Abstract
Ferulic acid ethyl ester (FAEE) is a derivate from ferulic acid which reportedly has antioxidant effect; however, its role on inflammation was unknown. In this study, we investigated the orally administered FAEE anti-inflammatory activity on experimental inflammation models and Complete Freund’s Adjuvant (CFA)-induced arthritis in rats. CFA-induced arthritis has been evaluated by incapacitation model and radiographic knee joint records at different observation time. FAEE (po) reduced carrageenan-induced paw edema (p < 0.001) within the 1st to 5th hours at 50 and 100 mg/kg doses. FAEE 50 and 100 mg/kg, po inhibited leukocyte migration into air pouch model (p < 0.001), and myeloperoxidase, superoxide dismutase, and catalase activities (p < 0.001) increased total thiol concentration and decreased the TNF-α and IL-1β concentrations, NO, and thiobarbituric acid reactive species. In the CFA-induced arthritis, FAEE 50 and 100 mg/kg significantly reduced the edema and the elevation paw time, a joint disability parameter, since second hour after arthritis induction (p < 0.001). FAEE presented rat joint protective activity in radiographic records (p < 0.001). The data suggest that the FAEE exerts anti-inflammatory activity by inhibiting leukocyte migration, oxidative stress reduction, and pro-inflammatory cytokines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is a chronic, inflammatory, autoimmune disease with a 0.5–1.0 % prevalence around the world. It is associated with joint destruction, pain and impaired movement, progressive disability, systemic complications, high socioeconomic costs, and early death (McInnes and Schett 2011). Notably, arthritic joints have polymorpho-nucleate cell infiltration that release cytokines, mainly tumor necrosis factor alpha (TNF-α) and interleukin-1β (IL-1β), metalloproteinase, and free radicals that together are responsible for cartilage and bone damage (Dinarello 2007; Salvemini et al. 2011; Brieger et al. 2012).
Current anti-inflammatory therapy comprises the non-steroidal anti-inflammatory drugs (NSAIDs), corticosteroids, disease-modifying antirheumatic agents, and monoclonal antibodies (Aaltonen et al. 2012). However, these drugs are associated with gastrointestinal or cardiovascular side effects and either are expensive (Ashley et al. 2012). Thereby, it is necessary to find new drugs for RA treatment and other chronic diseases such as lupus and Crohn’s disease.
Severous natural compounds extracted from plants and essential oils have been used for inflammatory condition treatment (Sousa 2011). Essential oils are natural origin volatile constituent mixtures, which have anti-inflammatory and antioxidant activity (Turek and Stintzing 2012). Essential oils have been identified as important free radical scavenging due to its phenolic constituents (phenylpropanoids, flavonoids, and terpenoids) that act by direct or indirect means (Amorati et al. 2013).
Phenylpropanoids and its derivatives are among the foods, flavors, fragrances, essential oils, wines, beers, and various segments most common active components in Chinese traditional medicine (Vogt 2010; Sá et al. 2014). It exerts important anti-inflammatory activity through inhibiting pro-inflammatory enzyme expressions (COX-2, 5-LOX, iNOS, NADPH oxidase, and PLA2), signal transduction inflammatory response pathways (NF-kβ, AP-1, Akt, and ERK), and receptors and gene transcription inhibition (PPARs and ARE) (Gautam and Jachak 2009; Korkina et al. 2011).
Ferulic acid ethyl ester (ethyl-3-hydroxy-4-methoxycinnamate) (C12H14O4), a phenylpropanoid, is a ferulic acid derivative widely present in plants and especially in grains such as corn and rice (Zhang et al. 2010) (Fig. 1). Steric ferulic acid derivatives exert free radical scavenger mainly through increase antioxidant protein expressions such as glutathione, quinone reductase, HO-1, and HSP-70 protein (Calabrese et al. 2008a; Bolling et al. 2011). The mechanisms involved in this response have been related to the Nrf-2 pathway activation, NF-kB, COX-2, and iNOS inhibition (Islam et al. 2009; Sultana 2012). Front to ferulic acid ethyl ester (FAEE) antioxidant activity evidences, we investigated the orally administrated FAEE effect on inflammatory models and CFA-induced arthritis in rats.
Materials and methods
Reagents
Carrageenan (Sigma, USA), indometacin (Sigma, USA), Tween 80 (Sigma, USA), Turk’s solution, sodium bicarbonate (MERCK, Brasil), Complete Freund’s Adjuvant (Sigma, USA), acetic acid (Sigma, USA), l-methionine (Sigma, USA), Triton X-100 (Sigma, USA), hydroxylamine hydrochloride (Sigma, USA), EDTA, riboflavin (Sigma, USA), Griess’ reagent (Sigma, USA), thiobarbituric acid (TBA) (Sigma, USA), sodium dodecil sulfate (SDS) (Fluka, USA), diazepan (Sigma, USA), hexadeciltrimetilamonium bromide (HTAB) (Sigma, USA), o-dianisidine (Sigma, USA), sodium phosphate (Merck, Germany), potassium phosphate (Merck, Germany), zinc sulfate (Merck, Germany), hydrogen peroxide (Merck, Germany), Tris base (Merck, Germany), sodium hydroxide (Merck, Germany), tricloroacetic acid (Merck, Germany), 5,5′-dithiobis acid (2-nitrobenzoic acid) (DTNB) (Merck, Germany), sodium nitrite (NaNO2) (Sigma, USA), enzyme-linked immunosorbent assay kits TNF-α and IL-1β (Enzo LifeSciences®) were provided. FAEE was provided by Dr. Damião Pergentino de Sousa, Pharmaceutical Sciences Department, Federal University of Paraíba. The FAEE was used in 12.5, 25, 50, and 100 mg/kg doses diluted in a 3 % Tween 80 in 0.9 % NaCl solution. The used doses in this study were chosen according to the average lethal dose LD50 already established in the literature for mice (980 mg/kg, po) plus the other phenylpropanoid effectiveness at similar doses (Ballabeni et al. 2010; Yin et al. 2012).
Animals and ethics
Male and female Wistar rats (150–210 g) and Swiss mice (25–30 g) (n = 6 per group) were kept at 24 ± 1 °C ambient temperature, with 12 h light/dark cycles, received standard chow and water ad libitum. The animals were fasted for 12 h and received FAEE, vehicle (3 % Tween 80 in 0.9 % NaCl solution), or indomethacin. After the experimental procedures, the animals were euthanized with a thiopental sodium (100 mg/kg, ip) overdose, in accordance with June 20, 2002 Resolution No. 714. The experimental protocols used were approved by Federal University of Piauí, Brazil Ethics Committee on Animal Experimentation (EAEC/UFPI; No. 082/14). All experimental research on animals in this study followed internationally recognized guidelines.
FAEE effect in the carrageenan-induced paw edema
Wistar rats were previously treated orally with vehicle, FAEE (12.5, 25, 50, and 100 mg/kg), or indomethacin (10 mg/kg), and 60 min after, intra-plantar carrageenan (1 %, 0.1 mL) into the right hind paw was performed. Paw volumes were measured by plethysmometer (Insight®, Brazil) and expressed in milliliter at before carrageenan administration and 1, 2, 3, 4, and 6 h after the carrageenan application (Winter et al. 1962).
FAEE effect in cell migration on carrageenan-activated air pouch
Air pouches were produced by subcutaneous 20-mL sterile air injection into the intra-scapular back area and maintained by air re-inflation with 10 mL 3 days later, as described earlier (Muller et al. 1999). On the 60 days, animals were previously treated with vehicle, indomethacin (10 mg/kg, po), and FAEE (25, 50, and 100 mg/kg po). One hour after treatment, 1 % carrageenan dissolved in saline 100 μL was injected directly into the pouch. Six hours after, a small incision was made in the pouch wall and the air pouch contents were carefully removed using a sterile Pasteur pipette after 10-mL ice-cold PBS injection containing 0.1 % EDTA. Thereafter, a 1:20 dilution in Turk solution was performed, proceeding to the total leukocyte count in a Neubauer chamber. Another exudate aliquot was separated by centrifugation and stored in a freezer −80 °C for further analysis.
Myeloperoxidase assay
Myeloperoxidase activity was determined by o-dianisidine-H2O2 method (Bradley et al. 1982). Briefly, exudate samples (100 μL) were added to 1 mL 0.5 % HTAB buffer (pH 6.0) followed by centrifugation at 4500g for 10 min at 4 °C. After centrifugation, supernatant 10 μL was removed and added to 96-well plate with subsequent 200-μL solution addition containing 0.167 mg/mL o-dianisidinedihydrochloride and 0.15 mmol/L H2O2 in 50 mmol/L potassium phosphate buffer (pH 6.0). The change in absorbance was measured at 450 nm. Results were expressed as myeloperoxidase (MPO) per microliter units.
Nitrite concentration
Briefly, 200 μL of collected exudate from the air pouches was diluted in distilled water (1:4 v/v) for subsequent deproteinization by addition of zinc sulfate solution (300 g/L) 1/20 volume to achieve a 15 g/L final concentration. Then, the samples were centrifuged at 1000g for 15 min at room temperature (Romitelli et al. 2007). For the nitric oxide determination, supernatant 100 μL was pipetted into a well of 96-well plate followed by 100-μL Griess reagent addition, and after 10 min at room temperature, the optical density was measured by spectrophotometry in ELISA Reader (BioTek EL800) using a 550-nm filter (Green et al. 1982).
Cytokines concentration
TNF-α and IL-1β quantification were carried out taking as the sample air pouch exudate and using captured ELISA kits. The procedure was performed according to the manufacturer’s instructions (Enzo LifeSciences®), and results were expressed as TNF-α or IL-1β pg/mL according to the standard curve obtained.
Superoxide dismutase activity
To determine if the superoxide dismutase activity was used, the method was described by Das et al. (2000), in which 100-μL exudate sample is added to 1110 μL phosphate buffer (50 mM, pH 7.4), 75 μL L-methionine (20 mM), 40 μL Triton X-100 (1 % v/v), 75 μL hydroxylamine hydrochloride (10 mM), and 100 μL EDTA (50 mM), followed by incubation in a water bath at 37 °C for 5 min. Then, 80 μL riboflavin (50 mM) was added and samples were exposed to light for 10 min. At the end, 100-μL sample was added to the 96-well plate plus 100-μL Griess reagent, and after 10 min at room temperature, the optical density was measured by spectrophotometry in ELISA Reader (BioTek EL800) using a 550-nm filter (Green et al. 1982). Enzyme activity unit is defined as the superoxide dismutase (SOD) amount able to inhibit the nitrite formation by 50 %. The calculation is done using the following formula: SOD = v0/v1, where v0 is the control absorbance and v1 is the test absorbance. In this method, 36 ng SOD inhibits 50 % nitrite formation.
Glutathione concentration
One-hundred microliter sample was precipitated by adding 200 μL of 5 % TCA and then stirred. The mixture was then centrifuged at 3000g for 15 min at 4 °C. Seventy-five microliter supernatant was added to 150 μL of 0.4 M EDTA, 0.2 M Tris (pH 8.9), and 40 μL of DTNB. The absorbance was measured in visible light spectrophotometer at 412 nm. The reduced glutathione concentration is given in milligrams per deciliter (Ellman 1959).
Catalase activity
Two-hundred microliter exudate was added to 1.2 mL phosphate buffer (50 mM, pH 7.0), and then, 1 mL H2O2 solution (30 mM) was added. The absorbance was measured in UV–Vis spectrophotometer at 240 nm and characterized by decreased absorbance due to H2O2 degradation. The absorbance was recorded every minute for 6 min. Catalase is expressed as H2O2 decomposed iemmoles per minute per microliter serum (Takahara et al. 1960).
Thiobarbituric acid reactive species determination
For thiobarbituric acid reactive species (TBARS) determination, 200-μL exudate sample was added to 350 μL of 20 % acetic acid (pH 3.5) and 600 μL of 0.5 % TBA (dissolved in acetic acid). The samples were boiled for 45 min, immediately succeeded by an ice bath for 15 min, 50-μL SDS addition (8.1 %), and centrifugation at 12,000g for 15 min at 25 °C. The optical density was measured by spectrophotometry in ELISA Reader (BioTek EL800) using filters of 420, 490, and 550 nm. The value was expressed in nanomole per millimeter (Ohkawa et al. 1979).
FAEE effect on CFA-induced arthritis
For Complete Freund’s Adjuvant-induced arthritis, Wistar rats received an initial 50-μL CFA sc dose containing 0.5 mg/mL Mycobacterium tuberculosis in the tail base. After 21 days, the animals were divided into six groups (n = 6) and treated orally with vehicle or indomethacin (5 mg/kg) and three groups were given FAEE orally at 25, 50, and 100 mg/kg doses and immediately after the CFA booster injection similar to first in the right knee joint to induce arthritis (Woodruff et al. 2002). The second dose day was considered the initial arthritis day (D0). Animals were evaluated immediately before the booster dose application every hour for the first 5 h and the first (D1) day (acute protocol); third (D3), fifth (D5), and seventh (D7) days (phase protocol sub-acute); and tenth (D10) and 14 days (D14) (chronic phase). Upon induction, the animals were orally treated daily until the 14th day. After 14 treatment days, the animals were euthanized for evaluation and sample collection for analysis. Radiographic records were made in D1, D7, and D14 to evolutionary control.
FAEE effect on CFA-induced chronic inflammatory edema in rats
The rats were evaluated immediately before the booster dose application, each 1/1 h in the 5 h (D0) and (D1) (acute phase protocol); in (D3), (D5), and (D7) (sub-acute phase protocol); and (D10) and (D14) (chronic phase). In this trial, the incidence and arthritis severity were assessed by measuring the right knee diameter with a digital caliper (Digimess®), with all the measurements made by the same observer and in duplicate. The dates were expressed in millimeters (mm).
FAEE effect on CFA-induced incapacitation in rats
Concurrently with the CFA-induced edema evaluation, the joint motor disability degree was rated. For this, we used the coordinate impairment model in rats described by Tonussi and Ferreira (1994). The registration system consists of a stainless steel cylinder which has a continuous rotation at a 3 rpm speed. The cylinder surface is divided into three equal rails, each connected to grounding a computer, and allows the simultaneous three animal analyses. Metallic sneakers were adjusted in both hind legs, and only the right hind paw was connected to the computer’s input port. After shoe placement, the animals were kept in their respective cages for at least 1 h for adaptation. To register the functional joint status, the animals were submitted to forced march, on the rotating cylinder, for a 60 s period. The computer records the total time that the right hind animal paw remains not touching the cylinder surface at this time (elevation paw time (TEP); second). In all experimental procedures, the animals were trained to walk on the recording device the day before the test. Data were presented as mean values between the TEP of at least five animals obtained every hour after CFA injection. The animals were euthanized at 14th day for assessment and material collection for analysis.
Radiographic records
After the disabling evaluation at D1, D7, and D14, animals were anesthetized by intra-muscular administration solution composed of ketamine (50 mg/kg) and xylazine (5 mg/kg) to perform the radiographic recording. The animals were positioned supine with abduction and external femoral shaft rotation with its fixed paws and tail to the table to display the knees and incidence X-ray beam profile at a 120 cm distance from the radiating source and 5 ms exposure time in the fine focus mode (Siemens Raex RC 300 D, 45 kV, 100 mA) (Yu et al. 2006). The images were analyzed by a radiologist blindly observing soft tissue edema presence, bone matrix resorption, and bone erosion and classified according to clinical signs, where 0 = no bone injury, 1 = soft tissue edema, 2 = articular cartilage erosion, and 3 = bone erosion and osteophytes. The higher the factors, the severe the arthritis sum (Cuzzocrea et al. 2000).
FAEE effect on spontaneous motor activity in mice
The motor animal activity was verified by an open field square (30 cm × 30 cm × 15 cm), with its base divided into nine equal diameter squares. Swiss male mice (n = 5 per group) were treated with vehicle (0.9 % saline, 10 mL/kg, po) FAEE (50 and 100 mg/kg, po), and diazepam (4 mg/kg, 0.4 mg/mL, ip). After 45 administration minutes, the animals were individually brought into the open field and observed for a 4 min period, with 1 min for adaptation and movement frequency based on the model described by Capaz et al. (1981).
Statistical analysis
To verify the sample distribution normality, Shapiro-Wilk test was performed. We used ANOVA followed by Tukey’s post-test to compare groups with one independent variable and two-way ANOVA followed by Bonferroni post-test between groups with more than one independent variable. Kruskal-Wallis followed by Dunn post-test was used to non-parametric analyzed variables. Values are expressed as mean ± standard error (SE), and the significance level was p < 0.05. GraphPad Prism® 6.01 Software was used.
Results
FAEE effect in the carrageenan-induced paw edema
Animal pretreatment with FAEE 100 mg/kg reduced significantly (p < 0.001) the paw edema induced by carrageenan from first to fifth observation hours compared to group treated with vehicle (Fig. 2). Similarly, animal pretreatment with FAEE at 25 and 50 mg/kg doses also significantly reduced edema formation, from second hour (p < 0.01) and obtaining maximum inhibition from third to fifth hours (p < 0.001) compared to the control group. The FAEE at a 12.5 mg/kg dose showed no significant paw edema reduction induced by carrageenan (p > 0.05).
FAEE effect on carrageenan-induced paw edema (1 %, 0.1 mL, ipl) in rats (six animals/group). AUC showing that FAEE significantly inhibited edema formation compared to the control group. Values are expressed as mean ± SE. **p < 0.01 and ***p < 0.001 versus vehicle (one-way ANOVA followed by Tukey’s post-test)
FAEE effect in cell migration on carrageenan-activated air pouch
The carrageenan in the air pouch resulted in an increase in the number of total leukocytes in the vehicle group when compared to groups pretreated with FAEE at 25 (p < 0.01), 50 (p < 0.01), and 100 mg/kg (p < 0.001) doses (Fig. 3).
FAEE effect on a, b TNF-α and IL-1β levels, c the total leukocytes number count, and d myeloperoxidase activity in air pouch activated by carrageenan (six animals/group). Values are expressed as mean ± SE. *p < 0.05, **p < 0.01, and ***p < 0.001 versus vehicle (one-way ANOVA followed by Tukey’s test)
Myeloperoxidase assay
MPO activity was reduced in animals pretreated with FAEE at 25, 50, or 100 mg/kg doses (p < 0.001) than from the control group. The same was observed in animals treated with the standard anti-inflammatory indomethacin (p < 0.001) (Fig. 4).
FAEE effect on a nitrite concentration (NO2 −), b superoxide dismutase activity (SOD) expressed indirectly by inhibition of the nitrite formation (NO2 −), c reduced glutathione concentration expressed in terms of total thiols, d catalase activity, and e concentration of thiobarbituric acid reactive species (TBARS) in the air pouch activated by carrageenan (six animals/group). Values are expressed as mean ± SE. *p < 0.05, **p < 0.01, and ***p < 0.001 versus vehicle (one-way ANOVA followed by Tukey’s test)
Nitrite concentration
Pretreatment with FAEE at 25, 50, and 100 mg/kg doses (p < 0.001) and indomethacin at 10 mg/kg (p < 0.01) resulted in a significant decrease in the NO2 − concentration (nitrite) compared to the vehicle group (Fig. 5).
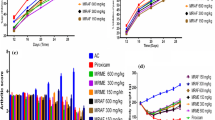
FAEE effect on a knee edema and b time of elevation paw in CFA-induced monoarthritis. AUC showing that FAEE significantly inhibited the formation of knee swelling and incapacitation compared to the control group (six animals/group). Values are expressed as mean ± SE. *p < 0.05, **p < 0.01, and ***p < 0.001 versus vehicle (one-way ANOVA and Tukey’s post-test)
Cytokine concentration
The animals treated with FAEE exhibited significantly lower TNF-α and IL-1β concentrations (p < 0.001) when compared to the vehicle group at all doses with similar results to those found for the pretreatment with anti-inflammatory pattern (Fig. 6).
FAEE effect on radiographic changes in CFA-induced knee monoarthritis (six animals/group). Values are expressed as mean ± SE. *p < 0.05 versus vehicle, **p < 0.01 versus vehicle and FAEE at 50 mg/kg dose, and ***p < 0.001 versus vehicle and FAEE at 25 mg/kg dose, respectively (Kruskal-Wallis and Dunn post-test)
Superoxide dismutase activity
Regarding the SOD activity, it was observed that both groups pretreated with different FAEE and indomethacin doses were equally effective in enhancing the superoxide dismutase activity compared to the vehicle group (p < 0.001) (Fig. 7).
FAEE effect on radiographic images of CFA-induced knee monoarthritis on the day of induction (D1) and after 7 (D7) and 14 days (D14) (six animals/group). Legend F femur, f fibula, T tibia, and P patella. Green arrows represent areas of erosion, and yellow arrows represent areas of arthritis/joint wear
Glutathione concentration
The previous administration of FAEE, at 50 and 100 mg/kg doses, significantly (p < 0.01) increased the total thiol concentration in the air pouch exudate in rats. Animal treatment with FAEE at 25 mg/kg dose and indomethacin at 10 mg/kg dose resulted in no significant difference from the vehicle group (Fig. 8).
Catalase activity
Catalase activity was significantly increased in the groups treated with FAEE at 50 (p < 0.05) and 100 mg/kg (p < 0.01) doses compared to vehicle group. Animal treatment with FAEE and indomethacin at 25 mg/kg dose resulted in no significant increase in enzyme activity compared to the vehicle group.
Determination of TBARS
FAEE at 50 (p < 0.05) and 100 mg/kg (p < 0.01) doses were effective in reducing thiobarbituric acid reactive species concentrations.
FAEE effect on CFA-induced chronic inflammatory edema in rats
Animal pretreatment with FAEE at 100 mg/kg dose resulted in significant knee-swelling reduction in all observation times from the fourth hour. Similarly, animal treatment with FAEE at 50 mg/kg dose resulted in a significant edema reduction at all hours (p < 0.01), except for the second day, from the fifth hour to D14. Ferulic ester (FE) at 25 mg/kg dose resulted in no edema reduction. The group treated with indomethacin significantly reduced edema from the fifth observation hour and lasted until the 14th observation day (p < 0.01).
FAEE effect on CFA-induced incapacitation in rats
The intra-articular CFA resulted in TEP increased in animals treated with vehicle relative to the baseline TEP of the first hour after CFA administration until the 14th evaluation day (p < 0.001). Animal treatment with indomethacin and FE at 100 mg/kg dose decreased the TEP significantly in all observation points from the second hour compared to vehicle-treated animals. Daily treatment with FAEE at a 50 mg/kg dose resulted in decreased TEP only from the fourth hour assessment by the 14th day from the vehicle-treated group.
FAEE effect on radiographic records in CFA-induced arthritis in rats
Based on radiograph records displayed, it is noted that the FAEE at 100, 50, and 25 mg/kg doses and indomethacin at 5 mg/kg dose exerted different protection degrees on the rat tibio-femoral joint with CFA-induced monoarthritis. Thus, the FAEE at 100 mg/kg dose showed little bone erosion at the end of 14 experiment days, as well as animals treated with FAEE at 50 mg/kg dose and indomethacin at 5 mg/kg dose. The animals treated with FAEE at 25 mg/kg dose showed multiple bone injury site and osteoarthritis similarly to the animals treated with vehicle alone.
FAEE effect on spontaneous motor activity in mice
FAEE resulted in no change in the exploratory mouse capacity in any tested doses (p > 0.05). Of diazepam, 0.4 mg/kg resulted in a significant decrease in the invasion number from the vehicle group.
Discussion
Among the experimental models, carrageenan-induced paw edema has been the choice model for screening anti-inflammatory drugs since their description by Winter et al. (1962). The carrageenan injection produced a biphasic inflammatory swelling through bradykinin, serotonin (5-HT), and histamine release during the first hour after induction (Kirchhoff et al. 1990) followed by the prostaglandins and nitric oxide production (NO), neutrophil infiltration, COX-2 induction, free radical production, and oxidative stress until sixth hour after induction (Garrelly et al. 1991; Salvemini et al. 1996a; Nantel et al. 1999).
In this study, FAEE inhibited the swelling from the second hour after the induction. These results suggest FAEE action on both carrageenan phase-induced inflammation through mediator inhibition such as 5-HT, bradykinin and histamine, antioxidant activity or by COX, iNOS, prostaglandins, and nitric oxide release inhibition (Salvemini et al. 1996b). Similar results were found to other phenylpropanoids as eugenol, anethole, cinnamaldehyde, dilapiolle, and diidrodilapiolle, which inhibit the edema formation induced by carrageenan by mechanisms related to the prostaglandins, arachidonic acid pathway, and NO inhibition (Ballabeni et al. 2010; Parise-Filho et al. 2011; Ritter et al. 2013; Domiciano et al. 2013).
Given the positive FAEE effect in the early and late paw edema phases induced by carrageenan, we investigated the mechanisms involved in FAEE anti-inflammatory activity on leukocyte migration, cytokine concentrations (TNF-α and IL-1β), and antioxidant activity. For this, we used the air pouch model.
The air pouch mimics many joint inflammation aspects characterized by a non-granulomatous immune reaction (Martin et al. 1994). The results show that FAEE was effective in reducing the total leukocyte number migration to the air pouch. Similar action has been reported in the literature for other phenylpropanoids such as siringina, caffeic acid, and caffeic acid phenethyl ester (Michaluart et al. 1999; Liz et al. 2008; Yin et al. 2012).
In order to confirm the FAEE inhibitory effects on leukocyte migration, then for the indirect transmigration, and quantity leukocyte assessment by MPO, the most abundant pro-inflammatory enzyme stored in the azurophilic neutrophil granules provides an intimate relationship with the cell number which was extracted (Bradley et al. 1982; Pulli et al. 2013).
Thus, the FAEE significantly reduced the myeloperoxidase amount in subcutaneous air pouch exudate in rats. A similar result was found for animals treated with Solidago chilensis Meyen butanolic extract, rich in caffeic acid and chlorogenic acid (Liz et al. 2008). In colitis model induced by dextran sulfate sodium (DSS), the У-oryzanol, a fenilpropanoids mixture, resulted in myeloperoxidase activity reduction after six and eight oral treatment days (Islam et al. 2008). The decrease in total cells count and MPO, as observed in FAEE treatment, can be related to decreased adhesion molecule expression as observed in the treatment with syringin, a glycosylated phenylpropanoid, which significantly reduces the ICAM-1 expression (Yin et al. 2012).
Recent studies have shown that phytochemicals (terpenes, flavonoids, and phenylpropanoids) with antioxidant activity play a key role in the NF-kB inactivation signaling pathway by detoxifying enzyme complex expressions such as catalase, glutathione peroxidase system/glutathione S-transferase, and quinone reductase through the Nrf2-Keap1 (Mollace 2005; Nagasaka et al. 2007; Surh 2008; Calabrese et al. 2008b; Brigelius-Flohé and Kipp 2012).
FAEE significantly increased SOD and catalase activity and GSH concentration. A similar result was found in sensitized macrophage cultures treated with LPS and У-oryzanol or cycloartenil ferulate and where there SOD increased transcription, suggesting that the FAEE can also induce this enzyme transcription (Nagasaka et al. 2007). During the inflammatory process, free radical degradation by antioxidant agents prevents it from inducing the cytokine production, adhesion molecules, and chemotactic agent expression (Jang et al. 2004; Niki et al. 2005; Jang et al. 2005; Li and Zhou 2011; Morgan and Liu 2011).Thus, FAEE anti-inflammatory action part is due to its antioxidant activity as already demonstrated. Accordingly, Perluigi et al. (2006) and San Miguel et al. (2012) observed a decrease in the carbonil protein concentrations, the reactive oxygen species amount, and increase in cell viability by reducing free radicals on cell culture treated with FAEE.
The access to behavioral patterns in human arthritis animal models is a valuable step in the alternative development for the pharmacological pain treatment and inflammation (Min et al. 2001). Among the models that mimic this disease, there is the CFA-induced arthritis. This procedure generates inflammatory response with strong cellularity, markedly T lymphocytes, and circulating antigen-specific antibodies. Various cytokines, such as TNF-α, IL-1β, IL-12, and IFN-γ, and numerous chemokines are re-induced by aberrant crypt foci, mimicking in many ways the chronic inflammatory process pathophysiology (Chillingworth and Donaldson 2003).
These results demonstrate that FAEE exerts attenuating CFA-induced arthritis function by negative modulating IL-1β concentrations and especially TNF-α. This action can be explained by the lower leukocyte migration and consequent lower cytokine release. IL-1β and TNF-α are potent pro-inflammatory cytokines that are produced primarily by macrophages and neutrophils and may act on the endothelium inducing integrin expression and stimulating the pro-inflammatory cytokine release by other immune cells (Bradley 2008; Broggi and Granucci 2015).
Referring to decrease the cytokine concentration, regulatory activity exerted by NO may be involved on NF-kB, COX-2, and PGE2 activation. Nitric oxide is continuously synthesized by nitric oxide synthase (NOS) (Dejam et al. 2004). This molecule exhibits diverse physiological functions; however, if at high concentrations released by the iNOS action, NO has cytotoxic and cytostatic actions (Salvemini 1996a; D’acquisto et al. 2001).
The FAEE significantly decreased the nitrite concentration (NO2 −). Similar results involving the iNOS transcription suppression have been demonstrated to other phenylpropanoids such as anethole (Kang et al. 2013), 2-hydroxy cinnamaldehyde (Lee et al. 2009), and trans-cinnamaldehyde (Ballabeni et al. 2010) and S. chilensis Meyen extract (Liz et al. 2008). The results expressed here and data published by Perluigi et al. (2006) and Joshi et al. (2006), in various studies, where the FAEE significantly decreased the iNOS expression in neuronal cultures, support such an action mechanism for the FAEE.
Concomitant to assessments of TEP and knee swelling, radiographic records were performed to control the structural changes developed during the 14 arthritis-induced days ACF. We adopted the basal recording protocol (on the induction day) to demonstrate the joint structure integrity and two records on day 7 and day 14 after induction in order to assess progress in the middle period and outcome of the protocol.
Important factor about CFA-induced monoarthritis is the similarity between the joint destruction degree and gait patterns. Thus, animal groups with worse gait patterns; that is, higher TEP have higher joint wear (Boettger et al. 2009). This statement is in accordance to the results shown here based on the TEP and joint radiographic records on the induction day, after 7 and 14 days where the animals treated with FAEE showed less joint damage compared to the control group. Similar results were reported here found for Careya arborea Roxb extracts and Terminalia paniculata Roth, rich in phenylpropanoids, which showed less edema and bone resorption areas (Talwar et al. 2011; Rayhana et al. 2014).
Front to inflammatory process and all attached to him for the free radical consequence production, which are highly damaging to chondrocytes and increase intra-articular oxidative stress DNA p53 subunit triggering apoptosis activation, especially peroxynitrite (ONOO−) (Henrontin 2005; Grishko et al. 2009). Arthritic joint hallmark, bone matrix erosion, or resorption areas are the osteoclastogenesis stimulation results by TNF-α (Afonso et al. 2007; Lam et al. 2000).
FAEE dose–response effect absence appears to be a standard for phenylpropanoids. FAEE in the middle and higher doses showed no statistical differences between them in inflammation models used as demonstrated by the area under the curve (AUC). Other studies have shown that the phenylpropanoids such as anethole and eugenol (Daniel et al. 2008; Domiciano et al. 2013) also showed no dose–response effect.
On the other hand, FAEE has shown a potential antioxidant and anti-inflammatory in vitro (Scapagnini et al. 2004; Perluigi et al. 2006; Joshi et al. 2006). Our work, however, proves the FAEE potentiality in vivo applied in arthritis model. These results are encouraging the therapeutic agent development for the inflammatory disease treatment and that have low toxicity.
Concerning this, the FAEE increased the catalase activity, SOD, and the total thiol concentrations (glutathione marker system), as well as cytokine influence as discussed above, listing it as a promising chondroprotective agent and anti-inflammatory for acute and chronic arthritic frames.
Conclusion
FAEE oral treatment exerts anti-inflammatory and chondroprotective activities. FAEE showed antiedematogenic activity in the carrageenan-induced paw edema in rats. Furthermore, FAEE reduced joint swelling and joint disability in CFA-induced knee monoarthritis. Chondroprotective activity observed through X-ray records possibly associated with TNF-α and IL-1β inhibition as well as antioxidant activity. This phenylpropanoid has the potential as a new lead compound for the future therapeutic intervention in inflammation development.
References
Aaltonen KJ, Virkki LM, Malmivaara A, Konttinen YT, Nordström DC, BLOM M (2012) Systematic review and meta-analysis of the efficacy and safety of existing TNF blocking agents in treatment of rheumatoid arthritis. Plos One 7:1–14. doi:10.1371/journal.pone.0030275
Afonso V, Champy R, Mitrovic D, Colin P, Lomri A (2007) Reactive oxygen species and superoxide dismutases: role in joint diseases. Joint Bone Spine 74:324–329
Amorati R, Foti MC, Valgimigli L (2013) Antioxidant activity of essential oils. J Agric Food Chem 61:10835–10847. doi:10.1021/jf403496k
Ashley NT, Weil ZM, Nelson RJ (2012) Inflammation: mechanisms, costs, and natural variation. Annu Rev Ecol Evol Syst 43:385–406. doi:10.1146/annurev-ecolsys-040212-092530
Ballabeni V, Tognolini M, Giorgio C, Bertoni S, Bruni R, Barocelli E (2010) Ocotea quixos Lam. essential oil: in vitro and in vivo investigation on its anti-inflammatory properties. Fitoterapia 81:289–295. doi:10.1016/j.fitote.2009.10.002
Boettger MK, Weber K, Schmidt M, Mieczyslaw G, Bräuer R, Schaible H (2009) Gait abnormalities differentially indicate pain or structural joint damage in monoarticular antigen-induced arthritis. Pain 145:142–150. doi:10.1016/j.pain.2009.06.006
Bolling BW, Ji LL, Lee C, Parkin KL (2011) Dietary supplementation of ferulic acid and ferulic acid ethyl ester induces quinone reductase and glutathione-S-transferase in rats. Food Chem 124:1–6. doi:10.1016/j.foodchem.2010.05.093
Bradley JR (2008) TNF-mediated inflammatory disease. J Pathol 214:149–160
Bradley PP, Priebat DA, Christensen RD, Rothstein G (1982) Mesurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol 78:206–209
Brieger K, Schiavonea S, Miller JRFJ, Krause K (2012) Reactive oxygen species: from health to disease. Swiss Med Wkly 142:1–14. doi:10.4414/smw.2012.13659
Brigelius-Flohé R, Kipp AP (2012) Physiological functions of GPx2 and its role in inflammation-triggered carcinogenesis. AnN N Y Acad Sci 1259:19–25. doi:10.1111/j.1749-6632.2012.06574.x
Broggi A, Granucci F (2015) Microbe- and danger-induced inflammation. Mol Immunol 63:127–133. doi:10.1016/j.molimm.2014.06.037
Calabrese V, Calafato S, Puleo E, Cornelius C, Sapienza M, Morganti P, Mancuso C (2008) Redox regulation of cellular stress response by ferulic acid ethyl ester in human dermal fibroblasts: role of vitagenes. Clin Dermatol 26:358–363. doi:10.1016/j.clindermatol.2008.01.005
Capaz FR, Vanconcellos LE, De Moraes S, Neto JP (1981) The open field: a simple method to show ethanol withdrawal symptoms. Arch Int Pharmacodyn Ther 251:228–36
Chillingworth NL, Donaldson LF (2003) Characterisation of a Freund’s complete adjuvant-induced model of chronic arthritis in mice. J Neurosci Methods 128:45–52
Cuzzocrea S, Mazzon E, Bevilaqua C, Costantino G, Britti D, Mazzullo G, De Sarro A, Caputi AP (2000) Cloricromene, a coumarine derivative, protects against collagen-induced arthritis in Lewis rats. Br J Pharmacol 131:1399–1407. doi:10.1038/sj.bjp.0703695
D’acquisto F, Maiuri MC, Cristofaro F, Carnuccio R (2001) Nitric oxide prevents inducible cyclooxygenase expression by inhibiting nuclear factor-κB and nuclear factor-interleukin-6 activation. Naunyn-Schmiedeberg’s Arch Pharmacol 364:157–165. doi:10.1007/s002100100435
Daniel AN, Sartoretto SM, Schmidt G, Caparroz-Assef SM, Bersani-Amado CA, Cuman RKN (2008) Anti-inflammatory and antinociceptive activities of eugenol essential oil in experimental animal models. Braz J Pharmacog 19(1B):212–217
Das K, Samanta L, Chainy GBN (2000) A modified spectrophotometric assay of superoxide dismutase using nitrite formation by superoxide radicals. IJBB 37:201–204
Dejam A, Hunter CJ, Schechter AN, Glaadwin MT (2004) Emerging role of nitrite in human biology. Blood Cells Mol Dis 32:423–429
Dinarello CA (2007) Historical review of cytokines. Eur J Immunol 37:S34–S45. doi:10.1002/eji.200737772
Domiciano TP, Dalalio MMO, Silva EL, Ritter AMV, Estevão-Silva CF, Ramos F, Caparroz-Assef SM, Cuman RKN, Bersani-Amado CA (2013) Inhibitory effect of anethole in nonimmune acute inflammation. Naunyn Schmiedebergs Arch Pharmacol 386:331–338. doi:10.1007/s00210-012-0820-5
Ellman GL (1959) Tissue sulphydril groups. Arch Biochem Biophys 82:70–77
Garrelly L, Bureau P, Labreque G (1991) Temporal study of carrageenan-induced PMN migration in mice. Agents Actions 33:225–228
Gautam R, Jachak SM (2009) Recent developments in anti-infammatory natural products. Med Res Rev 29:767–820. doi:10.1002/med.20156
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analyses of nitrate, nitrite and [15N]nitrate in biological fluids. Anal Biochem 126:131–138
Grishko VI, Ho R, Wilson GL, Pearsall AW (2009) Diminished mitochondrial DNA integrity and repair capacity in OA chondrocytes. Osteoarthr Cartil 17:107–113. doi:10.1016/j.joca.2008.05.009
Henrontin Y, Kurz B, Aigner T (2005) Oxygen and reactive oxygen species in cartilage degradation: friends or foes? Osteoarthr Cartil 13:643–654
Islam MS, Murata T, Fujisawa M, Nagasaka R, Ushio H, Bari AM, Hori M, Ozaki H (2008) Anti-inflammatory effects of phytosteryl ferulates in colitis induced by dextran sulphate sodium in mice. Br J Pharmacol 154:812–824. doi:10.1038/bjp.2008.137
Islam S, Yoshida H, Matsuki N, Ono K, Nagasaka R, Ushio H, Guo Y, Hiramatsu T, Hosoya T, Murata T, Hori M, Ozaki H (2009) Antioxidant, free radical–scavenging, and NF-κB–inhibitory activities of phytosteryl ferulates: structure–activity studies. J Pharmacol Sci 111:328–337
Jang B, Paik J, Kim S, Bae J, Mun K, Song D, Cho C, Shin D, Kwon TK, Park J, BaekW SM, Lee SH, Baek S, Lee I, Suh S (2004) Catalase induces the expression of inducible nitric oxide synthase through activation of NF-kB and PI3K signaling pathway in RAW 264.7 cells. Biochem Pharmacol 68:2167–2176
Jang B, Paik J, Kim S, Shin D, Song D, Park J, Suh M, Park J, Suh S (2005) Catalase induced expression of inflammatory mediators via activation of NF-nB, PI3K/AKT, p70S6K, and JNKs in BV2 microglia. Cell Signaling 17:625–633
Joshi G, Perluigi M, Sultana R, AgrippinoR CVD, Butterfield A (2006) In vivo protection of synaptosomes by ferulic acid ethyl ester (FAEE) from oxidative stress mediated by 2,2-azobis(2-amidino-propane) dihydrochloride (AAPH) or Fe2+/H2O2: insight into mechanisms of neuroprotection and relevance to oxidative stress-related neurodegenerative disorders. Neurochem Internat 48:318–327. doi:10.1016/j.neuint.2005.11.006
Kang P, Kim KY, Lee HS, Min SS, Seol GH (2013) Anti-inflammatory effects of anethole in lipopolysaccharide-induced acute lung injury in mice. Life Sci 93:955–961. doi:10.1016/j.lfs.2013.10.014
Kirchhoff C, Jung S, Reeh PW, Handwerker HO (1990) Carrageenan inflammation increases bradykinin sensitivity of rat cutaneous nociceptors. Neurosci Lett 111:206–210
Korkina L, Kostyuk V, De Luca C, Pastore S (2011) Plant phenylpropanoids as emerging anti-inflammatory agents. Mini Rev Med Chem 11:823–835
Lam J, Takeshita S, Barker JE, Kanagawa O, Ross FP, Titelbawm SL (2000) TNF-α induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J Clin Invest 106:1481–1488
Lee SH, Lee SY, Son DJ, Lee H, Yoo HS, Song S, Oh KW, Han DC, Kwon BM, Hong JT (2009) Inhibitory effect of 2′-hydroxycinnamaldehyde on nitric oxide production through inhibition of NF-kappaB activation in RAW 264.7 cells. Biochem Pharmacol 69:791–799. doi:10.1016/j.bcp.2004.11.013
Li C, Zhou H (2011) The role of manganese superoxide dismutase in inflammation defense. Enzyme Res 2011:01–06. doi:10.4061/2011/387176
Liz R, Vigil SVG, Goulart S, Moritz MIG, Schenkel EP, Fröde TS (2008) The anti-inflammatory modulatory role of Solidago chilensis Meyen in the murine model of the air pouch. J Pharm Pharmacol 60:515–521. doi:10.1211/jpp.60.4.0015
Martin SW, Stevens AJ, Brennan BS, Davies D, RowlandM HJB (1994) The six-day-old rat air pouch model of inflammation: characterization of the inflammatory response to carrageenan. J Pharmacol Toxicol Methods 32:139–147
McInnes IB, Schett J (2011) The pathogenesis of rheumatoid arthritis. N Engl J Med 365:2205–19. doi:10.1056/NEJMra1004965
Michaluart P, Masferrer JL, Carothers AM, SubbaramaiahK ZBS, Koboldt C, Mestre JR, Grunberger D, Sacks PG, Tanabe T, Dannenberg AJ (1999) Inhibitory effects of caffeic acid phenethyl ester on the activity and expression of cyclooxygenase-2 in human oral epithelial cells and in a rat model of inflammation. Cancer Res 59:2347–2352
Min SS, Han JS, Kim YI, Na HS, Yoon YW, Hong SK, Han HC (2001) A novel method for convenient assessment of arthritic pain in voluntarily walking rats. Neurosci Lett 308:95–98
Mollace V (2005) Modulation of prostaglandin biosynthesis by nitric oxide and nitric oxide donors. Pharmacol Rev 57:217–252
Morgan MJ, Liu Z (2011) Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res 21:103–115. doi:10.1038/cr.2010.178
Muller AA, Reiter SA, Heider KG, Wagner H (1999) Plant-derived acetophenones with antiasthmatic and antiinflammatory properties: inhibitory effects in chemotaxis, right angle scatter and actin polimeryzation of polymorphonuclear granulocytes. Planta Med 65:590–594
Nagasaka R, Chotimarkorn C, Shafiqul I, Hori M, Ozaki H, Ushio H (2007) Anti-inflammatory effects of hydroxycinnamic acid derivatives. Biochem Biophys Res Commun 358:615–619
Nantel F, Denis D, Gordon R, NortheyA CM, Metters KM, Chan CC (1999) Distribution and regulation of cyclooxygenase-2 in carrageenan-induced inflammation. Br J Pharmacol 128:853–859
Niki E, Yoshida Y, Saito Y, Noguchi N (2005) Lipid peroxidation: mechanisms, inhibition, and biological effects. Biochem Biophys Res Commun 338:668–676
Ohkawa K, Ohishi N, Yagi K (1979) Assay of lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Parise-Filho R, Pastrello M, Camerlingo CEP, Silva GJ, Agostinho LA, Souza T, Magri FMM, Ribeiro RR, Brandt CA, Polli MC (2011) The anti-inflammatory activity of dillapiole and some semisynthetic analogues. Pharm Biol 49:1173–1179. doi:10.3109/13880209.2011.575793
Perluigi M, Joshi G, Sultana R, Calabrese V, De Marco C, Coccia R, Cini C, Butterfield A (2006) In vivo protective effects of ferulic acid ethyl ester against amyloid-beta peptide 1–42-induced oxidative stress. J Neurosci Res 84:418–426
Pulli B, Ali M, Forghani R, Schob S, Hsieh KLC, Wojtkiewicz G, Linnoila JJ, Chen JW (2013) Measuring myeloperoxidase activity in biological samples. PLoS ONE 8:01–10. doi:10.1371/journal.pone.0067976
Rayhana B, Sheliya MA, Pillai KK, Aeri V, Sharma M (2014) Evaluation of anti-inflammatory effect of Careya arborea in CFA induced chronic inflammation. Int J Pharm Sci Rev Res 26:292–298
Ritter AMV, Domiciano TP, Verri WAJ, Zarpelon AC, Silva LG, Barbosa CP, Natali MRM, Cuman RKN, Bersani-Amado CA (2013) Antihypernociceptive activity of anethole in experimental inflammatory pain. Inflammopharmacology 21:187–197. doi:10.1007/s10787-012-0152-6
Romitelli F, Santini AS, Chierica E, Pitocco D et al (2007) Comparison of nitrite/nitrate concentration in human plasma and serum samples measured by the enzymatic batch Griess assay, ion-pairing HPLC and ion-trap GC–MS: the importance of a correct removal of proteins in the Griess assay. J Chromatogr B Analyt Technol Biomed Life Sci 851:257–267
Sá RCS, Andrade LN, Oliveira RRB, Sousa DP (2014) A review on anti-inflammatory activity of phenylpropanoids found in essential oils. Molecules 19:1459–1480
Salvemini D, Wang Z, Bourdon DM, Stern MK, Currie MG, Manning PT (1996a) Nitric oxide: a key mediator in the early and late phase of carrageenan-induced rat paw inflammation. Br J Pharmacol 118:829–838
Salvemini D, Wang Z, Bourdon DM, Stern MK, Currie MG, Manning PT (1996b) Evidence of peroxynitrite involvement in the carrageenan-induced rat paw edema. Eur J Pharmacol 303:217–220. doi:10.1016/0014-2999(96)00140-9
Salvemini D, Little JW, Doyle T, Neumann WL (2011) Roles of reactive oxygen and nitrogen species in pain. Free Radic Biol Med 51:951–966. doi:10.1016/j.freeradbiomed.2011.01.026
San Miguel SM, Opperman LA, Allen EP, Zielinski J, Svodoba KKH (2012) Bioactive polyphenol antioxidants protect oral fibroblasts from ROS-inducing agents. Arch Oral Biol 57:1657–1667. doi:10.1016/j.archoralbio.2012.04.021
Scapagnini G, Butterfield A, Colombrita Cl, Sultana R, Pascale A, Calabrese V (2004) Ethyl Ferulate, a Lipophilic Polyphenol, Induces HO-1 and Protects Rat Neurons Against Oxidative Stress. Antioxid. Redox Signal. 6:811–818
Sousa DP (2011) Analgesic-like activity of essential oils constituents. Molecules 16:2233–2252. doi:10.3390/molecules16032233
Sultana R (2012) Ferulic acid ethyl ester as a potential therapy in neurodegenerative disorders. Biochim Biophys Acta 1822:748–752. doi:10.1016/j.bbadis.2011.10.015
Surh Y (2008) NF-κB and Nrf2 as potential chemopreventive targets of some anti-inflammatory and antioxidative phytonutrients with anti-inflammatory and antioxidative activities. Asia Pac J Clin Nutr 17:269–272
Takahara S, Hamilton BH, Nell JV, Ogura Y, Nishimura ET (1960) Hypocatalasemia, a new genetic carrier states. J Clin Invest 29:610–619
Talwar S, Nandakumar K, Nayak PG, Bansal P, Mudgal J, Mor V, Rao CM, Lobo R (2011) Anti-inflammatory activity of Terminalia paniculata bark extract against acute and chronic inflammation in rats. J Ethnopharmacol 134:323–328. doi:10.1016/j.jep.2010.12.015
Tonussi CR, Ferreira SH (1994) Mechanism of diclofenac analgesia: direct blockade of inflammatory sensitization. Eur J Pharmacol 251:173–179
Turek C, Stintzing FC (2012) Stability of essential oils: a review. Compr Rev Food Sci Food Saf 12:40–53. doi:10.1111/1541-4337.12006
Vogt T (2010) Phenylpropanoid biosynthesis. Mol Plant 03:2–20. doi:10.1093/mp/ssp106
Winter CA, Risley EA, Nuss GW (1962) Carrageenin-induced oedema in hind paw of the rats as an assay for anti-inflammatory drugs. Proc Soc Exp Biol Med 111:544–551
Woodruff TM, Strachan AJ, Dryburgh N, Shiels IA, Reid RC, Fairlie DP, Taylor SM (2002) Antiarthritic activity of an orally active C5a receptor antagonist against antigen-induced monarticular arthritis in the rat. Arthritis Rheum 46:2476–2485
Yin L, Yang Y, Wang M, Zhang M, Duan J (2012) Effects of syringin from Phellodendron chinensis on monosodium urate crystal-induced inflammation and intercellular adhesion molecule-1 (ICAM-1) expression. African J Pharm Pharmacol 6:1515–1519. doi:10.5897/AJPP12.081
Yu Y, Xiong Z, Lv Y, Qian, Y, Jiang S, Tian Y (2006) In vivo evaluation of early disease progression by X-ray phase-contrast imaging in the adjuvant-induced arthritic rat. Skelet Radiol 35:156–164
Zhang L, Al-Suwayeh SA, Hsiehc P, Fang J (2010) A comparison of skin delivery of ferulic acid and its derivatives: evaluation of their efficacy and safety. Int J Pharm 399:44–51. doi:10.1016/j.ijpharm.2010.07.054
Acknowledgments
Our thanks to Federal University of Piauí (UFPI), the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for their financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cunha, F.V.M., Gomes, B.d.S., Neto, B.d.S. et al. Ferulic acid ethyl ester diminished Complete Freund’s Adjuvant-induced incapacitation through antioxidant and anti-inflammatory activity. Naunyn-Schmiedeberg's Arch Pharmacol 389, 117–130 (2016). https://doi.org/10.1007/s00210-015-1180-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-015-1180-8