Abstract
l-Threonine is an important amino acid, which can be added in food, medicine or feed. In this paper, an l-threonine-producing strain was constructed using a modified CRISPR gene editing technology. Here, it was verified that the mutation of glycine at position 433 of aspartate kinase AKI to arginine (thrAG1297A) relieve effectively the feedback inhibition of AKI by l-threonine. The trc promoter replaced the native promoter of thrA in the Escherichia coli XQ-12 genome, and thus increasing its expression level. Moreover, by modifying the glycolytic pathway, disruption of phosphofructokinase encoded by the gene pfkA and pyruvate kinase encoded by the gene pykF increased l-threonine production. Then, the ppc gene encoding phosphoenolpyruvate carboxylase was overexpressed by replacing its native promoter with the core-trc promoter, which slightly improved the growth of the l-threonine-producing strain E. coli XQ-12 as well as the l-threonine production. In addition, it was found that further absence of the gene crr in the PTS system and the gene tdh encoding threonine dehydrogenase improved significantly l-threonine production. The final amounts of l-threonine produced by plasmid-free, antibiotic-free and inducer-free strains E. coli XQ-12.4 were 127.3 g/L and 3.536 g/L/h, respectively, in fed-batch fermentation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
l-Threonine, one of the nine essential amino acids, is widely used in animal production husbandry, food, medicine, health products and cosmetics [15]. For a long time, with the continuous and rapid growth of the demand for l-threonine in domestic and international markets, it is undoubtedly very important to study the efficient and economical production of l-threonine [3]. l-Threonine production methods mainly include proteolysis, chemical synthesis and microbial fermentation. At present, the industrial production of l-threonine mainly adopts microbial fermentation, which has almost completely replaced the traditional chemical synthesis and proteolytic method due to its absolute advantages in production cost, production efficiency and environmental pollution [5]. Escherichia coli has become a popular strain for transforming l-threonine with high yield in recent years because of the clear genetic background and short fermentation time.
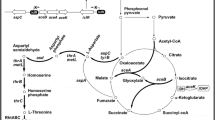
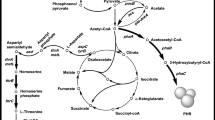
The l-threonine biosynthetic pathway in E. coli using glucose as carbon source can be divided into three modules: glycolysis, the tricarboxylic acid (TCA) cycle, and the l-threonine synthesis module [31]. Glucose enters the cell through the phosphoenolpyruvate-phosphoglucose transferase (PTS) system and generates phosphoenolpyruvate (PEP). Studies have shown that oxaloacetate supply is one of the limiting factors in the biosynthesis of l-aspartate family amino acids. Therefore, to optimize oxaloacetate supply, Wei used artificial promoter regulatory elements to modulate the expression of ppc, which encodes phosphoenolpyruvate carboxylase [27]. In the biosynthetic pathway of l-threonine, l-aspartate undergoes a five-step enzymatic reaction to produce l-threonine [30]. The first step is the conversion of l-aspartate to l-aspartyl-phosphate catalyzed by aspartokinase (AK) (Fig. 1). There are three isoenzymes of this enzyme in E. coli: AKI, AKII and AKIII, which are encoded by thrA, metL and lysC, respectively [30]. Intracellular AKI content is much higher than the other two enzymes, and its activity is feedback-inhibited by l-threonine [9]. Study has demonstrated that the regulatory domain of the bacterial enzyme is located in the intermediary region between the AK catalytic domain and the HSDH catalytic domain [18]. As shown in Fig. 2, Arabidopsis AK/HSDH showed similar domain organization with E. coli AK/HSDH, with two small regulatory domains located between the N-terminal AK catalytic domain and the C-terminal HSDH catalytic domain [7]. The second step is the reduction of l-aspartyl-phosphate to l-aspartate-4-semialdehyde catalyzed by aspartate dehydrogenase (ASD), which is encoded by asd in E. coli. In the third step, l-aspartate-4-semialdehyde is converted to l-homoserine catalyzed by homoserine dehydrogenase (HD). In the fourth step, l-homoserine is converted to homoserine phosphate catalyzed by homoserine kinase (HK). This reaction is encoded by thrB. In the fifth step, the homoserine phosphate is converted into l-threonine by threonine synthetase, which is encoded by thrC. l-threonine is catalyzed by threonine dehydrogenase (TDH, encoded by tdh) to produce 2-amino-3-keto butyrate [9].
With the maturing of technology, systems metabolic engineering has become an ideal method for transforming strains to produce amino acids. Lee et al. enhanced the l-threonine biosynthetic pathway by removing the feedback inhibition of aspartokinase I and III, deleting the transcriptional decay regulator (located in the thrL operon) and tdh as well as mutating ilvA to remove the l-threonine degradation pathway and deleting metA and lysA gene to make more precursors available for l-threonine biosynthesis [9]. Ding et al. found that the deletion of the genes arcA, iclR and tdcC can effectively increase the production of l-threonine in E. coli [1]. Xie et al. increased l-threonine accumulation by modification of glycolysis in E. coli THRD because of reducing acetate content [13, 26]. In addition, Su et al. found that when betaine was added to the fermentation medium, the metabolic fluxes into the pentose phosphate pathway (HMP) and the l-threonine biosynthesis pathway were increased by 57.3% and 10.1%, respectively [22].
However, maintaining the stable existence of intracellular plasmids mostly relies on the addition of antibiotics, which will cause problems such as environmental pollution, bacterial resistance and increased production costs [25]. How to construct an antibody-free screening system suitable for efficiently modifying E. coli is an important scientific problem to be solved in this thesis. In recent years, a new generation of artificial endonuclease technology, Clustered regularly interspaced short palindromic repeats/CRISPR-associated protein (CRISPR/Cas9), has been widely used in editing the genomes of a variety of organisms rapidly and efficiently [16]. Although E. coli has a shorter fermentation cycle than other strains, the engineered bacteria grow more slowly than the model bacteria of E. coli. To achieve rapid gene editing, we modified the pCas/pTargetF system in this study according to the method in the literature [11]. This study establishes a fast-acting genome editing tool that can be used with a wider range of E. coli strains and will also be useful for other Enterobacteriaceae species. As an example, E. coli XQ-12, a l-threonine-producing strain derived from E. coli MG1655 by conventional breeding methods, was used as the original strain to modify the l-threonine biosynthetic pathway and to reduce the content of by-products using an improved CRISPR-Cas9 platform to obtain a l-threonine high-producing strain E. coli XQ-12.4. The resultant strain XQ-12.4 produced 127.3 g/L of l-threonine with productivity of 3.536 g/L/h and yield of 0.31 g/(g glucose) after 36 h in fed-batch fermentation. These results demonstrate the effectiveness of combining protein engineering and metabolic pathway engineering to construct l-threonine high-producing strain from E. coli, and the resulted strain XQ-12.4 showed the potential to produce l-threonine in industry.
Materials and methods
Strains and plasmid
l-Threonine-producing strain E. coli XQ-12 (E. coli AHVhr AECr MFr Rifr Metl Thr−) was derived from the wild-type strain E. coli MG1655, which was mutagenized by multiple rounds of nitrosoguanidine (NTG) and ultraviolet (UV). E. coli XQ-12 was an l-threonine-auxotrophic (Thr−) and l-methionine-attenuated (Metl) mutant as well as resistant to α-amino-β-hydroxyvalerate (AHVhr), S-2-aminoethyl-l-cysteine (AECr), monofluoroacetate (MFr) and rifampicin (Rifr). Strains and plasmids involved in this study are listed in Table 1. The E. coli BL21(DE3) and the E. coli JM109 used for plasmid expression and plasmid construction, respectively, were aerobically cultivated at 37 °C in Luria–Bertani (LB) broth, containing (g/L) 10 NaCl, 5 yeast extract and 10 tryptone. Kanamycin (50 μg/mL) was added to the medium as required. Plasmids were constructed via homologous recombination. In addition, restriction endonucleases (Takara) and the DNA Ligase Kit Ver. 2.0 (Takara) were used to construct plasmids similarly. DNA sequencing and services of primer were provided by GENEWIZ Inc. (Suzhou, China) and EXSYN-BIO, respectively.
Medium and culture conditions
The single colonies of the recombinant strains were inoculated into LB liquid medium at 37 °C with a rotation speed of 100 r/min for 10–12 h. Then, 3 mL of the seed culture was transferred to 30 mL of fermentation medium in a 500 mL shake flask. Fermentation medium contains (per liter) 10 g glucose, 15 g (NH4)2SO4, 2 g KH2PO4, 0.4 g MgSO4·7H2O, 0.6 g KCl, 50 mg FeSO4·7H2O, 50 mg MnSO4·4H2O, 0.5 g corn steep powder, 18 mL beet molasses, 1 g betaine, 0.5 mL defoamer, 10 g CaCO3, adjust pH to 7.3 with dilute sodium hydroxide solution and the inoculum size was 10%. Fermentation was performed at 37 °C for 36 h.
Genome editing
As explained earlier, we used CRISPR/Cas9 genome editing system to perform general DNA manipulations in the E. coli genome. The process of gene pfkA deletion was presented as an example of genome manipulation. First, to obtain the donor DNA fragment, using the genomic DNA of E. coli XQ-12 strain as the template and the pfkA-up-F/pfkA-up-R and pfkA-down-F/pfkA-down-R as primers, the upstream and downstream homology arms were amplified, respectively, and then ligated by overlapping PCR to obtain the donor DNA fragment. Then, we got the 20-bp sgRNA spacer sequences were obtained from CRISPR RGEN Tools (http://www.rgenome.net/). The pTargetF plasmid was used as the template, and the pair of above complementary primers sgRNA-pfkA-F and sgRNA-pfkA-R were used for constructing the pTatargF plasmid. Then, 100 ng of pTargetF series DNA and 400 ng of donor DNA were transformed to the electroreceptor cells of E. coli XQ-12 which harbored the pRED-Cas9 plasmid. After electroporation, 1 mL of LB medium was added, and the cells were recovered at 30 °C for 2 h, then spread onto LB agar plates containing kanamycin and spectinomycin, incubating at 30 °C for appropriate time. Single colonies were identified by colony PCR before DNA sequencing. After that, the correct transformant was cultured at 30 °C in the LB medium containing kanamycin and IPTG to cure the plasmid pTarget. The colonies cured of plasmid pTarget were used for subsequent genome editing. Finally, the plasmid pCas9 in the strain was cured by cultivating overnight at 42 °C nonselectively.
Enzymatic analysis of the recombinant ThrA
AK enzyme activity was measured according to a previously reported method [4]. The amount of l-aspartate isohydroxamic acid produced by the reaction of l-aspartate, hydroxylamine and ATP was determined spectrophotometrically to indicate the enzymatic activity of AKI. Reaction system 1 mL: Tris·HCl buffer (pH 8.1) 94 mM, ATP 10.4 mM, l-aspartate 10 mM, MgSO4 1.6 mM, β-mercaptoethanol 10 mM, NH2OH 10 mM, KCl 0.8 M, 1/10 volume of enzyme. React at 26 °C for 30 min, add 1.0 mL FeCl3 (5% FeCl3 in 0.1 M HCl solution, 12% trichloroacetic acid, 3 M HCl in a volume ratio of 1:1:1 mixed) to terminate the reaction, centrifuge at 12,000 r min−1 for 5 min, the absorbance (OD540) of hydroxamic acid-Fe at 540 nm wavelength was measured spectrophotometrically, and the enzyme activity was expressed as OD540 × 1000. Different concentration of inhibitors l-threonine were added to the reaction system (the final concentrations of inhibitors were 0.2, 1.0, 5.0, and 10.0 mM, respectively), to study the effect of inhibitors on AK activity. The same amount of distilled water distilled water served as the control, and its enzymatic activity was defined as 100%.
Analytical techniques
Add 200 µL of fermentation broth to a test tube containing 4.8 mL of dilute hydrochloric acid (0.01 mmol L−1) to dilute 25 times, and mix by vortex. Using distilled water as a blank control, the cell concentration (OD600) was measured in a pre-warmed UV spectrophotometer. The samples were diluted 100 times and centrifuged at 12,000 r min−1 for 2 min. The glucose standard solution was used as a control, and the SBA-40E biosensor was used to analyze and determine the glucose concentration in the fermentation broth. Take 1 mL of fermentation broth, centrifuge at 10,000 r min−1 for 5 min to remove bacteria, use 2,4-dinitrofluorobenzene to derivatize l-threonine first, and filter it through a 0.22 μm pore size filter. Samples were run on a high performance liquid chromatography (HPLC) system (Agilent 1290 series; Agilent, Palo Alto, CA, USA) equipped with a C18 column (250 mm × 4.6 mm, 5 μm Waters, Milford, MA, USA). The column temperature was 33 °C, and detection was performed with a UV detector at a wavelength of 360 nm. The mobile phase was acetonitrile-sodium acetate buffer at a flow rate of 1 mL min−1.
Acetate was quantified by 1200 series HPLC system (Agilent Technology, USA) equipped with an aminex HPX-87H column (300 × 7.8 mm), and 5 mM H2SO4 was used as a mobile phase with a flow rate of 0.6 mL/min. The column temperature was maintained at 50 °C, and the UV absorption was determined at 210 nm [31].
Results and discussion
The modification of pCas/pTargetF system
As the initial strain E. coli XQ-12 is an engineered strain with relatively slow growth, it has low efficiency in gene editing. Therefore, we improved our laboratory’s pCas/pTargetF system according to previous literature reports [11] (Fig. 3). We replaced the temperature-sensitive replicator pSC101ts on pCas with the pSC101 replicator from pMW119, so that the E. coli strain XQ-12 could grow at optimal temperatures (37 °C). The mechanism of action is as follows: sacB gene encodes the enzyme levansucrase which can catalyze the hydrolysis of sucrose into levan. Excessive accumulation of levan is toxic to E. coli, thus allowing the addition of sacB gene to the plasmid pCas as an anti-selection marker. We call this updated system pEcCas/pTargetF [6, 23].
Screening of aspartokinase mutant for l-threonine production
It is well-recognized that the end-product of normal cellular anabolism has a feedback inhibitory effect on the first enzyme of the synthetic pathway, because the end-product can bind to allosteric enzymes [19]. Since this binding is reversible, when the final product of metabolism participates in metabolism and its concentration in the cell decreases, it will no longer bind to the allosteric enzyme, and the catalysis of the enzyme can continue [2]. Therefore, releasing the feedback inhibition of thrA by l-threonine is a key step to improve l-threonine production [20].
The AKI nucleotide sequence of the key enzyme in the l-threonine synthesis process of strain XQ-12 was sequenced and analyzed (Fig. 4a), indicating that there was one missense mutation in the amino acid sequence of thrA encoding gene of AKI in XQ-12 as compared with E. coli MG1655. Therefore, we evaluated this mutant to analyze whether its contribution to increased l-threonine production came from alleviation of feedback inhibition or from improved enzymatic activity. AKI and AKIG433R were simultaneously expressed, the enzyme concentration and the enzymatic property were determined. The SDS-PAGE results of wild-type AKI and AKIG433R are shown in Fig. 4b. It can be observed from Fig. 4b that the difference in expression level between wild-type AKI and AKIG433R was not significant.
Site-directed mutation of AKI of E. coli for improved l-threonine production via CRISPR/Cas9 gene editing technology. a Conservation analysis of AKI. The G433 residues mutated in this study are highlighted in red. Aspartate kinase sequences used for conservation analysis are provided in Supplementary Data 1. b SDS-PAGE of plasmids pEC-XK99E-AKI. M protein marker, 1–6 crude enzymes, 7 blank control. The target molecular weight is 68 kDa which was marked by red box. c, d The molecular docking results. The backbone of AKI is shown in a ribbon model on which several key residues are shown in a stick model. l-threonine is indicated in yellow. e Growth and l-threonine production of strains XQ-12, XQ-12.1 and XQ-12 AKIR433G. These data represent average values and standard deviations achieved from three independent fermentation experiments. f Enzyme activities of AKIs. These data represent average values and standard deviations achieved from three independent experiments
Then, the enzymatic property was done. 10 mΜ l-aspartate was used as substrate and the system was spiked with different concentration gradients of l-threonine used as inhibitor, and enzyme activity was calculated based on the observed formation of l-aspartic acid-β-hydroxamate. We found that AKIG433R enzyme activity increased by about 15% as compared to wild-type AKI in the presence of 0.2–10 mM l-threonine (Fig. 4f). Simultaneously, we mutated the base A at position 1297 of thrA back to G in the strain XQ-12 genome, and observed the changes in l-threonine production of this derived strain XQ-12 AKIR433G. As shown in Fig. 4e, the l-threonine production of XQ-12 AKIR433G strain after mutation was marginally lower than that of strain XQ-12, confirming that the mutation thrAA1297G may improve l-threonine production because of the alleviating feedback inhibition. We simultaneously aligned and analyzed 400 thrA sequences in the UniProt database (Fig. 4a). The predicted binding site G433 of AKI from E. coli. MG1655 is highly conserved among thrA enzymes from different sources. The protein structure of AKI (PDBID: 6MX1) was downloaded from the PDB website and the structure of the small molecule l-threonine from the website PubChem. We used the molecular docking software Schrödinger to dock AKI with the l-threonine for visual analysis of the interaction (Fig. 4c, d). As shown in Fig. 4d, the hydrogen bond between AKI and the product l-threonine at Gly433 can promote the interaction between l-threonine and AKI. Hydrogen bonding plays a key role between proteins and small molecule ligands, which makes the more stable binding between l-threonine and AKI [17], whereas the side chain of the amino acid residue was enlarged after mutating amino acid 433 in AKI from Gly to Arg. It is speculated that replacement of Gly by Arg at 433 in AKI may have a greater impact on the catalytic steric configuration of the enzyme and thus resulting in steric hindrance, which may be an important reason for the reduced feedback inhibition of the AKIG433R mutant [10]. This result indicated that the binding of AKIG433R to l-threonine is more unstable than that of wild-type AK1 protein. Therefore, we combined the CRISPR-Cas9 gene editing technology to replace the thrA promoter with a stronger trc promoter and increase its copy number on the XQ-12 genome, resulting in the strain XQ-12.1. Compared with the original strain XQ-12, the l-threonine production of strain XQ-12.1 increased by about 1.4 times (from 9.5 ± 0.3 to 13.2 ± 0.3 g/L) in shake-flasks fermentation (Fig. 4e).
Optimization expression of PPC improve l-threonine biosynthetic metabolic flux
The availability of oxaloacetate is one of the limiting factors for the production of amino acids in the l-aspartic acid family [27]. Therefore, the phosphoenolpyruvate carboxylase-coding gene ppc was cloned into pEC-XK99E, and thus resulted plasmid pEC-XK99E-PPC was transformed into the strain XQ-12.1. The resulted strain XQ-12.1/pEC-XK99E-PPC was batch cultured in fermentation medium containing 10 g L−1 glucose. However, it was observed that the yield of l-threonine in the strain XQ-12.1/pEC-XK99E-PPC was 3.8% lower than that in the control strain XQ-12.1 (Fig. 5b). Previous studies have shown that moderate expression of PPC favors l-threonine biosynthesis, while excess PPC activity has a negative impact [9]. Therefore, in order to optimize the supply of oxaloacetate and improve the production of l-threonine, we replaced the native promoter of the gene ppc with the trc promoter, resulted in strain XQ-12.1 Pppc::Ptrc. The flask fermentation results showed that the yield of l-threonine in strain XQ-12.1Pppc::Ptrc was 0.7 g/L higher than that in strain XQ-12.1, reaching to 14.1 g/L. Meanwhile, the growth of strain XQ-12.1 Pppc::Ptrc was significantly higher than that of strain XQ-12.1 (i.e., OD600 = 16.3 ± 0.35 vs. OD600 = 8.9 ± 0.3) (Fig. 5b). It may be that the replacement of the promoter allows the strain XQ-12.1 Pppc::Ptrc to modulating the expression of ppc to increase the oxaloacetate supply. The strain XQ-12.1 Pppc::Ptrc was also known as strain XQ-12.2. Compared with strain XQ-12.1, strain XQ-12.2 showed a slight increase in l-threonine production, while the biomass (OD600) increased to 16.3. By regulating the expression of PPC to increase the supply of oxaloacetate and to promote the biosynthesis of the precursor l-aspartate, the production of l-threonine was increased. This result is consistent with previous reports in the literature, in which the appropriate expression of PPC is beneficial to the biosynthesis of l-threonine because of the enhance of the flux from oxaloacetate to l-threonine synthesis [27].
Effect of PPC expression on l-threonine production. Variation of OD600 and l-threonine concentration during fermentation in strain XQ-12.1, XQ-12.1/pEC-XK99E-PPC and XQ-12.1 Pppc::Ptrc. These data represent average values and standard deviations achieved from three independent fermentation experiments
Modification of glycolytic pathway through the knockdown of genes pfkA and pykF
Phosphofructokinase (PFK) is an allosteric enzyme, and the rate of glycolysis is strictly dependent on the activity level of PFK [21]. E. coli contains two phosphofructokinase isoenzymes: PFKI and PFKII (encoded by pfkA and pfkB, respectively), and PFKI accounts for 90% of the entire enzyme activity while PFKII only accounts for 5–10% [12]. Deletion of pfkA and pfkB results in that fructose 6-phosphate cannot enter glycolysis and must be metabolized during the Pentose Phosphate Pathway (PPP) (Fig. 1). Phosphofructokinase I single deletion mutant (ΔpfkA) or phosphofructokinase I and II double deletion mutants (ΔpfkAΔpfkB) provide 6 mol of NADPH per mole of glucose in theory in [21].
In addition, NADPH flux through PPP was also found to be upregulated in pykF-deficient mutants [21]. It is widely known that NADPH is mainly formed by PPP in E. coli and many other organisms. Therefore, deletion of pfkA or pykF may increase NADPH synthesis, which may increase the production of NADPH-dependent products such as l-threonine [28]. Therefore, we combined CRISPR-cas9 technology to knock out pfkA or/and pykF in strain XQ-12.2. To investigate whether pykF and pfkA deletions affect E. coli XQ-12 biomass and l-threonine production, we performed shake-flask fermentations. After 36 h of fermentation, the l-threonine yield of strain XQ-12.2ΔpfkAΔpykF (19.3 ± 0.26 g/L) was significantly higher than that of strain XQ-12.2 (14.1 ± 0.29 g/L) (Fig. 6). At the same time, the l-threonine production of strain XQ-12.2ΔpfkAΔpykF was also increased as compared with the single-gene knockout strains XQ-12.2ΔpfkA and XQ-12.2ΔpykF. However, the growth of strain XQ-12.2ΔpfkAΔpykF was significantly decreased as compared with XQ-12.2 and single-gene knockout strains XQ-12.2ΔpfkA and XQ-12.2ΔpykF. Among them, the OD600 of strain XQ-12.2ΔpfkAΔpykF was 8.15 ± 0.3, while the strain XQ-12.2 was 16.34 ± 0.26. The strain XQ-12.2ΔpfkAΔpykF was named XQ-12.3.
Effect of knockout of genes crr and tdh on l-threonine production
Glucose enters cells through the phosphoenolpyruvate-phosphoglucotransferase (PTS) system, and generates pyruvate (PYR) through the glycolysis (Fig. 7a) [31]. When the cellular energy supply is sufficient, acetyl-CoA eventually generates acetate co-catalyzed by phosphate acetyltransferase (Pta) and acetate kinase (AckA). It should be note that 5 g L−1 of acetate are toxic to E. coli because of the decrease in intracellular pH [24]. In addition, the accumulation of excess acetate will waste the substrate and cause an increase in fermentation cost. In order to resolve this problem, PTS system was modified to reduce the accumulation of acetate in this study.
Effect of knocking out the crr on l-threonine production and acetate secretion. a Schematic of the PTS sugar transport system. Transport systems capable of internalizing glucose in E. coli. The PTSGlc is composed of the soluble non-sugar-specific protein component Enzyme I (EI, ptsI) and phosphohistidine carrier protein (HPr, ptsH), soluble glucose-specific enzyme IIAGlc (crr), and membrane-integral glucose-specific permease IICBGlc (ptsG). b Variation of OD600 and l-threonine concentration during fermentation in strain XQ-12.3 and XQ-12.4. These data represent average values and standard deviations achieved from three independent fermentation experiments. c Acetate secretion of XQ-12.3 and XQ-12.3Δcrr with 10 g L−1 glucose under aerobic conditions. The pH was not controlled
The three types of proteins that constitute the PTS system are: phosphoenolpyruvate-dependent protein kinaseI (PEP-dependent protein kinase enzyme I, EI), phosphohistidine transport protein HPr (Heat-stable histidine-phosphoryl protein, HPr) and the multi-protein complex EIIS [14]. EIIS is a multi-protein complex, and different carbohydrates are phosphorylated by specific EIIS involved in the transport process. The EIIS used for transporting glucose consists of EIIAGlc and EIIBCGlc, which are encoded by the genes crr and ptsG, respectively. It has been reported in the literature that although the deletion of the crr gene causes a decrease in the ability of the strain to consume glucose, the resulting decrease in the acetate content is beneficial to the synthesis of l-threonine [31].These positive results were also found in this study, in which the maximum acetate content of strain XQ-12.3Δcrr was 1.65 g L−1, only 42.85% of the control strain XQ-12.3 (Fig. 7c). In addition, the growth of strain XQ-12.3Δcrr was not significantly inhibited during fermentation as shown in Fig. 7b. Compared with the control strain XQ-12.3, the l-threonine production of strain XQ-12.3Δcrr was increased by 47.5%, reached to 20.5 g L−1. In conclusion, deletion of the gene crr reduces the acetate accumulation during shake-flask fermentation. Apart from this, deletion of the gene crr encoding domain A of the glucose-specific enzyme II of PTS reduced the consumption of the precursor substance PEP by PTS, ultimately leading to an increase in l-threonine production. What is more, with the prolongation of fermentation time, the biomass of strain XQ-12.3Δcrr was significantly higher than that of the control strain XQ-12.3 (Fig. 7b). The above results indicated that the main pathway of acetate synthesis was weakened after the gene knockout of the crr genome, which not only reduced the production of acetate, but also increased the growth of the strain and the synthesis of l-threonine.
In E. coli, TDH is involved in the catabolism of l-threonine to form 2-amino-3-ketobutyric acid [29]. To improve the efficiency of l-threonine production, we reduced the l-threonine catabolic pathway to construct a tdh-deficient strain XQ-12.3ΔcrrΔtdh in this study. We found that double knockout of crr and tdh genes reduced the l-threonine degradation pathway and increased l-threonine production. The resulted strain XQ-12.3ΔcrrΔtdh produced 23.8 g L−1 in shake-flask fermentation. The strain XQ-12.3ΔcrrΔtdh strain was named XQ-12.4.
Production performance of strain XQ-12.4 in fed-batch fermentation
To assess the ability of engineered strain XQ-12.4 in producing l-threonine, the fed-batch fermentation was carried out in a 5-L jar fermenter containing 2 L fermentation medium. Figure 8 shows the time profiles of the fed-batch fermentations. The final titer and productivity of strain XQ-12.4 reached 127.3 g/L and 3.536 g/L/h, respectively, with a maximum OD600 of 35.5 in the 5-L bioreactor (Fig. 8).
Overall, the final strain XQ-12.4 showed an efficient l-threonine production under fed-batch fermentation, making it a very promising platform for l-threonine production.
Conclusion
l-Threonine is widely used in the pharmaceutical industry and has broad prospects. Previous researchers have reported how to produce l-threonine using a plasmid system. However, plasmid-based production can imply complications such as increased the coexistence of multiple plasmids and genetic instability [8]. In this study, we worked to achieve gene editing of the target gene in the genome to construct a high l-threonine yielding strain E. coli XQ-12.4 from an l-threonine-producing strain E. coli XQ-12. The resulted strain E. coli XQ-12.4 produced 127.30 g/L of l-threonine with productivity of 3.536 g/L/h and yield of 0.31 g/g glucose after 36 h in fed-batch fermentation. These results demonstrate the effectiveness of combining protein engineering and metabolic pathway engineering to construct l-threonine high-producing strain from E. coli, and the resulted strain XQ-12.4 showed the potential to produce l-threonine in industry. Although E. coli XQ-12.4 has a high sugar-to-acid conversion rate (i.e., 0.31 g/g glucose), it is still lower than the theoretical maximum value, indicating that there is still a lot of room to boost l-threonine synthesis. For example, previous reports (Supplementary Data 2) have reported that thrAC1034T can also relieve the feedback inhibition of AKI by l-threonine. Therefore, we have the following prospects for this study, comparing AKIGly433Arg, AKISer345Phe and AKIGly433Arg/Ser345Phe combination mutations, and selecting the mutant with the best feedback inhibition effect of l-threonine on AKI in E. coli XQ-12 strain.
Data availability
The authors declare that all the data supporting the findings of this study are available within the paper and its Supplementary Information or are available from the corresponding author on request.
References
Ding ZX, Fang Y, Zhu LF, Wang JL, Wang XY. Deletion of arcA, iclR, and tdcC in Escherichia coli to improve l-threonine production. Biotechnol Appl Bioc. 2019;66:794–807.
Dong X, Zhao Y, Zhao J, Wang X. Characterization of aspartate kinase and homoserine dehydrogenase from Corynebacterium glutamicum IWJ001 and systematic investigation of l-isoleucine biosynthesis. J Ind Microbiol Biotechnol. 2016;43:873–85.
Dong XY, Quinn PJ, Wang XY. Metabolic engineering of Escherichia coli and Corynebacterium glutamicum for the production of l-threonine. Biotechnol Adv. 2011;29:11–23.
Fan Z, Fang L, Wu L, Wang Z, Wang Y, Han C, Liu X. Improved catalytic activity of a novel aspartate kinase by site-directed saturation mutagenesis. Bioprocess Biosyst Eng. 2022;45:541–51.
Fang Y, Wang JL, Ma WJ, Yang J, Zhang HL, Zhao L, Chen SS, Zhang SY, Hu XQ, Li Y, Wang XY. Rebalancing microbial carbon distribution for l-threonine maximization using a thermal switch system. Metab Eng. 2020;61:33–46.
Huang C, Guo L, Wang J, Wang N, Huo YX. Efficient long fragment editing technique enables large-scale and scarless bacterial genome engineering. Appl Microbiol Biotechnol. 2020;104:7943–56.
Isogai S, Takagi H. Enhancement of lysine biosynthesis confers high-temperature stress tolerance to Escherichia coli cells (vol 105, pg 6899, 2021). Appl Microbiol Biotechnol. 2021;105:7547–7547.
Kachroo AH, Jayaram M, Rowley PA. Metabolic engineering without plasmids. Nat Biotechnol. 2009;27:729–31.
Lee KH, Park JH, Kim TY, Kim HU, Lee SY. Systems metabolic engineering of Escherichia coli for l-threonine production. Mol Syst Biol. 2007;3:149.
Li L, Liao Y, Luo Y, Zhang G, Liao X, Zhang W, Zheng S, Han S, Lin Y, Liang S. Improved efficiency of the desulfurization of oil sulfur compounds in Escherichia coli using a combination of desensitization engineering and DszC overexpression. ACS Synth Biol. 2019;8:1441–51.
Li Q, Sun B, Chen J, Zhang Y, Jiang Y, Yang S. A modified pCas/pTargetF system for CRISPR-Cas9-assisted genome editing in Escherichia coli. Acta Biochim Biophys Sin (Shanghai). 2021;53:620–7.
Li Y, Xian H, Xu Y, Zhu Y, Sun Z, Wang Q, Qi Q. Fine tuning the glycolytic flux ratio of EP-bifido pathway for mevalonate production by enhancing glucose-6-phosphate dehydrogenase (Zwf) and CRISPRi suppressing 6-phosphofructose kinase (PfkA) in Escherichia coli. Microb Cell Fact. 2021;20:32.
Liu JH, Li HL, Xiong H, Xie XX, Chen N, Zhao GR, Caiyin Q, Zhu HJ, Qiao JJ. Two-stage carbon distribution and cofactor generation for improving l-threonine production of Escherichia coli. Biotechnol Bioeng. 2019;116:110–20.
Long CP, Au J, Sandoval NR, Gebreselassie NA, Antoniewicz MR. Enzyme I facilitates reverse flux from pyruvate to phosphoenolpyruvate in Escherichia coli. Nat Commun. 2017;8: 14316.
Lv YY, Wu ZH, Han SY, Lin Y, Zheng SP. Construction of recombinant Corynebacterium glutamicum for l-threonine production. Biotechnol Bioproc E. 2012;17:16–21.
Ma YW, Zhang LF, Huang XX. Genome modification by CRISPR/Cas9. FEBS J. 2014;281:5186–93.
Meyer M, Wilson P, Schomburg D. Hydrogen bonding and molecular surface shape complementarity as a basis for protein docking. J Mol Biol. 1996;264:199–210.
Paris S, Viemon C, Curien G, Dumas R. Mechanism of control of Arabidopsis thaliana aspartate kinase-homoserine dehydrogenase by threonine. J Biol Chem. 2003;278:5361–6.
Park JH, Lee SY. Metabolic pathways and fermentative production of l-aspartate family amino acids. Biotechnol J. 2010;5:560–77.
Petit C, Kim Y, Lee SK, Brown J, Larsen E, Ronning DR, Suh JW, Kang CM. Reduction of feedback inhibition in homoserine kinase (ThrB) of Corynebacterium glutamicum enhances l-threonine biosynthesis. ACS Omega. 2018;3:1178–86.
Siedler S, Bringer S, Bott M. Increased NADPH availability in Escherichia coli: improvement of the product per glucose ratio in reductive whole-cell biotransformation. Appl Microbiol Biotechnol. 2011;92:929–37.
Su YW, Guo QQ, Wang S, Zhang X, Wang J. Effects of betaine supplementation on l-threonine fed-batch fermentation by Escherichia coli. Bioproc Biosyst Eng. 2018;41:1509–18.
Subburaj S, Chung SJ, Lee C, Ryu SM, Kim DH, Kim JS, Bae S, Lee GJ. Site-directed mutagenesis in Petunia × hybrida protoplast system using direct delivery of purified recombinant Cas9 ribonucleoproteins. Plant Cell Rep. 2016;35:1535–44.
Trcek J, Mira NP, Jarboe LR. Adaptation and tolerance of bacteria against acetic acid. Appl Microbiol Biotechnol. 2015;99:6215–29.
Tyo KEJ, Ajikumar PK, Stephanopoulos G. Stabilized gene duplication enables long-term selection-free heterologous pathway expression. Nat Biotechnol. 2009;27:760-U115.
Wang J, Cheng LK, Chen N. High-level production of l-threonine by recombinant Escherichia coli with combined feeding strategies. Biotechnol Biotechnol Equip. 2014;28:495–501.
Wei L, Wang Q, Xu N, Cheng J, Zhou W, Han G, Jiang H, Liu J, Ma Y. Combining protein and metabolic engineering strategies for high-level production of O-acetylhomoserine in Escherichia coli. ACS Synth Biol. 2019;8:1153–67.
Xie XX, Liang Y, Liu HL, Liu Y, Xu QY, Zhang CL, Chen N. Modification of glycolysis and its effect on the production of threonine in Escherichia coli. J Ind Microbiol Biotechnol. 2014;41:1007–15.
Xu J, Yu H, Chen X, Liu L, Zhang W. Accelerated green process of 2,5-dimethylpyrazine production from glucose by genetically modified Escherichia coli. ACS Synth Biol. 2020;9:2576–87.
Zhao H, Fang Y, Wang XY, Zhao L, Wang JL, Li Y. Increasing l-threonine production in Escherichia coli by engineering the glyoxylate shunt and the l-threonine biosynthesis pathway. Appl Microbiol Biotechnol. 2018;102:5505–18.
Zhu LF, Fang Y, Ding ZX, Zhang SY, Wang XY. Developing an l-threonine-producing strain from wild-type Escherichia coli by modifying the glucose uptake, glyoxylate shunt, and l-threonine biosynthetic pathway. Biotechnol Appl Biochem. 2019;66:962–76.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2021YFC2100900), the National Natural Science Foundation of China (No. 32271534), and the Top-Notch Academic Programs Project of Jiangsu Higher Education Institutions, the 111 project (Grant number 111-2-06).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, H., Hou, YJ., Xu, JZ. et al. Metabolic engineering of Escherichia coli for the efficient production of l-threonine. Syst Microbiol and Biomanuf 4, 810–819 (2024). https://doi.org/10.1007/s43393-023-00183-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43393-023-00183-2