab 19:234–243
Taslimi P, Işık M, Türkan F, Durgun M, Türkeş C, Gülçin İ, Beydemir Ş (2021) Benzenesulfonamide derivatives as potent acetylcholinesterase, α-glycosidase, and glutathione S-transferase inhibitors: biological evaluation and molecular docking studies. J Biomol Struct Dyn 39:5449–5460. https://doi.org/10.1080/07391102.2020.1790422
Tohidi B, Rahimmalek M, Arzani A (2017) Essential oil composition, total phenolic, flavonoid contents, and antioxidant activity of Thymus species collected from different regions of Iran. Food Chem 220:153–161. https://doi.org/10.1016/j.foodchem.2016.09.203
Tohidi B, Rahimmalek M, Trindade H (2019) Review on essential oil, extracts composition, molecular and phytochemical properties of Thymus species in Iran. Ind Crops Prod 134:89–99. https://doi.org/10.1016/j.indcrop.2019.02.038
Uysal S, Senkardes I, Mollica A, Zengin G, Bulut G, Dogan A, Glamočlija J, Soković M, Lobine D, Mahomoodally FM (2019) Biologically active compounds from two members of the Asteraceae family: Tragopogon dubius Scop. and Tussilago farfara L. J Biomol Struct Dyn 37:3269–3281. https://doi.org/10.1080/07391102.2018.1506361
Viacava F, Ortega-Hernández E, Welti-Chanes J, Cisneros-Zevallos L, Jacobo-Velázquez DA (2020) Using high hydrostatic pressure processing come-up time as an innovative tool to induce the biosynthesis of free and bound phenolics in whole carrots. Food Bioprocess Technol 13:1717–1727. https://doi.org/10.1007/s11947-020-02512-y
Villa-Rodriguez JA, Kerimi A, Abranko L, Tumova S, Ford L, Blackburn RS, Rayner C, Williamson G (2018) Acute metabolic actions of the major polyphenols in chamomile: an in vitro mechanistic study on their potential to attenuate postprandial hyperglycaemia. Sci Rep 8(1):1–4. https://doi.org/10.1038/s41598-018-23736-1
Xu H (2010) Inhibition kinetics of flavonoids on yeast α-glucosidase merged with docking simulations. Protein Pept Lett 17:1270–1279. https://doi.org/10.2174/092986610792231492
Yan J, Zhang G, Pan J, Wang Y (2014) α-Glucosidase inhibition by luteolin: kinetics, interaction and molecular docking. Int J Biol Macromol 64:213–223. https://doi.org/10.1016/j.ijbiomac.2013.12.007
Zhao W, Zhang W, Chen Y, Yang F, Cao Q, Chen L, Dai K (2015) Identification and purification of novel chlorogenic acids in Artemisia annua L. J Exp Biol Agric Sci 3:415–422. https://doi.org/10.18006/2015.3(5).415.422
Zhu F, Wang J, Takano H, Xu Z, Nishiwaki H, Yonekura L, Yang R, Tamura H (2019) Rosmarinic acid and its ester derivatives for enhancing antibacterial, α-glucosidase inhibitory, and lipid accumulation suppression activities. J Food Biochem 43(2):12719. https://doi.org/10.1111/jfbc.12719
Zhu C, Niu H, Nie A, Bian M (2021) Bioactivity-guided separation of potential α-glycosidase inhibitor from clerodendranthus spicatus based on HSCCC coupled with molecular docking. Sci Rep 11(1):1–2. https://doi.org/10.1038/s41598-021-86379-9
Funding
This research was supported by Tehran University of Medical Sciences and Health Services Grants (No. 99-03-33-39646).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mohammadi-Liri, A., Parsa-Khankandi, H., Dehnoee, A. et al. α-Glucosidase inhibitors from the aerial part of Thymus fedtschenkoi: isolation, kinetic and molecular docking study. Chem. Pap. 77, 571–581 (2023). https://doi.org/10.1007/s11696-022-02511-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-022-02511-7