Abstract
Huntington’s disease (HD) is an inherited neurodegenerative disorder caused by an expansion in CAG repeat on huntington (Htt) gene, leading to a degeneration of GABAergic medium spiny neurons (MSNs) in the striatum, resulting in the generation of reactive oxygen species, and decrease antioxidant activity. These pathophysiological alterations impair mitochondrial functions, leading to an increase in involuntary hyperkinetic movement. However, researchers investigated the neuroprotective effect of antioxidants using various animal models. Still, their impact is strictly limited to curtailing oxidative stress and increasing the antioxidant enzyme in the brain, which is less effective in HD. Meanwhile, researchers discovered Mitochondria-targeted antioxidants (MTAXs) that can improve mitochondrial functions and antioxidant activity through the modulation of mitochondrial signaling pathways, including peroxisome proliferator-activated receptor (PPAR)-coactivator 1 (PGC-1α), dynamin-related protein 1 (Drp1), mitochondrial fission protein 1 (Fis1), and Silent mating type information regulation 2 homolog 1 (SIRT-1), showing neuroprotective effects in HD. The present review discusses the clinical and preclinical studies that investigate the neuroprotective effect of MTAXs (SS31, XJB-5–131, MitoQ, bezafibrate, rosiglitazone, meldonium, coenzyme Q10, etc.) in HD. This brief literature review will help to understand the relevance of MTAXs in HD and enlighten the importance of MTAXs in future drug discovery and development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
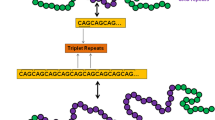
Huntington’s disease (HD) is a hereditary disorder characterized by expanded repeated sequences of CAG on exon 1 of the huntingtin (Htt) gene in chromosome 4, which leads to the production and deposition of mutant Htt (mHtt) protein in the cytoplasm [1,2,3]. These mHtt proteins alter mitochondrial function and ubiquitination process, followed by excitotoxicity [4]. Various preclinical and clinical studies suggest that mutation in the Htt gene is a prognostic factor of HD and is responsible for behavioral abnormalities such as jerking movement, postural imbalance, difficulty in walking, and cognitive impairments [5,6,7]. The incidence of HD is greater among people aged between 30 and 50 years. The literature reported that approximately 2.7 million population globally suffer from HD, and the prevalence rate differs between countries [8, 9]. The incidence of HD is continuously increasing because of complex pathology and unexplored causative molecular signaling pathways [10]. The statement reflects the necessity of developing therapeutic agents that halt the disease progression and exert neuroprotection for HD.
In physiological conditions, the Htt gene retains 7–35 CAG repeats and produces wild-type Htt protein in the nucleus and cytoplasm, regulating embryonic development and neurogenesis [11]. In disease conditions, significant accumulation of mHtt, oxidative stress, and mitochondrial abnormalities have been reported to contribute to HD progression. However, clinical evidence has also proposed a direct link between mitochondria and HD [12]. Studies show that in HD, the CAG repeats are more than 35, which is accountable for the mutation in the Htt protein.
Further, the mHTT protein translocates into the nucleus, interacts with several transcription factors/cofactors, and interferes with their normal functioning, resulting in abnormal cellular activities, including a reduction in mitochondrial oxidative phosphorylation (OXPHOS), membrane permeability, an increase in inner mitochondrial membrane translocation (MMT), and a decrease axonal transport in the neurons [13, 14]. Additionally, significant accumulation of mHtt enhances mitochondrial permeability transmission pore, leading to leakage of Ca2+ and promoting neuronal excitotoxicity [14,15,16]. Similarly, an in-vitro study observed that 3-nitropropionic acid (3-NP) treatment fluctuates Ca2+ levels in neurons and astrocytes, leading to Ca2+ overload and excitotoxicity [17, 18]. Likewise, 3-NP inhibits mitochondrial complex II activity, significantly increasing reactive oxygen species (ROS) in GABAergic striatal neurons to generate HD-like symptoms [19, 20].
On the other hand, free radicals generated during the adenosine triphosphate (ATP) production process are neutralized by antioxidant enzymes within mitochondria, maintaining the redox and antioxidant balance [21, 22]. Further, the mitochondria accomplished ATP are utilized by neurons to regulate several processes, including regulation of membrane potential, vesicle recycling, exoplasmic transport, neurotransmitter synthesis, and synaptic plasticity [23]. Mitochondrial biogenesis is also an important phenomenon that regulates the transcription and translation process of mitochondrial and nuclear DNA-encoded proteins. Which facilitates multiple intracellular signaling pathways, including peroxisome proliferator-activated receptor (PPAR)-coactivator 1 (PGC-1α), Dynamin-related protein-1 (DRP-1), and Silent mating type information regulation 2 homolog) 1 (SIRT-1) [12, 24].
Moreover, mitochondria have an antioxidant defense system that maintains the oxidative stress level by activating several enzymes, including superoxide dismutase (SOD), Glutathione peroxidase (GPx), and peroxiredoxins [25]. A meta-analysis study documented that the generation of ROS and decreased antioxidant enzyme levels in the brain significantly contribute to HD pathophysiology [26, 27]. Previously, it was found that loss of membrane potential, reduction in ca2+ buffering capacity, lowering an expression of oxidative phosphorylation, and mitochondrial dysfunction contribute to the progression of HD [28, 29]. Meanwhile, clinical studies also documented abnormal mitochondrial complex activity and impaired mitochondrial dynamics in HD brains [30, 31]. However, several natural extracts, phytomolecules, and synthetic drugs have been reported to reduce oxidative stress against various In-vivo and In-vitro experimental models. Still, their mechanisms are limited to oxidative stress-mediated neuroprotection [32,33,34]. Nevertheless, target-based therapy is needed to prevent HD pathological conditions that improve the quality of life.
To resolve this issue, researchers investigated MTAXs, which exert a mitochondria-mediated neuroprotective effect on HD. However, there is still a lack of awareness about MTAXs and related molecular mechanisms [35]. However, the present review compiled various pre-clinical and clinical studies investigating MTAXs and their possible signaling pathways in HD. For a critical analysis of study outcomes, a comprehensive literature search was conducted to explore the neuroprotective mechanisms of various MTAXs in HD [36, 37]. Moreover, this review encourages further investigation of antioxidants as novel approaches to treat HD.
Physiological role of mitochondria
Mitochondria is a unit that generates ATP in the cells, which is utilized by the body to regulate multiple functions, including metabolism, Ca2+ homeostasis, and apoptosis. Moreover, these ATP are used by neurons to release neurotransmitters, maintaining synaptic plasticity and remyelination [38, 39]. The mitochondrial membrane contains iron, which is required for energy metabolism, the formation of respiratory complexes, and the regulation of mitochondrial potential equilibrium [40]. The mitochondria have complexes (I, III, and IV) that transfer the protons from the mitochondrial matrix into the intermembrane space. These reactions can change the potential between matrix and intermembrane space, leading to proton transmission, which further increases ATP levels in complex V. Alongside, the transferring of protons during OXPHOS increases the generation of superoxide anions (O2-), results oxidative stress [41]. Coenzyme Q10 (CoQ10) and ubiquinone help transfer electrons from complex I to II in the electron transport chain (ETC). Over these, reduced forms of CoQ10 are increased, which shows an antioxidant effect by decreasing the damage to lipids, DNA, and proteins [41].
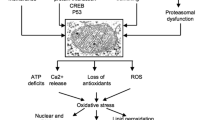
On the contrary, free radicals generated during these processes act as a redox signaling molecule, which transmits signals to the mitochondria at lower concentrations. This is advantageous to the human body [42, 43]. Under physiological conditions, mitochondria maintain homeostasis, stimulate Ca2+ associated ATP synthesis, and regulate apoptotic pathways. Meanwhile, Ca2+ enhances respiratory rates, OXPHOS, and ATP production by enhancing pyruvate dehydrogenase, isocitrate dehydrogenase, and ATP synthase enzymes [44]. Moreover, the inner mitochondria protein has a voltage-gated channel together with Ca2+ dependent transporters that regulate the entry of Ca2+ into the matrix, and these Ca2+ regulate the buffering system, maintain membrane potential, cellular integrity, and mitophagy, essential to remove the mitochondrial waste [45]. Furthermore, mitochondria regulate extracellular homeostasis, which is necessary for neuronal survival. Simultaneously, the rough endoplasmic reticulum (ER) supplies essential lipids and amino acids for protein synthesis and regulates Ca2+ production and storage, which cells use to maintain an optimal cellular environment [46, 47] (Fig. 1).
Mitochondria ATP formation under the physiological condition After glycolysis process, the pyruvate is obtained as an end product, which is utilized by the mitochondrial TCA cycle for the formation of NADH and FADH2; thus, reduced coenzyme NADH binds to complex I, transferred the electron to complex II, where FADH2 convert to FAD and transfer the elector to complex III and reduced two molecules of cytochrome C, then complex IV finally transfer the electron to oxygen and simultaneously release two proton across, this proton involved in the phosphorylation of ADP to ATP. During ATP synthesis, + O2 is generated, which is converted to H2O2 by the enzyme Superoxide Dismutase, and this H2O2 is converted to H2O + O2. Neurons utilize the mitochondrial-generated ATP to perform various activities, including generating the action potential and maintaining homeostasis through calcium signaling, mitochondrial membrane potential, antioxidant defense system, and synaptic plasticity. The figure is drawn with the help of biorender software and a PowerPoint presentation. [Abbrevations; Tricarboxylic acid cycle (TCA), nicotinamide adenine dinucleotide (NAD) + hydrogen (H) (NADH), Flavin adenine dinucleotide hydride (FADH), Flavin Adenine Dinucleotide (FAD), Adenosine diphosphate (ADP), Adenosine triphosphate (ATP), Hydrogen Peroxide (H2O2)]
Conversely, mitochondrial dynamics are engaged in cellular metabolism, apoptosis, and synaptic plasticity. Alterations in dynamics are regulated by the DRP-1 mitochondrial fission protein, which divides the mitochondria cells into daughter cells and another. Mitochondrial fusion allows the mitochondria to join into a giant tubular organelle [48, 49]. This process enables the mitochondrial network to move, maintain its shape, and establish physical connections between organelles at the synaptic level [50].
The fusion and fission are essential processes; the outer membrane proteins [mitofusin1 (Mfn1) and mitofusin2 (Mfn2)] and inner membrane proteins [optic atrophy1 (OPA1), mitochondrial fission protein 1 (Fis1), and DRP-1] are highly preserved GTPase that is essential for maintaining homeostasis and protect the cells from Ca2+ mediated excitotoxicity [49, 51, 52]. Similarly, the PGC-1α is a transcriptional coregulator that activates mitochondrial biogenesis by expressing the nuclear respiratory and mitochondrial transcription factors that enhance mitochondria DNA replication and gene transcription [53]. All these mitochondrial proteins are significantly involved in maintaining and regulating neuronal survival.
Several other signaling pathways are also interlinked with mitochondria, and their impairment causes neurodegenerative disorders [54, 55]. To overcome this issue, researchers target mitochondrial protein as a therapeutic target for preventing neurological disorders, including HD [56, 57].
Evidence of ROS and oxidative stress in HD
ROS are oxygen-carrying radicals that have the potency to exist independently with one or more unpaired electrons [58]. Mitochondrial antioxidants further decompose these free radicals, but an alteration in the mitochondrial complex activity enhances the production of ROS; these ROS activate endoplasmic ryanodine receptors and block sarcoplasmic reticulum Ca2+ ATPase (SERCA) pumps that lead to higher Ca2+ levels into the cytoplasm results resulting in excitotoxicity [70, 71]. Moreover, the frequent ROS generation causes oxidative stress by suppressing the mitochondrial enzyme activity, including SOD, and catalase results in impairment in mitochondrial activity followed by cell death [27, 59]. Another study indicates an increase in oxidative damage and altered antioxidant signaling molecules involved in the degeneration of GABAergic striatal neurons [60]. On the other hand, a case report found a significant increase in oxidative stress levels in the brain of HD patients, showing their involvement in disease progression [26, 61].
Moreover, researchers observed that mutation in the HTT protein enhanced oxidative stress, resulting in damage of DNA and proteins in the brains of HD rodents [62]. Increased oxidative stress was also found in the brains of transgenic HD animals [5, 63]. Similarly, several studies utilized a 3-NP-induced HD model to investigate the neuroprotective effect of compounds, and their findings indicate that 3-NP treatment significantly enhanced oxidative stress by inhibiting mitochondrial complex II enzyme in the striatum [60, 64]. Our previous study also observed a significant increase in nitrite and malonaldehyde levels in 3-NP-treated rats related to the progression of various pathological processes, including apoptosis and neuroinflammation [20, 63]. It was found that 3-NP treatment impaired the activity of the antioxidant defense system and increased the oxidative stress, followed by downregulation of the nuclear factor erythroid 2 related factor 2 (Nrf2)/Hemeoxydenase-1 (HO-1) signaling pathway, which is majorly responsible for the oxidative balance [65, 66].
Moreover, oxidative stress impaired mitochondrial signaling molecules, including PGC-1α, DRP-1, and SIRT-1, maintaining mitochondrial biogenesis, whereas their alteration induced HD-like pathophysiological condition in rodents [67,68,69]. To resolve this issue, researchers are now focusing on developing MTAXs because mitochondrial proteins and their associated signaling pathways are directly linked with oxidative stress and could be a treatment option for HD.
Mitochondrial dysfunction in HD
HD is an autosomal dominant neurodegenerative disorder. Almost nine distinct inherited neurodegenerative diseases are triggered by the repetition of a CAG trinucleotide that causes disease by encoding extended polyglutamine (poly Q) tracts in each protein product, and HD is one of them [70, 71]. Several studies have reported that mHtt protein is extensively expressed in the striatum and degenerates the GABAergic MSNs [5]. Earlier in 1993, Beal et al. and their team showed that impairment in mitochondrial function is a prognostic factor for HD. However, preclinical studies revealed that administering 3-NP, a mitochondrial toxin, selectively degenerates the striatal MSNs in rodents and induces HD-like symptoms [63, 64, 72]. This investigation shows that impairing mitochondrial activity could contribute to HD pathogenesis [2].
Additionally, an earlier report found a substantial reduction in mitochondrial OXPHOS and mitochondrial complex (II, III, and IV) activities significantly damage the caudate nucleus and putamen [73, 74]. Similarly, another study found remarkable mitochondrial dysfunction and the formation of mHtt proteins in HD patient’s brain [62]. Moreover, the substantial reduction in membrane potential, homeostasis, and elevation of Ca2+ mediated permeability with the activation of apoptotic pathways have been reported as a causative factor for HD [20, 27, 75].
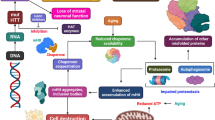
Similarly, the preclinical study reported that mHtt proteins altered mitochondria complex activity, incredibly complex II and IV, resulting in ROS generation and cell death. Besides this, the mHtt protein interacts with the external membrane of mitochondria and enhances transcriptional changes associated with HD [66, 76]. Furthermore, PGC-1α is expressed in mitochondria, and its downregulation has been documented in mHtt-expressed HD mice, indicating its role in HD [77]. Other than these impaired mitochondria, subsequently enhance the protease activity that cleaves HTT protein, increasing the production of N-terminal HTT fragments and enhancing disease progression [13]. Several clinical and preclinical studies reported mitochondrial dysfunction, ROS generation, transcriptional dysfunction, and impaired mitochondrial antioxidant enzyme as pathological markers of HD [78,79,80]. To address this problem, researchers started investigating antioxidants as a treatment option for HD (Fig. 2).
Mitochondrial dysfunction associated HD Pathophysiology The abnormal mHtt formation and 3-Nitropropionic Acid inhibits mitochondrial complex II activity and leads to an increase in the ROS, which crosses the mitochondrial membrane pores and releases the endoplasmic stored Ca2+ into the cytoplasm that initiates the excitotoxicity. Alongside, ca2+ disrupts the inner mitochondrial membrane, leading to the release of caspases, resulting in cell death. Meanwhile, mHtt protein altered various mitochondrial proteins, including PGC-1α, SIRT-1, DRP-1, and NF-kβ; this abnormal genetic expression is responsible for abnormal mitochondrial biogenesis, antioxidant system, and neurodegeneration leads to progression of HD. The figure is drawn with the help of biorender software and a PowerPoint presentation. [Abbrevations; Reactive Oxygen Species (ROS), peroxisome proliferator-activated receptor (PPAR)-coactivator 1 (PGC-1α), silent mating type information regulation 2 homolog 1 (SIRT-1), Dynamin-related protein 1 (DRP-1), and Nuclear factor kappa B (NF-kβ)]
MTAX-regulated signaling pathways in HD
Mitochondria regulates several antioxidant targets, including SIRT 1, DRP-1, and PGC-1α, a transcription coactivator factor involved in regulating energy metabolism, mitochondrial biogenesis, and homeostasis. In addition, the PGC-1α regulates the expression of nuclear-encoded subunits of the ETC complexes, mitochondrial antioxidant defense proteins, and nuclear respiratory factors 1 and 2 (Nrf1 and Nrf2); all these factors are essential for mitochondrial redox balance and neuronal survival [24]. Previously it was found that an increase in H2O2 can promote the mRNA expression of PGC-1α and enhance the level of detoxifying enzymes, including SOD, GPx, and GSH [67, 69]. In contrast, an excessive release of H2O2 can downregulate the PGC-1α activity and increase oxidative stress-mediated cell death [69]. Furthermore, a substantial reduction of PGC-1α has been recorded in transgenic HD mice [71].
Similarly, a study reported spongiform degeneration of striatal neurons in PGC-1α knockout mice and showed abnormal hyperkinetic motor activities; the results indicate that it could be used as a therapeutic target for treating HD [81,82,83]. The evidence confirmed that mutation in the Htt protein downregulates the expression of PGC-1α, leading to evoked HD pathogenesis [12]. For this reason, the researcher investigated photoactive molecules that can enhance the expression of PGC-1α and show neuroprotective effects against the HD model [12, 68]. A study examined a PGC-1α mediated activation of an HO-1 signaling pathway; the study used PGC-1α knockout 3T3-L1 cells and found downregulation of HO-1 levels, study concluded that activation of PGC-1α could be implemented as an antioxidant therapy (Singh et al., 2016, Waldman et al., 2016).
In addition, SIRTs are class-III histone deacetylases that depend on NAD + and are divided into seven different forms (SIRT-1–7) [81]. The SIRT-1 and SIRT-3 specifically correlated to HD. The SIRT-1 is a NAD + dependent histone deacetylase primarily found in mitochondria and their lower levels in HD patients’ brains [84, 85]. Additionally, the downregulation or knockdown of SIRT-1 elevates the oxidative stress in cultured C2C12 myoblasts [81, 86]. The decrease in NAD + levels damages the DNA, which inactivates the SIRT-1 gene expression in the mitochondria and encourages neuronal inflammation and cognitive dysfunction in HD [87]. Meanwhile, the upregulation of SIRT-1 has been demonstrated to strengthen locomotor activity, decreasing cortical and striatal neuronal lesions in the N171-82Q and BACHD transgenic HD models [88, 89]. Another study examined the fact that mHtt protein interferes with the CREB-regulated transcription coactivator 1 (TORC-1) with CREB interaction, which suppresses the transcription of BDNF and SIRT-1. In contrast, activation of SIRT-1 promotes the survival of neurons in HD mice [88, 90, 91]. Similarly, R6/2 mice with brain-specific SIRT-1 knockout exacerbate HD pathophysiology. However, activation of SIRT-1 in HD brains overexpresses Brain derived neurotrophic factor (BDNF), demonstrating neuroprotective action against HD [81, 88].
SIRT-3 is also found in the mitochondria and exerts NAD + dependent activity, but their downregulation or knockout increases the free radicals. However, activation of SIRT-3 restricts the development of the mitochondrial permeability transition pore by deacetylating cyclophilin D [86, 92]. SIRT-3 plays an essential role in maintaining mitochondrial integrity via the regulation of succinate dehydrogenase [93], while SIRT-3 reduces the ROS levels through stimulation of antioxidant enzymes, including SOD2, which is triggered by SIRT-3 activation [94, 95].
According to Cheng et al., mice lacking SIRT-3 exhibit glutamate-induced excitotoxicity, oxidative stress, and mitochondrial damage in cortical neurons. Also, 3-NP injected rats showed significant striatal neuronal loss in SIRT-3 deficient mice compared to mice with the SIRT-3 gene [96]. Furthermore, researchers tested various SIRT-3 activators and inhibitors against in-vitro and in-vivo models. They found that SIRT-3 modulators exert neuroprotection by alleviating mitochondrial abnormalities and improving rodents’ HD-like pathological conditions [65, 97, 98]. These findings suggest that SIRT-1 could be a potential mitochondrial target for HD.
Moreover, DRP-1 is a cytoplasmic GTPase that frequently regulates mitochondrial fission. The DRP-1 gets translocated into the mitochondria by a set of adaptor proteins found in the outermost layer of mitochondria, including Fis1, Mff, and mitochondrial elongation factor 1 [99, 100]. After entering the mitochondria, the DRP-1 binds to the specific site of the endoplasmic reticulum (ER) and then oligomerizes to form an outer mitochondrial membrane. These events can cleave mitochondria into two daughter mitochondria [99, 101, 102]. Earlier researchers found abnormal expression of DRP-1 proteins in several neurological disorders [49], including AD [103], PD [104], and Epilepsy [105].
Moreover, the role of DRP-1 is well reported in HD pathophysiology; earlier researchers observed that mHtt protein erratically interacts with Drp 1 mediated mitochondrial fission in rodents and humans to stimulate enzymatic activity. This process further promotes mitochondrial fragmentation and lowers the mitochondrial transport activity, leading to neuronal damage [106]. Furthermore, the mHtt protein interacts with DRP-1 and enhances the activity of GTPase DRP-1 enzymes, resulting in abnormal anterograde mitochondrial dynamics and synaptic modification in the transgenic BACHD mouse model of HD [107]. Additionally, a study found elevated levels of S-nitroso-DRP-1 in the striatum of postmortem brains of HD patients and transgenic mice models. This elevated S-nitroso-DRP-1 could be responsible for excessive mitochondrial fragmentation, dendritic spine loss, and synaptic alterations [108]. Another study’s results indicate that DRP-1 heterozygous knockout mice had no differences in mitochondrial proteins, synaptic plasticity, or dendritic functions compared to wild-type mice [109]. Several in-vitro and in-vivo experiments demonstrated that inhibiting DRP-1 could decrease mitochondrial fragmentation and improve synaptic activity [110,111,112], suggesting that it could be used as a therapeutic strategy for HD. However, there is a contradiction about DRP-1, which shows that DRP-1 knockout mice showed higher levels of H2O2 and lipid peroxidation than wild-type mice (Manczak et al., 2012). So, there is still a conflict over DRP-1-mediated mitochondrial regulation. To resolve this puzzle, further studies must target Drp 1 against HD.
More so, coenzyme Q10 is another protein that regulates MTAX and is a crucial transporter of electrons from complex I to II, involved in ATP generation and neuron survival [113]. The reduction in COQ10 expression was reported in the striatum region of the HD patient’s brain [114, 115]. In-vivo experimental findings indicate that administration of COQ10 improves mitochondrial ATP generation and prevents lipid peroxidation, as well as DNA damage in the R6/2 transgenic mouse model of HD [116, 117]. Moreover, in another study, COQ10 restored the motor performance and declined behavioural abnormalities in N171-82Q transgenic HD mice [118]. Additionally, it has been proven that activation of the COQ10 enzyme enhances grip strength, locomotor function, and cognition in a 3-NP-induced HD model [119, 120]. Research indicates that administering COQ10 or triggering the COQ10 exerts neuroprotection by reducing oxidative stress and improving mitochondria activity in HD models [121, 122]. Although many signaling pathways are linked to mitochondria, the focus of this review is on MTAXs because they have been extensively studied and have been shown to have important neuroprotective effects against HD. Thus, MTAXs could be implemented as a treatment option for HD.
Preclinical and clinical studies targeting MTAX in HD
Mitochondria contains a variety of antioxidant proteins that regulate the physiological function of neurons via balancing the ROS and antioxidant levels in the brain, which is discussed in the above section [123]. The abnormal expression of mitochondrial proteins is also reported in HD patients and rodents’ brains [6, 61]. Furthermore, researchers started discovering MTAXs that activate or inhibit the specific mitochondrial signaling pathways to halt the progression of neurodegenerative disorders [1, 124]. Afterward, several natural and synthetic compounds were tested to investigate the molecular mechanism that provides neuroprotective effects against animal models of HD. Their results indicate that MTAXs can reduce oxidative damage, mitochondrial dysfunction, and neurodegeneration, improving locomotor performance in HD rodents [1, 124]. This section discusses the selective MTAXs that modulate specific mitochondrial targets and regulate their downstream signaling pathways, demonstrating a neuroprotective effect against HD models [35].
Previous, plenty of evidence found that downregulation of PGC-1α is involved in the progression of HD [125]. As a result, a study was carried out to investigate the beneficial effect of resveratrol, a polyphenol mainly extracted from grapes and cranberries, against YAC128 transgenic HD mice. The study findings revealed that resveratrol treatment boosts the activity of ETC complexes and the expression of PGC-1α, demonstrating neuroprotection against YAC128 HD mice [126]. Resveratrol also showed neuroprotection via upregulation of PGC-1α in C. elegans HD model [127]. Similarly, rosiglitazone, a PPARgamma agonist, reduced mHtt-induced striatal neurotoxicity, strengthened motor activity, and reduced hyperglycaemic condition in N171-82Q HD mice [71]. B-lapachone is a naturally occurring substance derived from the inner bark of the Lapacho tree that has medicinal properties. For this reason, it was tested against HD; the B-Lapachone treatment reduced the elevated level of ROS in the In-vitro HD model. In contrast, its oral administration improves muscular strength via upregulation of SIRT-1 mediated PGC-1α expression in R6/2 HD mice [97].
Moreover, nicotinamide, a prominent water-soluble vitamin with antioxidant properties, was explored in HD. Nicotinamide (250 mg/kg) administration intensified BDNF mRNA levels and enhanced PGC-1α activation in B6.HDR6/1 transgenic mice. Thus, activation of PGC-1α could enhance locomotor activity and neuromuscular coordination, but no effect on mHtt aggregation was observed [128]. Meldonium is a fatty acid oxidation inhibitor primarily used to treat cardiovascular diseases. It alters the pathways for carnitine, a nutrient that aids fat breakdown. Later, Cristo et al., and his colleagues investigated the effect of meldonium against a transgenic Drosophila HD model; the results suggest that meldonium significantly increases the expression of PGC-1α exerting neuroprotective effect by restoring motor function and reduces ROS-mediated apoptotic process in transgenic Drosophila HD model [59]. Bezafibrate is a lipid-lowering drug that has poor blood–brain barrier permeability, and its high dose and prolonged duration exerts severe side effects, including gastrointestinal disturbances, liver enzyme abnormalities, myopathy, and, in rare cases, rhabdomyolysis. Apart from this, the earlier researchers investigated its potential against HD; the study administered 0.5% of bezafibrate with diet for 10 months–16 months showed a substantial decrease in oxidative stress and apoptotic damage in the striatum of BACHD mice. Additionally, benzafibrate increased PGC-1α expression in the striatum, which may be responsible for improved motor coordination in the BACHD mouse model of HD [129]. Aside from that, several compounds activate PGC-1α, demonstrating a possible neuroprotective effect by halting the progression of HD-like symptoms in rodents. These outcomes indicate that PGC-1α may be a more precise target for HD drug development.
SIRT is the second most common mitochondrial target involved in HD modulation. Its activation has a beneficial effect on neurodegenerative disorders [89, 130]. Previously, researchers investigated the neuroprotective potential of diapocynin in a 3-NP-induced animal model. Their results indicated that Diapocynin activates the SIRT-1-mediated downstream pathways, including the Nrf2/BDNF signaling, which reduces oxidative stress and increases antioxidants. Apocynin also inhibited the NF-kB and P53-mediated apoptotic pathways and restored histopathological changes caused by 3-NP [65].
Furthermore, researchers discovered SRT2104, a synthetic compound, and found that its administration increases SIRT-1 expression in HD mice and improves motor abilities and neuronal survival in N171-82Q HD mice [131]. Similarly, selisistat reversed the toxic effect of mHtt fragments via activation of SIRT signaling and ameliorated neurotoxicity in drosophila and mouse models of HD [132]. Melatonin has also been tested against HD; the results show that melatonin treatment activated multiple signaling pathways, including SIRT-1 and PGC-1α, and inhibits the NF-kB, demonstrating neuroprotective potential against HD [133]. Similarly, viniferin, a natural phytomolecule, was tested against cells overexpressing the mHtt protein. Viniferin appears to upregulate SIRT3, increasing antioxidant capacity and mitochondrial dynamic while decreasing fission 1 and DRP-1 levels in mitochondria, increasing cell survival [98]. There have been very few studies on SIRT-1 and SIRT3 activators against HD, so more drugs must be developed that improve HD via the activation of SIRT signaling pathways.
Earlier researchers reported abnormal expression of DRP-1 in the HD rodent models, and it was found that the peptide inhibitor P110 specifically inhibited DRP-1 under stress conditions and played a crucial role in HD pathology. Also, it reduced mitochondrial fragmentation and enhanced neuronal survival against the mHtt-induced zQ175 knock-in mouse model of HD [134]. Previously, researchers investigated the neuroprotective potential of N-acetylcysteine and edaravone against preclinical animal models. The findings suggest that both compounds can reduce mitochondrial membrane potential dysfunction, apoptosis, and neuronal death in 3-NP treated striatal cells [135]. However, another study developed synthetic compound 3-(2,6-diethylphenyl)quinazoline-2,4-dione (PAQ-22) for HD, and the result indicated that PAQ-22 could inhibit DRP-1 and it could be used in the treatment of HD [112] Moreover, several studies reported that downregulation of DRP-1 reduced apoptotic markers and increased antioxidants, followed by a decrease in histopathological alteration in HD rodents [48, 104, 107, 136].
Researchers also developed synthetic compounds and evaluated their impact on mitochondria protein; the study investigated the effect of XJB-5–131 on a transgenic mouse model of HD [137]. Findings indicate that XJB-5–131 restored mitochondrial function and improved locomotor functions and neuronal survival [137, 138]. Similarly, Coenzyme Q, a mitochondrial enzyme tested against R6/2 HD, the Coenzyme Q treated mice showed a substantial reduction in oxidative stress and improved motor control compared to R6/2 HD mice [139]. With this, coenzyme Q alleviates overactive autophagy induced in the R6/2 muscle, which has been interlinked with muscle wasting, but coenzyme Q10 treatment did alter autophagic markers in the brain. Thereby limiting the risk of neuronal impairments [139].
In another study, Ying et al. investigated the treatment of MitoQ and SS31, which improved neuronal survival by enhancing mitochondrial function in neurons with mHtt-induced mitochondrial and synaptic damage. Their results indicate that it could be a potent therapy for treating HD [140]. Furthermore, a study explored the structure–activity relationship of four different mitochondrial-targeted tetrapeptides in which SPN10 and SS-31 showed a strong relation with oxidative stress. The SPN10 and SS-31 demonstrate neuroprotective potential by restoring the mitochondrial membrane potential, increasing ATP content, and neuronal survival [141]. The elamipretide is another mitochondrion-targeted tetrapeptide explored against neurodegenerative disorders, and their outcomes suggest that elamipretide increases mitochondrial respiration and neural mitochondrial biogenesis through mitochondrial biogenesis regulators (PCG-1α and TFAM) and translocate factors (TOM-20). In addition, it activates mitochondrial fusion (MNF-1, MNF-2, and OPA1), downregulates the expression of mitochondrial fission proteins (Fis-1 and DRP-1), and enhances mitochondria autophagy. Evidence suggests that treatment with elamipretide reduced oxidative stress and neuroinflammation and promoted neural pro-survival by increasing the expression of BDNF and TrkB proteins [142] (Fig. 3).
Mitochondrial-targeted antioxidants showing neuroprotection against HD In physiological conditions, calcium releases in response to stimuli such as physical exercise cause activation of CAMK, which further activates MAPK, followed by expression of PCG-1α. Similarly, adrenergic stimulation increases cAMP level, then expression of CREB, which leads to activation of PCG-1α. The NAD + and cAMP levels also enhance PCG-1α activity via SIRT-1 acetylation and AMPK phosphorylation, respectively. Including this activation of eNOS increases cGMP and PCG-1α expression. However, PCG-1α activates NRF2 signalling to regulate oxidative phosphorylation, increases SIRT-3 to promote GSH, SOD, and GPx levels, also facilitate the activity of PPAR-γ to maintain Bax/Bcl ratio, and mitochondrial oxidation, also inhibits Drp-1 to reduce mitofusion and exert neuroprotection. The figure is drawn with the help of biorender software and a PowerPoint presentation. [Abbrevations; Reactive Oxygen Species (ROS), Calmodulin-dependent protein kinase (CAMK), Mitogen-activated protein kinase (MAPK), peroxisome proliferator-activated receptor (PPAR)-coactivator 1 (PGC-1α), cyclic adenosine monophosphate (cAMP), cAMP-response element binding protein (CREB), silent mating type information regulation 2 homolog 1 (SIRT-1), endothelial nitric oxide synthase (eNOS), nuclear factor erythroid 2–related factor 2 (Nrf2), and Nuclear factor kappa B (NF-kβ), superoxide dismutase (SOD), glutathione (GSH), glutathione peroxidases (GPx),]
Moreover, researchers developed different types of nano-formulations that increase the target specificity of mitochondria, increasing the drug’s bioavailability. Apocynin nanoformulation is the best example; the exposure of apocynin nanoformulation reduces oxidative stress-induced mitochondrial dysfunction and promotes dopaminergic neuronal survival [143]. There is a list of preclinical studies investigating the effect of compounds that target the mitochondrial proteins and exert the neuroprotective effect against HD (Table 1).
All the promising drug candidates and phytochemicals that show positive outcomes from preclinical studies are utilized in clinical trials being conducted in humans. According to the U.S National Library of Medicine, approximately 172 studies have been registered focused on investigating the effect of potential molecules in HD patients. Out of 172 studies, six studies were found (By searching mesh words Huntington disease and mitochondrial antioxidants) on MTAXs this includes Melatonin (NCT04421339), Resveratrol (NCT02336633), (2)-epigallocatechin-3-gallate (EGCG) ( NCT01357681), Complete symptomatic treatment therapy (Haloperidol 2 mg Tab, Risperidone 1 mg, Zoloft 50 mg Tab) (NCT04071639), and N-Acetyl Cysteine (NAC) (NCT05509153), The study’s results could provide a new treatment therapy for HD.
Conclusion
HD is mainly caused by mHtt aggregation and abnormal mitochondrial functioning, which are directly related to the progression of HD. However, targeting mitochondrial proteins may be an effective drug discovery and development strategy. Thus, discussing various aspects of mitochondrial dysfunction and its targeted therapy can be essential in managing HD pathophysiology. Some mitochondrial signaling targets, such as PGC-1α, SIRT-1 & 3, and DRP-1, are being investigated by researchers to demonstrate the neuroprotective effect of various synthetic and natural molecules by employing an HD model. The outcomes of both clinical and preclinical studies evidence that mHtt and mitochondrial dysfunction increase ROS production, oxidative stress, and apoptosis, which can be done by modulating specific mitochondrial proteins. As a result, several MTAXs (SS31, CDDO-ethyl amide, XJB-5–13, MitoQ, bezafibrate, rosiglitazone, meldonium, and coenzyme Q10) have shown neuroprotection against HD models. Based on the evidence, MTAXs could be a beneficial target for mitochondrial homeostasis and might be used as a treatment strategy for HD. However, further research is required to identify mitochondrial-associated downstream pathways relevant to HD and whose modulation can slow disease progression. Additionally, more natural and synthetic compounds should be tested to explore their molecular mechanism and neuroprotective effect against HD. Furthermore, preclinical tested MTAXs should be evaluated for their efficacy and safety potential in clinical trials.
Data availability
Not applicable.
Abbreviations
- 3-NP:
-
3-Nitropropionic acid
- AD:
-
Alzheimer’s disease
- ARE:
-
Antioxidant response element
- ATP:
-
Adenosine triphosphate
- BDNF:
-
Brain-derived neurotrophic factor
- CAG:
-
Cytosine adenine guanine
- CDDO:
-
2-Cyano-3,12-dioxooleana-1,9-dien-28-oic acid
- CoQ10:
-
Coenzyme Q10
- DRP-1:
-
Dynamin-related protein 1
- EGCG:
-
Epigallocatechin-gallate
- ETC:
-
Electron transport chain
- Fis1:
-
Fission protein 1
- GSH:
-
Glutathione peroxidase
- H2O2 :
-
Hydrogen peroxide
- HD:
-
Huntington’s disease HD
- HTT:
-
Huntingtin
- IP3R:
-
Inositol trisphosphate receptor
- Mfn1:
-
Mitofusin1
- MMT:
-
Mitochondrial membrane translocation
- MSNs:
-
Medium spiny neurons
- MTAXs:
-
Mitochondria-targeted antioxidants
- NDGA:
-
Norhihydroguaiaretic acid
- NF-κβ:
-
Nuclear factor kappa-β
- Nrf1:
-
Nuclear respiratory factors 1
- Nrf2:
-
Nuclear factor erythroid 2-related factor 2
- OH- :
-
Hydroxyl radical
- OXPHOS:
-
Oxidative phosphorylation
- PGC-1 α:
-
Peroxisome proliferator-activated receptor (PPAR)-coactivator 1
- Poly Q:
-
Polyglutamine
- ROS:
-
Reactive oxygen species
- SERCA:
-
Sarcoplasmic reticulum Ca2+ ATPase
- SIRT-1:
-
Silent mating type information regulation 2 homolog 1
- SOD:
-
Superoxide dismutase
References
Chaturvedi RK, Beal MF. Mitochondria targeted therapeutic approaches in Parkinson’s and Huntington’s diseases. Mol Cell Neurosci. 2013;55(1):101–14.
Roze E, Saudou F, Caboche J. Pathophysiology of Huntington’s disease: from huntingtin functions to potential treatments. Curr Opin Neurol. 2008;21(4):497–503.
Mestre TA. Recent advances in the therapeutic development for Huntington disease. Parkinsonism Relat Disord. 2019;59:125–30.
Upadhayay S, Jamwal S, Kumar P. Animal models of Huntington’s disease and their applicability to novel drug discovery and development. Expert Opin Drug Discov. 2023;18(5):527–38.
Khan MQ, Mubeen H, Khan ZQ, Masood A, Zafar A, Wattoo JI, et al. Computational insights into missense mutations in HTT gene causing Huntington’s disease and its interactome networks. Ir J Med Sci. 2023;192(3):1435–45.
Kaye J, Reisine T, Finkbeiner S. Huntington’s disease iPSC models-using human patient cells to understand the pathology caused by expanded CAG repeats. Fac Rev. 2022;11:16.
Wiggins R, Feigin A. Emerging therapeutics in Huntington’s disease. Expert Opin Emerg Drugs. 2021;26(3):295–302.
Upadhayay S, Gupta R, Singh S, Mundkar M, Singh G, Kumar P. Involvement of the G-protein-coupled estrogen receptor-1 (GPER) signaling pathway in neurodegenerative disorders: a review. Cell Mol Neurobiol. 2023;43:1833–1847.
Papanna B, Lazzari C, Rabottini M. The prevalence of Huntington disease in Asia highlights needs in clinical, genetic and instrumental diagnosis: a systematic review and meta-analysis. Psychiatr Danub. 2022;34(Suppl 10):13–23.
Alqahtani T, Deore SL, Kide AA, Shende BA, Sharma R, Chakole RD, et al. Mitochondrial dysfunction and oxidative stress in Alzheimer’s disease, and Parkinson’s disease, Huntington’s disease and amyotrophic lateral sclerosis-an updated review. Mitochondrion. 2023;71:83–92.
Gatto EM, Rojas NG, Persi G, Etcheverry JL, Cesarini ME, Perandones C. Huntington disease: advances in the understanding of its mechanisms. Clin Parkinsonism Related Dis. 2023;71:83–92.
Intihar TA, Martinez EA, Gomez-Pastor R. Mitochondrial dysfunction in Huntington’s disease; interplay between HSF1, p53 and PGC-1α transcription factors. Front Cell Neurosci. 2020;3:100056.
Damiano M, Galvan L, Déglon N, Brouillet E. Mitochondria in Huntington’s disease. biochimica et biophysica acta BBA- biophys. Acta - Mol Basis Dis. 2010;1802(1):52–61.
Milakovic T, Quintanilla RA, Johnson GV. Mutant Huntingtin expression induces mitochondrial calcium handling defects in clonal striatal cells. J Biol Chem. 2006;281(46):34785–95.
Verma M, Lizama BN, Chu CT. Excitotoxicity, calcium and mitochondria: a triad in synaptic neurodegeneration. Transl neurodegener. 2022;11(1):1–14.
Kalani K, Yan SF, Yan SS. Mitochondrial permeability transition pore: a potential drug target for neurodegeneration. Drug Discov Today. 2018;23(12):1983–9.
Fukuda A, Deshpande SB, Shimano Y, Nishino H. Astrocytes are more vulnerable than neurons to cellular Ca2+ overload induced by a mitochondrial toxin, 3-nitropropionic acid. Neuroscience. 1998;87(2):497–507.
Cirillo G, Maggio N, Bianco MR, Vollono C, Sellitti S, Papa M. Discriminative behavioral assessment unveils remarkable reactive astrocytosis and early molecular correlates in basal ganglia of 3-nitropropionic acid subchronic treated rats. Neurochem Int. 2010;56(1):152–60.
Okada N, Nakamura S, Shimazawa M. 3-Nitropropionic acid enhances ferroptotic cell death via NOX2-mediated ROS generation in STHdhQ111 striatal cells carrying mutant huntingtin. Biol Pharm Bull. 2023;46(2):177–86.
Kalonia H, Kumar P, Kumar A. Licofelone attenuates quinolinic acid induced Huntington like symptoms: possible behavioral, biochemical and cellular alterations. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(2):607–15.
Lee KH, Cha M, Lee BH. Neuroprotective effect of antioxidants in the brain. Int J Mol Sci. 2020;21(19):7152.
Lopes C, Ferreira IL, Maranga C, Beatriz M, Mota SI, Sereno J, et al. Mitochondrial and redox modifications in early stages of Huntington’s disease. Redox Biol. 2022;56:102424.
Carmo C, Naia L, Lopes C, Rego AC. Mitochondrial dysfunction in Huntington’s disease. polyglutamine disorders. Adv Exp Med Biol. 2018. https://doi.org/10.1007/978-3-319-71779-1_3.
Chen M, Yan R, Luo J, Ning J, Zhou R, Ding L. The role of PGC-1α-mediated mitochondrial biogenesis in neurons. Neurochem Res. 2023;48:1–12.
Wei Y-H, Lu C-Y, Wei C-Y, Ma Y-S, Lee H-C. Oxidative stress in human aging and mitochondrial disease-consequences of defective mitochondrial respiration and impaired antioxidant enzyme system. Chin J Physiol. 2001;44(1):1–12.
Peña-Sánchez M, Riverón-Forment G, Zaldívar-Vaillant T, Soto-Lavastida A, Borrero-Sánchez J, Lara-Fernández G, et al. Association of status redox with demographic, clinical and imaging parameters in patients with Huntington’s disease. Clin Biochem. 2015;48(18):1258–63.
Binawade Y, Jagtap A. Neuroprotective effect of lutein against 3-nitropropionic acid-induced Huntington’s disease-like symptoms: possible behavioral, biochemical, and cellular alterations. J Med Food. 2013;16(10):934–43.
Sindhu RK, Kaur P, Kaur P, Singh H, Batiha GE, Verma I. Exploring multifunctional antioxidants as potential agents for management of neurological disorders. Environ Sci Pollut Res Int. 2022;29(17):24458–77.
Johri A, Beal MF. Mitochondrial dysfunction in neurodegenerative diseases. J Pharmacol Exp Ther. 2012;342(3):619–30.
Shirendeb U, Reddy AP, Manczak M, Calkins MJ, Mao P, Tagle DA, et al. Abnormal mitochondrial dynamics, mitochondrial loss and mutant huntingtin oligomers in Huntington’s disease: implications for selective neuronal damage. Hum Mol Genet. 2011;20(7):1438–55.
Uddin MH, Rumman M, Sarowar T. Mitochondrial Dysfunction in Huntington Disease. Antioxidants and Functional Foods for Neurodegenerative Disorders: CRC Press; 2021. p. 151–74.
Bin-Jumah MN, Gilani SJ, Alabbasi AF, Al-Abbasi FA, AlGhamdi SA, Alshehri OY, et al. Protective effect of fustin against Huntington’s disease in 3-nitropropionic treated rats via downregulation of oxidative stress and alteration in neurotransmitters and brain-derived neurotrophic factor activity. Biomedicines. 2022;10(12):3021.
Kaur K, Devi B, Agrawal V, Kumar R, Sandhir R. Identification of potential inhibitors of brain-specific CYP46A1 from phytoconstituents in Indian traditional medicinal plants. J Proteins Proteom. 2022;13(4):227–45.
Kaur P, Attri S, Singh D, Rashid F, Singh S, Kumar A, et al. Neuromodulatory effect of 4-(methylthio)butyl isothiocyanate against 3-nitropropionic acid induced oxidative impairments in human dopaminergic SH-SY5Y cells via BDNF/CREB/TrkB pathway. Sci Rep. 2023;13(1):4461.
Jurcau A, Jurcau CM. Mitochondria in Huntington’s disease: implications in pathogenesis and mitochondrial-targeted therapeutic strategies. Neural Regen Res. 2023;18(7):1472–7.
Gonchar OO, Maznychenko AV, Klyuchko OM, Mankovska IM, Butowska K, Borowik A, et al. C-60 fullerene reduces 3-nitropropionic acid-induced oxidative stress disorders and mitochondrial dysfunction in rats by modulation of p53, Bcl-2 and Nrf2 targeted proteins. Int J Mol Sci. 2021;22(11):24.
Kumar A, Sharma N, Mishra J, Kalonia H. Synergistical neuroprotection of rofecoxib and statins against malonic acid induced Huntington’s disease like symptoms and related cognitive dysfunction in rats. Eur J Pharmacol. 2013;709(1–3):1–12.
Deitmer JW, Theparambil SM, Ruminot I, Noor SI, Becker HM. Energy dynamics in the brain: contributions of astrocytes to metabolism and pH homeostasis. Front Neurosci. 2019;13:1301.
Nakamura Y, Park JH, Hayakawa K. Therapeutic use of extracellular mitochondria in CNS injury and disease. Exp Neurol. 2020;324:113114.
Jurcau A. Insights into the pathogenesis of neurodegenerative diseases: focus on mitochondrial dysfunction and oxidative stress. Int J Mol Sci. 2021;22(21):1184.
Kalra J. Crosslink between mutations in mitochondrial genes and brain disorders: implications for mitochondrial-targeted therapeutic interventions. Neural Regen Res. 2023;18(1):94.
Di Meo S, Napolitano G, Venditti P. Physiological and pathological role of ros: benefits and limitations of antioxidant treatment. Int J Mol Sci. 2019;20(19):4810.
Poljsak B, Šuput D, Milisav I. Achieving the balance between ROS and antioxidants: when to use the synthetic antioxidants. Oxid Med Cell Longev. 2013;2013:956792.
Feno S, Butera G, Vecellio Reane D, Rizzuto R, Raffaello A. Crosstalk between calcium and ROS in pathophysiological conditions. Oxid Med Cell Longev. 2019;2019:1–18.
Szabo I, Szewczyk A. Mitochondrial Ion channels. Annu Rev Biophys Biomol Struct. 2023;52:229–54.
Vicente-Gutierrez C, Bonora N, Bobo-Jimenez V, Jimenez-Blasco D, Lopez-Fabuel I, Fernandez E, et al. Astrocytic mitochondrial ROS modulate brain metabolism and mouse behaviour. Nat Metab. 2019;1(2):201–11.
Yan XH, Guo XY, Jiao FY, Liu X, Liu Y. Activation of large-conductance Ca(2+)-activated K(+) channels inhibits glutamate-induced oxidative stress through attenuating ER stress and mitochondrial dysfunction. Neurochem Int. 2015;90:28–35.
Shi W, Tan C, Liu C, Chen D. Mitochondrial fission mediated by Drp1-Fis1 pathway and neurodegenerative diseases. Rev Neurosci. 2023;34(3):275–94.
Qi Z, Huang Z, Xie F, Chen L. Dynamin-related protein 1: a critical protein in the pathogenesis of neural system dysfunctions and neurodegenerative diseases. J Cell Physiol. 2019;234(7):10032–46.
Kim YY, Um JH, Yoon JH, Lee DY, Lee YJ, Kim DH, et al. p53 regulates mitochondrial dynamics by inhibiting Drp1 translocation into mitochondria during cellular senescence. FASEB J. 2020;34(2):2451–64.
Losón OC, Song Z, Chen H, Chan DC. Fis1, Mff, MiD49, and MiD51 mediate Drp1 recruitment in mitochondrial fission. Mol Biol Cell. 2013;24(5):659–67.
Grel H, Woznica D, Ratajczak K, Kalwarczyk E, Anchimowicz J, Switlik W, et al. Mitochondrial dynamics in neurodegenerative diseases: unraveling the role of fusion and fission processes. Int J Mol Sci. 2023;24(17):13033.
Gureev AP, Shaforostova EA, Popov VN. Regulation of mitochondrial biogenesis as a way for active longevity: interaction between the Nrf2 and PGC-1α signaling pathways. Front genet. 2019;10:435.
Akbar M, Essa MM, Daradkeh G, Abdelmegeed MA, Choi Y, Mahmood L, et al. Mitochondrial dysfunction and cell death in neurodegenerative diseases through nitroxidative stress. Brain Res. 2016;1637:34–55.
Islam MT. Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol Res. 2017;39(1):73–82.
Bono-Yagüe J, Gómez-Escribano AP, Millán JM, Vázquez-Manrique RP. Reactive species in Huntington disease: are they really the radicals you want to catch? Antioxidants. 2020;9(7):577.
Liang Z, Currais A, Soriano-Castell D, Schubert D, Maher P. Natural products targeting mitochondria: emerging therapeutics for age-associated neurological disorders. Pharmacol Ther. 2021;221:107749.
Elfawy HA, Das B. Crosstalk between mitochondrial dysfunction, oxidative stress, and age related neurodegenerative disease: Etiologies and therapeutic strategies. Life Sci. 2019;218:165–84.
Di Cristo F, Finicelli M, Digilio FA, Paladino S, Valentino A, Scialò F, et al. Meldonium improves Huntington’s disease mitochondrial dysfunction by restoring peroxisome proliferator-activated receptor γ coactivator 1α expression. J Cell Physiol. 2019;234(6):9233–46.
Kumar P, Kalonia H, Kumar A. Role of LOX/COX pathways in 3-nitropropionic acid-induced Huntington’s disease-like symptoms in rats: protective effect of licofelone. Br J Pharmacol. 2011;164(2B):644–54.
Jedrak P, Mozolewski P, Wegrzyn G, Wieckowski MR. Mitochondrial alterations accompanied by oxidative stress conditions in skin fibroblasts of Huntington’s disease patients. Metab Brain Dis. 2018;33(6):2005–17.
Krach F, Stemick J, Boerstler T, Weiss A, Lingos I, Reischl S, et al. An alternative splicing modulator decreases mutant HTT and improves the molecular fingerprint in Huntington’s disease patient neurons. Nat Commun. 2022;13(1):6797.
Shawki SM, Saad MA, Rahmo RM, Wadie W, El-Abhar HS. Liraglutide improves cognitive and neuronal function in 3-NP rat model of Huntington’s disease. Front Pharmacol. 2021;12:731483.
Sharma P, Kumar M, Bansal N. Ellagic acid prevents 3-nitropropionic acid induced symptoms of Huntington’s disease. Naunyn Schmiedebergs Arch Pharmacol. 2021;394(9):1917–28.
Ibrahim WW, Abdel Rasheed NO. Diapocynin neuroprotective effects in 3-nitropropionic acid Huntington’s disease model in rats: emphasis on Sirt1/Nrf2 signaling pathway. Inflammopharmacology. 2022;30(5):1745–58.
Calkins MJ, Jakel RJ, Johnson DA, Chan KM, Kan YW, Johnson JA. Protection from mitochondrial complex II inhibition in vitro and in vivo by Nrf2-mediated transcription. Proc Natl Acad Sci U S A. 2005;102(1):244–9.
Kröller-Schön S, Jansen T, Schüler A, Oelze M, Wenzel P, Hausding M, et al. Peroxisome proliferator-activated receptor γ, coactivator 1α deletion induces angiotensin II-associated vascular dysfunction by increasing mitochondrial oxidative stress and vascular inflammation. Arterioscler Thromb Vasc Biol. 2013;33(8):1928–35.
Johri A, Chandra A, Flint BM. PGC-1α, mitochondrial dysfunction, and Huntington’s disease. Free Radic Biol Med. 2013;62:37–46.
St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jäger S, et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127(2):397–408.
Podvin S, Reardon HT, Yin K, Mosier C, Hook V. Multiple clinical features of Huntington’s disease correlate with mutant HTT gene CAG repeat lengths and neurodegeneration. J Neurol. 2019;266:551–64.
Jin J, Albertz J, Guo Z, Peng Q, Rudow G, Troncoso JC, et al. Neuroprotective effects of PPAR-γ agonist rosiglitazone in N171–82Q mouse model of Huntington’s disease. J Neurochem. 2013;125(3):410–9.
Beal MF, Brouillet E, Jenkins BG, Ferrante RJ, Kowall NW, Miller JM, et al. Neurochemical and histologic characterization of striatal excitotoxic lesions produced by the mitochondrial toxin 3-nitropropionic acid. J Neurosci. 1993;13(10):4181–92.
Upadhayay S, Yedke NG, Rahi V, Singh S, Kumar S, Arora A, et al. An overview of the pathophysiological mechanisms of 3-nitropropionic acid (3-NPA) as a neurotoxin in a Huntington’s disease model and its relevance to drug discovery and development. Neurochem Res. 2023;48(6):1631–47.
Mahdi WA, AlGhamdi SA, Alghamdi AM, Imam SS, Alshehri S, Almaniea MA, et al. Neuroprotectant effects of hibiscetin in 3-nitropropionic acid-induced Huntington’s Disease via subsiding oxidative stress and modulating monoamine neurotransmitters in rats brain. Molecules. 2023;28(3):1402.
Sorolla MA, Reverter-Branchat G, Tamarit J, Ferrer I, Ros JQ, Cabiscol E. Proteomic and oxidative stress analysis in human brain samples of Huntington disease. Free Radic Biol Med. 2008;45(5):667–78.
Sharma A, Behl T, Sharma L, Aelya L, Bungau S. Mitochondrial dysfunction in Huntington’s disease: pathogenesis and therapeutic opportunities. Curr Drug Targets. 2021;22(14):1637–67.
Ho DJ, Calingasan NY, Wille E, Dumont M, Beal MF. Resveratrol protects against peripheral deficits in a mouse model of Huntington’s disease. Exp Neurol. 2010;225(1):74–84.
Bains M, Kaur J, Akhtar A, Kuhad A, Sah SP. Anti-inflammatory effects of ellagic acid and vanillic acid against quinolinic acid-induced rat model of Huntington’s disease by targeting IKK-NF-Kappa B pathway. Eur J Pharmacol. 2022;934:13.
Albekairi TH, Kamra A, Bhardwaj S, Mehan S, Giri A, Suri M, et al. Beta-boswellic acid reverses 3-nitropropionic acid-induced molecular, mitochondrial, and histopathological defects in experimental rat model of Huntington’s Disease. Biomedicines. 2022;10(11):27.
Kumar D, Hasan GM, Islam A, Hassan MI. Therapeutic targeting of Huntington’s disease: molecular and clinical approaches. Biochem Biophys Res Commun. 2023;655:18–24.
Lloret A, Beal MF. PGC-1α, Sirtuins and PARPs in Huntington’s disease and Other neurodegenerative conditions: NAD+ to rule them all. Neurochem Res. 2019;44(10):2423–34.
Lin J, Wu PH, Tarr PT, Lindenberg KS, St-Pierre J, Zhang CY, et al. Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1alpha null mice. Cell. 2004;119(1):121–35.
Leone TC, Lehman JJ, Finck BN, Schaeffer PJ, Wende AR, Boudina S, et al. PGC-1alpha deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol. 2005;3(4):e101.
Tulino R, Benjamin AC, Jolinon N, Smith DL, Chini EN, Carnemolla A, et al. SIRT1 activity is linked to its brain region-specific phosphorylation and is impaired in Huntington’s Disease mice. PLoS ONE. 2016;11(1):e0145425.
Pallas M, Pizarro J, Gutierrez-Cuesta J, Crespo-Biel N, Alvira D, Tajes M, et al. Modulation of SIRT1 expression in different neurodegenerative models and human pathologies. Neuroscience. 2008;154(4):1388–97.
Jing E, Emanuelli B, Hirschey MD, Boucher J, Lee KY, Lombard D, et al. Sirtuin-3 (Sirt3) regulates skeletal muscle metabolism and insulin signaling via altered mitochondrial oxidation and reactive oxygen species production. Proc Natl Acad Sci U S A. 2011;108(35):14608–13.
Zhang S, Zhao J, Quan Z, Li H, Qing H. Mitochondria and other organelles in neural development and their potential as therapeutic targets in neurodegenerative diseases. Front Neurosci. 2022;16:853911.
Jeong H, Cohen DE, Cui L, Supinski A, Savas JN, Mazzulli JR, et al. Sirt1 mediates neuroprotection from mutant huntingtin by activation of the TORC1 and CREB transcriptional pathway. Nat Med. 2011;18(1):159–65.
Jiang M, Wang J, Fu J, Du L, Jeong H, West T, et al. Neuroprotective role of Sirt1 in mammalian models of Huntington’s disease through activation of multiple Sirt1 targets. Nat Med. 2011;18(1):153–8.
Conkright MD, Canettieri G, Screaton R, Guzman E, Miraglia L, Hogenesch JB, et al. TORCs: transducers of regulated CREB activity. Mol Cell. 2003;12(2):413–23.
Zuccato C, Ciammola A, Rigamonti D, Leavitt BR, Goffredo D, Conti L, et al. Loss of huntingtin-mediated BDNF gene transcription in Huntington’s disease. Science. 2001;293(5529):493–8.
Hafner AV, Dai J, Gomes AP, Xiao CY, Palmeira CM, Rosenzweig A, et al. Regulation of the mPTP by SIRT3-mediated deacetylation of CypD at lysine 166 suppresses age-related cardiac hypertrophy. Aging (Albany NY). 2010;2(12):914–23.
Qiu X, Brown K, Hirschey MD, Verdin E, Chen D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 2010;12(6):662–7.
Tao R, Coleman MC, Pennington JD, Ozden O, Park SH, Jiang H, et al. Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol Cell. 2010;40(6):893–904.
Chen Y, Zhang J, Lin Y, Lei Q, Guan KL, Zhao S, et al. Tumour suppressor SIRT3 deacetylates and activates manganese superoxide dismutase to scavenge ROS. EMBO Rep. 2011;12(6):534–41.
Cheng A, Yang Y, Zhou Y, Maharana C, Lu D, Peng W, et al. Mitochondrial SIRT3 mediates adaptive responses of neurons to exercise and metabolic and excitatory challenges. Cell Metab. 2016;23(1):128–42.
Lee M, Ban JJ, Chung JY, Im W, Kim M. Amelioration of Huntington’s disease phenotypes by beta-Lapachone is associated with increases in Sirt1 expression, CREB phosphorylation and PGC-1α deacetylation. PLoS ONE. 2018;13(5):e0195968.
Fu J, Jin J, Cichewicz RH, Hageman SA, Ellis TK, Xiang L, et al. trans-(-)-ε-Viniferin increases mitochondrial sirtuin 3 (SIRT3), activates AMP-activated protein kinase (AMPK), and protects cells in models of Huntington Disease. J Biol Chem. 2012;287(29):24460–72.
Cho B, Choi SY, Cho HM, Kim HJ, Sun W. Physiological and pathological significance of dynamin-related protein 1 (drp1)-dependent mitochondrial fission in the nervous system. Exp Neurobiol. 2013;22(3):149–57.
Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F, et al. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell. 2001;1(4):515–25.
Zerihun M, Sukumaran S, Qvit N. The Drp1-mediated mitochondrial fission protein interactome as an emerging core player in mitochondrial dynamics and cardiovascular disease therapy. Int J Mol Sci. 2023;24(6):5785.
Seo BJ, Yoon SH, Do JT. Mitochondrial dynamics in stem cells and differentiation. Int J Mol Sci. 2018;19(12):3893.
Oliver D, Reddy PH. Dynamics of dynamin-related protein 1 in Alzheimer’s disease and other neurodegenerative diseases. Cells. 2019;8(9):961.
Feng ST, Wang ZZ, Yuan YH, Wang XL, Sun HM, Chen NH, et al. Dynamin-related protein 1: a protein critical for mitochondrial fission, mitophagy, and neuronal death in Parkinson’s disease. Pharmacol Res. 2020;151:104553.
Lee DS, Kim TH, Park H, Kim JE. CDDO-Me attenuates clasmatodendrosis in CA1 astrocyte by inhibiting HSP25-AKT mediated DRP1-S637 phosphorylation in chronic epilepsy rats. Int J Mol Sci. 2022;23(9):4569.
Song W, Chen J, Petrilli A, Liot G, Klinglmayr E, Zhou Y, et al. Mutant huntingtin binds the mitochondrial fission GTPase dynamin-related protein-1 and increases its enzymatic activity. Nat Med. 2011;17(3):377–82.
Shirendeb UP, Calkins MJ, Manczak M, Anekonda V, Dufour B, McBride JL, et al. Mutant huntingtin’s interaction with mitochondrial protein Drp1 impairs mitochondrial biogenesis and causes defective axonal transport and synaptic degeneration in Huntington’s disease. Hum Mol Genet. 2012;21(2):406–20.
Haun F, Nakamura T, Shiu AD, Cho DH, Tsunemi T, Holland EA, et al. S-nitrosylation of dynamin-related protein 1 mediates mutant huntingtin-induced mitochondrial fragmentation and neuronal injury in Huntington’s disease. Antioxid Redox Signal. 2013;19(11):1173–84.
Manczak M, Sesaki H, Kageyama Y, Reddy PH. Dynamin-related protein 1 heterozygote knockout mice do not have synaptic and mitochondrial deficiencies. Biochim Biophys Acta. 2012;1822(6):862–74.
Kornfeld OS, Qvit N, Haileselassie B, Shamloo M, Bernardi P, Mochly-Rosen D. Interaction of mitochondrial fission factor with dynamin related protein 1 governs physiological mitochondrial function in vivo. Sci Rep. 2018;8(1):14034.
Roe AJ, Qi X. Drp1 phosphorylation by MAPK1 causes mitochondrial dysfunction in cell culture model of Huntington’s disease. Biochem Biophys Res Commun. 2018;496(2):706–11.
Numadate A, Mita Y, Matsumoto Y, Fujii S, Hashimoto Y. Development of 2-thioxoquinazoline-4-one derivatives as dual and selective inhibitors of dynamin-related protein 1 (Drp1) and puromycin-sensitive aminopeptidase (PSA). Chem Pharm Bull (Tokyo). 2014;62(10):979–88.
Hidalgo-Gutiérrez A, González-García P, Díaz-Casado ME, Barriocanal-Casado E, López-Herrador S, Quinzii CM, et al. Metabolic targets of coenzyme Q10 in mitochondria. Antioxidants. 2021;10(4):520.
Manzar H, Abdulhussein D, Yap TE, Cordeiro MF. Cellular consequences of coenzyme Q10 deficiency in neurodegeneration of the retina and brain. Int J Mol Sci. 2020;21(23):9299.
Devadiga SJ, Bharate SS. Recent developments in the management of Huntington’s disease. Bioorg Chem. 2022;120:105642.
Sawant N, Morton H, Kshirsagar S, Reddy AP, Reddy PH. Mitochondrial abnormalities and synaptic damage in Huntington’s disease: a focus on defective mitophagy and mitochondria-targeted therapeutics. Mol Neurobiol. 2021;2:1–28.
Ferrante RJ, Andreassen OA, Dedeoglu A, Ferrante KL, Jenkins BG, Hersch SM, et al. Therapeutic effects of coenzyme Q10 and remacemide in transgenic mouse models of Huntington’s disease. J Neurosci. 2002;22(5):1592–9.
Schilling G, Coonfield ML, Ross CA, Borchelt DR. Coenzyme Q10 and remacemide hydrochloride ameliorate motor deficits in a Huntington’s disease transgenic mouse model. Neurosci Lett. 2001;315(3):149–53.
Mehan S, Monga V, Rani M, Dudi R, Ghimire K. Neuroprotective effect of solanesol against 3-nitropropionic acid-induced Huntington’s disease-like behavioral, biochemical, and cellular alterations: restoration of coenzyme-Q10-mediated mitochondrial dysfunction. Ind J Pharmacol. 2018;50(6):309–19.
Yang L, Calingasan NY, Wille EJ, Cormier K, Smith K, Ferrante RJ, et al. Combination therapy with coenzyme Q10 and creatine produces additive neuroprotective effects in models of Parkinson’s and Huntington’s diseases. J Neurochem. 2009;109(5):1427–39.
Beal MF, Shults CW. Effects of Coenzyme Q10 in Huntington’s disease and early Parkinson’s disease. BioFactors. 2003;18(1–4):153–61.
Orsucci D, Mancuso M, Ienco EC, LoGerfo A, Siciliano G. Targeting mitochondrial dysfunction and neurodegeneration by means of coenzyme Q10 and its analogues. Curr Med Chem. 2011;18(26):4053–64.
Brondani M, Roginski AC, Ribeiro RT, de Medeiros MP, Hoffmann CIH, Wajner M, et al. Mitochondrial dysfunction, oxidative stress, ER stress and mitochondria-ER crosstalk alterations in a chemical rat model of Huntington’s disease: potential benefits of bezafibrate. Toxicol Lett. 2023;381:48–59.
Bhatti JS, Thamarai K, Kandimalla R, Manczak M, Yin X, Kumar S, et al. Mitochondria-targeted small peptide, SS31 ameliorates diabetes induced mitochondrial dynamics in male TallyHO/JngJ mice. Mol Neurobiol. 2021;58(2):795–808.
Chaturvedi RK, Adhihetty P, Shukla S, Hennessy T, Calingasan N, Yang L, et al. Impaired PGC-1alpha function in muscle in Huntington’s disease. Hum Mol Genet. 2009;18(16):3048–65.
Naia L, Rosenstock TR, Oliveira AM, Oliveira-Sousa SI, Caldeira GL, Carmo C, et al. Comparative mitochondrial-based protective effects of resveratrol and nicotinamide in Huntington’s Disease models. Mol Neurobiol. 2017;54(7):5385–99.
Parker JA, Arango M, Abderrahmane S, Lambert E, Tourette C, Catoire H, et al. Resveratrol rescues mutant polyglutamine cytotoxicity in nematode and mammalian neurons. Nat Genet. 2005;37(4):349–50.
Hathorn T, Snyder-Keller A, Messer A. Nicotinamide improves motor deficits and upregulates PGC-1α and BDNF gene expression in a mouse model of Huntington’s disease. Neurobiol Dis. 2011;41(1):43–50.
Chandra A, Sharma A, Calingasan NY, White JM, Shurubor Y, Yang XW, et al. Enhanced mitochondrial biogenesis ameliorates disease phenotype in a full-length mouse model of Huntington’s disease. Hum Mol Genet. 2016;25(11):2269–82.
Pallàs M, Casadesús G, Smith MA, Coto-Montes A, Pelegri C, Vilaplana J, et al. Resveratrol and neurodegenerative diseases: activation of SIRT1 as the potential pathway towards neuroprotection. Curr Neurovasc Res. 2009;6(1):70–81.
Jiang M, Zheng J, Peng Q, Hou Z, Zhang J, Mori S, et al. Sirtuin 1 activator SRT2104 protects Huntington’s disease mice. Ann Clin Transl Neurol. 2014;1(12):1047–52.
Smith MR, Syed A, Lukacsovich T, Purcell J, Barbaro BA, Worthge SA, et al. A potent and selective Sirtuin 1 inhibitor alleviates pathology in multiple animal and cell models of Huntington’s disease. Hum Mol Genet. 2014;23(11):2995–3007.
Tajes M, Gutierrez-Cuesta J, Ortuño-Sahagun D, Camins A, Pallàs M. Anti-aging properties of melatonin in an in vitro murine senescence model: involvement of the sirtuin 1 pathway. J Pineal Res. 2009;47(3):228–37.
Zhao Y, Sun X, Qi X. Inhibition of Drp1 hyperactivation reduces neuropathology and behavioral deficits in zQ175 knock-in mouse model of Huntington’s disease. Biochem Biophys Res Commun. 2018;507(1–4):319–23.
Okada N, Yako T, Nakamura S, Shimazawa M, Hara H. Reduced mitochondrial complex II activity enhances cell death via intracellular reactive oxygen species in STHdhQ111 striatal neurons with mutant huntingtin. J Pharmacol Sci. 2021;147(4):367–75.
Chaturvedi RK, Beal MF. Mitochondria targeted therapeutic approaches in Parkinson’s and Huntington’s diseases. Mol Cell Neurosci. 2013;55:101–14.
Xun Z, Rivera-Sánchez S, Ayala-Peña S, Lim J, Budworth H, Skoda EM, et al. Targeting of XJB-5-131 to mitochondria suppresses oxidative DNA damage and motor decline in a mouse model of Huntington’s disease. Cell Rep. 2012;2(5):1137–42.
Polyzos A, Holt A, Brown C, Cosme C, Wipf P, Gomez-Marin A, et al. Mitochondrial targeting of XJB-5-131 attenuates or improves pathophysiology in HdhQ150 animals with well-developed disease phenotypes. Hum Mol Genet. 2016;25(9):1792–802.
Pinho BR, Duarte AI, Canas PM, Moreira PI, Murphy MP, Oliveira JMA. The interplay between redox signalling and proteostasis in neurodegeneration: in vivo effects of a mitochondria-targeted antioxidant in Huntington’s disease mice. Free Radic Biol Med. 2020;146:372–82.
Yin X, Manczak M, Reddy PH. Mitochondria-targeted molecules MitoQ and SS31 reduce mutant huntingtin-induced mitochondrial toxicity and synaptic damage in Huntington’s disease. Hum Mol Genet. 2016;25(9):1739–53.
Mitchell W, Tamucci JD, Ng EL, Liu S, Birk AV, Szeto HH, et al. Structure-activity relationships of mitochondria-targeted tetrapeptide pharmacological compounds. Elife. 2022. https://doi.org/10.7554/eLife.75531.
Nhu NT, Xiao SY, Liu Y, Kumar VB, Cui ZY, Lee SD. Neuroprotective effects of a small mitochondrially-targeted tetrapeptide elamipretide in neurodegeneration. Front Integr Neurosci. 2021;15:747901.
Brenza TM, Ghaisas S, Ramirez JEV, Harischandra D, Anantharam V, Kalyanaraman B, et al. Neuronal protection against oxidative insult by polyanhydride nanoparticle-based mitochondria-targeted antioxidant therapy. Nanomedicine. 2017;13(3):809–20.
Maldonado PD, Molina-Jijón E, Villeda-Hernández J, Galván-Arzate S, Santamaría A, Pedraza-Chaverrí J. NAD(P)H oxidase contributes to neurotoxicity in an excitotoxic/prooxidant model of Huntington’s disease in rats: protective role of apocynin. J Neurosci Res. 2010;88(3):620–9.
Quintanilla RA, Jin YN, Fuenzalida K, Bronfman M, Johnson GVW. Rosiglitazone treatment prevents mitochondrial dysfunction in mutant huntingtin-expressing cells: possible role of peroxisome proliferator-activated receptor-gamma (PPARgamma) in the pathogenesis of Huntington disease. J Biol Chem. 2008;283(37):25628–37.
Acknowledgements
The authors would like to express gratitude to the Central University of Punjab (CUPB), Bathinda, India, for the assistance and would like to acknowledge the Indian Council of Medical Research, New Delhi, India, for providing a Senior Research Fellowship (45/16/2022-PHA/BMS) to Mr. Shubham Upadhayay to pursue his research at the department of Pharmacology, CUPB.
Funding
This review did not receive any funding.
Author information
Authors and Affiliations
Contributions
SU: Manuscript writing, data acquisition, and manuscript drafting. PK: concept design, supervision, editing, and final approval of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Upadhayay, S., Kumar, P. Mitochondrial targeted antioxidants as potential therapy for huntington’s disease. Pharmacol. Rep 76, 693–713 (2024). https://doi.org/10.1007/s43440-024-00619-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43440-024-00619-z