Abstract
Background
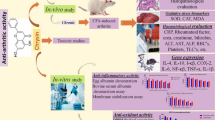
Astaxanthin (ATX), a natural xanthophyll carotenoid, has shown to exert significant protective effects against various diseases via its antioxidant and anti-inflammatory properties. However, its potential role in arthritis is still not reported. Therefore, the aim of the present study was to investigate the potential anti-arthritic properties of ATX against complete Freund’s adjuvant (CFA)-induced arthritis rats.
Methods
Adjuvant arthritis was induced by single intraplantar injection of complete Freund’s adjuvant (CFA) in the left hind paw of adult female Wistar rats. ATX (25, 50 and 100 mg/kg) and indomethacin (5 mg/kg) were given orally from days 14 to 28. The anti-arthritic activity was evaluated through various nociceptive behavioral tests (mechanical allodynia, mechanical hyperalgesia, cold allodynia, and thermal hyperalgesia), paw edema assessment, and arthritis scores. Serum tumor necrosis factor-α (TNF-α), C-reactive protein (CRP) and cyclic citrullinated peptide (CCP) antibody levels were assessed. Moreover, malondialdehyde (MDA), nitrite, glutathione (GSH), superoxide dismutase (SOD) and catalase (CAT) levels were also evaluated.
Results
Oral administration of ATX (50 and 100 mg/kg) exhibited significant anti-arthritic activity via enhancing the nociceptive threshold, reducing paw edema and improving arthritis scores. Moreover, ATX treatment also markedly suppressed inflammatory and oxidative mediators in adjuvant-administered rats.
Conclusions
Our findings suggest that ATX possesses potential anti-arthritic activity, which could be attributed to its anti-inflammatory and antioxidant properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA), is a chronic inflammatory disease characterized by joint swelling, pain, articular cartilage erosion and bone destruction [1, 2]. It is known to affect approximately 0.5–2% of the adult population, with women being two to three times more susceptible to RA than men [3]. While the pathogenesis of RA remains unclear, the involvement of genetic and environmental factors may lead to inappropriate immunomodulation, thereby resulting in inflammation-mediated damage of the synovial structures. Indeed, enhanced expression of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6 have been observed in the rheumatoid joint [4]. Moreover, studies have also supported an association between oxidative/nitrosative stress and RA [5]. Free radicals are directly or indirectly involved in joint damage as they not only degrade the joint cartilage via attacking its proteoglycan and inhibiting its synthesis but also serve as secondary messengers in various inflammatory and immunological cellular responses [6]. Currently, drug therapy employed to treat RA includes nonsteroidal anti-inflammatory drugs (NSAIDs) disease-modifying anti-rheumatic drugs (DMARDs), corticosteroids, and biologics. However, due to severe side effects and limited clinical efficacy of these treatments, there has been a renewed interest in effective alternative therapeutic approaches which may halt the progression of disease without any unwanted side effects.
Astaxanthin (3, 3′-dihydroxy-b, b’-carotene-4, 4′-dione, ATX) is a naturally occurring carotenoid known to be present in various marine organisms such as algae, shrimps, crabs and salmon [7]. It is a powerful biological antioxidant possessing stronger free radical scavenging activity than vitamin E and other carotenoids [8]. Owing to its antioxidant ability and cell signal modulating properties, in various studies, ATX has shown to exhibit a full range of health benefits such as potent anti-inflammatory, anti-apoptotic, immunomodulatory, anti-tumor, anti-diabetic, and hepatoprotective effects [9]. ATX is known to prevent oxidative stress-induced cell or tissue damage via effectively scavenging intracellular free radicals and destroying peroxide chain reactions [10]. Previous studies have shown that it also decreases serum inflammatory mediators and cytokines levels and inhibits NF-κβ-cell activation, as well as ROS accumulation in RAW264.7 cells, stimulated with LPS [11, 12]. In addition, dietary supplementation of ATX in humans has been proven to be effective in various diseases and conditions, such as Alzheimer’s disease, type-2 diabetes, fatigue, functional dyspepsia, dyslipidemia and age-related macular degeneration [13,14,15,16,17,18].
However, no studies have yet reported the protective effects of ATX against RA. Consequently, the current study was designed to investigate the anti-arthritic activity of ATX in a CFA-induced RA rat model.
Materials and methods
Animals
Female Wistar rats (200–250 g) bred at the central animal house, Panjab University, Chandigarh were used in this study. Animals were housed under standard conditions (25 ± 2 °C, 60–70% humidity) maintained on natural 12-h light and dark cycle with free access to food and water. Animals were acclimatized to the laboratory conditions for 1 week before initiating the experiments. The experimental protocols were approved by the Institutional Animal Ethical Committee (PU/IAEC/S/16/73) and conducted in accordance with the guidelines of Committee for the purpose of control and supervision of experiments on animals (CPCSEA) for the use and care of experimental animals.
Drugs and chemicals
Astaxanthin and Indomethacin were purchased from Pragati Biocare Pvt. Ltd, Bangalore, India and Jagsonpal Pharmaceuticals Ltd, New Delhi, India, respectively. Complete Freund-Adjuvant (CFA) was purchased from Difco Laboratories, Detroit, MI, USA. Enzyme-Linked Immunosorbent Assay (ELISA) kit for TNF-α was obtained from Ray Biotech, USA. CRP and CCP ELISA kits were supplied by BioVendor-Laboratorní medicína a.s. and Wkea Med supplies, respectively. All other chemicals used were of analytical grade.
Experimental design
Arthritis was induced on day 0 by a single injection of complete Freund’s adjuvant (CFA) consisting of 0.5 mg of Mycobacterium butyricum suspended in 0.1 ml of paraffin oil into the subplantar region of left hind paw of each animal under mild anesthesia with a combination of ketamine/xylazine (80:10 mg/kg, ip) [19]. On day 15, animals were randomly divided into six experimental groups (6–8 animals per group). Group I comprised of animals injected with paraffin oil (0.1 ml) in the left hind paw; Group II consisted of animals injected with 0.1 ml of adjuvant (CFA) in the left hind paw; Groups III, IV, V were kept as test treatment groups and received ATX orally at different treatment dosages of 25, 50 and 100 mg/kg dissolved in olive oil. Group VI was kept as a reference treatment group and received 5 mg/kg indomethacin (IM) via oral gavage. The doses of ATX and IM were selected on the basis of previous studies conducted on rodents [20, 21]. The daily doses of vehicle/ATX/IM were administered for fourteen consecutive days, starting from day 15 and continuing till day 28 post-injection. Bodyweight, nociceptive behavioral assays, paw volume assessment and arthritic index were evaluated on days 0, 7, 14, 21, and 28. A schematic representation of the entire protocol is depicted in Fig. 1.
Nociceptive behavioral tests
Mechanical hyperalgesia
Mechanical nociceptive stimulus-induced hyperalgesia was assessed using the Randall–Selitto paw pressure analgesia meter (IITC Life Science, Woodland Hills, CA). Increasing pressure with a rate of 10 g/s was applied to the plantar region of the hind paw. The cut-off threshold was determined as 250 g to avoid any potential injury to the paw. Paw withdrawal threshold was expressed in mass units (grams). The mean value was recorded after repeating the test three times at an interval of 10 min [22].
Thermal hyperalgesia
Assessment of thermal nociceptive threshold, an index of thermal hyperalgesia, was conducted by placing animals on a preheated hot plate surface (55 ± 1 °C). The time latency for paw licking/jumping was recorded. A cut-off time of 10 s was taken to avoid tissue damage [22].
Mechanical allodynia
The electronic Von Frey anesthesiometer was used to assess mechanical allodynia. Rats were allowed to acclimatize for at least 30 min in plexiglass boxes on top of a wire mesh grid. The amount of force required to initiate a withdrawal response on applying a semi-flexible probe (IITC Life Science, Woodland Hills, CA) vertically to the plantar surface of the hind paw was digitally recorded. A maximum force of 90 g was taken as the cut-off limit. The test was repeated three times, and the average measurement was finally calculated and recorded [23].
Cold allodynia
Application of cold stimuli to the plantar surface of the paw was associated with the presence of foot withdrawal responses (paw licking/lifting). Each animal was placed in a plexiglass chamber consisting of an aluminum plate (21 × 21 cm of surface and 2-cm thick) coiled with PVC tubes that circulate water with 50% methanol (Instrumental Manufacturing Corporation, Ambala, Haryana). The cold plate was maintained at 5 ± 1 °C with a cut-off time of 3 min. The average measurement was calculated by repeating the test three times at 5 min intervals [24].
Evaluation of paw edema
Paw volume of animals was ascertained by the volume displacement method using water Plethysmometer (UgoBasile 7140, Milan, Italy). On marking the hind paw at the tibiotarsal joint, it was subsequently immersed in water up to the marked point, and the paw volume was expressed in milliliters.
Arthritis score
Arthritis severity was evaluated according to a well-established scoring system, as previously described [25]. Animal paws were examined and scored using a 5-point ordinal scale (scores 0–4), as follows: 0 = absence of erythema or swelling, 1 = mild erythema or swelling of the digits, 2 = moderate swelling and erythema, 3 = severe swelling and erythema involving the ankle and 4 = ankylosis, and inability to bend the ankle.
Serum sampling
At the end of the experiment, animals were lightly anesthetized, and blood was collected from the retro-orbital plexus. Serum separated from the clotted blood was kept at room temperature in the test tubes which was subsequently centrifuged at 12,000 × g for 15 min at 4 °C using a cooling centrifuge (Eppendorf, model 5430R). Thereafter, serum samples obtained were stored at − 80 °C for further estimations. Tumour necrosis factor-α (TNF-α), cyclic citrullinated peptide antibodies (CCP), and C-reactive protein (CRP) levels were estimated using enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturers’ instructions.
Biochemical estimations
Estimation of lipid peroxidation
Malondialdehyde content, a marker of lipid peroxidation, was assessed in the serum by a method previously described by Wills [26]. Briefly, a mixture consisting of 0.5 ml of Tris–HCl and serum (0.5 ml) was incubated for 2 h at 37 °C. To this incubated mixture, 1 ml of 10% trichloroacetic acid was added and centrifuged for 10 min at 1000 g. Thereafter, 1 ml of thiobarbituric acid was added to 1 ml of supernatant obtained after centrifugation and kept in boiling water for 10 min. Finally, on cooling, 1 ml of distilled water was added and the absorbance was measured at 532 nm. TBARS were calculated using the molar extinction coefficient of 1.56 × 105 M−1 cm−1 and was expressed as nanomole of malondialdehyde per milligram protein.
Estimation of nitrite
Serum nitrite levels were estimated using the Greiss reagent. To 100 μl of homogenate, 500 µl of Greiss reagent (1:1 solution of 1% sulfanilamide in 5% phosphoric acid and 0.1% naphthylamine diamine hydrochloric acid in water) was added and the absorbance was measured at 546 nm. A standard curve for sodium nitrite was used to calculate nitrite levels, and results were expressed as µg per ml [27].
Estimation of reduced glutathione levels (GSH)
Reduced glutathione level was assayed by a method previously described [28]. A mixture of equal volumes of serum and 5% sulphosalicylic acid was kept at 4 °C for 1 h followed by centrifugation at 4500 g for 10 min at 4 °C. Thereafter, 450 µl of phosphate buffer and 1.5 ml of 5, 5, dithiobis-(2-nitro benzoic acid) in 0.1 M phosphate buffer (pH 8.0) were added to 50 µl of supernatant obtained earlier. The reaction mixture was then incubated for 10 min at 37 °C, and the color developed was analyzed at 412 nm. GSH levels were calculated using molar extinction coefficient of the chromophore, i.e. 1.36 × 104 M−1 cm−1 and results were expressed as μM per mg protein.
Estimation of superoxide dismutase activity
Superoxide dismutase activity was assayed by the method described by [29]. Briefly, the assay system consisted of equal volumes of serum and hydroxylamine hydrochloride (pH 6.0) along with 0.1 mM ethylene diamine tetraacetic acid (EDTA), 50 mM sodium carbonate, and 96 mM of nitro blue tetrazolium (NBT). Change in absorbance, which indicates auto-oxidation of hydroxylamine, was measured for 2 min at 30-s intervals by measuring absorbance at 560 nm.
Estimation of catalase activity
Catalase activity was assessed by the method described by [30]. The assay mixture consisted of 0.05 ml serum, 1.0 ml hydrogen peroxide (0.019 M), 1.95 ml phosphate buffer (0.05 M, pH 7.0), in a final volume of 3.0 ml. Changes in optical density were recorded at 240 nm. Using the millimolar extinction coefficient of H2O2 (0.07 mM), catalase activity was calculated and expressed as µM of H2O2 decomposed per minute per milligram protein.
Protein estimation
Protein concentration was estimated using the Lowry method using bovine serum albumin (BSA) as standard [31].
Statistics analysis
Results were statistically analyzed using Graph pad prism (Version 6.0). Results were expressed as mean ± SEM. Behavioral data were analyzed by two-way analysis of variance (ANOVA) followed by post hoc Bonferroni multiple comparison tests. For arthritic scores, Kruskal–Wallis one-way ANOVA followed by Dunn’s multiple comparison tests were used. Biochemical and ELISA data were analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s test. p < 0.05 was considered to be statistically significant.
Results
Effect of ATX on body weight in CFA-induced arthritic rats
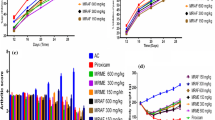
As shown in Fig. 2, CFA-treated rats did not display any significant change in body weight throughout the study. However, ATX (100 mg/kg) treated animals gained body weight on day 28 (p < 0.01). In contrast, ATX (25 and 50 mg/kg) and IM (5 mg/kg) had no significant effect on body weight during the course of study.
Effect of ATX on mechanical hyperalgesia in CFA-induced arthritic rats
On day 0, CFA-injected rats did not exhibit any significant difference in the mean baseline paw withdrawal threshold when compared to control animals. However, as depicted in Fig. 3, weekly assessments of nociceptive threshold indicate that it progressively decreased in CFA administered rats throughout the study (p < 0.001). While ATX (100 mg/kg) caused significant reduction in mechanical hyperalgesia as assessed on day 21 and 28 (p < 0.05, p < 0.001), ATX (50 mg/kg) demonstrated similar results on day 28 only (p < 0.05). IM (5 mg/kg) also demonstrated statistically significant inhibition in mechanical hyperalgesia on day 21 and day 28 (p < 0.01, p < 0.001) (Fig. 3).
Effect of ATX on thermal hyperalgesia in CFA-induced arthritic rats
As shown in Fig. 4, a significant decrease in thermal hyperalgesia threshold was observed in CFA-injected rats on day 7, which continued till day 28 (p < 0.001). However, two-way ANOVA followed by Bonferroni’s test revealed that highest dose of ATX (100 mg/kg) and IM (5 mg/kg) attenuated CFA-induced thermal hyperalgesia on day 21 and on day 28 (p < 0.001). Treatment with ATX (50 mg/kg) also led to a significant improvement (increased paw withdrawal threshold) (p < 0.01, p < 0.001). The lowest dose of ATX (25 mg/kg) failed to attenuate the thermal hyperalgesia at any time point.
Effect of ATX on mechanical allodynia in CFA-induced arthritic rats
CFA-injected rats did not exhibit any significant difference in mean baseline paw withdrawal threshold when compared to control animals on day 0 (Fig. 5). However, CFA-induced arthritic animals displayed a significant decrease in paw withdrawal mechanical threshold (mechanical allodynia) as compared to normal control animals which continued to worsen till the end of the experiment (p < 0.001). While treatment with ATX (25 mg/kg) failed to show any significant difference compared to CFA-induced arthritic rats, ATX (50 and 100 mg/kg) demonstrated a statistically significant increase in paw withdrawal threshold in CFA-treated rats on the day 21 and 28. Similarly, IM (5 mg/kg) also significant increased paw withdrawal threshold in CFA-treated group on day 28 (p < 0.001) (Fig. 5).
Effect of ATX on thermal allodynia in CFA-induced arthritic rats
Paw withdrawal latency (mean baseline reaction time) displayed no significant difference among the groups on day 0. However, as depicted in Fig. 6, starting from day 7 to day, 28 we observed a progressive decrease in paw withdrawal latency (i.e., thermal allodynia) in CFA-injected arthritic rats as assessed in cold plate test (p < 0.001). ATX (100 mg/kg) and IM(5 mg/kg) exhibited a significant increase in paw withdrawal latency on day 21 and on day 28 when compared to CFA-injected arthritic rats (p < 0.01, p < 0.001). Moreover, ATX (50 mg/kg)also demonstrated significant inhibition in thermal allodynia (p < 0.05, p < 0.01). No significant difference was found with a low dose of ATX (25 mg/kg) in paw withdrawal latency compared to CFA-injected arthritic rats.
Effect of ATX on paw volume in CFA-injected arthritic rats
A paw edema assay was conducted to investigate the anti-inflammatory activity of ATX. As shown in Fig. 7, a progressive increase in paw volume on day 7 was observed on day 7 in CFA-induced rats which sustained till the end of the protocol (p < 0.001). Two-way ANOVA followed by post hoc Bonferroni’s multiple comparison test revealed that ATX (100 mg/kg) administration led to a significant decrease in paw volume on day 21 and 28 (p < 0.01, p < 0.001). Similarly, treatment with IM (5 mg/kg) also led to a significant decrease in paw volume (p < 0.001). Furthermore, ATX (50 mg/kg) administration led to a significant decrease in arthritic scores on day 28 (p < 0.001).
Effect of ATX on arthritis scores in CFA-injected arthritic rats
As depicted in Fig. 8, during the course of the study CFA treated animals demonstrated a significant increase in the mean arthritic scores (p < 0.001). Treatment with ATX (100 mg/kg) caused a significant reduction in the overall arthritic index on post-arthritis day 21 and 28 as compared to the disease control group (p < 0.05, p < 0.001). Similarly, treatment with IM (5 mg/kg) also led to significant improvement in arthritis scores (p < 0.001). Meanwhile, ATX (50 mg/kg) also led to a significant decrease in arthritic scores on day 28 (p < 0.001). In contrast, ATX (25 mg/kg) did not significantly improve arthritis score in arthritic rats.
Effect of ATX on oxidative stress markers in CFA-induced arthritic rats
As depicted in Table 1, a statistically significant increase in serum MDA and nitrite levels were observed in CFA-inoculated arthritis rats when compared to the control group (p < 0.001). However, oral treatment with ATX (100 mg/kg) and the positive control IM (5 mg/kg) reversed these alterations by decreasing serum MDA (p < 0.01) and nitrite (p < 0.001) levels (Table 1). While ATX (50 mg/kg) significantly lowered serum LPO levels (p < 0.05), it failed to decrease serum nitrite levels in arthritic rats.
Effect of ATX on antioxidant indices in CFA-induced arthritic rats
The effect of ATX supplementation on enzymatic antioxidants (GSH, SOD, and Catalase) in arthritic rats was investigated (Table 1). CFA-induced arthritic rats demonstrated a significant decrease in serum GSH, SOD, and CAT activities as compared to control rats (p < 0.001). However, compared to the untreated CFA model group, ATX (100 mg/kg) and IM (5 mg/kg)treatment replenished the depleted pool of key antioxidant enzymes by restoring GSH (p < 0.001), SOD (p < 0.001, p < 0.01) and CAT (p < 0.05) activities (Table 1). ATX (50 mg/kg) also demonstrated a significant improvement in serum GSH (p < 0.05) and SOD (p < 0.05) levels, but failed to enhance serum CAT activity. These findings indicate a possible role of ATX in maintaining cellular redox status.
Effect of ATX on serum TNF-α level in CFA-induced arthritic rats
As depicted in Fig. 9, arthritic non-treated rats exhibited a significant increase in the serum levels of TNF-α, as compared to the normal group (p < 0.01). In contrast, treatment with ATX (50 and 100 mg/kg) exhibited a significant increase in serum TNF-α levels when compared to the untreated CFA group (p < 0.05, p < 0.01). Similarly, IM (5 mg/kg) administration also led to a significant decrease in serum TNF-α level in CFA treated rats (p < 0.01). These findings highlight the inhibitory effect of ATX on inflammatory responses.
Effect of ATX on serum CRP and CCP levels in CFA-induced arthritic rats
Results presented in Fig. 10a, b show that that serum CRP and CCP antibody levels were significantly elevated CFA group as compared to the control group (p < 0.001). ATX (50 and 100 mg/kg) administration led to a significant decrease in serum CRP and CCP levels. Moreover, IM (5 mg/kg) also exhibited significant decrease in serum CRP and CCP levels (p < 0.001).
Discussion
The CFA-induced arthritis model is known to share several features of human RA, such as joint swelling, pain, cartilage degradation, and loss of joint function. It is widely used for studying disease pathogenesis and evaluation of potential therapeutic targets useful for arthritis treatment. In recent years, a large body of evidence has highlighted the importance of natural products as potential therapeutic alternatives for treating numerous inflammatory diseases including rheumatoid arthritis. Recently, the natural carotenoid ATX via its multiple biological activities has gained significant attention as a potential remedy for the prevention and treatment of various diseases. Therefore, in the current study, the possible protective effects of ATX were investigated against CFA-induced RA in rats.
In the current study, CFA-inoculated rats demonstrated a marked decrease in nociceptive threshold as accessed via various behavioral tests. It is well reported that CFA produces chronic inflammatory pain due to the release of numerous inflammatory mediators that may trigger sensitization of the nociceptive neurons, thereby leading to reduced pain thresholds. Interestingly, ATX not only restored threshold of various pain assessments (mechanical allodynia, cold allodynia, mechanical hyperalgesia, and thermal hyperalgesia) in CFA-injected rats but also showed a significant reduction in paw volume and arthritic scores. These findings are in agreement with previous studies. Indeed, in a recent study, ATX suppressed thermal hyperalgesia, mechanical allodynia, and depressive-like behavior of CCI mice [32]. Similarly, in another study, rats undergoing chronic constriction of the sciatic nerve demonstrated a significant reduction in von Frey mechanical hyperalgesia and both thermal and mechanical hypersensitivity when treated with ATX [33]. The beneficial effects of ATX might be associated with suppression of CFA-induced release of inflammatory markers. Moreover, as previous studies have indicated a positive correlation between disease severity and oxidative stress in RA, ATX ability to alleviate oxidative damage and restore endogenous antioxidant levels may have reduced tissue damage thereby improving arthritic scores [34].
Tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), interferon-γ (IFN-γ), IL-6, and IL-17 are the key cytokines directly implicated in the pathogenesis of RA. These cytokines are known to maintain chronic inflammation via the recruitment of leukocytes into the joints; trigger pannus formation via activation of synovial fibroblasts; promote angiogenesis as well as stimulate osteoclast formation leading to cartilage and bone degradation [35, 36]. Moreover, elevated TNF-α levels have been reported in serum and synovial fluid of RA patients when compared to the healthy controls [37, 38]. In the rheumatoid synovial tissue, TNF-α, the principal cytokine, not only regulates the activation of pro-inflammatory cytokine (IL-1, IL-6 as well as GM-CSF) but also inhibits regulatory T (Treg) cells functions and resistance to Treg-mediated suppression via induction of effector T cells [39]. In addition, TNF-α-mediated phosphorylation of inhibitor kinase beta is known to induce transcription of inflammatory mediators by allowing migration of NF-κβ dimers to the nucleus [40]. In the present study, CFA-injected rats demonstrated a significant increase in serum TNF-α levels, which were reversed on ATX and IM. The anti-inflammatory properties of ATX have been shown in various studies [7, 41]. Indeed, in LPS-stimulated RAW264.7 cells, FlexPro MD® (FP-MD), a dietary supplement formulation containing ATX as one of its ingredients, has shown to inhibit pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6) and elevate anti-inflammatory cytokine IL-10 levels. In addition, FP-MD also suppressed LPS-induced phosphorylation levels of nuclear factor-κB (NF-κB) p65 and inhibitor of κB-α (IκB-α) [42].
Oxidative stress is known to be involved in the pathophysiology of RA. In the present study, CFA-induced arthritis caused a significant rise in oxidative stress, which was evident by increased levels of MDA and nitrite and reduced levels of SOD, catalase, and GSH activities. An imbalance between oxidants and antioxidants results in increased oxidative stress, which damages biological macromolecules. Evidence of increased levels of intracellular ROS production, lipid peroxidation, protein oxidation, and DNA damage have been reported in RA patients [43, 44]. Accumulation of oxidized cellular components and damaged/degradation products may initiate an augmented synovial inflammatory response. In addition, it may form neoepitopes leading to autoimmune responses [45]. Moreover, impaired enzymatic (GPx, SOD, CAT, GR, AE, NADPH ox, and MPO) and non-enzymatic antioxidants (β-carotene, Vitamin-C, GSH) defense system has also been reported in active RA patients [6]. Thus, antioxidants may serve as a possible therapeutic strategy for treating this chronic inflammatory disorder.
In the current study, ATX demonstrated potent antioxidant effects, as it reduced the levels of MDA and nitrite; accompanied by an increase in antioxidant molecules (GSH, SOD, and Catalase). Indeed, numerous studies have reported the potent antioxidant effects of ATX against lipid peroxidation and oxidative stress in various in vitro and in vivo systems. In a previous study, ATX treatment alleviated oxidative/nitrosative stress, inflammation levels, and pulmonary apoptosis in lung tissues [46]. Similarly, ATX suppressed oxidative stress via attenuating oxidative stress-induced inflammation and mitochondria-related apoptosis in a rat deep-burn model [46]. In humans, dietary ATX has shown to enhance immune response and decrease DNA oxidative damage, an acute-phase protein, and inflammation [47]. In addition, the beneficial effects of ATX supplementation could also be closely related to the Nrf2–ARE signaling pathway that is known to regulate the expression of a large battery of genes involved in the cellular antioxidant and anti-inflammatory defense [41, 48]. Indeed, in a recent study, ATX enhanced HO-1 expression and improved the expression and activity of GSH-Px via activating the ERK-Nrf-2/HO-1 antioxidant pathway in human umbilical vein endothelial cells [49].
Serum CRP and anti-CCP levels were significantly elevated in CFA-injected rats. C-reactive protein (CRP) level is a widely used clinical marker of inflammation that is positively correlated disease activity, synovial histological changes, and radiological progression in RA patients [50,51,52]. Indeed, CRP is involved in bone destruction in RA via its ability to induce RANKL expression and stimulate osteoclastogenesis [53]. Pro-inflammatory cytokines such as IL-6 and to a lesser extent IL-1β and tumor necrosis factor are known to induce expression of CRP via the transcription factors C/EBPβ and NF-κβ [54]. Furthermore, in recent years, the anti-cyclic citrullinated peptide has generated increased interest as a valuable serological marker in RA. In the inflamed synovium, anti-CCP antibodies might contribute to the perpetuation of the inflammation and the chronicity of the disease [55]. In the present study, administration of ATX reversed elevated CRP and anti-CCP levels in CFA-induced arthritic rats. Indeed, in a previous study, ATX has shown to suppress CRP, thereby promoting cardiovascular health in rats fed a high-fat diet [56]. Similarly, dietary ATX have demonstrated the potent anti-inflammatory activity by decreasing CRP levels in adult female human subjects [47].
In the present study, the positive control indomethacin was effective in improving arthritis symptoms in the CFA treated group. Indomethacin blocks the enzymes that make prostaglandins (cyclooxygenases 1 and 2) and thereby reduces the levels of prostaglandins leading to a reduction in pain and inflammation. However, the use of NSAIDs in RA is currently limited due to gastrointestinal irritation, diarrhea, rectal irritation, skin rash, hematologic toxicity, and loss of response with chronic use [57]. Research has so far reported no significant adverse effects of ATX consumption in animals and humans [58]. These results support the safety of ATX for future clinical studies.
Conclusion
From our findings, we conclude that ATX demonstrates significant anti-arthritic activity which could be attributed to its ability to suppress levels of pro-inflammatory cytokine (TNF-α), oxidative stress markers and markers of acute inflammation (CRP and CCP). Other than this, ATX treatment also prevented the decrease in anti-oxidant levels. Collectively, these findings demonstrate the therapeutic potential of ATX in the treatment of RA.
References
Lan Z, Wei M, Chen L, Xie G, Liu X, Zhang X. Role of sinomenine on complete freund’s adjuvant-induced arthritis in rats. IUBMB Life. 2016;68:429–35.
Chang Y, Jia X, Wei F, Wang C, Sun X, Xu S, et al. CP-25, a novel compound, protects against autoimmune arthritis by modulating immune mediators of inflammation and bone damage. Sci Rep. 2016;6:26239.
Mazzucchelli R, Fernandez EP, Crespí-Villarías N, Quirós-Donate J, Vadillo AG, Espinosa M, et al. Trends in hip fracture in patients with rheumatoid arthritis: results from the Spanish National Inpatient Registry over a 17-year period (1999–2015). TREND-AR study. RMD Open. 2018;4:e000671.
Umar S, Sarwar AHMG, Umar K, Ahmad N, Sajad M, Ahmad S, et al. Piperine ameliorates oxidative stress, inflammation and histological outcome in collagen induced arthritis. Cell Immunol. 2013;284:51–9.
Veselinovic M, Barudzic N, Vuletic M, Zivkovic V, Tomic-Lucic A, Djuric D, et al. Oxidative stress in rheumatoid arthritis patients: relationship to diseases activity. Mol Cell Biochem. 2014;391:225–32.
Quiñonez-Flores CM, González-Chávez SA, Del Río Nájera D, Pacheco-Tena C. Oxidative stress relevance in the pathogenesis of the rheumatoid arthritis: a systematic review. BioMed Res Int. 2016;13(Supplement):1–14.
Fang Q, Guo S, Zhou H, Han R, Wu P, Han C. Astaxanthin protects against early burn-wound progression in rats by attenuating oxidative stress-induced inflammation and mitochondria-related apoptosis. Sci Rep. 2017;7:41440.
Yeh P-T, Huang H-W, Yang C-M, Yang W-S, Yang C-H. Astaxanthin inhibits expression of retinal oxidative stress and inflammatory mediators in streptozotocin-induced diabetic rats. PLoS One. 2016;11:e0146438.
Yamagishi R, Aihara M. Neuroprotective effect of astaxanthin against rat retinal ganglion cell death under various stresses that induce apoptosis and necrosis. Mol Vis. 2014;20:1796–805.
Xue X-L, Han X-D, Li Y, Chu X-F, Miao W-M, Zhang J-L, et al. Astaxanthin attenuates total body irradiation-induced hematopoietic system injury in mice via inhibition of oxidative stress and apoptosis. Stem Cell Res Ther. 2017;8:7.
Farruggia C, Kim M-B, Bae M, Lee Y, Pham TX, Yang Y, et al. Astaxanthin exerts anti-inflammatory and antioxidant effects in macrophages in NRF2-dependent and independent manners. J Nutr Biochem. 2018;62:202–9.
Lee S, Bai S, Lee K, Namkoong S, Na H, Ha K, et al. Astaxanthin inhibits nitric oxide production and inflammatory gene expression by suppressing I(kappa)B kinase-dependent NF-kappaB activation. Mol Cells. 2003;16:97–105.
Ito N, Saito H, Seki S, Ueda F, Asada T. Effects of composite supplement containing astaxanthin and sesamin on cognitive functions in people with mild cognitive impairment: a randomized, double-blind, placebo-controlled trial. J Alzheimer’s Dis. 2018;62:1767–75.
Mashhadi NS, Zakerkish M, Mohammadiasl J, Zarei M, Mohammadshahi M, Haghighizadeh MH. Astaxanthin improves glucose metabolism and reduces blood pressure in patients with type 2 diabetes mellitus. Asia Pac J Clin Nutr. 2018;27:341–6.
Imai A, Oda Y, Ito N, Seki S, Nakagawa K, Miyazawa T, et al. Effects of dietary supplementation of astaxanthin and sesamin on daily fatigue: a randomized, double-blind, placebo-controlled, two-way crossover study. Nutrients. 2018;10:281.
Kupcinskas L, Lafolie P, Lignell Å, Kiudelis G, Jonaitis L, Adamonis K, et al. Efficacy of the natural antioxidant astaxanthin in the treatment of functional dyspepsia in patients with or without Helicobacter pylori infection: a prospective, randomized, double blind, and placebo-controlled study. Phytomedicine. 2008;15:391–9.
Barrios V, Escobar C, Cicero AFG, Burke D, Fasching P, Banach M, et al. A nutraceutical approach (Armolipid Plus) to reduce total and LDL cholesterol in individuals with mild to moderate dyslipidemia: review of the clinical evidence. Atheroscler Suppl. 2017;24:1–15.
Group CS. Carotenoids in age-related maculopathy Italian study (CARMIS): two-year results of a randomized study. Eur J Ophthalmol. 2012;22:216–25.
Arora R, Kuhad A, Kaur I, Chopra K. Curcumin loaded solid lipid nanoparticles ameliorate adjuvant-induced arthritis in rats. Eur J Pain. 2015;19:940–52.
Preuss HG, Echard B, Yamashita E, Perricone NV. High dose astaxanthin lowers blood pressure and increases insulin sensitivity in rats: are these effects interdependent? Int J Med Sci. 2011;8:126–38.
Ali EA, Barakat BM, Hassan R. Antioxidant and angiostatic effect of Spirulina Platensis suspension in complete Freund’s adjuvant-induced arthritis in rats. PLoS One. 2015;10:e0121523.
Kuhad A, Chopra K. Tocotrienol attenuates oxidative–nitrosative stress and inflammatory cascade in experimental model of diabetic neuropathy. Neuropharmacology. 2009;57:456–62.
Mittal R, Kumar A, Singh DP, Bishnoi M, Nag TC. Ameliorative potential of rutin in combination with nimesulide in STZ model of diabetic neuropathy: targeting Nrf2/HO-1/NF-kB and COX signalling pathway. Inflammopharmacology. 2018;26:755–68.
Marwaha L, Bansal Y, Singh R, Saroj P, Sodhi RK, Kuhad A. Niflumic acid, a TRPV1 channel modulator, ameliorates stavudine-induced neuropathic pain. Inflammopharmacology. 2016;24:319–34.
Taksande BG, Gawande DY, Chopde CT, Umekar MJ, Kotagale NR. Agmatine ameliorates adjuvant induced arthritis and inflammatory cachexia in rats. Biomed Pharmacother. 2017;86:271–8.
Wills E. Mechanisms of lipid peroxide formation in animal tissues. Biochem J. 1966;99:667–76.
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15 N] nitrate in biological fluids. Anal Biochem. 1982;126:131–8.
Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–7.
Kono Y. Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophys. 1978;186:189–95.
Bansal Y, Singh R, Saroj P, Sodhi RK, Kuhad A. Naringenin protects against oxido-inflammatory aberrations and altered tryptophan metabolism in olfactory bulbectomized-mice model of depression. Toxicol Appl Pharmacol. 2018;355:257–68.
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75.
Jiang X, Yan Q, Liu F, Jing C, Ding L, Zhang L, et al. Chronic trans-astaxanthin treatment exerts antihyperalgesic effect and corrects co-morbid depressive like behaviors in mice with chronic pain. Neurosci Lett. 2018;662:36–43.
Sharma K, Sharma D, Sharma M, Sharma N, Bidve P, Prajapati N, et al. Astaxanthin ameliorates behavioral and biochemical alterations in in vitro and in vivo model of neuropathic pain. Neurosci Lett. 2018;674:162–70.
Datta S, Kundu S, Ghosh P, De S, Ghosh A, Chatterjee M. Correlation of oxidant status with oxidative tissue damage in patients with rheumatoid arthritis. Clin Rheumatol. 2014;33:1557–64.
Kim EY, Moudgil KD. Immunomodulation of autoimmune arthritis by pro-inflammatory cytokines. Cytokine. 2017;98:87–96.
Chen Z, Bozec A, Ramming A, Schett G. Anti-inflammatory and immune-regulatory cytokines in rheumatoid arthritis. Nat Rev Rheumatol. 2018;15:9–17.
Altomonte L, Zoli A, Mirone L, Scolieri P, Magaro M. Serum levels of interleukin-1b, tumour necrosis factor-a and interleukin-2 in rheumatoid arthritis. Correlation with disease activity. Clin Rheumatol. 1992;11:202–5.
Tetta C, Camussi G, Modena V, Di Vittorio C, Baglioni C. Tumour necrosis factor in serum and synovial fluid of patients with active and severe rheumatoid arthritis. Ann Rheum Dis. 1990;49:665–7.
Brzustewicz E, Bryl E. The role of cytokines in the pathogenesis of rheumatoid arthritis–Practical and potential application of cytokines as biomarkers and targets of personalized therapy. Cytokine. 2015;76:527–36.
Mateen S, Moin S, Shahzad S, Khan AQ. Level of inflammatory cytokines in rheumatoid arthritis patients: correlation with 25-hydroxy vitamin D and reactive oxygen species. PLoS ONE. 2017;12:e0178879.
Feng Y, Chu A, Luo Q, Wu M, Shi X, Chen Y. The protective effect of astaxanthin on cognitive function via inhibition of oxidative stress and inflammation in the brains of chronic T2DM rats. Front Pharmacol. 2018;9:748.
Park DR, Ko R, Kwon SH, Min B, Yun SH, Kim MH, et al. FlexPro MD, a Mixture of Krill Oil, Astaxanthin, and Hyaluronic Acid, Suppresses Lipopolysaccharide-Induced Inflammatory Cytokine Production Through Inhibition of NF-κB. J Med Food. 2016;19:1196–203.
Altindag O, Karakoc M, Kocyigit A, Celik H, Soran N. Increased DNA damage and oxidative stress in patients with rheumatoid arthritis. Clin Biochem. 2007;40:167–71.
Mateen S, Moin S, Khan AQ, Zafar A, Fatima N. Increased reactive oxygen species formation and oxidative stress in rheumatoid arthritis. PLoS ONE. 2016;11:e0152925.
Phull A-R, Nasir B, ul Haq I, Kim SJ. Oxidative stress, consequences and ROS mediated cellular signaling in rheumatoid arthritis. Chem Biol Interact. 2018;281:121–36.
Bi J, Cui R, Li Z, Liu C, Zhang J. Astaxanthin alleviated acute lung injury by inhibiting oxidative/nitrative stress and the inflammatory response in mice. Biomed Pharmacother. 2017;95:974–82.
Park JS, Chyun JH, Kim YK, Line LL, Chew BP. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr Metab. 2010;7:18.
Zhu X, Chen Y, Chen Q, Yang H, Xie X. Astaxanthin promotes Nrf2/ARE signaling to alleviate renal fibronectin and collagen IV accumulation in diabetic rats. J Diabetes Res. 2018;18:1–7.
Niu T, Xuan R, Jiang L, Wu W, Zhen Z, Song Y, et al. Astaxanthin induces the Nrf2/HO-1 antioxidant pathway in human umbilical vein endothelial cells by generating trace amounts of ROS. J Agric Food Chem. 2018;66:1551–9.
Mallya R, Berry H, Hamilton E, Mace B, Pepys M. Correlation of clinical parameters of disease activity in rheumatoid arthritis with serum concentration of C-reactive protein and erythrocyte sedimentation rate. J Rheumatol. 1982;9:224–8.
Matsuno H, Yudoh K, Nakazawa F, Koizumi F. Relationship between histological findings and clinical findings in rheumatoid arthritis. Pathol Int. 2002;52:527–33.
Plant MJ, Williams AL, O’Sullivan MM, Lewis PA, Coles EC, Jessop JD. Relationship between time-integrated C-reactive protein levels and radiologic progression in patients with rheumatoid arthritis. Arthritis Rheum. 2000;43:1473–7.
Kim K-W, Kim B-M, Moon H-W, Lee S-H, Kim H-R. Role of C-reactive protein in osteoclastogenesis in rheumatoid arthritis. Arthritis Res Ther. 2015;17:41.
Rhodes B, Fürnrohr BG, Vyse TJ. C-reactive protein in rheumatology: biology and genetics. Nat Rev Rheumatol. 2011;7:282–9.
Vossenaar ER, van Venrooij WJ. Anti-CCP antibodies, a highly specific marker for (early) rheumatoid arthritis. Clin Appl Immunol Rev. 2004;4:239–62.
Xu J, Gao H, Zhang L, Chen C, Yang W, Deng Q, et al. A combination of flaxseed oil and astaxanthin alleviates atherosclerosis risk factors in high fat diet fed rats. Lipids Health Dis. 2014;13:63.
Li Y, Kakkar R, Wang J. In vivo and in vitro approach to anti-arthritic and anti-inflammatory effect of crocetin by alteration of nuclear factor-E2-related factor 2/hem oxygenase (HO)-1 and NF-κB expression. Front Pharmacol. 2018;9:1341.
Ambati R, Phang S-M, Ravi S, Aswathanarayana R. Astaxanthin: sources, extraction, stability, biological activities and its commercial applications—a review. Mar Drugs. 2014;12:128–52.
Acknowledgements
All the authors acknowledge the funding provided by the University Grants Commission (UGC), New Delhi, India. Grant Number is F.38-11/2016/(SA-III).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumar, A., Dhaliwal, N., Dhaliwal, J. et al. Astaxanthin attenuates oxidative stress and inflammatory responses in complete Freund-adjuvant-induced arthritis in rats. Pharmacol. Rep 72, 104–114 (2020). https://doi.org/10.1007/s43440-019-00022-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43440-019-00022-z