Abstract
The biorefinery approach ensures a sustainable source of valuable fatty acids and opens up new avenues for their application in healthcare industries. Recent studies highlight the health benefits of omega-PUFAs, spurring the search for cost-effective production methods. Microbial platforms are promising for high-yield PUFA production, with ω-3 dominating the market. ω-3 PUFAs offer antioxidant and anti-inflammatory effects, reducing illness risk, while all PUFAs contribute to cardiovascular health, diabetes prevention, cancer risk reduction, and more. ω-6 PUFAs, particularly linoleic acid (LA) and arachidonic acid (ARA), play vital roles in various aspects of health, making them high-demand bioavailable compounds. Additionally, docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) exhibit potential benefits in brain development and COVID-19 prevention. This comprehensive review provides insights into the state-of-the-art microbial biorefinery strategies for ω-3 and ω-6 PUFA production and their wide-ranging health-related benefits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With growing health concerns, the urban population is greatly inclined to a healthy lifestyle, which includes functional foods and healthy food supplements in their daily diets to lower their common health risks related to nutrition and age. Among several healthy diets, essential (omega) ω-fatty acid consumption is rising with increasing awareness and benefits offered by the ω-3 and ω-6 group of fatty acids [112, 116, 117, 119, 122, 123, 126]. The human body lacks some genes, including fatty acid desaturase 2 (FADS2), Δ5-desaturase, and Δ12-desaturase, which help convert lipids into essential ω-3 and ω-6 polyunsaturated fatty acids (PUFAs). Due to this, they cannot convert lipids into essential PUFAs, which must be obtained from their diet [42, 62]. These essential fatty acids have a crucial role to produce prostaglandins, leukotrienes, and thromboxanes by involving a cyclooxygenase, lipoxygenase, and CYP450 enzymes. These enzymes functioning would be compromised without a source of arachidonic acid which commonly have a significant impact on many regular metabolic processes [68]. Therefore, they must be obtained from daily diet to overcome the complexes and illnesses caused by the deficiency. Thus, both the above-mentioned ω-groups are called essential fatty acids.
Essential fatty acids
The ω-3 and ω-6 fatty acids cannot be produced by the human body in sufficient levels, therefore, they are essential to obtain from the diet, and are referred to by the term "essential fatty acids" (EFA). EFA refers to PUFA and is classified into two types: ω-3 and ω-6 fatty acids [43]. EFA are the components of lipids and consist of hydrocarbon chains with carboxyl groups at one end and methyl groups at the opposite end. Different types of fatty acids can be identified based on criteria such as length, number, and position of double bonds in the hydrocarbon chain. The presence of double bonds defines the saturation and unsaturation of fatty acids. If there is one double bond, it is known as monounsaturated fatty acids or MUFAs, and if two or more double bonds exist, it is termed PUFA [99]. PUFAs play an important role in human nutrition, as ω-3 fatty acids carry a double bond between C-3 and C-4 and ω-6 fatty acids carry a double bond between C-6 and C-7 from the methyl end of the fatty acid chain. Based on the double bond position, α-linolenic acid (ALA), eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA), and docosahexaenoic acid (DHA) are placed in the ω-3 fatty acid pool, whereas mainly linoleic acid (LA), γ-arachidonic acid (ARA), and linoleic acid (GLA) are placed in the ω-6 fatty acids pool [112, 116, 117, 119, 122, 123, 126]. Many scientists have classified PUFAs based on their carbon chain length. A long chain PUFA consists of 20–24 carbon atoms, and a very long chain contains ≥ 26 carbon atoms. Moreover, notation is very crucial to nominate any naturally occurring fatty acids or unsaturated fatty acids; “n-minus” is commonly used as notation. It designates the position of the first double bond that is closest to the methyl group of the hydrocarbon chain. For example, linoleic acid is designated as C18:2n-6 since the first double bond is present at carbon 6 atoms from the methyl end, but with the help of this nomenclature, the position of the remaining double bonds present in the molecular structure cannot be specified [131]. The ω-PUFAs from fish and microbial sources are mainly DHA and EPA, both of which are more readily bioavailable than PUFAs (mainly ALA from plant sources upon consumption). Plant LA and ALA are relatively less bioavailable, and hence animal sources mainly fish are preferred. A study shows that microalgal PUFAs are the main source of fish-derived PUFAs [75]. Microalgae and thraustochytrids can accumulate lipid 35–70% of their dry biomass in which 15–29% DHA is reported [25,26,27]. Hence, developing the microbial platform for PUFA production is the current research’s major focus. The biorefinery concept is a sustainable approach to maximize the value obtained from biomass resources while reducing waste. It optimizes resource utilization by extracting value from multiple fractions of biomass and involves the integrated processing of various biomass feedstocks to produce a range of valuable products, including PUFAs, from different fractions of biomass [3]. This environmentally sustainable approach aligns with the principles of the circular economy, where resources are used efficiently.
ω-3 and ω-6 PUFA and health prospects
Since humans lack enzymes that support the production of ω-3 and ω-6 PUFA, it is a must to obtain them from their diet. It is important to find rich sources of these EFA. Both ω-3 and ω-6 fatty acids are possessed in numerous plant and animal products at varying levels such as chia seed, flaxseed, sunflower oil, walnuts, kidney beans, leafy greens, fish, pork, and beef. [46]. Buglossoides arvensis is the richest natural plant-based source and is considered an emerging oilseed crop for nutritional ω-3 fatty acid [105]. There are four major ω-3 fatty acids: alpha-linolenic acid (ALA-C18:3n3), stearidonic acid (SDA-C18:4n3), eicosapentaenoic acid (EPA-C20:5n3), and docosahexaenoic acid (DHA-C22:6n3), which are long-chain fatty acids and EFA [164]. During the last decades, many studies have recommended the positive health effects of consuming EPA and DHA in the diet, such as reducing the weakening of eyesight, lowering the risk of blood vessel stiffness, and bringing down the risk of anxiety and inflammation. It has also been observed that low DHA content in the diet causes many psychological problems [7]. Recent research has established that the consumption of seed oils rich in SDA offers superior nutritional benefits compared to ALA, primarily due to the more efficient conversion of SDA to EPA in the human body. SDA serves as a highly effective alternative precursor for the synthesis of ω-3 LC-PUFAs because it circumvents the rate-limiting step in EPA production. Moreover, recent studies have uncovered the major plant sources of SDA and its potential health advantages, which include preventive effects on inflammation, cardiovascular disease, and cancer [132]. Moreover, the three ω-6 fatty acids are also important in health prospects such as linoleic acids (LA-C18:2n-6), gamma-linolenic acid (GLA-C18:3n6), and arachidonic acids (ARA-C20:4n-6). Among them, LA and ARA help in blood clotting, wound healing, cholesterol maintenance, membrane fluidity, and hypertension, as well as lowering the risk of vision loss and improving bone and sperm quality [39]. To avoid complications and maintain good health, and a balanced metabolic process of the body, a ratio of 1:1–4:1 between ω-6 and ω-3 is recommended. The proportion of both ω-3 and ω-6 required in the diet is 16–17 g/day and 11–12 g/day for males and females, respectively; however, western diets possess rather high ω-6 (> 4:1), which causes several health risks. If the final product ALA and ARA is eicosanoids, it may cause contrasting effects on health. It mainly causes inflammatory diseases such as obesity. Obtaining a high amount of ω-6 in diets may cause pro-inflammatory diseases such as arthritis and atherosclerosis, leading to the narrowing of blood vessels known as vasospasm, causing an increase in blood pressure [46].
A balanced diet is crucial for human health, as it helps to prevent many adverse health issues. Both ω-3 and ω-6 PUFA help to maintain numerous biological functions, such as reproductive, physiological, and developmental. These PUFAs are the main energy source and have a rich caloric value. EFA and liposoluble bioactive compounds are the most important components of vegetable oils such as soybean oil, sunflower oil, mustard oil, and olive oil [5]. Botanical organisms, microalgae, and microorganisms constitute the primary origins of these fatty acids. The escalating demand for oils and fats in recent times has prompted the food industry to explore alternative sources for these essential components. [40]. According to research, Amazonian fruits are high in bioactive components, particularly unsaturated fatty acids, carotenoids, and sterols. It has been observed that high levels of linoleic acid are mainly found in passion fruit seeds and Brazil nut oil [143]. There are some natural producers, such as marine microalgae, yeast, bacteria, diatoms, and phytoplankton, and protists such as thraustochytrids that can produce DHA, EPA, and ARA. For obtaining high lipid content, scientists mainly focused on the thraustochytrid genus Thraustochytrium, Aurantiochytrium, and Schizochytrium, a single-celled eukaryotic organism found in the marine environment and with the potential to produce lipids and carotenoids [26, 87]. In thraustochytrids, the elongase–desaturase pathway and polyketide-like synthase pathway are the two important routes actively involved to produce PUFAs. The biosynthesis of PUFAs was carried out by various pathways in different species of thraustochytrids, some of which used the elongase–desaturase pathway, while others used the polyketide-like synthase (PKS) pathway, and others used both, e.g., Schizochytrium mangrovei PQ6 [41]. In fact, the PKS pathway is more efficient as it requires 14 NADPH molecules, while the elongase–desaturase pathway requires 26 NADP molecules to produce fatty acids [41]. In recent studies, researchers mainly focus on metabolic engineering techniques, and with the help of these techniques they can improve the amount of the target product [61]. In response to commercial demands and considerations regarding health-related requirements, microalgae have emerged as a viable alternative source of polyunsaturated fatty acids (PUFAs). Consequently, their potential in mitigating chronic diseases has garnered attention, primarily due to the substantial PUFA yields offered by microalgae in comparison to other microbial sources [83, 148]. Scientists primarily focus on large-scale nutritional PUFA production from various sources to prevent many heart-related diseases, inflammatory diseases, obesity, vasospasm, diabetes, etc. [21].
Emerging research trends on microbial ω-PUFA production
To understand the co-occurrence of specific keywords in the field of emerging omega and their production by using the microbial platform, the following terms were searched: “microorganism”, “polyunsaturated fatty acid”, and “omega” in the Scopus database. These terms were searched in the article title, keywords, and abstract; moreover, the timeline was limited to the years 2017–2022. The published document type was research article, review article, and book chapter. The language selected as English in scopus; and articles in the final publication stage were selected and a total of 116 were retrieved and saved in comma-separated values (CSV) Excel format. The data were further analyzed for duplicate entries and incomplete entries. Thereafter, all 116 entries remained and were named dataset A.
Subsequently, the dataset was analyzed for duplicate entries and finalized and analyzed in VOSviewer for the co-occurrence of related index keywords under the above-mentioned combined dataset. The minimum number of occurrences of a keyword was 5 for all 2309 keywords found with 144 meeting thresholds. Due to fewer occurrences of novel index keywords, the threshold for keyword occurrence was set to two, i.e., if any keyword was repeated two or more times, it would be considered for analysis. A total of 2309 index keywords were listed and out of these, the top 140 keywords were selected based on their total link strength. Thereafter, the remaining keywords were analyzed, and most related keywords were manually screened and selected based on the relatedness to our interests and focus. A total of 140 keywords were selected and classified into five clusters, where clusters 1, 2, 3, 4, and 5 had 40, 33, 33, 28, and 6 items in descending order. Based on this analysis, Fig. 1 shows the dataset’s co-occurrence network of specific keywords. The larger dots in the figure show popular terms which have been greatly used in past works and smaller dots show emerging platforms to produce ω-PUFAs. ω-3 fatty acid, polyunsaturated fatty acid, and microorganisms were the dominant models for ω production. Microalgae, fatty acid, DHA, ω-6, and EPA model were extensively used in Fig. 1.
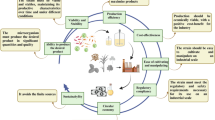
These datasets indicate the increasing research trend and awareness toward polyunsaturated fatty acid groups, mainly ω-3 fatty acid-containing diets for maintaining a healthy lifestyle. The larger dots (red dots) represent the microorganisms that comprise the most fascinating platform for omega production, which mainly consist microalgae and macroalgae groups, followed by smaller dots of emerging Schizochytrium thraustochytrid. Moreover, research trends illustrate that the biosynthesis and metabolism aspects of these microbes are adequately covered. Based on the above search, the current article reviews research advances on dietary PUFA sources and current updates on PUFA production from potential microorganisms, mainly thraustochytrids, and microalgae groups as emerging alternative platforms that are promising among other groups reported for PUFA production in reasonable quantities such as fungi, yeasts, and bacteria. However, the demand for PUFAs has been on the rise, driven by increasing consumer awareness of their health benefits. Microbial biorefinery approaches have emerged as promising solutions due to their potential for high yield and scalability while minimizing environmental impact. This article seeks to provide valuable insights into sustainable PUFA production and its applications in healthcare industries, addressing a critical need for both scientific knowledge and practical solutions in the fields of biotechnology and health. Especially, it includes a specific ω-PUFA role for the development of bone, skin, eye, brain, and fetus development and to cure several diseases such as CVDs, diabetes, cancer, and hypertension. The overview of natural sources and microbial bioprocess of PUFAs along with their health beneficiary effect on human life is schematically shown in Fig. 2.
The growing microbial platform for ω-PUFA production
The demand for ω-PUFAs as a necessary diet is growing because of their leading role in health promotion. Under the suggested daily nutritional design for managing health, regular essential PUFA uptake of 0.2–0.3 g seems quite promising. Prevention is better than cure is an emerging mindset for urban lives who cannot afford adequate time for collecting and consuming several essential food types for essential nutrients. The microbial platforms are alternate greater PUFA sources that must be developed due to rising demand, notably for vegetarians. The microbial platform could be promising for developing health supplements and functional food to fulfill essential fatty acid requirements and bypass the health risks due to deficiencies. Several oleaginous microbes such as thraustochytrids, microalgae, bacteria, yeast, and fungu, are reported for high PUFA and offer a promising supply of ω-3 and ω-6 PUFAs. Somewhere, diatoms and phytoplanktons are also found to be PUFAs-producing organisms. Due to their great capacities for substrate consumption and lipid production, as well as their rapid growth rates, many oleaginous microorganisms are exploited for lipid and PUFAs production [10, 112, 116, 117, 119, 122, 123, 126]
Thraustochytrids
In recent years, thraustochytrids have served as commercial bio-factories for ω-3 and ω-6 PUFAs [25, 26]. In the eukaryotic group of marine protists, thraustochytrids are non-photosynthetic creatures possessing monocentric thalli with an ectoplasmic net and biflagellate zoospores. They can be found globally in habitats like seawater, seagrass, coral reefs, estuaries, mangrove forests, sediment, and oceans [69]. Numerous thraustochytrid strains have beeen specifically introduced for high lipid content and ω-PUFAs. Notable strains include Thraustochytrium ANVKK, Schizochytrium mangrovei and Thraustochytrium kinnei, all recognized for their remarkable lipid content, ranging from 40% to 71.2%. In particular, Schizochytrium mangrovei stands out for its rich ω-3 (DHA) up to 2.8 g/L [112, 116, 117, 119, 122, 123, 126]. To improve the DHA purity, the metabolic engineering technique was used in some studies such as in Aurantiochytrium. Through the disruption of one copy of the fatty acid synthase gene, the competitive pathway of DHA biosynthesis is partially deactivated. Acetyl-CoA carboxylase and diacylglycerol acyltransferase are overexpressed, boosting the substrate supply and triacylglycerol production. This method produced a final mutant with a 61% DHA purity in total PUFAs [168]. Several other wild-type strains also exhibited high ω-3 PUFA production from this group of protists [112, 116, 117, 119, 122, 123, 126]. Thraustochytridhis group is the most fascinating due to their fast growth rate and highly oleaginous nature (up to 70% total lipid accumulation) of biomass with approx. 30% ω-3 PUFA contents [25,26,27]. However, there is lack of reports to confirm their ability to produce ω-6 PUFA [112, 116, 117, 119, 122, 123, 126].
Microalgae
Microalgae are a growing research area for ω-3 and ω-6 biosynthesis and a fascinating platform based on PUFA productivity, scale-up potential, and other sustainable features. The latest cultivation method improves three to five times the biomass and five to ten times the product yields [115, 149] (Choi et al. 2019); however, its advantages are not limited to a wide range of products to offset cultivation and harvesting costs [111, 116,117,118,119, 121, 124, 126, 127] (Patel et al. 2022g), but widely covers high CO2 capturing ability, bioremediation ability, greenhouse gas emission (GHGs) reduction, carbon footprint improvement, etc. [109, 113, 122, 123, 125]. Emerging nanobubble, nanoparticle, and other existing auxiliary technologies can further enhance this platform’s performance [117, 120]. The four microalgae species, Chlamydomonas variabilis, Chlorella vulgaris, Haematococcus pluvialis, and Spirulina platensis, are recognized for producing essential PUFAs. The findings disclosed that Chlorella vulgaris has significant lipid content with 21.17% of ω-3, whereas C. variabills has the highest concentration of ω-6 (30.24%) and the highest lipid (21%), respectively. Spirulina platensis, an important species of cyanobacteria, has the highest percentage of total lipids (15.8%) and ω-3 PUFAs (4.9%). In contrast, Haematococcus pluvialis, a bio-platform that can generate the highest percentage of ω-6 PUFAs (14.83%), has the lowest percentage of total lipid (10%) [140]. H. pluvialis increased the generation of 31% ω-3 content by improving CO2 capturing and lipid productivities through Ca-driven membrane fluidity adaptation and biomineralization [175]. By introducing the 5-elongase from the green algae Ostreococcus tauri into the host cells, Phaeodactylum tricornutum was genetically modified to accumulate the high-value ω-3 long-chain polyunsaturated fatty acid docosahexaenoic acid (DHA). The amount of DHA increased eightfold due to heterologous elongase expression [58]. Table 1 provides detailed information on various groups of microalgae efficient in both ω-3 and ω-6 PUFA production.
The most promising platform for producing ω-6 PUFAs is fast-growing oleaginous microalgae such as Porphyridium purpureum. They are also effective producers of ω-3 PUFAs. Commercial models are primarily fungi now, but microalgae are becoming increasingly popular because of fast-growing strain development, mixotrophic cultivation for supporting additional biomass, and their sustainable properties such as carbon capturing, GHG emission reduction, O2 evolution, and carbon footprint [157].
Bacteria
Bacteria are not thought to be effective producers of EFAs when compared to microalgae and fungi. However, some barophilic and psychrophilic organisms found in the ocean depths, such as members of the Shewanella genus, have been shown to be significant PUFA producers [145]. Acinetobacter sp., Rhodococcus sp., Gordonia sp., Arthrobacter sp., Kocuria sp., and Bacillus alcalophilus are a few notable genera of oleaginous bacteria. Oleaginous bacteria are also a rich source of TAGs. Rhodococcus sp. has received the most attention among them due to its capacity to flourish on various substrates. R. opacus PD630 has been reported for lipid content of 70% w/w using the substrate as dextrose and dairy wastewater [75, 109, 113, 114]. Colwellia strains, heterotrophs occurring in cold habitats, produce DHA, up to 17% of TFAs. DHA concentrations in Colwellia psychrerythraea 34H were raised by the addition of cerulenin, an inhibitor of FAS. Another deep-sea bacterium, Shewanella sp., amassed enough EPA to account for up to 36% of TFAs. Shewanella electrodiphila MAR441 produced more EPA on adding cerulenin, and chemical mutagenesis increased production at low temperatures. Aetherobacter sp. produced all major PUFAs in terrestrial myxobacteria, whereas Sorangium cellulosum only produced LA, and Phaselicystis flava was enriched in ARA. Several studies have also demonstrated the viability of manufacturing PUFAs in heterologous bacteria such as Escherichia coli, lactic acid bacteria, and Pseudomonas putida. Still, the output did not exceed milligram levels [72].
Moreover, for suitability of lipid fraction, their fatty acid content from oleaginous bacteria is preferable for biofuels due to less PUFA contents and generation from waste fraction. Moreover, the fascinating part is that it can effectively biotransform high-carbon wastes into lipids. Certain chemolithotrophic bacteria use CO2 as a carbon source and generate large amounts of extracellular lipids. Unfortunately, the fraction still represents an inadequate essential ω-PUFA fraction compared to the microalgae group and hence can be used to make biodiesel and reduce CO2 emissions effectively [80].
Yeast and fungi
Due to the faster ability of carbon utilization, effective lipogenesis, the ability of higher lipid production, and the capability to adopt genomic alterations to have prevailing micro-factories, oleaginous yeasts have emerged as the utmost advantageous models for lipid and PUFA production among several investigated microbes [161]. Several yeasts are reported for PUFAs production such as Rhodotorula, and Rhodosporidium genera. In contrast, the most prominent novel yeast that can store plenty of PUFAs (largely EPA) in cells is Yarrowia lipolytica. Many biotechnological approaches have been applied to Y. lipolytica for enhancement of PUFAs by optimizing and reconstructing the pathways, such as inhibiting the difficult pathway, enhancing the supply of precursors for NADPH and acetyl CoA, suppressing the β-oxidation metabolic pathway, manipulating a gene responsible for the metabolism of fatty acids, etc. These modifications were made by using gene editing technology called CRISPR/Cas-9 in Y. lipolytica to increase the generation of PUFA. Two yeasts, Lodderomyces elongisporus, and Rhodotorula mucilaginosa, have also been capable of producing high ω-fatty acids. Yeast models are promising for genomic and omics studies with adequate genomic information. The major disadvantage is that they are less oleaginous (30–40%) than thraustochytrids and microalgae (30–70%), and hence reported for less total essential PUFA yield. Therefore, other high-yielding microbial platforms are more attractive for genomic alteration and enhance PUFA production [70, 112, 116, 117, 119, 122, 123, 126].
Fungi constitute a significant alternative source of microbial oils that are now being researched. Zhao et al. [182] found several fungal strains among 669 strains that are capable of PUFAs. A promising phylum for producing microbial-rich oil that contains essential PUFAs is the Mucoromycota. Genus Backusella has been observed to have higher oil accumulation rates of up to 59.08 ± 2.24%, Pilaria is known to synthesize a high LA, while Rhizopus and Thamnostylum synthesize normally greater GLA. An excellent fungus model that produces a lot of ARA is Mortierella alpina. Numerous fungus genera such as Trichosporon, Cryptococcus, and Lipomyces, and Yarrowia have been found to be a good source of PUFAs generation. Various strains of Mortierella alpina were promising in higher essential PUFA production, according to a recent study [28]. Out of the three strains they looked at, M. alpina has the maximum total lipid (around 26%) and ARA ratio (about 35%). Higher ARA, dihomo-γ-linolenic acid (DGLA), and EPA fractions were examined in M. Alpina. Mucor circinelloides, an oleaginous filamentous fungus, has gained attention for its superior ability to produce and store lipids like large amounts of GLA [47]. The lipid yield of the fungal system was improved by adding nanoparticles. M. alpine, an oleaginous fungus, has been studied for its use in enhancing the lipid fraction (by increasing the glucose intake) rich in high ARA. In a recent work, the Mg2+ ions act as cofactors for numerous enzymes involved in the induction of lipids [95]. Promising results were found on the effect of TiO2 nanoparticles in a fungus Pichia pastoris for increased synthesis of total lipid and PUFA. The model yeast experiences certain stress due to the mild toxicity of the TiO2 nanoparticles, which leads to vacuolic membrane penetration, cellular membrane damage, and cell wall destruction by reactive O2 radicals. To combat the pressures, these circumstances also caused a larger buildup of ω-6 PUFA besides total lipid content [176]. MgONPs may undoubtedly be tested to increase lipid production in thraustochytrid strains and oleaginous algae, as both organisms are eukaryotic, like fungus. The use of TiO2 nanoparticles was directly investigated in the yeast expression model to improve total lipid and GLA fractions. The above work also supported the impact of TiO2 on the upregulation of lipid-related genes, particularly those involved in GLA production [112, 116, 117, 119, 122, 123, 126].
Although some oleaginous fungi are promising in PUFA content, total lipid content is not competitive with the microalgae and thraustochytrid groups. Oleaginous fungi are promising to produce ω-6 PUFA (ARA and GLA); however, biofuel can be produced from remaining saturated and monosaturated fatty acids fraction. They also slightly produce EPA and DHA fractions. The drawback of fungi are their often long development periods and the need of an organic carbon source as compared to CO2-utilizing phototrophic microbes [146].
Diatoms and phytoplankton
Many more species of diatoms and phytoplankton are significant producers of PUFAs. Phaeodactylum tricornutum, a photosynthetic diatom, may accumulate ω-3 fatty acids through the metabolic pathway. P. tricornutum naturally assembles up to 36% EPA in its total fatty acids (TFAs). A mutant strain that accumulated 36.5% EPA and 23.6% DHA, both reported as fractions of the TFAs, was created by the simultaneous introduction of glucose transporter and the 5 elongase. As further evidenced, endogenous 6-desaturase overexpression improved EPA selectivity. With a large increase in TAGs components (15.2% EPA, 7.5% ARA, and 1.2% DHA), the total lipid content was increased by 1.8 times [72]. Hamilton et al. [58] increased the endogenous fatty acid pathway by expressing δ-5-elongase from O. tauri. When heterologous δ-5-elongase was expressed in P. tricornutum, the concentration of DHA increased eightfold and the overall number of fatty acids increased by 10.4%, which is a useful and important modification in the PUFA profile of this algae. Later, in the subsequent round of metabolic engineering, the co-expression of acyl CoA-driven δ-6-desaturase and δ-5-elongase led to an even greater rise in DHA contents (Jakhwal et al. 2022). According to reports, the marine oleaginous diatom Fistulifera solaris grown under photoautotrophic conditions can produce large amounts of EPA. F. solaris was found to produce 135.7 mg/L/day of EPA under optimal production circumstances, which is on par with the output of heterotrophic cultivation. In contrast, it has been noted that the addition of glucose for the heterotrophic growth of Nitzschia laevis produced EPA at a rate of 174.6 g/L/day [109, 113, 114].
The microscopic primary producers known as phytoplankton play a key role in the transformation such as the cycling of energy and biomolecules in aquatic food webs. For the benefit of consumers, it produces PUFA. While all phytoplankton taxa can produce shorter-chain ω-3 and ω-6 PUFA, only specific phytoplankton taxa can produce EPA and DHA [163]. A meta-analysis of more than 160 fatty acid profiles from seven different marine phytoplankton phyla demonstrates that marine phytoplankton produces PUFAs that are not only phylum specific, but also substantially class specific. Dinophyta and the Haptophyte Emiliana huxleyi exhibit the highest production of DHA, whereas the two groups of Haptophyta and Ochrophyta produce the highest amounts of EPA relative to total fatty acids. High concentrations of an essential EPA and stearidonic acid (SDA) precursor are observed in Cryptophyta and the Chlorophyta class Pyramimonadophyceae [71]. Table 1 summarizes the ω-3 and ω-6 PUFAs content along with total lipid determined in different microorganisms’ dried biomass. Omega content can vary depending on the specific biomass source and its processing method. Omega-3 fatty acids include EPA, DHA, and ALA, and their content may be different in each source. This environmentally sustainable approach aligns with the principles of the circular economy, where resources are used efficiently. In the context of PUFA production, it allows for the generation of not only valuable fatty acids, but also a range of co-products with economic and environmental benefits which could be further used for bio-oil production [108].
ω-6 PUFAs role in human health management
ω-6 and the brain, blood cholesterol, and CVDs
For normal growth and development of the human body, LA is a necessary polyunsaturated fatty acid. During the 1930s, western diets was popular for PUFA rich food due to agricultural shifts. Many researches found that high cultivation of soybean and corn oil are rich in ω-6 PUFAs [5]. It has been observed that ARA plays an essential role in neurodevelopment and many other physiological processes [152]. LA is also known as oxidized LA metabolites (OXLAMs), as it is a precursor to oxidized products. OXLAMs are lipid moderators that aid in the maintenance of peripheral tissue function, as well as the regulation of pain and inflammatory signaling [134]. The association between food and sickness is an important factor in identifying diverse diseases in the human body, as PUFAs in the diet are linked to neurodegenerative diseases. Intake of ARA, ALA, EPA, and DHA in the diet aids in preventing neural cell death, also known as neurodegeneration [165]. Several studies show the role of LA in breast milk. LA is an important component for developing infants, as it accumulates in the mother’s breast milk, a primary source from which the infant gets nutrition during the first few months. However, some researchers discovered an inverse relationship between breast milk and linoleic acid. Some believe that if the consumption of LA is not in the proper amount, there is a high chance of reduced motor control in the age group of 2- to 3-year-old infants and also loss of verbal IQ at the age of 5–6 years [162]. Brain tissues are highly rich in PUFAs, such as ARA and DHA, which are essential constituents of the phospholipid membrane. The mammalian brain comprises approx. 20% ARA and DHA [129]. COX2, cyclooxygenase-2, accelerates the production of thromboxane, levuloglandins, and prostaglandins, but the overexpression of COX2 causes neural damage and various brain injuries, which result in the interruption of the cell membrane and activation of phospholipases, leading to the release of ARA from the membrane. ARA, then converted into eicosanoids, produces reactive oxygen products that are required for preventing neural damage [14]. Alzheimer’s disease (AD) is the most common neurological disease resulting in brain shrinkage, tissue damage, and cell death. Amyloid precursor protein (β-amyloid peptide), βA, plays a major role in Alzheimer’s disease and causes progressive memory loss. The generation of toxicity by βA has not been completely researched; it leads to neuroinflammation, a vital feature of AD. However, GLA plays an important role in AD, as it inhibits the action of pro-inflammatory cytokines and aids in the prevention of amyloid-induced damage through the nuclear factor kappa B (NF-KB) signaling pathway [6, 174].
It was studied that saturated fatty acids are not beneficial for human health as they cause many chronic diseases like heart failure, high blood pressure, and increased cholesterol levels. The diet replaces unsaturated fatty acids with saturated fatty acids to overcome this. Basically, polyunsaturated acids are commonly used, including ω-6 PUFA (LA, ARA, and GLA) and ω-3 PUFA (ALA, EPA, and DHA). It has also been studied that linoleic-rich foods and foods that contain both linoleic and linolenic fatty acids show different results for diseases like coronary artery disease and nonfatal myocardial infarction [16]. Many hypotheses have suggested that consumption of linoleic acids (ω-6) along with linolenic acid (ω-3) decreases the accumulation of cholesterol and triglycerides in artery walls and also reduces the risk of cardiovascular diseases (CVD) [52]. Triglycerides are lipids that provide us energy, and a high number of triglycerides along with high cholesterol levels cause many different types of cardiovascular diseases. Intake of LA with fish oil that is rich in EPA and DHA maintains the proper number of triglycerides in the human body, but LA does not show the same output as olive oil capsules that are rich in oleic acid [20]. Similarly, LA-containing diet decreases total cholesterol concentrations as compared to diets having stearic acid (SA), saturated fatty acid (SFA), monounsaturated fatty acid (MUFA), and medium-chain fatty acid (MCFA). Some theories demonstrate the mechanisms by which polyunsaturated fatty acid (PUFA) decreases CVD. High-density lipoproteins (HDL) are the particles also known as good cholesterol, as a high level of HDL in the body results in a lower risk of CVD [136]. The concept behind this is inhibiting gene transcription (sterol-regulatory element-binding protein-1) responsible for cholesterol and lipogenesis synthesis in the liver [66]. Intake of PUFAs results in the activation of gene transcription of liver X receptor alpha (LXRα), which shows upregulation of cholesterol 7α-hydroxylase (CYP7) expression. It transforms cholesterol into bile acids; hence, PUFAs aid in the catabolism of cholesterol by increasing CYP7 activity [39]. ARA and its derivatives, along with ω-3 PUFAs, are collectively known as eicosanoids. They also play a crucial role in preventing many cardiovascular diseases [59]. The release of endogenous ARA from the phospholipid cell membrane is induced by inflammation and stimulation of various receptors such as tumor necrosis factor receptor (TNFR) and toll-like receptor 4 (TLR4), catalyzed by phospholipase enzyme. It has been observed that ARA has an important relationship with growing chronic conditions in the human body, as an imbalance of hypercholesterolemia and lipoprotein leads to an increase in the low-density lipoprotein cholesterol (LDL-C). This results in the deformation of arteries and blood flow passages becoming thick, which narrows the passage and reduces the blood flow due to which cardiovascular conditions exist and contribute to mortality worldwide. Foam cells and polymorphonuclear leukocytes (PMNs) also accelerate inflammation and atherosclerosis (deformation of blood vessels). Still, the process conducted with the help of ARA metabolites, known as efferocytosis, majorly focuses on the resolution of inflammation and foam cells [155].
ω-6 PUFA and the health of skin and eye
Skin is the most important and largest organ in our body. The outermost layer of skin is known as the epidermis. It acts as a shield that protects our body from various chemical, physical, and biological stresses that frequently result in tissue damage. Under the epidermis, the dermis layer is present, which consists of hair follicles and sweat glands. Then the layer of subcutaneous tissue, fatty tissue, and connective tissue present is known as the hypodermis (deeper layer). Each layer of skin has the potential to activate an important process that helps in wound repair. The development of some chronic conditions such as aging, diabetes, rheumatoid arthritis, CVD, etc., causes the prevention of wound repair efficiently. Many studies have been conducted that demonstrate that PUFAs (ω-3 and ω-6) are crucial factors in skin healing and also help in wound repair [142]. Similarly, the skin barrier is also very important for the body as it protects from different types of allergens and irritants, including chemical, microbial, and UV radiation. To strengthen the skin barrier, the contribution of certain dietary changes has been researched [18]. GLA helps in building up the skin barrier, as human skin does not consist of the δ-6-desaturase enzyme, which is responsible for the production of GLA from LA. As a result, the skin is primarily dependent on GLA produced by the liver. But oral intake of evening primrose oil (EPO) and borage oil (BO) provides GLA to the skin in an appropriate amount. GLA and its metabolites possess anti-inflammatory functions and also lead to an increase in ceramide production. It also prevent the synthesis of leukotriene (LT) B4, which is the main cause of rigidness of muscles and having shortening of breathing [107]. Nowadays, it has been observed that 20% of children are affected by a disease known as atopic dermatitis, in which the skin becomes usually irritated with red and itchy patches, leading to a noticeable reduction in the patient's quality of life as a failure of the skin's natural barrier and also an increase of transdermal absorption [82]. These changes allow the malfunction of type 1 IgE antibodies. Abnormalities in immune responses mediated by cells and the skin antimicrobial barrier are also compromised. These changes collectively result in the loss of water from the skin, which leads to skin dehydration. For skin restoration and defense, hydration of the skin is crucial, which it gets from skin moisturizers and various types of natural oils such as evening primrose oil, borage oil, hemp seed oil, and blackcurrant seed oil, which contain significant amounts of beneficial GLA. Compared to other oils, blackcurrant oil is the most crucial for skin hydration. Blackcurrant seed oil is one of the richest sources of GLA, containing 10.9–16.7% compared to the fruit. It has been observed that when blackcurrant seed oil is consolidated with electrospun patches, it enables the release of the oil through the skin surface in a desired and regulated manner. So, we can say that GLA delivery to atopic skin improves skin blockade and lowers trans-epidermal dehydration [156].
The stratum corneum (SC), the outermost layer of the epidermis, acts as a skin barrier. The epidermal keratinocytes undergo a multi-step differentiation process to form the SC, and both the structural and homeostatic aspects of lipid metabolism are crucial to this process. Basically, linoleic acid is involved in these processes by the metabolic pathway. The basal layer of the epidermis, where cells are multiplying, is where this route is most active. The breakdown of phospholipids is seen during the skin's differentiation process, and keratinocytes tend to create more neutral lipids such as triglycerides and ceramides. The SC lipid matrix, specifically made up of a combination of 45% ceramides, 30% cholesterol, and 15% free fatty acids, will be formed by the newly generated lipid [150]. Derivates of LA such as hydroxy-epoxy- and trihydroxy also play an essential role in the building up of the skin permeability barrier by providing a structural role when protein binding is eased by derivates of linoleic acid [76]. Apart from ω-6 PUFAs, ω-3 also has an essential role in skin-related problems. It has been observed that recipients of organ transplants may suffer from cutaneous squamous cell carcinoma (SCC) due to UV exposure, which may cause inflammation and affect the skin’s immune system. Recently, it has been noted that skin cancer has been reduced by the intake of ω-3 PUFAs by recipients of lung transplants. Patients have been advised to take care of their skin from direct sun rays by applying sunscreen all over their bodies [96]. The process of skin wound healing is a highly efficient mechanism involving the migration of various cell types into the wound, the generation of fresh epithelial cells, and the formation of new capillaries. This results in the emergence of pink, granular new tissue, a phenomenon commonly referred to as granulation [88]. Many treatments are involved in skin wound healing, including cytokines growth factors that are fabricated by hematopoietic cells and immune cells such as interleukins and interferons. An additional medical approach, recognized as cell-based therapy, has gained significance, involving the utilization of stem cells. The present study has demonstrated that ARA instigates the movement of human umbilical cord blood-derived mesenchymal stem cells (HUCB-MSCs) to improve stem cell mobility and recruitment into the wound site. Basically, ARA amplifies the skin wound healing process by boosting up the mTORC2 signaling pathway through the GPR40 coupling reaction mechanism which promotes cell proliferation and angiogenesis, the formation of new blood vessels [104].
When tears cannot adequately lubricate your eyes, dry eye disease (DED), a common illness, develops. It is an ocular surface disease in which the eye’s surface layer has been damaged, namely, the cornea and conjunctiva. Neurosensory dysfunctions, in addition to inflammation and damage to the ocular surface, can lead to ocular symptoms such as discomfort, stinging sensation, irritation, a feeling of a contaminant in the eye, and blurry vision, which can interfere with everyday activities. For treatment of DED symptoms, clinical trials were reported with food items rich in PUFAs. It has been observed that the symptoms of DED and tear osmolarity were found to be improved by the oral consumption of 2000 mg of sea buckthorn oil, which contains both ω-3 and ω-6 PUFAs [67]. Arachidonic acid’s efficient bioactive metabolite prostaglandins (PGs) may control various biological reactions in different tissues including the eye. ARA helps in obstructing glaucoma, which is the third most common global cause of blindness and visual impairment. Damage to the head of the optic nerve and the visual field characterizes the diverse illness known as glaucoma. High intraocular pressure is a significant risk factor and the cause of glaucoma optic neuropathy [170]. Premature birth leads to a major issue where the retina is not properly developed, commonly known as retinopathy of prematurity (ROP), which results in blindness. Premature infants who consumed fish oil and soybean oil high in DHA and LA reduced their risk of ROP. It has been reported that both ω-3 and ω-6 PUFAs are required for fetal eye growth. DHA and EPA aid in vascularizing the retina, whereas ARA is essential for the growth and metabolism of retinal neurons [53].
Significant contribution of ω-6 PUFA in cancer treatment
PUFAs have major implications in breast cancer and help inhibit mammary tumor cells by inducing morphological changes in the cell membrane and affecting gene expression, and signaling pathways of cells [177]. c9, t11-CLA, and t10, c12-CLA are two conjugated linoleic acid (CLA) isomers having chemotherapeutic properties. The findings demonstrated that apoptosis of colorectal (MIP-101) and prostate (PC-3) cancerous cells were more effectively induced by the t10, c12-CLA isomer. It has been observed that daily consumption of dairy products rich in high fat reduced the risk of distal colon cancer by 34% and colorectal cancer by 13%. Clinical trials showed a positive influence between breast cancer and the intake of CLA by female patients aged 55–69 years [33]. One of the most popular parenteral formulations used in advanced breast cancer treatment is docetaxel (DTX) solution. DTX causes the mitotic catastrophe of cancerous cells by preventing dynamic microtubule activity at the mitotic spindle. Docetaxel–linoleic acid conjugate (DTX–LA), a new DTX-based prodrug, was successfully designed by the esterification of 2’-OH of DTX and the R-COOH group of LA to target breast cancerous cells and increase drug loading [180]. In 2020, more than 50,000 fatalities from colorectal cancer and 150,000 new cases were recorded in the USA. Obesity, Westernized eating patterns, and a lack of exercise are all recognized risk factors for this malignancy. In contrast, 15-lipoxygenase-1 metabolizes LA resulting in the activation of PPARγ, inhibiting the proliferation of cancerous cells. Furthermore, long-term administration with LA has resulted in dormancy and senescence in cancer cells [103]. Bleomycin is a popular antineoplastic antibiotic used as an anticancer medication. ARA when combined with bleomycin shows the tumoricidal effects of ARA and its capacity to boost the cytotoxic effects of several anticancer medications. ARA intensifies the action of destruction of tumor-causing cells on human neuroblastoma cells (IMR-32) and results in activation of the extrinsic pathway for apoptosis by expression of FAS, caspases 3 and 8. Caspases play a part in inflammation, cell growth, and the prevention of tumors. As compared to other PUFAs, ARA was discovered to be the most effective in reducing the viability of human neuroblastoma cells [130]. Non-small cell lung cancer (NSCLC) is the most common kind of lung cancer. It has been reported that GLA inhibits the growth of NSCLC cells by down-regulating the effects of HIF-1α and VEGF. HIF-1α and HIF-1β are two common subunits of hypoxia-inducible factor 1 (HIF-1), which is responsible for hypoxia and promotes tumor malignancy [167]. GLA increases the level of cellular lipid peroxidation, which causes the release of cytochrome c from the mitochondrial intermembrane space, activating caspase-3, and blocking the signaling pathway for protein kinase B (PKB, commonly known as AKT). The protein kinase B pathway plays an important role in cell proliferation [138]. GLA regulates cell apoptosis and anti-inflammatory activities by altering the composition of the mitochondrial membrane,e thus reducing hexokinase’s ability to attach to the outer mitochondrial membrane [166].
Effect of ω-3 and ω-6 PUFAs supplements in health promotion
Obesity and diabetes
Although short-term measures to combat obesity, such as exercise and calorie restriction, have been successful, obesity still exists due to a high propensity for weight gain [37]. Obesity continues to be a growing health issue, especially for young people. One of the top ten health concerns that may be avoided, according to the World Health Organization, is obesity. Americans’ diets have significantly increased in LA, mostly because of recommendations made over the past several decades to reduce saturated fat consumption in the hopes of reducing cardiovascular disease [94]. When 3.2–3.4 g/day of CLA was taken for at least 6 months, meta-analyses of three human trials found that CLA supplementation caused a substantial decrease in body weight and BFM. There are several health benefits that the CLA isomers cis-9, trans-11-(c9, t11) are known to have on the body. For humans, CLA is considered an anti-obesity agent [13]. Madry et al. [91] have studied the relationship between body fat content in overweight and obese women and CLA intake in the diet. 3 g/day CLA for 12 weeks was randomly allocated to 74 obese or overweight women. His trials demonstrated decreases in a variety of fat metrics, including the amount of fat in the visceral, android, and gynoid tissues, and the ratio of lean body mass to height increased significantly by p = 6.1 × 10−11. Obesity brought on by a high-fat diet (HFD) affects the reverse cholesterol transfer from macrophages to feces (RCT). Conjugated linoleic acid (CLA) and alpha-linolenic acid (ALA) are thought to protect HFD-impaired RCT by altering hepatic protein pathways [101]. Obesity occurs in mice fed a high-fat diet due to activating inflammatory responses via TLR4. It has been discovered that saturated fatty acids are responsible for activating toll-like receptor-4 (TLR4) and induce pro-inflammatory cell signaling pathways, therefore, producing pro-inflammatory cytokines. Saturated fatty acids are prevented from activating the pro-inflammatory signaling pathway of TLR4 by ARA, which binds directly to the TLR4 co-receptor, myeloid differentiation factor 2 [181]. Nuts and green leafy vegetables contain trace levels of preformed GLA. Breast milk is the main source of GLA for babies. Dihomogamma linolenic acid (DGLA), formed from GLA, is metabolized through oxidative metabolism by cyclooxygenases and lipoxygenases to create anti-inflammatory eicosanoids [11].
Increasing LA intake is one of the dietary suggestions for preventing type 2 diabetes. However, in persons with atherogenic dyslipidemia, a low-carb diet led to a significant rise in ω-6 PUFAs and a significant drop in serum saturated fat. Thus, the levels of adipose linoleic acid in the USA are significantly greater than those in European nations, where the prevalence of type 2 diabetes is lower [64]. Because it involves both insulin resistance and abnormal pancreatic beta-cells, type 2 diabetes mellitus may be distinguished from other forms of the disease. Chronic feeding with CLA slightly raises fasting blood glucose and/or insulin in non-diabetic pigs and mice, despite the fact that it improves impaired glucose tolerance and insulin levels in diabetic rats [17]. Finding inhibitors for PTPN1, PTPN9, or PTPN11 is seen as a successful technique for the prospective treatment of type-2 diabetes, since these PTPs are linked to the negative control of insulin activity. Studies have shown that LA inhibits PTPN1, PTPN9, and PTPN11’s catalytic activity, showing that LA specifically targets these three proteins to prevent type 2 diabetes. It has also been observed that the improvement of glucose homeostasis and insulin sensitivity that results from AMPK activation, suggesting that AMPK should be a target for the treatment of metabolic syndrome and type 2 diabetes [173]. Research conducted in the USA showed that both men and women who consume a lot of LA have a decreased chance of developing type 2 diabetes, especially when LA is used to substitute SFAs, trans fats, or carbs [183].
Bones and semen quality
Both ω-3 and ω-6 are crucial factors for bone development. An ideal ω-6/ω-3 ratio in the diet can also help with age-related bone health issues including osteoporosis. It may be because ω-3 PUFAs do not induce adipogenesis as strongly as ω-6 PUFAs do, enabling osteoblastogenesis to occur while also promoting an inhibitory impact on osteoclastogenesis, which aids in preserving bone mineral density [15]. Dietary fats, important for bones and joints, have been noted in states where essential fatty acid deficiencies were at their peak, due to abnormal calcification. In the signaling pathway of bone turnover, lipid mediators are crucial. Prior to the mechanical stress of bone tissue, mature osteoblasts and osteocytes both emit prostaglandin E2 (PGE2). The cyclooxygenase 2 (COX-2) enzyme, which transforms ARA into PGE2, is expressed more often when phospholipase-mediated membrane releases of ARA, the substrate for PGE2 production, occur. This increases the turnover of bone [15, 133]. Numerous metabolic processes can influence the development of bones. In fatty acid metabolism, it has been noticed that LA and ALA control calcification and bone resorption processes in the body. Bone disorders are associated with metabolic problems, but nutrition and medication may be able to modify these pathways to prevent or treat them [160]. In Odutuga et al. [102] study, the young growing rats were tested for high calcification of bones which was influenced by the consumption of zinc and vital PUFAs as part of their dietary intake. Soybean oil contains 53.7% linoleic acid and 7.6% linolenic acid, which were used in the research. The present study indicates that a lack of zinc and EFA in the diet results in a reduction of an enzyme known as alkaline phosphate (ALP) present in the rat femur. As part of the calcification process, alkaline phosphatase is involved in cleaving phosphate ions from organic ester bonds. Both EFA and zinc deficiency have comparable consequences observed in both humans and young growing rats. Comparative studies have been conducted between Antarctic krill oil, rich in ω-3 PUFAs, and ARA, rich in ω-6PUFAs. The study revealed that the enhancement of bone mineral density (BMD) by AKO was more as compared to ARA-rich oil (AKO) administered to mice (220 mg/kg) for 30 days [178].
The intricate process of spermatogenesis involves the growth of spermatozoa in the seminal tubules. A number of cell types must participate in the differentiation of spermatogonia into spermatozoa, and the proper essential fatty acid profile is necessary for a healthy spermatogenetic process [29]. These PUFAs are also important elements of the sperm membrane, which has to be fluid and active to encourage fertilization. In fact, an increase in mitochondrial energy metabolism and a decrease in oxidative damage were seen in response to PUFA delivery, particularly ω-3 PUFA [48]. Changes in testes as well as a decrease in testosterone production have been observed in animal studies administered by trans-fat-rich diets. ω-6 PUFAs are also important, especially if their supply is excessive compared to that of ω-3 PUFAs. They may have a negative impact on fertility since they are likely to cause mild inflammation, oxidative stress, endothelial dysfunction, and atherosclerosis [154]. Table 2 summarizes the recommended ω-3 and ω-6 amounts for consumption by humans per day, along with their prevention mechanism.
Therapeutic effects of ω-3 and ω-6 PUFA
Role of ω-3 and ω-6 PUFAs in fetal programming
The mother’s health during pregnancy is important for the development of the fetus, and negative impacts on the mother's physiology are closely linked to the offspring's poor health. Therefore, proper maintenance of diet intake is essential. Both LA and ALA are significant ω-6 and ω-3 PUFAs and are considered to be a crucial part of the maternal diet. Since fetal growth is rapid, both ω-6 and ω-3 from the mother's diet are necessary for optimal heart, neurological, immunomodulation, and immune development and function [147]. Members of many transmembrane protein families, including fatty acid transport proteins (FATPs), fatty acid translocase (FAT/CD36), and intracellular FA binding proteins, are hypothesized to transfer FAs from the maternal to the fetal circulation [63]. It has been noted that the fetus’s epigenetic processes are influenced by its surroundings in gestation. Food intake can affect fetal development and placental function in ways that are either decreased or boosted. Changes in fetal growth and development can result from endothelial dysfunction brought on by altered placental function [73]. The programming of the epigenetic machinery in the child can be affected by the maternal consumption of various SFA, MUFA, and PUFA types through histone modifications, DNA methylation, and miRNA regulation. To assess variations between the sexes, further research, including both in male and female children, is required, as well as epigenetic investigations in the descendants of male progenitors exposed to various kinds of fatty acids [144]. Research has demonstrated that the programming of health may involve ALA. More specifically, it may go beyond being converted metabolically into DHA and EPA to possess inherent regulatory qualities on gene expression throughout fetal development [86]. The frequency of physiological inflammatory reactions is closely associated with the incidence of ovulation, menstruation, pregnancy, and delivery. As a result, the lipoxygenase-5 (5-LOX) and cyclooxygenase (COX) pathways for ARA transformation are turned on. It has been discovered that improper embryo implantation and a decidual response occur in COX-2 deficient circumstances [79].
ω-3 and ω-6 role in psychological, psychiatric, and behavioral disorders
Psychological distress is a mental health condition that affects general functioning and raises the risk of death from all causes [34]. ω-3 and ω-6 groups of PUFAs found in various food products have been suggested as potential treatment for psychological, psychiatric, and behavioral disorders, behavioral management issues and mental health issues. Numerous epidemiological studies have connected aggressive behavior to a lack of seafood consumption or low levels of ω-3 PUFAs in the diet [36]. DHA is a crucial essential fatty acid with brain function for the formation of neuronal cells, according to animal and cell research. Pharmacologically, these ω-3 PUFAs enhance psychological well-being and lower the risk of brain-related illnesses such as Alzheimer's disease, moderate cognitive impairment, depressive symptoms, epilepsy, schizophrenia, stroke, Parkinson's disease, and autism spectrum disorders [77]. Yau et al. [172] have demonstrated, in their research on 5-week-old male and female offspring who underwent behavioral tests to gauge social interaction and anxiety- and depression-like behavior, that a prolonged high linoleic acid or HLA (ω-6) diet did not alter friendliness or social memory, but it did cause depressive-like behavior in male offspring but not in female offspring. Therefore, HLA under a recommended safe ratio with ω-3 would be encouraging for the offspring’s normal behavioral development.
Effect of ω-3 PUFA on COVID-19
Acute respiratory distress syndrome (ARDS), a serious lung ailment that is sometimes fatal, complicates coronavirus disease-2019 (COVID-19) symptoms in around 10% of SARS-CoV-2-infected individuals [12]. ARDS, mainly caused by cytokine stroke syndrome, causes systemic inflammation and multiple organ failure due to the excessive production of immune cells and cytokines. This disorder appears between 7 and 15 days after the onset of symptoms [55]. It has been noticed that PUFAs (ω-3 LC-PUFAs) may contribute to a better resolution of the inflammatory balance. Based on prior clinical trials that suggested adding ω-3 supplements to the diets of critically sick patients in the acute stage of ARDS might enhance their clinical outcomes [169]. The main goals of current therapies are to treat thrombosis and inflammation. ω-3 fatty acids, particularly EPA and DHA, are anti-inflammatory, encourage the production of pre-resolving mediators, and control platelet aggregation and thrombosis. These results imply that EPA and DHA may be helpful in COVID-19 treatment [56]. Macrophages, natural killer cells, mast cells, basophils, and eosinophils are only a few of the innate immune system's cells whose capabilities have been discovered to be enhanced by ω-3 fatty acids. Additionally, they support T cell and B cell-mediated antigen-specific responses that produce antibodies and develop an immunological memory specific to recurrent infections with the same pathogen [8]. According to research, people with an ω-3 index of more than 5.7% had a roughly 75% reduced mortality risk than those with a lower index. Based on the results of the COVID-19 symptom study app, those who consume ω-3 PUFA supplements have a modestly reduced risk of being infected by the virus [39]. Both ω-3 and ω-6 metabolites are essential in synthesizing mediators such as prostaglandins, leukotrienes, thromboxanes, protectins, and resolvins [35]. The research demonstrates that fish oil boosts the antiviral response by triggering interferon (IFN), which prevents virus multiplication. By integrating into the cell membrane, ω-3 fatty acids (specifically, DHA and EPA) contribute to immune regulation, hinder the aggregation of toll-like receptors, block signaling pathways that activate NF-B, and reduce the production of pro-inflammatory molecules. These actions collectively aid in mitigating the complications associated with COVID-19 [60, 159]. Protectin D1, DHA-derived mediators, have been shown to inhibit viral RNA replication, such as influenza viruses [98]. The UK, USA, and Sweden have reported lower rates of positive SARS-CoV-2 infection in people who took ω-3 fatty acid supplements more than three times a week [90]. An additional study concluded that ω-3 fatty acid supplementation has a variable effect on infection depending on the pathogen, the dose, and the frequency of supplementation [100].
Industrial scope and health applications
PUFAs will play a crucial role in human health, especially in the hectic urban lifestyle. They have intriguing anti-oxidative, anti-inflammatory, or anticancer capabilities and have been demonstrated to favorably affect a variety of disorders [135]. Many developed nations raising investments in bio-based platform development for providing functional foods and health supplements to consumers. These nations make up the bulk of the market for ω-3 PUFAs globally and are mostly found in North America (the USA, Canada, and Mexico). PUFAs are also found in various nutraceutical items intended for direct human ingestion, often with positive benefits. Like animal feed, fish oils are the primary source of PUFAs in nutraceuticals. Overall, it has been projected that fish oils account for most of the PUFA market volume [139]. However, fish ingest PUFAs primarily through their food, which includes zooplankton and other PUFA-rich creatures as well as microalgae or microalgal grazers. Terrestrial plants, microalgae, and certain heterotrophic protists are additional sources of PUFA. Short-chain PUFAs, such as LA, linolenic acid, and ALA, are common in plants and are especially prevalent in linseeds and nuts.
EPA and DHA PUFAs are notably abundant in microalgae. The primary producers of PUFAs are microalgae and other protists like thraustochytrids or heterotrophic dinoflagellates [22]. Currently, the practices of ω PUFA are reported for various industries due to their wide range of health applications: food additives, feed for farm animals and birds, aquaculture, cosmetic products, food products, etc. [93]. PUFAs from these sources are major components of nutraceuticals. Nutraceutical is a broad term that originated from the term nutrition and pharmaceutical. Herbs, nutrients, and dietary supplements are the three basic subcategories of nutraceuticals. Dietary fiber, prebiotics, probiotic supplement or symbiotic, PUFAs, antioxidants, spices, and other natural herbs and plants are among the food products utilized as nutraceutical foods. Using ω-3 PUFA lowers cholesterol, and excessive cholesterol levels in several inflammatory illnesses and cardiovascular conditions have been observed [54]. Marine fish have long been considered a nutritious food option. Still, recent advances in the fields of nutraceuticals and functional foods reveal that their value goes beyond nutritive, as they provide our diet with molecules that have a significant therapeutic role in the treatment of human diseases or their prevention. The commercial demand and pricing of ω-6 polyunsaturated fatty acids (PUFAs) typically exceed that of ω-3 PUFAs. This is due to their easy digestibility in an infant's digestive system, a crucial factor for proper cognitive development during pregnancy [112, 116, 117, 119, 122, 123, 126, 152].
The development of microbial platforms is gaining importance mainly due to their scaleup potential, more sustainable features, utilization of waste fraction for growth, structural simplicity, ease of process, continuation in supply chain irrespective of climate condition and season, and of offer by-products such as sterols, enzymes, carotenoids, exopolysaccharides, and flavonoids to offset processing costs among others [106, 112, 116, 117, 119, 122, 123, 126]. Especially, oleaginous thraustochytrids and microalgae could be a sustainable substitute for existing plant and animal sources to produce ω-3 and ω-6 at a commercial scale due to the fascinating growth rate, productivity, and yield [89, 112, 116, 117, 119, 122, 123, 126]. Currently, microalgae are a better source of ω-6 PUFAs production, and thraustochytrids are the preferred platform for ω-3 [112, 116, 117, 119, 122, 123, 126]. Moreover, microbial platform yields are greater than from any other sources, have easy production at controlled fermentation conditions, offer more oxidative stability of the product, and reduce the dependency on plant and animal sources [30].
No report provides a comparative account of the bioactivity of microbial and nonmicrobial PUFAs. However, some studies have shown how native PUFAs bioactivity can be enhanced. The direct transformation of microbial lipids results in PUFA salts with stronger bioactivity than their parent PUFAs. For example, EPA and GLA-containing lipids produced by Thamnidium elegans and Nanochloropsis salina were transformed into water-soluble fatty acid potassium salts (FAPS). FAPS were found to be potent inhibitors even at low doses against several Gram-positive and Gram-negative bacteria, as well as breast cancer cells [141].
Future research direction and enhancement strategies of ω-PUFAs
The first research question that comes to mind is how this microbial platform can be efficiently developed replacing the existing ω-PUFA production platforms from plant and animal sources. Moreover, would ω-PUFA offer the same level of bioactivities and health benefits? Would microbial ω-PUFA be acceptable for a vegetarian diet, and above all, on what production scale would it be cost-effective and sustainable, etc.? These are future questions that will be dependent on the preliminary outcome of their yield and productivity in an acceptable range. Research advances show that one group of microorganisms are not able to produce all kinds of ω-PUFA and their production range greatly lies on their genetic capabilities; however, nutrition factor is the most affecting parameter for ω-PUFA enhancement. Several reports have been published over the past decades addressing enhanced ω-3 production strategies by thraustochytrids. None of the reports show a significant accumulation of ω-6 PUFAs which are intermediates of ω-3 PUFAs in the metabolic pathway [109, 113, 114]. It is a prerequisite to target enhanced production of ω-6 PUFAs from these strains by omics (genomics, proteomics, miRNAomics, transcriptomics, nutrigenomics, etc.) and mutation approaches to enhance ω-6 fraction in microbial cells [84]. Moreover, recent studies also tried some exogenous supplementation strategies to enhance ω-PUFA from their baseline production range. Some strategies to improve ω-6 PUFA, supplementation of polyoxyethylene sorbitan monooleate and nanoparticles, UV treatments, and inhibition of delta-15-desaturase, delta-17-desaturase, delta-4-desaturase, and delta-19-desaturase must be adopted [112, 116, 117, 119, 122, 123, 126]. Furthermore, for enhancing ω-3 PUFA, potential strategies may include the addition of 1:1 of external oils or waste oils and linseed oil or garden cress oil. Additionally, supplementation of natural antioxidants such as α-tocopherol, ascorbic acid, mannitol, tea extract, sesamol, melatonin, butylated hydroxytoluene, and others could be considered. Inhibition of the delta-17-desaturase gene and the overexpression of heterologous delta-5-elongase are also feasible approaches [85, 112, 116, 117, 119, 122, 123, 126, 179].
Conclusions
The demand for ω-PUFAs as a necessary diet is growing due to their leading role in health promotion. The microbial platforms are alternate sources of PUFA that must emerge to meet the rising demand, especially for vegetarians who do not prefer foods from animal sources. Several oleaginous microbes are known for higher PUFA content and offer an adequate proportion of ω-3 and ω-6 groups of essential PUFAs. Microalgae stand out as a fascinating source of high polyunsaturated fatty acid (PUFA) production, offering scalability and sustainability with a reduced carbon footprint and greenhouse gas emissions. PUFAs, including ω-3 and ω-6, play critical roles in health by providing antioxidant and anti-inflammatory effects, lowering the risk and severity of illnesses. They contribute to reducing the risk of cardiovascular diseases, diabetes, cancer, and hypertension through various mechanisms. ω-6 PUFA, linoleic acid (LA), positively influences LDL cholesterol levels, while EPA and DHA support cardiovascular function, blood flow, and inflammation regulation. Recent studies highlight LA and ARA’s impact on bone, skin, and eye development. Despite some controversy over the ideal ω-6 to ω-3 ratio in diets, the combination of these PUFAs consistently reduces inflammation and supports various biological processes. The microbial production platform would offer to design the preferred ratio of ω content at the production and co-extraction or sequential extraction stages. Early visual and brain development depends on DHA. The main functions of the plant ω-3 PUFA, ALA are to regulate the conversion of LA to ARA and to serve as a substrate for EPA production. ω-3 PUFAs (EPA + DHA) appear to affect COVID-19 patients positively based on preliminary data positively, but further clinical research is required to validate these advantages.
Data availability
NA.
Change history
07 November 2023
A Correction to this paper has been published: https://doi.org/10.1007/s43393-023-00221-z
References
Abdelhamid A, Hooper L, Sivakaran R, Hayhoe RPG, Welch A, PUFAH Group. The relationship between omega-3, omega-6 and total polyunsaturated fat and musculoskeletal health and functional status in adults: a systematic review and meta-analysis of RCTs. Calcif Tissue Int. 2019;105(4):353–72. https://doi.org/10.1007/s00223-019-00584-3.
Adel A, El-Baz A, Shetaia Y, Sorour NM. Biosynthesis of polyunsaturated fatty acids by two newly cold-adapted Egyptian marine yeast. 3 Biotech. 2021;11(11):461. https://doi.org/10.1007/s13205-021-03010-4.
Adeleke AA, Ikubanni PP, Orhadahwe TA, Christopher CT, Akano JM, Agboola OO, Ibikunle RA. Sustainability of multifaceted usage of biomass: a review. Heliyon. 2021;7(9):08025.
Ahmadniay Motlagh H, Aalipanah E, Mazidi M, Faghih S. Effect of flaxseed consumption on central obesity, serum lipids, and adiponectin level in overweight or obese women: a randomised controlled clinical trial. Int J Clin Pract. 2021;75(10): e14592. https://doi.org/10.1111/ijcp.14592.
Alagawany M, Elnesr SS, Farag MR, Abd El-Hack ME, Khafaga AF, Taha AE, et al. Omega-3 and omega-6 fatty acids in poultry nutrition: effect on production performance and health. Animals (Basel). 2019;9(8):573. https://doi.org/10.3390/ani9080573.
Albensi BC. What is nuclear factor kappa B (NF-κB) doing in and to the mitochondrion? Front Cell Dev Biol. 2019;7:154. https://doi.org/10.3389/fcell.2019.00154.
Armenta RE, Valentine MC. Single-cell oils as a source of omega-3 fatty acids: an overview of recent advances. J Am Oil Chem Soc. 2013;90(2):167–82. https://doi.org/10.1007/s11746-012-2154-3.
Asher A, Tintle NL, Myers M, Lockshon L, Bacareza H, Harris WS. Blood omega-3 fatty acids and death from COVID-19: A pilot study. Prostaglandins Leukot Essent Fatty Acids. 2021;166: 102250. https://doi.org/10.1016/j.plefa.2021.102250.
Azin E, Moghimi H, Dastgheib SMM, Darvishi F. Biovalorization of wastewater of fish canning process by Yarrowia lipolytica for biodiesel and animal feed supplement production. Biomass Convers Biorefin. 2022. https://doi.org/10.1007/s13399-022-03025-8.
Bai M, Sen B, Wen S, Ye H, He Y, Zhang X, et al. Culturable diversity of thraustochytrids from coastal waters of Qingdao and their fatty acids. Mar Drugs. 2022;20(4):229. https://doi.org/10.3390/md20040229.
Balić A, Vlašić D, Žužul K, Marinović B, Bukvić MZ. Omega-3 versus omega-6 polyunsaturated fatty acids in the prevention and treatment of inflammatory skin diseases. Int J Mol Sci. 2020;21(3):741. https://doi.org/10.3390/ijms21030741.
Ballout RA, Sviridov D, Bukrinsky MI, Remaley AT. The lysosome: a potential juncture between SARS-CoV-2 infectivity and Niemann–-Pick disease type C, with therapeutic implications. FASEB J. 2020;34(6):7253–64. https://doi.org/10.1096/fj.202000654R.
Basak S, Duttaroy AK. Conjugated linoleic acid and its beneficial effects in obesity, cardiovascular disease, and cancer. Nutrients. 2020. https://doi.org/10.3390/nu12071913.
Basak S, Mallick R, Banerjee A, Pathak S, Duttaroy AK. Maternal supply of both arachidonic and docosahexaenoic acids is required for optimal neurodevelopment. Nutrients. 2021;13(6):2061. https://doi.org/10.3390/nu13062061.
Basak S, Vilasagaram S, Duttaroy AK. Maternal dietary deficiency of n-3 fatty acids affects metabolic and epigenetic phenotypes of the developing fetus. Prostaglandins Leukot Essent Fatty Acids. 2020;158: 102109. https://doi.org/10.1016/j.plefa.2020.102109.
Bazinet RP, Chu MW. Omega-6 polyunsaturated fatty acids: is a broad cholesterol-lowering health claim appropriate? CMAJ. 2014;186(6):434–9. https://doi.org/10.1503/cmaj.130253.
Belury MA. Conjugated linoleic acids in type 2 diabetes mellitus: implications and potential mechanisms. In: Advances in conjugated linoleic acid research. AOCS Publishing; 2020. p. 302–15.
Berger A. Oil, containing the anti-inflammatory fatty acid sciadonic acid, improves skin barrier function in a skin irritation model in healthy female subjects. Lipids Health Dis. 2022;21(1):1–15.
Bhattacharjya R, Kiran Marella T, Tiwari A, Saxena A, Kumar Singh P, Mishra B. Bioprospecting of marine diatoms Thalassiosira, Skeletonema and Chaetoceros for lipids and other value-added products. Bioresour Technol. 2020;318: 124073. https://doi.org/10.1016/j.biortech.2020.124073.
Birkic N, Azar T, Maddipati KR, Minic Z, Reynolds CA. Excessive dietary linoleic acid promotes plasma accumulation of pronociceptive fatty acyl lipid mediators. Sci Rep. 2022;12(1):17832. https://doi.org/10.1038/s41598-022-21823-y.
Bishnoi S, Mudgil D. Current concepts and prospects of herbal nutraceutical. Handb Nutraceuticals Nat Prod Biol Med Nutr Prop Appl. 2022;1:189–204.
Blasio M, Balzano S. Fatty acids derivatives from eukaryotic microalgae, pathways and potential applications. Front Microbiol. 2021;12:2689. https://doi.org/10.3389/fmicb.2021.718933.
Brault HS. Dietary intakes of saturated, polyunsaturated, monounsaturated, omega-6, and omega-3 fatty acids in relation to self-reported anxiety, self-reported depression, and risk for clinical depression in the civilian, noninstitutionalized adult population in the United States [doctoral dissertation]; 2021.
Castejón N, Luna P, Señoráns FJ. Microencapsulation by spray drying of omega-3 lipids extracted from oilseeds and microalgae: effect on polyunsaturated fatty acid composition. LWT. 2021;148: 111789. https://doi.org/10.1016/j.lwt.2021.111789.
Chauhan AS, Chen CW, Tambat VS, Singhania RR, Chang JS, Dong CD, Patel AK. Bioprocess engineering to produce essential polyunsaturated fatty acids from Thraustochytrium sp. Bioresour Technol. 2023;23: 129209. https://doi.org/10.1016/j.biortech.2023.129209.
Chauhan AS, Chen CW, Yadav H, Parameswaran B, Singhania RR, Dong CD, Patel AK. Assessment of thraustochytrids potential for carotenoids, terpenoids and polyunsaturated fatty acids biorefinery. J Food Sci Technol. 2023;28:1–3. https://doi.org/10.1007/s13197-023-05740-0.
Chauhan AS, Patel AK, Chen CW, Chang JS, Michaud P, Dong CD, Singhania RR. Enhanced production of high-value polyunsaturated fatty acids (PUFAs) from potential thraustochytrid Aurantiochytrium sp. Bioresour Technol. 2023;1(370): 128536. https://doi.org/10.1016/j.biortech.2022.128536.
Chen YH, Ong CC, Lin TY. Effect of sea salt and taro waste on fungal mortierella alpina cultivation for arachidonic acid-rich lipid production. Fermentation. 2022;8(2):81. https://doi.org/10.3390/fermentation8020081.
Collodel G, Castellini C, Lee JCY, Signorini C. Relevance of fatty acids to sperm maturation and quality. Oxid Med Cell Longev. 2020;2020:7038124. https://doi.org/10.1155/2020/7038124.
Colonia BSO, de Melo Pereira GV, Soccol CR. Omega-3 microbial oils from marine thraustochytrids as a sustainable and technological solution: a review and patent landscape. Trends Food Sci Technol. 2020;99:244–56. https://doi.org/10.1016/j.tifs.2020.03.007.
Cui Y, Thomas-Hall SR, Chua ET, Schenk PM. Development of a Phaeodactylum tricornutum biorefinery to sustainably produce omega-3 fatty acids and protein. J Clean Prod. 2021;300: 126839. https://doi.org/10.1016/j.jclepro.2021.126839.
da Rochaa ACF, Cavalcantea JLP. Evaluation of the consumption of omega 3 fatty acid in Brazilian pregnant women: a cross-sectional study.
Dachev M, Bryndová J, Jakubek M, Moučka Z, Urban M. The effects of conjugated linoleic acids on cancer. Processes. 2021;9(3):454. https://doi.org/10.3390/pr9030454.
Davison KM, Lung Y, Lin SL, Tong H, Kobayashi KM, Fuller-Thomson E. Psychological distress in older adults linked to immigrant status, dietary intake, and physical health conditions in the Canadian Longitudinal Study on Aging (CLSA). J Affect Disord. 2020;265:526–37. https://doi.org/10.1016/j.jad.2020.01.024.
De Cosmi V, Mazzocchi A, Turolo S, Syren ML, Milani GP, Agostoni C. Long-chain polyunsaturated fatty acids supplementation and respiratory infections. Ann Nutr Metab. 2022;78(1):8–15.
Dean AJ, Bor W, Adam K, Bowling FG, Bellgrove MA. A randomized, controlled, crossover trial of fish oil treatment for impulsive aggression in children and adolescents with disruptive behavior disorders. J Child Adolesc Psychopharmacol. 2014;24(3):140–8. https://doi.org/10.1089/cap.2013.0093.
den Hartigh LJ. Conjugated linoleic acid effects on cancer, obesity, and atherosclerosis: a review of pre-clinical and human trials with current perspectives. Nutrients. 2019;11(2):370. https://doi.org/10.3390/nu11020370.
Didrihsone E, Dubencovs K, Grube M, Shvirksts K, Suleiko A, Suleiko A, et al. Crypthecodinium cohnii growth and omega fatty acid production in mediums supplemented with extract from recycled biomass. Mar Drugs. 2022;20(1):68. https://doi.org/10.3390/md20010068.
Djuricic I, Calder PC. Beneficial outcomes of omega-6 and omega-3 polyunsaturated fatty acids on human health: an update for 2021. Nutrients. 2021;13(7):2421. https://doi.org/10.3390/nu13072421.
Dorni C, Sharma P, Saikia G, Longvah T. Fatty acid profile of edible oils and fats consumed in India. Food Chem. 2018;238:9–15. https://doi.org/10.1016/j.foodchem.2017.05.072.
Du F, Wang YZ, Xu YS, Shi TQ, Liu WZ, Sun XM, et al. Biotechnological production of lipid and terpenoid from thraustochytrids. Biotechnol Adv. 2021;48: 107725. https://doi.org/10.1016/j.biotechadv.2021.107725.
Dyal SD, Narine SS. Implications for the use of Mortierella fungi in the industrial production of essential fatty acids. Food Res Int. 2005;38(4):445–67. https://doi.org/10.1016/j.foodres.2004.11.002.
Dyall SC, Balas L, Bazan NG, Brenna JT, Chiang N, da Costa SF, Taha AY. Polyunsaturated fatty acids and fatty acid-derived lipid mediators: recent advances in the understanding of their biosynthesis, structures, and functions. Prog Lipid Res. 2022. https://doi.org/10.1016/j.plipres.2022.101165.
Epure A, Anastasoi V, Cheta DM. Correlation between the presence of metals with potential for intoxication, omega 3 deficiency, increased omega 6: omega 3 ratio and their associated symptoms. Rom Med J. 2022;69(2):72–9. https://doi.org/10.37897/RMJ.2022.2.5.
Estupiñán M, Hernández I, Saitua E, Bilbao ME, Mendibil I, Ferrer J, Alonso-Sáez L. Novel Vibrio spp. strains producing omega-3 fatty acids isolated from coastal seawater. Mar Drug. 2020;18(2):99. https://doi.org/10.3390/md18020099.
Fabiani H, Mudjihartini N, Lestari W. Low dietary omega-6 to omega-3 fatty acid intake ratio enhances adiponectin level in obesity. World Nutr J. 2021;5(1):30–9. https://doi.org/10.25220/WNJ.V05.i1.0005.
Fazili ABA, Shah AM, Zan X, Naz T, Nosheen S, Nazir Y, et al. Mucor circinelloides: a model organism for oleaginous fungi and its potential applications in bioactive lipid production. Microb Cell Factories. 2022;21(1):1–19.
Ferramosca A, Zara V. Diet and male fertility: the impact of nutrients and antioxidants on sperm energetic metabolism. Int J Mol Sci. 2022;23(5):2542. https://doi.org/10.3390/ijms23052542.
Ferreira GF, Ríos Pinto LF, Carvalho PO, Coelho MB, Eberlin MN, Maciel Filho R, et al. Correction to: biomass and lipid characterization of microalgae genera Botryococcus, Chlorella, and Desmodesmus aiming high-value fatty acid production. Biomass Convers Biorefin. 2022;12:2333.
Freese E, Rütters H, Köster J, Rullkötter J, Sass H. Gammaproteobacteria as a possible source of eicosapentaenoic acid in anoxic intertidal sediments. Microb Ecol. 2009;57:444–54. https://doi.org/10.1007/s00248-008-9443-2.
Frøkiær H, Andersen AD, Damsgaard LL, C.T. Fish oil in combination with high or low intakes of linoleic acid lowers plasma triacylglycerols but does not affect other cardiovascular risk markers in healthy men. J Nutr. 2008;138(6):1061–6.
Froyen E, Burns-Whitmore B. The effects of linoleic acid consumption on lipid risk markers for cardiovascular disease in healthy individuals: a review of human intervention trials. Nutrients. 2020;12(8):2329. https://doi.org/10.3390/nu12082329.
Fu Z, Yan W, Chen CT, Nilsson AK, Bull E, Allen W, et al. Omega-3/Omega-6 long-chain fatty acid imbalance in Phase I retinopathy of prematurity. Nutrients. 2022;14(7):1333. https://doi.org/10.3390/nu14071333.
Ghani U, Naeem M, Rafeeq H, Imtiaz U, Amjad A, Ullah S, et al. A novel approach towards nutraceuticals and biomedical applications. Sch Int J Biochemist. 2019;2:245–52.
Guo YR, Cao QD, Hong ZS, Tan YY, Chen SD, Jin HJ, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak–an update on the status. Mil Med Res. 2020;7(1):1.
Gutiérrez S, Svahn SL, Johansson ME. Effects of omega-3 fatty acids on immune cells. Int J Mol Sci. 2019;20(20):5028. https://doi.org/10.3390/ijms20205028.
Ha NC, Hong DD. Optimization of cultural conditions for omega 3–6 fatty acids and carotenoids production by \textit {Schizochytrium mangrovei} TB17. Acad J Biol Sci. 2022;44(1):11–28.
Hamilton ML, Haslam RP, Napier JA, Sayanova O. Metabolic engineering of Phaeodactylum tricornutum for the enhanced accumulation of omega-3 long chain polyunsaturated fatty acids. Metab Eng. 2014;22:3–9. https://doi.org/10.1016/j.ymben.2013.12.003.
Hanna VS, Hafez EAA. Synopsis of arachidonic acid metabolism: a review. J Adv Res. 2018;11:23–32. https://doi.org/10.1016/j.jare.2018.03.005.
Hathaway D, Pandav K, Patel M, Riva-Moscoso A, Singh BM, Patel A, et al. Omega 3 fatty acids and COVID-19: a comprehensive review. Infect Chemother. 2020;52(4):478–95. https://doi.org/10.3947/ic.2020.52.4.478.
Hayashi S, Satoh Y, Ogasawara Y, Maruyama C, Hamano Y, Ujihara T, et al. Control mechanism for cis double-bond formation by polyunsaturated fatty-acid synthases. Angew Chem Int Ed Engl. 2019;58(8):2326–30. https://doi.org/10.1002/anie.201812623.
Hayashi Y, Shimamura A, Ishikawa T, Fujiwara Y, Ichi I. FADS2 inhibition in essential fatty acid deficiency induces hepatic lipid accumulation via impairment of very low-density lipoprotein (VLDL) secretion. Biochem Biophys Res Commun. 2018;496(2):549–55. https://doi.org/10.1016/j.bbrc.2018.01.064.
He Q, Chen Y, Wang Z, He H, Yu P. Cellular uptake, metabolism and sensing of long-chain fatty acids. Front Biosci. 2023;28(1):10. https://doi.org/10.31083/j.fbl2801010.
Henderson G, Crofts C, Schofield G. Linoleic acid and diabetes prevention. Lancet Diabetes Endocrinol. 2018;6(1):12–3. https://doi.org/10.1016/S2213-8587(17)30404-7.
Hodge L, Salome CM, Hughes JM, Liu-Brennan D, Rimmer J, Allman M, et al. Effect of dietary intake of omega-3 and omega-6 fatty acids on severity of asthma in children. Eur Respir J. 1998;11(2):361–5. https://doi.org/10.1183/09031936.98.11020361.
Hong T, Zou J, Yang J, Liu H, Cao Z, He Y, Feng D. Curcumin protects against bisphenol A-induced hepatic steatosis by inhibiting cholesterol absorption and synthesis in CD-1 mice. Food Sci Nutr. 2023;11:5091–101.
Hyon JY, Han SB. The protective effect of polyunsaturated fatty acids against dry eye disease: a literature review. Appl Sci. 2021;11(10):4519. https://doi.org/10.3390/app11104519.
Innes JK, Calder PC. Omega-6 fatty acids and inflammation. Prostaglandins Leukot Essent Fatty Acids. 2018;132:41–8. https://doi.org/10.1016/j.plefa.2018.03.004.
Jaritkhuan S, Suanjit S. Species diversity and polyunsaturated fatty acid content of thraustochytrids from fallen mangrove leaves in Chon Buri Province, Thailand. Agric Nat Resour. 2018;52(1):24–32. https://doi.org/10.1016/j.anres.2018.05.002.
Jia YL, Wang LR, Zhang ZX, Gu Y, Sun XM. Recent advances in biotechnological production of polyunsaturated fatty acids by Yarrowia lipolytica. Crit Rev Food Sci Nutr. 2021;62:8920–34.
Jónasdóttir SH. Fatty acid profiles and production in marine phytoplankton. Mar Drugs. 2019;17(3):151. https://doi.org/10.3390/md17030151.
Jovanovic S, Dietrich D, Becker J, Kohlstedt M, Wittmann C. Microbial production of polyunsaturated fatty acids—high-value ingredients for aquafeed, superfoods, and pharmaceuticals. Curr Opin Biotechnol. 2021;69:199–211. https://doi.org/10.1016/j.copbio.2021.01.009.
Juras J, Lovrić B, Blajić M, Zmijanović I, Krištofić B. The role of inositol, folic acid and polyunsaturated fatty acids in pregnancy and fetal development. [Hrana u zdravlju i bolesti: znanstveno-stručni časopis za nutricionizam i dijetetiku, 10]. 2021;2:97–103.
Kalidasan K, Vinithkumar NV, Peter DM, Dharani G, Dufossé L. Thraustochytrids of Mangrove Habitats from Andaman Islands: species Diversity, PUFA Profiles and Biotechnological Potential. Drugs. 2021;19(10):571.
Kannan N, Rao AS, Nair A. Microbial production of omega-3 fatty acids: an overview. J Appl Microbiol. 2021;131(5):2114–30. https://doi.org/10.1111/jam.15034.
Keyes GS, Maiden K, Ramsden CE. Stable analogs of 13-hydroxy-9, 10-trans-epoxy-(11E)-octadecenoate (13, 9-HEL), an oxidized derivative of linoleic acid implicated in the epidermal skin barrier. Prostaglandins Leukot Essent Fatty Acids. 2021;174: 102357. https://doi.org/10.1016/j.plefa.2021.102357.
Khalid W, Gill P, Arshad MS, Ali A, Ranjha MMAN, Mukhtar S, et al. Functional behavior of DHA and EPA in the formation of babies brain at different stages of age, and protect from different brain-related diseases. Int J Food Prop. 2022;25(1):1021–44. https://doi.org/10.1080/10942912.2022.2070642.
Khalili L, Valdes-Ramos R, Harbige LS. Effect of n-3 (omega-3) polyunsaturated fatty acid supplementation on metabolic and inflammatory biomarkers and body weight in patients with type 2 diabetes mellitus: a systematic review and meta-analysis of RCTs. Metabolites. 2021;11(11):742. https://doi.org/10.3390/metabo11110742.
Kikut J, Komorniak N, Ziętek M, Palma J, Szczuko M. Inflammation with the participation of arachidonic (ARA) and linoleic acid (LA) derivatives (HETEs and HODEs) is necessary in the course of a normal reproductive cycle and pregnancy. J Reprod Immunol. 2020;141: 103177. https://doi.org/10.1016/j.jri.2020.103177.
Koreti D, Kosre A, Jadhav SK, Chandrawanshi NK. A comprehensive review on oleaginous bacteria: an alternative source for biodiesel production. Bioresour Bioproc. 2022;9(1):1–9. https://doi.org/10.1186/s40643-022-00527-1.
Kountouras J, Doulberis M, Kazakos E, Tzika SK, Vardaka E, Liatsos C, et al. Correspondence on’Omega-3 supplementation and cardiovascular disease: formulation-based systematic review and meta-analysis with trial sequential analysis’ by Rizos. Heart. 2022;108(8):657. https://doi.org/10.1136/heartjnl-2020-318776.
Kowalska-Olędzka E, Czarnecka M, Baran A. Epidemiology of atopic dermatitis in Europe. J Drug Assess. 2019;8(1):126–8. https://doi.org/10.1080/21556660.2019.1619570.
Krishna Perumal P, Dong CD, Chauhan AS, Anisha GS, Chen CW, Singhania RR, Patel AK. Advances in oligosaccharide production from algal sources and their emerging health prospects. Biotechnol Adv. 2023;67: 108195.
Kumar G, Shekh A, Jakhu S, Sharma Y, Kapoor R, Sharma TR. Bioengineering of microalgae: recent advances, perspectives, and regulatory challenges for industrial application. Front Bioeng Biotechnol. 2020;8:914. https://doi.org/10.3389/fbioe.2020.00914.
Laddha H, Pawar PR, Prakash G. Bioconversion of waste acid oil to docosahexaenoic acid by integration of ex novo and de novo fermentation in Aurantiochytrium limacinum. Bioresour Technol. 2021;332: 125062. https://doi.org/10.1016/j.biortech.2021.125062.
Leikin-Frenkel AI. Is there a role for alpha-linolenic acid in the fetal programming of health? J Clin Med. 2016;5(4):40. https://doi.org/10.3390/jcm5040040.
Leyton A, Flores L, Shene C, Chisti Y, Larama G, Asenjo JA, et al. Antarctic thraustochytrids as sources of carotenoids and high-value fatty acids. Mar Drugs. 2021;19(7):386. https://doi.org/10.3390/md19070386.
Límová M. Active wound coverings: bioengineered skin and dermal substitutes. Surg Clin N Am. 2010;90(6):1237–55. https://doi.org/10.1016/j.suc.2010.08.004.
Lin HC, Li WH, Chen CC, Cheng TH, Lan YH, Huang MD, Chang HY, et al. Diverse enzymes with industrial applications in four thraustochytrid genera. Front Microbiol. 2020;11:2574.
Louca P, Murray B, Klaser K, Graham MS, Mazidi M, Leeming ER, et al. Modest effects of dietary supplements during the COVID-19 pandemic: insights from 445 850 users of the COVID-19 Symptom Study app. BMJ Nutr Prev Health. 2021;4(1):149–57. https://doi.org/10.1136/bmjnph-2021-000250.
Mądry E, Malesza IJ, Subramaniapillai M, Czochralska-Duszyńska A, Walkowiak M, Miśkiewicz-Chotnicka A, et al. Body fat changes and liver safety in obese and overweight women supplemented with conjugated linoleic acid: a 12-week randomised, double-blind, placebo-controlled trial. Nutrients. 2020;12(6):1811. https://doi.org/10.3390/nu12061811.
Maliszewska K, Adamska-Patruno E, Miniewska K, Bauer W, Mojsak M, Kretowski A. PET/MRI-evaluated brown adipose tissue activity may be related to dietary MUFA and omega-6 fatty acids intake. Sci Rep. 2022;12(1):4112. https://doi.org/10.1038/s41598-022-08125-z.
Maltsev Y, Maltseva K. Fatty acids of microalgae: diversity and applications. Rev Environ Sci Biotechnol. 2021;20:515–47. https://doi.org/10.1007/s11157-021-09571-3.
Manolis AA, Manolis TA, Melita H, Manolis AS. Features of a balanced healthy diet with cardiovascular and other benefits. Curr Vasc Pharmacol. 2023;21(3):163–84. https://doi.org/10.2174/1570161121666230327135916.
Mehni ME, Samadlouie HR, Rajaei A. Enhancement of oil productivity of mortierella alpine and investigation into the potential of pickering oil-in-water emulsions to improve its oxidative stability. Food Sci Nutr. 2021. https://doi.org/10.1002/fsn3.2651.
Miura K, Way M, Jiyad Z, Marquart L, Plasmeijer EI, Campbell S, et al. Omega-3 fatty acid intake and decreased risk of skin cancer in organ transplant recipients. Eur J Nutr. 2021;60(4):1897–905. https://doi.org/10.1007/s00394-020-02378-y.
Moreira ASP, Gonçalves J, Conde TA, Couto D, Melo T, Maia IB, et al. Chrysotila pseudoroscoffensis as a source of high-value polar lipids with antioxidant activity: a lipidomic approach. Algal Res. 2022;66: 102756. https://doi.org/10.1016/j.algal.2022.102756.
Morita M, Kuba K, Ichikawa A, Nakayama M, Katahira J, Iwamoto R, et al. The lipid mediator protectin D1 inhibits influenza virus replication and improves severe influenza. Cell. 2013;153(1):112–25. https://doi.org/10.1016/j.cell.2013.02.027.
Nascimento SM, Pisani LP. Update on the influence of fatty acids in epigenetic programming mechanisms. Nutrire. 2021. https://doi.org/10.1186/s41110-021-00142-8.
Nursyifa Fadiyah N, Megawati G, Erlangga LD. Potential of omega 3 supplementation for coronavirus disease 2019 (COVID-19): a scoping review. Int J Gen Med. 2022;15:3915–22. https://doi.org/10.2147/IJGM.S357460.
O’Reilly ME, Lenighan YM, Dillon E, Kajani S, Curley S, Bruen R, et al. Conjugated linoleic acid and alpha linolenic acid improve cholesterol homeostasis in obesity by modulating distinct hepatic protein pathways. Mol Nutr Food Res. 2020;64(7): e1900599. https://doi.org/10.1002/mnfr.201900599.
Odutuga AA, Adisa AO, Obaleye JA. Zinc and essential fatty acids modulate bone growth and metabolism in rats. Biokemistri. 2019;7(2).
Ogata R, Mori S, Kishi S, Sasaki R, Iwata N, Ohmori H, et al. Linoleic acid upregulates Microrna-494 to induce quiescence in colorectal cancer. Int J Mol Sci. 2021;23(1):225. https://doi.org/10.3390/ijms23010225.
Oh SY, Lee SJ, Jung YH, Lee HJ, Han HJ. Arachidonic acid promotes skin wound healing through induction of human MSC migration by MT3-MMP-mediated fibronectin degradation. Cell Death Dis. 2015;6(5): e1750. https://doi.org/10.1038/cddis.2015.114.
Parchuri P, Pappanoor A, Naeem A, Durrett TP, Welti R, Sreedhar RV. Lipidome analysis and characterization of Buglossoides arvensis acyltransferases that incorporate polyunsaturated fatty acids into triacylglycerols. Plant Sci. 2022;324: 111445. https://doi.org/10.1016/j.plantsci.2022.111445.
Park H, Kwak M, Seo J, Ju J, Heo S, Park S, et al. Enhanced production of carotenoids using a Thraustochytrid microalgal strain containing high levels of docosahexaenoic acid-rich oil. Bioprocess Biosyst Eng. 2018;41:1355–70. https://doi.org/10.1007/s00449-018-1963-7.
Parke MA, Perez-Sanchez A, Zamil DH, Katta R. Diet and skin barrier: the role of dietary interventions on skin barrier function. Dermatol Pract Concept. 2021;11(1): e2021132. https://doi.org/10.5826/dpc.1101a132.
Parsons S, Allen MJ, Chuck CJ. Coproducts of algae and yeast-derived single cell oils: a critical review of their role in improving biorefinery sustainability. Biores Technol. 2020;303(303): 122862. https://doi.org/10.1016/j.biortech.2020.122862.
Patel A, Karageorgou D, Rova E, Katapodis P, Rova U, Christakopoulos P, et al. An overview of potential oleaginous microorganisms and their role in biodiesel and omega-3 fatty acid-based industries. Microorganisms. 2020;8(3):434. https://doi.org/10.3390/microorganisms8030434.
Patel A, Pruthi V, Pruthi PA. Synchronized nutrient stress conditions trigger the diversion of CDP-DG pathway of phospholipids synthesis towards de novo TAG synthesis in oleaginous yeast escalating biodiesel production. Energy. 2017;139:962–74. https://doi.org/10.1016/j.energy.2017.08.052.
Patel AK, Albarico FPJB, Perumal PK, Vadrale AP, et al. Algae as an emerging source of bioactive pigments. Bioresour Technol. 2022;351: 126910.
Patel AK, Chauhan AS, Kumar P, Michaud P, Gupta VK, Chang JS, et al. Emerging prospects of microbial production of omega fatty acids: recent updates. Bioresour Technol. 2022;360: 127534. https://doi.org/10.1016/j.biortech.2022.127534.
Patel AK, Choi YY, Sim SJ. Emerging prospects of mixotrophic microalgae: way forward to bioprocess sustainability, environmental remediation and cost-effective biofuels. Bioresour Technol. 2020;300: 122741.
Patel AK, John J, Hong ME, Sim SJ. A sustainable mixotrophic microalgae cultivation from dairy wastes for carbon credit, bioremediation and lucrative biofuels. Bioresour Technol. 2020;313: 123681. https://doi.org/10.1016/j.biortech.2020.123681.
Patel AK, John J, Hong ME, Sim SJ. Effect of light conditions on mixotrophic cultivation of green microalgae. Bioresour Technol. 2019;282:245–53. https://doi.org/10.1016/j.biortech.2019.03.024.
Patel AK, Katiyar R, Chen CW, Singhania RR, Awasthi MK, Bhatia SK, Bhaskar T, Dong CD. Antibiotic bioremediation by new generation biochar: Recent updates. Bioresour Technol. 2022;358: 127384. https://doi.org/10.1016/j.biortech.2022.127384.
Patel AK, Kumar P, Chen CW, Tambat VS, Nguyen TB, Hou CY, Chang JS, Dong CD, Singhania RR. Nano magnetite assisted flocculation for efficient harvesting of lutein and lipid producing microalgae biomass. Bioresour Technol. 2022;363: 128009. https://doi.org/10.1016/j.biortech.2022.128009.
Patel AK, Singhania RR, Awasthi M, Varjani S, Bhatia SK, Tsai ML, Hseih SL, Chen CW, Dong CD. Emerging role of macro- and microalgae as prebiotic. Microb Cell Fact. 2021. https://doi.org/10.1186/s12934-021-01601-7.
Patel AK, Singhania RR, Chen CW, Dong CD. Algal polysaccharide: current status and future perspectives. Phytochem Rev. 2022. https://doi.org/10.1007/s11101-021-09799-5.
Patel AK, Singhania RR, Chen CW, Tseng YS, Kuo CH, Wu CH, Dong CD. Advances in micro-and nano bubbles technology for application in biochemical processes. Environ Technol Innov. 2021;23: 101729. https://doi.org/10.1016/j.eti.2021.101729.
Patel AK, Singhania RR, Dong CD, Obulisami PK, Sim SJ. Mixotrophic biorefinery: a promising algal platform for sustainable biofuels and high value coproducts. Renew Sust Energ Rev. 2021;152: 111669. https://doi.org/10.1016/j.rser.2021.111669.
Patel AK, Singhania RR, Frank Paolo JBA, Pandey A, Chen CW, Dong CD. Organic wastes bioremediation and its changing prospects. Sci Total Environ. 2022;824: 153889. https://doi.org/10.1016/j.scitotenv.2022.153889.
Patel AK, Singhania RR, Pal A, Chen CW, Dong CD. Advances on tailored biochars for bioremediation of antibiotics, pesticides and polycyclic aromatic hydrocarbon pollutants from aqueous and solid phases. Sci Total Environ. 2022;824(817): 153054.
Patel AK, Singhania RR, Sim SJ, Dong CD. Recent advancements in mixotrophic bioprocessing for production of high value microalgal products. Bioresour Technol. 2021;320: 124421. https://doi.org/10.1016/j.biortech.2020.124421.
Patel AK, Tseng YS, Singhania RR, Chen CW, Chang JS, Dong CD. Novel application of microalgae platform for biodesalination process: a review. Bioresour Technol. 2021;337: 125343. https://doi.org/10.1016/j.biortech.2021.125343.
Patel AK, Vadrale AP, Tseng YS, Chen CW, Dong CD, Singhania RR. Bioprospecting of marine microalgae from Kaohsiung seacoast for lutein and lipid production. Bioresour Technol. 2022;351: 126928. https://doi.org/10.1016/j.biortech.2022.126928.
Patel AK, Tambat VS, Chen CW, Chauhan AS, Kumar P, Vadrale AP, Dong CD, Singhania RR. Recent advancements in astaxanthin production from microalgae: a review. Bioresour Technol. 2022;364: 128030.
Peppone LJ, Inglis JE, Mustian KM, Heckler CE, Padula GDA, Mohile SG, et al. Multicenter randomized controlled trial of omega-3 fatty acids versus omega-6 fatty acids for the control of cancer-related fatigue among breast cancer survivors. JNCI Cancer Spectr. 2019;3(2):pkz005. https://doi.org/10.1093/jncics/pkz005.
Pifferi F, Laurent B, Plourde M. Lipid transport and metabolism at the blood–brain interface: implications in health and disease. Front Physiol. 2021;12: 645646. https://doi.org/10.3389/fphys.2021.645646.
Polavarapu S, Dwarakanath BS, Das UN. Arachidonic acid activates extrinsic apoptotic pathway to enhance tumoricidal action of bleomycin against IMR-32 cells. Prostaglandins Leukot Essent Fatty Acids. 2018;132:16–22. https://doi.org/10.1016/j.plefa.2018.04.001.
Ponnampalam EN, Sinclair AJ, Holman BWB. The sources, synthesis and biological actions of omega-3 and omega-6 fatty acids in red meat: an overview. Foods. 2021;10(6):1358. https://doi.org/10.3390/foods10061358.
Prasad P, Anjali P, Sreedhar RV. Plant-based stearidonic acid as sustainable source of omega-3 fatty acid with functional outcomes on human health. Crit Rev Food Sci Nutr. 2021;61(10):1725–37.
Quinn JM, Gillespie MT. Modulation of osteoclast formation. Biochem Biophys Res Commun. 2005;328(3):739–45. https://doi.org/10.1016/j.bbrc.2004.11.076.
Ramsden CE, Domenichiello AF, Yuan ZX, Sapio MR, Keyes GS, Mishra SK, et al. A systems approach for discovering linoleic acid derivatives that potentially mediate pain and itch. Sci Signal. 2017;10(493): eaal5241. https://doi.org/10.1126/scisignal.aal5241.
Remize M, Brunel Y, Silva JL, Berthon JY, Filaire E. Microalgae n-3 PUFAs production and use in food and feed industries. Mar Drugs. 2021;19(2):113. https://doi.org/10.3390/md19020113.
Rosenson RS, Brewer HB, Ansell BJ, Barter P, Chapman MJ, Heinecke JW, et al. Dysfunctional HDL and atherosclerotic cardiovascular disease. Nat Rev Cardiol. 2016;13(1):48–60. https://doi.org/10.1038/nrcardio.2015.124.
Saejung C, Puensungnern L. Evaluation of molasses-based medium as a low cost medium for carotenoids and fatty acid production by photosynthetic bacteria. Waste and Biomass Valoriz. 2020;11:143–52.
Samakova A, Gazova A, Sabova N, Valaskova S, Jurikova M, Kyselovic J. The PI3k/Akt pathway is associated with angiogenesis, oxidative stress and survival of mesenchymal stem cells in pathophysiologic condition in ischemia. Physiol Res. 2019;68(Suppl. 2):S131–8. https://doi.org/10.33549/physiolres.934345.
Santin A, Balzano S, Russo MT, Palma Esposito F, Ferrante MI, Blasio M, et al. Microalgae-based PUFAs for food and feed: current applications, future possibilities, and constraints. J Mar Sci Eng. 2022;10(7):844. https://doi.org/10.3390/jmse10070844.
Sayeda MA, Ali GH, El-Baz FK. Potential production of omega fatty acids from microalgae. Int J Pharm Sci Rev Res. 2015;34(2):210–5.
Sayegh F, Elazzazy A, Bellou S, Moustogianni A, Elkady AI, Baeshen MN, et al. Production of polyunsaturated single cell oils possessing antimicrobial and anticancer properties. Ann Microbiol. 2016;66:937–48. https://doi.org/10.1007/s13213-015-1176-0.
Serini S, Calviello G. New insights on the effects of dietary omega-3 fatty acids on impaired skin healing in diabetes and chronic venous leg ulcers. Foods. 2021;10(10):2306. https://doi.org/10.3390/foods10102306.
Serra JL, Rodrigues AMDC, de Freitas RA, Meirelles AJA, Darnet SH, Silva LHMD. Alternative sources of oils and fats from Amazonian plants: fatty acids, methyl tocols, total carotenoids and chemical composition. Food Res Int. 2019;116:12–9. https://doi.org/10.1016/j.foodres.2018.12.028.
Sertorio MN, de Souza EA, Pisani LP. Update on the influence of fatty acids in epigenetic programming mechanisms. Nutrire. 2021;46(2):1–11. https://doi.org/10.1186/s41110-021-00142-8.
Shah A, Yang W, Mohamed H, Zhang Y, Song Y. Microbe: a hidden treasure of polyunsaturated fatty acids. Front Nutr. 2022;9: 827837.
Shah AM, Mohamed H, Zhang Z, Song Y. Isolation, characterization and fatty acid analysis of Gilbertella persicaria DSR1: a potential new source of high value single-cell oil. Biomass Bioenerg. 2021;151: 106156. https://doi.org/10.1016/j.biombioe.2021.106156.
Shrestha N, Sleep SL, Cuffe JSM, Holland OJ, Perkins AV, Yau SY, et al. Role of Omega-6 and omega-3 fatty acids in fetal programming. Clin Exp Pharmacol Physiol. 2020;47(5):907–15. https://doi.org/10.1111/1440-1681.13244.
Silva MET, Martins MA, Leite MO, Milião GL, Coimbra JSR. Microalga Scenedesmus obliquus: extraction of bioactive compounds and antioxidant activity. Rev Cien Agronom. 2021. https://doi.org/10.5935/1806-6690.20210036.
Sim SJ, John J, Hong ME, Patel AK. Split mixotrophy: a novel mixotrophic cultivation strategy to improve mixotrophic effects in microalgae cultivation. Bioresour Technol. 2019;291: 121820.
Simard M, Tremblay A, Morin S, Martin C, Julien P, Fradette J, et al. α-linolenic acid and linoleic acid modulate the lipidome and the skin barrier of a tissue-engineered skin model. Acta Biomater. 2022;140:261–74. https://doi.org/10.1016/j.actbio.2021.11.021.
Šimat V. Nutraceuticals and pharmaceuticals from marine fish and invertebrates. Mar Drugs. 2021;19(7):401. https://doi.org/10.3390/md19070401.
Simopoulos AP. An increase in the omega-6/omega-3 fatty acid ratio increases the risk for obesity. Nutrients. 2016;8(3):128. https://doi.org/10.3390/nu8030128.
Singh P, Guldhe A, Kumari S, Rawat I, Bux F. Investigation of combined effect of nitrogen, phosphorus and iron on lipid productivity of microalgae Ankistrodesmus falcatus KJ671624 using response surface methodology. Biochem Eng J. 2015;94:22–9. https://doi.org/10.1016/j.bej.2014.10.019.
Skoracka K, Eder P, Łykowska-Szuber L, Dobrowolska A, Krela-Kaźmierczak I. Diet and nutritional factors in male (in) fertility—underestimated factors. J Clin Med. 2020;9(5):1400. https://doi.org/10.3390/jcm9051400.
Sonnweber T, Pizzini A, Nairz M, Weiss G, Tancevski I. Arachidonic acid metabolites in cardiovascular and metabolic diseases. Int J Mol Sci. 2018;19(11):3285. https://doi.org/10.3390/ijms19113285.
Sroczyk EA, Berniak K, Jaszczur M, Stachewicz U. Topical electrospun patches loaded with oil for effective gamma linoleic acid transport and skin hydration towards atopic dermatitis skincare. Chem Eng J. 2022;429: 132256. https://doi.org/10.1016/j.cej.2021.132256.
Su G, Jiao K, Chang J, Li Z, Guo X, Sun Y, Zeng X, Lu Y, Lin L. Enhancing total fatty acids and arachidonic acid production by the red microalgae Porphyridium purpureum. Bioresour Bioproc. 2016;3:33. https://doi.org/10.1186/s40643-016-0110-z.
Suhaimi N, Maeda Y, Yoshino T, Tanaka T. Effects of fatty acid synthase-inhibitors on polyunsaturated fatty acid production in marine diatom Fistulifera solaris JPCC DA0580. J Biosci Bioeng. 2022;133(4):340–6. https://doi.org/10.1016/j.jbiosc.2021.12.014.
Suraiya S, Ahmmed MK, Haq M. Immunity boosting roles of biofunctional compounds available in aquafoods: a review. Heliyon. 2022;8(5): e09547. https://doi.org/10.1016/j.heliyon.2022.e09547.
Suzuki A, Minamide M, Iwaya C, Ogata K, Iwata J. Role of metabolism in bone development and homeostasis. Int J Mol Sci. 2020;21(23):8992. https://doi.org/10.3390/ijms21238992.
Szczepańska P, Hapeta P, Lazar Z. Advances in production of high-value lipids by oleaginous yeasts. Crit Rev Biotechnol. 2022;42(1):1–22. https://doi.org/10.1080/07388551.2021.1922353.
Taha AY. Linoleic acid–good or bad for the brain? NPJ Sci Food. 2020;4(1):1. https://doi.org/10.1038/s41538-019-0061-9.
Taipale S, Peltomaa E, Salmi P. Variation in ω-3 and ω-6 polyunsaturated fatty acids produced by different phytoplankton taxa at early and late growth phase. Biomolecules. 2020;10(4):559. https://doi.org/10.3390/biom10040559.
Tong H, Zhang S, Shen W, Chen H, Salazar C, Schneider A, et al. Lung function and short-term ambient air pollution exposure: differential impacts of omega-3 and omega-6 fatty acids. Ann Am Thorac Soc. 2022;19(4):583–93. https://doi.org/10.1513/AnnalsATS.202107-767OC.
Wang DD, Li Y, Chiuve SE, Stampfer MJ, Manson JE, Rimm EB, et al. Association of specific dietary fats with total and cause-specific mortality. JAMA Intern Med. 2016;176(8):1134–45. https://doi.org/10.1001/jamainternmed.2016.2417.
Wang X, He MJ, Chen XJ, Bai YT, Zhou G. Glaucocalyxin A impairs tumor growth via amplification of the ATF4/CHOP/CHAC1 cascade in human oral squamous cell carcinoma. J Ethnopharmacol. 2022;290: 115100. https://doi.org/10.1016/j.jep.2022.115100.
Wang Y, Shi J, Gong L. Gamma linolenic acid suppresses hypoxia-induced proliferation and invasion of non-small cell lung cancer cells by inhibition of HIF1α. Genes Genom. 2020;42(8):927–35. https://doi.org/10.1007/s13258-020-00961-5.
Wang Z, Wang S, Feng Y, Wan W, Zhang H, Bai X, et al. Obtaining high-purity docosahexaenoic acid oil in thraustochytrid Aurantiochytrium through a combined metabolic engineering strategy. J Agric Food Chem. 2021;69(35):10215–22. https://doi.org/10.1021/acs.jafc.1c03781.
Weill P, Plissonneau C, Legrand P, Rioux V, Thibault R. May omega-3 fatty acid dietary supplementation help reduce severe complications in Covid-19 patients? Biochimie. 2020;179:275–80. https://doi.org/10.1016/j.biochi.2020.09.003.
Wu X, Yang X, Liang Q, Xue X, Huang J, Wang J, et al. Drugs for the treatment of glaucoma: targets, structure–activity relationships and clinical research. Eur J Med Chem. 2021;226: 113842. https://doi.org/10.1016/j.ejmech.2021.113842.
Yamaguchi A, Stanger L, Freedman JC, Prieur A, Thav R, Tena J, et al. Supplementation with omega-3 or omega-6 fatty acids attenuates platelet reactivity in postmenopausal women. Clin Transl Sci. 2022;15(10):2378–91. https://doi.org/10.1111/cts.13366.
Yau SY, Yip YSL, Formolo DA, He S, Lee THY, Wen C, et al. Chronic consumption of a high linoleic acid diet during pregnancy, lactation and post-weaning period increases depression-like behavior in male, but not female offspring. Behav Brain Res. 2022;416: 113538. https://doi.org/10.1016/j.bbr.2021.113538.
Yoon SY, Ahn D, Hwang JY, Kang MJ, Chung SJ. Linoleic acid exerts antidiabetic effects by inhibiting protein tyrosine phosphatases associated with insulin resistance. J Funct Foods. 2021;83: 104532. https://doi.org/10.1016/j.jff.2021.104532.
Youn K, Lee S, Jun M. Gamma-linolenic acid ameliorates Aβ-induced neuroinflammation through NF-κB and MAPK signalling pathways. J Funct Foods. 2018;42:30–7. https://doi.org/10.1016/j.jff.2017.12.065.
Yu BS, Sung YJ, Choi HI, Sirohi R, Sim SJ. Concurrent enhancement of CO2 fixation and productivities of omega-3 fatty acids and astaxanthin in Haematococcus pluvialis culture via calcium-mediated homeoviscous adaptation and biomineralization. Bioresour Technol. 2021;340: 125720. https://doi.org/10.1016/j.biortech.2021.125720.
Yu Q, Liu Z, Xu H, Zhang B, Zhang M, Li M. TiO2 nanoparticles promote the production of unsaturated fatty acids (UFAs) fighting against oxidative stress in pichia pastoris. RSC Adv. 2015;5(51):41033–40. https://doi.org/10.1039/C5RA02366A.
Zanoaga O, Jurj A, Raduly L, Cojocneanu-Petric R, Fuentes-Mattei E, Wu O, et al. Implications of dietary ω-3 and ω-6 polyunsaturated fatty acids in breast cancer. Exp Ther Med. 2018;15(2):1167–76. https://doi.org/10.3892/etm.2017.5515.
Zhan Q, Tian Y, Han L, Wang K, Wang J, Xue C. The opposite effects of Antarctic krill oil and arachidonic acid-rich oil on bone resorption in ovariectomized mice. Food Funct. 2020;11(8):7048–60. https://doi.org/10.1039/d0fo00884b.
Zhang S, Chen X, Sen B, Bai M, He Y, Wang G. Exogenous antioxidants improve the accumulation of saturated and polyunsaturated fatty acids in Schizochytrium sp. PKU#Mn4. Mar Drug. 2021;19(10):559. https://doi.org/10.3390/md19100559.
Zhang T, Li M, Yang R, Zhang D, Guan J, Yu J, et al. Therapeutic efficacy of lipid emulsions of docetaxel-linoleic acid conjugate in breast cancer. Int J Pharm. 2018;546(1–2):61–9. https://doi.org/10.1016/j.ijpharm.2018.05.032.
Zhang Y, Chen H, Zhang W, Cai Y, Shan P, Wu D, et al. Arachidonic acid inhibits inflammatory responses by binding to myeloid differentiation factor-2 (MD2) and preventing MD2/toll-like receptor 4 signaling activation. Biochim Biophys Acta Mol Basis Dis. 2020;1866(5): 165683. https://doi.org/10.1016/j.bbadis.2020.165683.
Zhao H, Lv M, Liu Z, Zhang M, Wang Y, Ju X, et al. High-yield oleaginous fungi and high-value microbial lipid resources from Mucoromycota. Bioenerg Res. 2021;14(4):1196–206. https://doi.org/10.1007/s12155-020-10219-3.
Zong G, Liu G, Willett WC, Wanders AJ, Alssema M, Zock PL, et al. Associations between linoleic acid intake and incident type 2 diabetes among US men and women. Diabetes Care. 2019;42(8):1406–13. https://doi.org/10.2337/dc19-0412.
Acknowledgements
The author acknowledges the Ph.D. program of the Institute of Aquatic Science and Technology, College of Hydrosphere, National Kaohsiung University and Science and Technology.
Funding
RRS acknowledges NSTC, Taiwan, for funding support (Ref. No. NSTC 112-2222-E-992-006-MY2) and the National Kaohsiung University of Science and Technology 112 Annual Marine Characteristics Sustainable Development Research Program (Program code 112A14).
Author information
Authors and Affiliations
Contributions
ASC: writing—original draft, literature review; AKP: supervision, writing—review and editing; VN: literature review, draft preparation; RRS: supervision, writing—review and editing, funding acquisition; C-WC: supervision, writing—review, and editing; AKP: literature review, draft preparation; supervision; TR: literature review, draft preparation; C-DD: supervision, writing—review and editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
In this article the affiliation details for Author Tirath Raj were incorrectly given as Center for Advanced Bioenergy and Bioproducts Innovation (CABBI), University of Illinois Urbana Champaign, AESB, 1304 West Pennsylvania Avenue I, Urbana, IL 61801, USA but should have been Department of Agricultural and Biological Engineering, University of Illinois Urbana-Champaign, 1304 West Pennsylvania Avenue, Urbana, IL, 61801, USA.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chauhan, A.S., Patel, A.K., Nimker, V. et al. Biorefining of essential polyunsaturated fatty acids from microbial sources: current updates and prospects. Syst Microbiol and Biomanuf 4, 425–447 (2024). https://doi.org/10.1007/s43393-023-00207-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43393-023-00207-x