Abstract
l-threonine is an important amino acid that can be added in food, medicine, or feed. Here, the influence of glyoxylate shunt on an l-threonine producing strain Escherichia coli TWF001 has been studied. The gene iclR was deleted, and the native promoter of the aceBA operon was replaced by the trc promoter in the chromosome of TWF001, the resulting strainTWF004 could produce 0.39 g l-threonine from1 g glucose after 36-h flask cultivation. Further replacing the native promoter of aspC by the trc promoter in the chromosome of TWF004 resulted in the strain TWF006. TWF006 could produce 0.42 g l-threonine from 1 g glucose after 36-h flask cultivation. Three key genes in the biosynthetic pathway of l-threonine, thrA* (a mutated thrA), thrB, and thrC were overexpressed in TWF006, resulting the strain TWF006/pFW01-thrA*BC. TWF006/pFW01-thrA*BC could produce 0.49 g l-threonine from 1 g glucose after 36-h flask cultivation. Next, the genes asd, rhtA, rhtC, or thrE were inserted into the plasmid TWF006/pFW01-thrA*BC, and TWF006 was transformed with these plasmids, resulting the strains TWF006/pFW01-thrA*BC-asd, TWF006/pFW01-thrA*BC-rhtA, TWF006/pFW01-thrA*BC-rhtC, and TWF006/pFW01-thrA*BC-thrE, respectively. These four strains could produce more l-threonine than the control strain, and the highest yield was produced by TWF006/pFW01-thrA*BC-asd; after 36-h flask cultivation, TWF006/pFW01-thrA*BC-asd could produce 15.85 g/l l-threonine, i.e., 0.53 g l-threonine per 1 g glucose, which is a 70% increase relative to the control strain TWF001. The results suggested that the combined engineering of glyoxylate shunt and l-threonine biosynthesis pathway could significantly increase the l-threonine production in E. coli.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
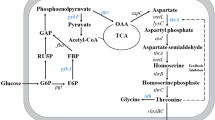
l-threonine produced by microbial fermentation has an increasing market demand (Dong et al. 2012; Liu et al. 2015). A high yield of l-lysine or l-glutamic acid can be produced by Corynebacterium glutamicum (Hashimoto 2017), but not l-threonine (Dong et al. 2016); therefore, Escherichia coli became the important strain for l-threonine production (Dong et al. 2011; Lee et al. 2006). Starting from oxaloacetate, an intermediate of the Krebs cycle, the biosynthesis of l-threonine in E. coli consists of six enzymatic steps (Fig. 1). First, oxaloacetate is converted to l-aspartate by aspartate aminotransferase encoded by aspC. The second reaction is catalyzed by three aspartate kinases (I, II, III). Aspartate kinase I encoded by thrA can be inhibited by l-threonine, and its synthesis is repressed by l-threonine and l-isoleucine; the feedback inhibition can be removed by replacing the 1034th C with T in thrA (Lee et al. 2003). Aspartate kinase II is encoded by metL, while aspartate kinase III is encoded by lysC. Both aspartate kinases I and II have the second enzymic activity of homoserine dehydrogenase; thus, they also catalyze the fourth step (Fig. 1). Aspartate kinase I is the most abundant one among the three aspartate kinases in E. coli. The third reaction is catalyzed by aspartate semialdehyde dehydrogenase encoded by asd. The last two reactions are catalyzed by homoserine kinase encoded by thrB and threonine synthase encoded by thrC. In E. coli, thrB and thrC are clustered with thrA in the thrABC operon. There are three export proteins for l-threonine in E. coli (Yuzbashev et al. 2013), which are encoded by rhtA (Livshits et al. 2003), rhtB (Zakataeva et al. 1999), and rhtC (Kruse et al. 2002), respectively. The expression of these three exporters can be induced at different conditions (Livshits et al. 2003). l-threonine export encoded by thrE in C. glutamicum could also efficiently function in E. coli (Kruse et al. 2002). Recently, the fourth l-threonine export encoded by yecC has been characterized in E. coli (Xu et al. 2017).
The glyoxylate bypass and l-threonine biosynthetic pathway in E. coli. IclR, the transcription factor encoded by iclR, represses the aceBAK operon and iclR, by binding to the IclR-binding boxes at the upstream of the aceBAK operon and the upstream of iclR. The Krebs cycle and the glyoxylate bypass are mediated by AceK. In the complete Krebs cycle, isocitrate is converted to α-ketoglutarate by the enzyme isocitrate dehydrogenase (IDH). AceK encoded by aceK phosphorylates IDH, causing its inactivation. The inactivation of IDH increases the conversion of isocitrate to glyoxylate. Glyoxylate is then combined with acetyl-CoA to form malate, completing the glyoxylate bypass. RhtABC, different l-threonine export proteins
Production of l-threonine in E. coli can be enhanced by inactivating the transcription factor IclR encoded by iclR (Lee et al. 2007). IclR is a repressor for the aceBAK operon, which encodes isocitrate lyase (aceB), malate synthase (aceA), and isocitrate dehydrogenase kinase/phosphorylase (aceK) in the glyoxylate bypass (Fig. 1), it also represses the expression of iclR in an autogenous manner (Yamamoto and Ishihama 2003). There are two IclR-binding boxes at the upstream of the aceBAK operon and one IclR-binding box at the upstream of iclR. Regulator AceK determines the carbon flux distribution between the Krebs cycle and the glyoxylate bypass (Zheng et al. 2012). In the complete Krebs cycle, isocitrate dehydrogenase (IDH) converts isocitrate to α-ketoglutarate and carbon dioxide; when acetate becomes the sole carbon source, AceK inactivates IDH, increasing the conversion of isocitrate to glyoxylate (Fig. 1). Then, glyoxylate reacts with acetyl-CoA, producing malate through the glyoxylate bypass. When E. coli is grown in the stationary phase, glucose is usually completely consumed while acetate produced during the exponential phase becomes the major carbon source; therefore, the glyoxylate bypass is activated (Kumari et al. 2000).
In this study, a series of l-threonine producing E. coli mutant strains was constructed through the metabolic engineering, the glyoxylate bypass, and the biosynthetic pathway of l-threonine. One of the strains, TWF006/pFW01-thrA*BC-asd, could produce 15.85 g/l l-threonine after 36-h flask fermentation, which is a 70% increase relative to the control strain TWF001.
Materials and methods
Bacteria and culture conditions
Bacteria and plasmids used in this work are listed in Table 1. E. coli cells were grown at 37 or 30 °C in an LB medium (5 g/l yeast extract, 10 g/l tryptone, and 10 g/l NaCl) with 200 rpm shaking. Kanamycin (50 mg/l), spectinomycin (50 mg/l), triclosan (100 mg/l), isopropyl-d-thiogalactopyranoside (IPTG; 0.5 mM), or arabinose (10 mM) was added when necessary. l-threonine-producing E. coli strain TWF001 (CCTCC no. M2017730) was originally isolated from soil and is closely related to E. coli MG1655, based on their 16S rDNA sequences.
Construction of E. coli mutant strains TWF002, TWF003, TWF004, TWF005, and TWF006
The primers used in this work are listed in Table 2. Most primer sequences were designed according to the genome sequence of E. coli MG1655. Genomic DNA of E. coli TWF001 was isolated and used as templates for PCR amplification of genes or their upstream and downstream fragments. Plasmids and genomic DNA were extracted by using the TIANGEN prep kit (Tiangen, Beijing, China) according to the manufacturer’s instruction. PrimeSTAR HS DNA Polymerase (Takara, Dalian, China) was used for PCR. Restriction endonucleases and T4 DNA ligase were purchased from Thermo Scientific (Waltham, USA). Both the in-frame gene deletion and the promoter replacement in the chromosome of E. coli were carried out according to the published method (Jiang et al. 2015).
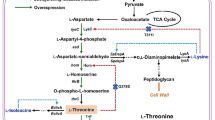
TWF002 was constructed from TWF001 by replacing the two IclR-binding boxes and the native promoter of the aceBAK operon with the trc promoter (Fig. 2a). The upstream fragment of aceBA gene was amplified using the primer pair aceBAf1 and aceBAr1. The downstream fragment of the aceBA gene was amplified using the primer pair aceBAf3 and aceBAr3. The trc promoter was amplified from pTrc99A (Amann et al. 1988) using the primers aceBAf2 and aceBAr2. These three PCR products were overlapped together by using the primers aceBAf1 and aceBAr3, resulting in the replacement fragment Ptrc-aceBA. The pTargetF-aceBA was obtained from pTargetF (Jiang et al. 2015) by inverse PCR using the primers sgRNA-aceBA-F and T-sgRNA-R, following by self-ligation, and was confirmed by using primers T-aceBA-F and T-sgRNA-R. The plasmid pCas (Jiang et al. 2015) was transformed into TWF001, and the competent TWF001/pCas cells were prepared as described previously (Sharan et al. 2009). Arabinose was added to the culture when preparing the competent TWF001/pCas cells to activate the Red enzymes for recombination. Then, 200 ng pTargetF-aceBA and 500 ng Ptrc-aceBA were mixed with 80 μl TWF001/pCas competent cells and electroporated. Cells were recovered at 30 °C for 2 h, then spread onto LB agar plates containing kanamycin and spectinomycin, and incubated for 48 h at 30 °C. Transformants were identified by colony PCR using the primers aceBAf1 and aceBAr2 and confirmed by DNA sequencing of the primer region of aceBA. At the end, pTargetF-aceBA was cured by adding IPTG, and pCas was cured by growing at 42 °C overnight.
TWF003 was constructed from TWF001 by deletion of iclR (Fig. 2a). The upstream fragment of iclR was amplified using the primer pair iclRf1 and iclRr1. The downstream fragment of iclR was amplified using the primersiclRf2 and iclRr2. These PCR products were linked together by overlapping PCR using the primersiclRf1 and iclRr2. The pTargetF-iclR was obtained from pTargetF by inverse PCR using the primers sgRNA-iclR-F and T-sgRNA-R, following by self-ligation, and was confirmed by using the primers T-iclR-F and T-sgRNA-R. Then, 200 ng pTargetF-iclR and 500 ng of the deletion fragment were mixed with 80-μl TWF001/pCas competent cells and electroporated. Cells were recovered, and the deletion mutant TWF003 was selected on LB plates containing kanamycin and spectinomycin. The correct iclR deletion in TWF003 was identified by PCR using the primers iclRf1 and iclRr2. At the end, pTargetF-iclR was cured by adding IPTG, and pCas was cured by growing at 42 °C overnight.
TWF004 was constructed from TWF003 by replacing the IclR-binding boxes and the native promoter of the aceBAK operon with the trc promoter, using the same method and primer pairs used for the construction of TWF002 (Fig. 2a).
TWF005 was constructed from TWF003 by inserting the trc promoter in the upstream of aspC (Fig. 2a). The upstream fragment of the aspC gene was amplified using the primers aspCf1 and aspCr1. The downstream fragment of the aspCgene was amplified using the primers aspCf3 and aspCr3. The trc promoter was amplified from pTrc99A using the primers aspCf2 andaspCr2. These three PCR products were overlapped together by using the primers aspCf1 and aspCr3, resulting in the replacement fragment Ptrc-aspC. The pTargetF-aspC was obtained from pTargetF by inverse PCR using the primers sgRNA-aspC-F and T-sgRNA-R, following by self-ligation, and was confirmed by using primers T-aspC-F and T-sgRNA-R. Arabinose was added to the culture when preparing the competent TWF003/pCas cells to activate the Red enzymes for recombination. Then, 200 ng pTargetF-aspC and 500 ng Ptrc-aspC were mixed with 80-μl TWF003/pCas competent cells and electroporated. Transformants were identified by colony PCR using the primers aspCf1 and aspCr2 and confirmed by DNA sequencing of the promoter region of aspC. At the end, pTargetF-aspC was cured by adding IPTG, and pCas was cured by growing at 42 °C overnight.
TWF006 was constructed from TWF004 by inserting the trc promoter in the upstream of aspC, using the same method and primer pairs used for the construction of TWF005 (Fig. 2a). The in-frame gene deletion and/or the promoter replacement in the chromosome of the above E. coli mutants was confirmed by DNA sequencing.
Construction of different plasmids
The vector pFW01 was constructed from pF2 (Jang and Magnuson 2013) by inserting the PJ23101 (5’-TTTACAGCTAGCTCAGTCCTAGGTATTATGCTAGC-3’) promoter (http://partsregistry.org/Part:BBa_J23101) and replacing mfabI with Pseudomonas aeruginosa fabV (Zhu et al. 2010). To insert the PJ promoter in pF2, a large linear DNA fragment was amplified with primers PJ-F and PJ-R using pF2 as a template and then self-ligated. The correct insertion of the PJ promoter in pF2 was confirmed by DNA sequencing. Using this new plasmid as a template, a linear DNA fragment was amplified with primers pJF-F and pJF-R; meanwhile, another DNA fragment was amplified with primers fabV-F and fabV-R using the genomic DNA of P. aeruginosa PAO1 as a template. These two PCR products were ligated by using the ClonExpress II One Step Cloning Kit C112-01 (Vazyme, Nanjing, China), resulting the plasmid pFW01 (Fig. 2b).
Three key genes for l-threonine biosynthesis in E. coli were inserted in pFW01, resulting in the plasmid pFW01-thrA*BC. In E. coli, thrA, thrB, and thrC are clustered in the thrABC operon. The thrA* indicates that the 1034th C in thrA is replaced with T. To create this point mutation, two DNA fragments were amplified by using primer pairs of thrA*BC-U-F/thrA*BC-U-R and thrA*BC-D-F/thrA*BC-D-R, respectively, using the genomic DNA of E. coli MG1655 as a template; then, they were ligated together by overlapping PCR using primers thrA*BC-U-F and thrA*BC-D-R, resulting the thrA*BC fragment. Next, the thrA*BC fragment was digested with BamHI and XbaI, and pFW01 was digested with BamHI and SacI; then, the two fragments were ligated together, resulting the plasmid pFW01-thrA*BC (Fig. 2b). The correct sequence in pFW01-thrA*BC was checked by using colony PCR, restriction enzyme digestion, and DNA sequencing.
The plasmids pFW01 and pFW01-thrA*BC were transformed into different E. coli strains, forming recombinant strains TWF001/pFW01, TWF001/pFW01-thrA*BC, TWF002/pFW01, TWF002/pFW01-thrA*BC, TWF003/pFW01, TWF003/pFW01-thrA*BC, TWF004/pFW01, TWF004/pFW01-thrA*BC, TWF005/pFW01, TWF005/pFW01-thrA*BC, TWF005/pFW01, and TWF005/pFW01-thrA*BC.
Then, asd, rhtA, rhtC, and thrE were separately inserted in pFW01-thrA*BC, resulting pFW01-thrA*BC-asd, pFW01-thrA*BC-rhtA, pFW01-thrA*BC-rhtC, and pFW01-thrA*BC-thrE. Using the genomic DNA of E. coli MG1655 as a template, the asd fragment was amplified by using primers asd-F and asd-R, the rhtA fragment was amplified by using primers rhtA-F and rhtA-R, the rhtC fragment was amplified by using primers rhtC-F and rhtC-R. The thrE fragment was amplified by using primers thrE-F and thrE-R and the genomic DNA of C. glutamicum ATCC13032 as a template. Next, the asd, rhtA, rhtC, thrE fragments, and the plasmid pFW01-thrA*BC were digested with XbaI and Sac I; then, the four fragments were separately ligated with pFW01-thrA*BC, resulting the plasmid pFW01-thrA*BC-asd, pFW01-thrA*BC-rhtA, pFW01-thrA*BC-rhtC, and pFW01-thrA*BC-thrE (Fig. 2b). The four different plasmids were transformed into TWF006, resulting in the recombinant strains TWF006/pFW01-thrA*BC-asd, TWF006/pFW01-thrA*BC-rhtA, TWF006/pFW01-thrA*BC-rhtC, and TWF006/pFW01-thrA*BC-thrE.
Quantification of mRNA using real-time PCR
Real-time PCR (RT-PCR) was used to quantify mRNA levels of aceA, aceB, thrA, thrB, thrC, rhtA, rhtB, rhtC, aspA, aspC, tyrB, icdA, and gltA in different E. coli strains. Total RNA was extracted from different E. coli cells grown at the mid-exponential phase using an RNA extraction kit (Bio Flux, Beijing, China). Residual DNA was removed from the RNA sample by DNaseI. The quality and amount of RNA were judged and quantified by electrophoresis. Using random hexamer primers, 500-ng RNA was reversely transcribed into cDNA using a Revert Aid™ First Strand cDNA synthesis kit (Fermentas, Shanghai, China). RT-PCR was performed using an ABI Step One RT-PCR system (Applied Biosystems, San Mateo, CA, USA) with a Real Master Mix kit (Tiangen, Beijing, China). Primers for detection of various genes are listed in Table 2. The following RT-PCR procedure was used:1 min at 94 °C, 40 cycles of 10 s at 94 °C, 30 s at 55 °C, and 15 s at 68 °C. The relative abundance of the targeted mRNAs was quantified based on the cycle threshold value, which is defined as the number of cycles required to obtain a fluorescent signal above the background and was calculated according to the published method (Livak and Schmittgen 2001; Nolden et al. 2001). To standardize the results, the relative abundance of 16S rRNA was used as an internal standard control. All assays were performed in triplicate.
Flask fermentation
The threonine-producing E. coli strains were grown on LB plates for 24 h and transferred to a test tube containing 5 ml LB medium. After growing for 4 h at 37 °C, the OD600 of the culture was measured. Then, the culture was transferred to a 250-ml flask containing 25 ml LB medium, with the initial OD600 of 0.1. After growing for 4 h at 37 °C, 5-ml culture was transferred to 30-ml fermentation medium (2 g/l yeast extract, 2 g/l citric acid, 25 g/l (NH4)2SO4, 7.46 g/l KH2PO4, 30 g/l glucose, 2 g/l MgSO4·7 H2O, 5 mg/l FeSO4·7H2O, 5 mg/l MnSO4·4 H2O, and 20 g/l CaCO3, pH 6.8) (Lee et al. 2009). The fermentation was run for 36 h at 37 °C, with vigorous agitation (200 rpm).
Analytical procedures
Biomass was determined by measuring OD600 with UV-1800 spectrophotometer (Shimadzu, Japan). During fermentation, 1 ml of the culture broth was taken at different time points and centrifuged at 12,000 rpm for 5 min. The supernatant was used to analyze the levels of glucose and amino acids. The glucose concentration was measured with a SBA-40C biosensor (Institute of Biology, Shan-dong Academy of Science, China). To determine the levels of amino acids, the supernatant was diluted 20-folds, filtered, and analyzed by the 1200 series HPLC system (Agilent Technology, USA); the separation and quantification of amino acids were performed on a Thermo ODS-2HYPERSIL C18 column (250 mm × 4.0 mm, USA) by using the orthophthalaldehyde precolumn derivatization method (Kőrös et al. 2008). All the solvents used in HPLC analysis were purchased from Sigma-Aldrich (Shanghai, China).
Results
Modifying the glyoxylate shunt to increase l-threonine production in E. coli TWF001
E. coli TWF001 can produce 9.32 g/l l-threonine from 30 g/l glucose after 36-h flask fermentation when its OD600 reached the maximum (15.94) and glucose was completely consumed (Fig. 3). To further increase l-threonine in TWF001, the two IclR-binding boxes and the native promoter of the aceBAK operon were replaced by the trc promoter, resulted in the strain TWF002 (Fig. 2). To our surprise, l-threonine production in TWF002 dramatically decreased, comparing to the control TWF001 (Fig. 3c). TWF002 cells grew faster than TWF001 cells, reaching the maximum OD600 17.94 after 36 h (Fig. 3a), and they also consumed more glucose than TWF001 cells at the same time point (Fig. 3b). This suggests that the glucose is consumed mainly for the growth rather than the production of l-threonine in TWF002.
Flask cultivation for l-threonine production in different E. coli strains TWF001, TWF002, TWF003, TWF004, TWF005, and TWF006. a Cell growth. b Glucose consumption. c l-threonine production. d Relative transcription levels of aceA, aceB, aspA, aspC, tyrB, and icdA analyzed by RT-PCR. The error bars indicate the standard deviations from three independent experiments
To understand the decrease of l-threonine production in TWF002, the transcriptional levels of aceA, aceB, thrA, thrB, thrC, rhtA, rhtB, rhtC, aspA, aspC, tyrB, icdA, and gltA were determined by RT-PCR. Although the transcriptional levels of aceA and aceB were significantly increased as expected in TWF002, while aspA, aspC, icdA, and tyrB were downregulated (Fig. 3d). The gene aspA encodes l-aspartase that catalyzes the reaction from fumarate to l-aspartate, while the genes tyrB and aspC encode the aromatic aminotransferase and aspartate aminotransferase, respectively, and both enzymes can catalyze the reaction from oxaloacetate to l-aspartate (Fig. 1). The downregulation of these three genes might explain why l-threonine production in TWF002 decreased, considering l-aspartate is the key precursor for l-threonine biosynthesis. The transcriptional levels of genes relevant to the biosynthesis pathway and transport of l-threonine (thrA, thrB, thrC, rhtA, rhtB, rhtC, and gltA) in TWF002 were the same to the control TWF001 (Data not shown), further confirming that l-aspartate shortage is the main reason for the decreased l-threonine production in TWF002. In addition, the glutamate (2.25 g/l) was significantly accumulated in TWF002, suggesting that the tricarboxylic acid cycle is enhanced in TWF002 since glutamate is mainly synthesized from α-ketoglutarate, an intermediate metabolite in the tricarboxylic acid cycle. This is consistent with the better growth of TWF002. This experiment demonstrates that replacing the IclR-binding boxes and the native promoter of the aceBAK operon with the trc promoter did not enhance the l-threonine biosynthesis but improved the cell growth by enhancing the tricarboxylic acid cycle.
Since IclR represses the transcription of the aceBAK operon in E. coli, the iclR gene was deleted inTWF001, resulting in the strain TWF003 (Fig. 2). After 36-h flask fermentation, glucose was completely consumed, 11.76 g/l l-threonine were produced from 30 g/l glucose in TWF003, which is a 26% increase compared to the control TWF001 (Fig. 3). The transcriptional levels of aceA and aceB in TWF003 were significantly increased, and icdA, aspA, and tyrB were also upregulated, but aspC was downregulated (Fig. 3d). The two IclR-binding boxes and the native promoter of the aceBAK operon in TWF003 were replaced with the trc promoter, resulting the strain TWF004 (Fig. 2a). l-threonine production in TWF004 was on the same level to TWF003 (Fig. 3c). The transcriptional levels of aceA and aceB in TWF004 were much higher than that in TWF001 but lower than in TWF003 (Fig. 3d). This indicates that the native promoter of the aceBAK operon in E. coli might be stronger than the trc promoter, considering that iclR has been deleted in both TWF003 and TWF004, and the only difference is the native promoter of the aceBAK operon in TWF003 has been replaced by the trc promoter in TWF004. Interestingly, compared to the control TWF001, the transcriptional levels of aspA, aspC, and tyrB were downregulated (Fig. 3d) even though the l-threonine production in TWF004 was increased.
Enhancing the expression of aspartate aminotransferase to increase l-threonine production in E. coli
The gene aspC encodes aspartate aminotransferase that catalyzes the reaction from oxaloacetate to l-aspartate, but its transcriptional levels were downregulated in TWF003 and TWF004. To draw the carbon flow from Krebs cycle to l-threonine biosynthesis, the trc promoter was inserted in the upstream of aspC inTWF003 and TWF004, resulting in TWF005 and TWF006, respectively (Fig. 2). Although l-threonine production in TWF005 was similar to that in TWF001, the cell growth of TWF005 is much better than of TWF003 (Fig. 3). Compared to the control TWF001, the transcriptional levels of aceA, aceB, and aspC were significantly increased in TWF005, and aspA, tyrB, and icdA were also upregulated (Fig. 3d). TWF006 produced 12.47 g/l l-threonine from 30 g/l glucose after 36-h flask fermentation (Fig. 3c), which is a 34% increase compared to the control TWF001. OD600 of TWF006 reached the maximum (13.9) at 24 h, but the l-threonine production continued to increase until 36 h when glucose was completely consumed (Fig. 3). The transcriptional levels of aceA, aceB, and aspC were significantly increased in TWF006, aspA and tyrB were also upregulated (Fig. 3d). This indicates that the carbon flux in TWF006 was efficiently directed to the l-threonine biosynthesis pathway.
Overexpressing the key enzymes in l-threonine biosynthesis pathway to further increase l-threonine production
Based on RT-PCR analysis, the transcriptional levels of the three key genes in l-threonine biosynthesis pathway, thrA, thrB, and thrC, in TWF002, TWF003, TWF004, TWF005, and TWF006, were the same to the control TWF001 (Data not shown). Therefore, the three genes thrA, thrB, and thrC were overexpressed in these E. coli strains to further increase l-threonine production (Fig. 4). The three genes were inserted in the vector pFW01 which was constructed by replacing the mfabI gene in the plasmid pF2 (Jang and Magnuson 2013) with the fabV gene (Zhu et al. 2010). Not like other plasmids which need addition of antibiotics to maintain in E. coli cells, pFW01 can maintain in E. coli by adding a tiny amount of triclosan. Triclosan specifically inhibits fatty acid synthesis in E. coli by inactivating an enoyl-acyl carrier protein reductase encoded by fabI. The pF2 contains a mutant fabI (mfabI) (Jang and Magnuson 2013), while pFW01 contains the gene fabV from Pseudomonas aeruginosa PAO1 (Fig. 2); fabV encodes a triclosan-resistant enoyl-ACP reductase in P. aeruginosa which is more sensitive to triclosan than that encoded by mfabI (Zhu et al. 2010). To enhance the expression of the genes cloned into the MCS, the promoter pJ has been inserted on the upstream of MCS in pFW01 (Fig. 2). E. coli harboring pFW01 grows much better than E. coli harboring pF2 in the presence of the same amount of triclosan (data not shown). Three key genes thrA*, thrB, and thrC in the l-threonine biosynthetic pathway in E. coli were inserted in pFW01, resulting pFW01-thrA*BC (Fig. 2). The thrA* is a thrA mutant in which the 1034th C has been replaced with T, and it encodes a mutant of aspartate kinase I which is resistant to feedback inhibition (Lee et al. 2003). The plasmids pFW01 and pFW01-thrA*BC were transformed into different E. coli strains, resulting in TWF001/pFW01, TWF001/pFW01-thrA*BC, TWF002/pFW01, TWF002/pFW01-thrA*BC, TWF003/pFW01, TWF003/pFW01-thrA*BC, TWF004/pFW01, TWF004/pFW01-thrA*BC, TWF005/pFW01, TWF005/pFW01-thrA*BC, TWF006/pFW01, and TWF006/pFW01-thrA*BC. The production of l-threonine in these strains was investigated (Fig. 4).
Flask cultivation for l-threonine production in different E. coli strains TWF001/pFW01, TWF001/pFW01-thrA*BC, TWF002/pFW01, TWF002/pFW01-thrA*BC, TWF003/pFW01, TWF003/pFW01-thrA*BC, TWF004/pFW01, TWF004/pFW01-thrA*BC, TWF005/pFW01, TWF005/pFW01-thrA*BC, TWF006/pFW01, and TWF006/pFW01-thrA*BC. a Cell growth. b Glucose consumption. c l-threonine production. The error bars indicate the standard deviations from three independent experiments
Compared to the vector control TWF001/pFW01, TWF001/pFW01-thrA*BC grew better, consumed less glucose, but l-threonine production (9.72 g/l after 36 h) was not increased (Fig. 4). Compared to the vector control TWF002/pFW01, TWF002/pFW01-thrA*BC grew better, consumed more glucose, and l-threonine production was higher (Fig. 4). Compared to the vector control TWF003/pFW01, TWF003/pFW01-thrA*BC grew better, consumed more glucose, and l-threonine production (12.5 g/l after 36 h) was increased (Fig. 4). Compared to the vector control TWF004/pFW01, TWF004/pFW01-thrA*BC grew worse, consumed more glucose, and l-threonine production (12.4 g/l after 36 h) was increased (Fig. 4). Compared to the vector control TWF005/pFW01, TWF005/pFW01-thrA*BC grew worse, consumed more glucose, and l-threonine production was higher (Fig. 4). Compared to the vector control TWF006/pFW01, TWF006/pFW01-thrA*BC grew better, consumed more glucose, and l-threonine production (14.7 g/l after 36 h) was increased (Fig. 4). Since TWF006/pFW01-thrA*BC produced the highest level of l-threonine among all the six strains, it was further investigated.
Comparison of l-threonine production in different E. coli TWF006 derivatives
To further increase l-threonine production, the gene asd encoding aspartate semialdehyde dehydrogenase and three genes encoding the l-threonine exporters (Yuzbashev et al. 2013) were overexpressed in TWF006, together with the genes thrA*BC, resulting in pFW01-thrA*BC-asd, TWF006/pFW01-thrA*BC-rhtA, TWF006/pFW01-thrA*BC-rhtC, and TWF006/pFW01-thrA*BC-thrE. RhtA and RhtC were two of the most efficient threonine exporters in E. coli (Diesveld et al. 2009; Livshits et al. 2003), while ThrE encoded by C. glutamicum thrE can efficiently export l-threonine in E. coli (Kruse et al. 2002).
Compared to the control TWF006/pFW01, the growth of pFW01-thrA*BC-asd, TWF006/pFW01-thrA*BC-rhtA, TWF006/pFW01-thrA*BC-rhtC, and TWF006/pFW01-thrA*BC-thrE in the medium containing 30 g/l glucose was improved, and glucose was completely consumed in 36 h. After 36 h, l-threonine production in TWF006/pFW01-thrA*BC-asd, TWF006/pFW01-thrA*BC-rhtA, TWF006/pFW01-thrA*BC-rhtC, and TWF006/pFW01-thrA*BC-thrE reached 15.85, 14.14, 14.34, and 14.11 g/l, respectively (Fig. 5c). The highest yield of l-threonine was produced by TWF006/pFW01-thrA*BC-asd, which is a 70% increase compared to the control TWF001.
Flask cultivation for l-threonine production in different E. coli strains TWF006/pFW01, TWF006/pFW01-thrA*BC-asd, TWF006/pFW01-thrA*BC-rhtA, TWF006/pFW01-thrA*BC-rhtC, and TWF006/pFW01-thrA*BC-thrE. a Cell growth. b Glucose consumption. c l-threonine production. The error bars indicate the standard deviations from three independent experiments
Discussion
Engineering the glyoxylate bypass, one of the important anaplerotic pathways, has been used to increase the production of various products. The iclR was knocked out in E. coli to overcome acetate overflow and improve the production of two acetyl-CoA-derived chemicals, phloroglucinol and 3-hydroxypropionate; the acetate concentrations were decreased by more than 50%, and the productions of the two chemicals were increased more than twice (Liu et al. 2017). Enhancing the glyoxylate shunt by deleting iclR has been used in E. coli to produce succinate from acetate as the sole carbon source (Li et al. 2016) and to accumulate oxaloacetate precursor to produce l-threonine (Lee et al. 2007), ectoine (Ning et al. 2016), fumaric acid (Zhang et al. 2015b; Song et al. 2013), or fatty acids (Lin et al. 2013). In addition, enhancing the glyoxylate bypass can also be achieved by replacing the native promoter of aceBAK with the trc promoter in the chromosome (Li et al. 2013), overexpressing the aceBA in plasmid (Li et al. 2014), or reducing the enzyme activity of IDH (Li et al. 2013). In this study, either deleting iclR or replacing the native promoter of aceBAK with the trc promoter was performed in l-threonine producing E. coli TWF001; l-threonine production was increased in the former case, but decreased significantly in the latter (Fig. 3). This indicates that the engineering of glyoxylate bypass could have different influence in different strains, and the l-threonine production in E. coli TWF001 might be regulated by iclR as well as other regulators such as crp and arcA (Yamamoto and Ishihama 2003; Zhang et al. 2005).
Aspartate aminotransferase encoded by aspC catalyzes the reaction from oxaloacetate to l-aspartate, drawing the carbon flux from Krebs cycle to the biosynthetic pathway of l-aspartate family. Deletion of aspC led to generation of small cells with slow growth, while overexpression of aspC exerted the opposite effect (Liu et al. 2014). Therefore, aspC was overexpressed in E. coli mutant strains TWF003 and TWF004, resulting in TWF005 and TWF006, respectively (Fig. 2). Interestingly, l-threonine production was increased in TWF006, but decreased in TWF005 (Fig. 3). It suggests that enhancing both the glyoxylate bypass and the aspartate aminotransferase could increase the production of threonine in E. coli.
Overexpressing the thrABC operon is the most often used approach to increase l-threonine production. Since aspartate kinase I encoded by thrA can be feedback inhibited, various mutants of thrA which can remove the feedback inhibition have been discovered (Dong et al. 2011; Lee et al. 2003; Livshits et al. 2003). The plasmid pFW01-thrA*BC (thrA*is a feedback-resistant thrA mutant in which the 1034th C is replaced with T) was transformed into E. coli strains TWF001, TWF002, TWF003, TWF004, TWF005, and TWF006. Compared to the vector control, the production of threonine increased in TWF002/pFW01-thrA*BC, TWF003/pFW01-thrA*BC, TWF004/pFW01-thrA*BC, TWF005/pFW01-thrA*BC, or TWF006/pFW01-thrA*BC; while the production of threonine did not increase in the control TWF001/pFW01-thrA*BC. This indicates that the various activation of glyoxylate bypass in E. coli TWF001 could increase the carbon flow to the l-threonine biosynthetic pathway.
Various strategies have been used to increase l-threonine production in E. coli. Based on transcriptome profiling and in silico flux response analysis, a genetically defined l-threonine overproducing E. coli strain has been constructed by using systems metabolic engineering (Lee et al. 2007). Firstly, feedback inhibitions of aspartate kinase I and III and transcriptional attenuation regulations were removed; secondly, pathways for Thr degradation were removed by deleting tdh and mutating ilvA; thirdly, metA and lysA were deleted to make more precursors available for Thr biosynthesis; finally, the expression levels of some target genes identified by transcriptome profiling combined with in silico flux response analysis were manipulated. The final strain was able to produce 0.393 g l-threonine from 1 g glucose after 50-h fed-batch fermentation (Lee et al. 2007). Another l-threonine hyper-producer MDS-205 was constructed from a reduced-genome E. coli strain MDS42, by deleting the gene tdh, replacing the genes sstT and tddC with the gene rhtA23, and overexpressing the feedback-resistant threonine operon thrA*BC, it could produce 0.401 g l-threonine from 1 g glucose after 30-h fermentation (Lee et al. 2009). The by-product acetate could be reduced in E. coli THRD by deleting the genes pykF and pykA to redirect the glycolysis flux to the pentose phosphate pathway, but the l-threonine yield on glucose (0.34 g/g) did not increase (Xie et al. 2014). Label-free proteomics technology combined with absolute protein expression measurements indicate that LysC has the largest flux control coefficient on l-threonine synthesis in strain Thr; therefore, overexpressing LysC can be used to increase l-threonine production (Zhang et al. 2015a). DNA scaffold system also can be used to improve l-threonine production in E. coli (Lee et al. 2013). A zinc finger protein was used as an adapter for the site-specific binding of enzymes involved in l-threonine production to a precisely ordered location on a DNA double helix, thus increasing the proximity of enzymes and the local concentration of metabolites in the cell. Such a DNA scaffold system enhanced the cell growth rate, significantly increased the efficiency of the threonine biosynthetic pathway in E. coli, substantially reducing the production time for l-threonine by over 50% (Lee et al. 2013). The fed-batch fermentation technique could also improve the yield of l-threonine in E. coli; under the optimal conditions, 0.46 g l-threonine could be obtained from 1 g glucose in E. coli TRFC after 38-h fermentation (Chen et al. 2009); combining the pseudo-exponential feeding and glucose-stat feeding strategies resulted in high cell density and more l-threonine production as well as low accumulation of by-products in E. coli TRFC (Wang et al. 2014). In this study, one of the strains we constructed, TWF006/pFW001-thrA*BC-asd, could produce 0.53 g l-threonine from 1 g glucose in 36-h fermentation. This suggests that enhancing the glyoxylate bypass followed by strengthening the l-threonine biosynthetic pathway is alternative strategy for increasing l-threonine production in E. coli. Since overexpression of genes encoding the pyruvate dehydrogenase complex (Skorokhodova et al. 2013), AspA (Chao et al. 2000; Leuchtenberger et al. 2005; Song et al. 2015), or LysC (Zhang et al. 2015a) could increase l-threonine production in E. coli, these genes should be overexpressed in TWF006/pFW001-thrA*BC-asd in further study to improve l-threonine production, together with fermentation optimization.
References
Amann E, Ochs B, Abel K-J (1988) Tightly regulated tac promoter vectors useful for the expression of unfused and fused proteins in Escherichia coli. Gene 69(2):301–315. https://doi.org/10.1016/0378-1119(88)90440-4
Chao Y-P, Lo T-E, Luo N-S (2000) Selective production of L-aspartic acid and L-phenylalanine by coupling reactions of aspartase and aminotransferase in Escherichia coli. Enzym Microbiol Technol 27(1):19–25. https://doi.org/10.1016/S0141-0229(00)00149-6
Chen N, Huang J, Feng ZB, Yu L, Xu QY, Wen TY (2009) Optimization of fermentation conditions for the biosynthesis of L-threonine by Escherichia coli. Appl Biochem Biotechnol 158(3):595–604. https://doi.org/10.1007/s12010-008-8385-y
Diesveld R, Tietze N, Furst O, Reth A, Bathe B, Sahm H, Eggeling L (2009) Activity of exporters of Escherichia coli in Corynebacterium glutamicum, and their use to increase L-threonine production. J Mol Microbiol Biotechnol 16(3–4):198–207. https://doi.org/10.1159/000142530
Dong X, Quinn PJ, Wang X (2011) Metabolic engineering of Escherichia coli and Corynebacterium glutamicum for the production of l-threonine. Biotechnol Adv 29(1):11–23. https://doi.org/10.1016/j.biotechadv.2010.07.009
Dong X, Quinn PJ, Wang X (2012) Microbial metabolic engineering for L-threonine production. In: Wang X, Chen J, Quinn P (eds) Reprog Microbiol Metab Path. Springer Netherlands, Dordrecht, pp 283–302
Dong X, Zhao Y, Hu J, Li Y, Wang X (2016) Attenuating l-lysine production by deletion of ddh and lysE and their effect on l-threonine and l-isoleucine production in Corynebacterium glutamicum. Enzym Microbiol Technol 93-94:70–78. https://doi.org/10.1016/j.enzmictec.2016.07.013
Hashimoto S-i (2017) Discovery and history of amino acid fermentation. In: Yokota A, Ikeda M (eds) Amino acid ferment. Springer Japan, Tokyo, pp 15–34
Jang C-W, Magnuson T (2013) A novel selection marker for efficient DNA cloning and recombineering in E. coli. PLoS One 8(2):e57075. https://doi.org/10.1371/journal.pone.0057075
Jiang Y, Chen B, Duan C, Sun B, Yang J, Yang S (2015) Multigene editing in the Escherichia coli genome via the CRISPR-Cas9 system. Appl Environ Microbiol 81(7):2506–2514. https://doi.org/10.1128/AEM.04023-14
Kőrös Á, Varga Z, Molnár-Perl I (2008) Simultaneous analysis of amino acids and amines as their o-phthalaldehyde-ethanethiol-9-fluorenylmethyl chloroformate derivatives in cheese by high-performance liquid chromatography. J Chromatogr A 1203(2):146–152. https://doi.org/10.1016/j.chroma.2008.07.035
Kruse D, Krämer R, Eggeling L, Rieping M, Pfefferle W, Tchieu J, Chung Y, Saier M, Burkovski A (2002) Influence of threonine exporters on threonine production in Escherichia coli. Appl Microbiol Biotechnol 59(2):205–210. https://doi.org/10.1007/s00253-002-0987-7
Kumari S, Beatty CM, Browning DF, Busby SJW, Simel EJ, Hovel-Miner G, Wolfe AJ (2000) Regulation of acetyl coenzyme a synthetase in Escherichia coli. J Bacteriol 182(15):4173–4179
Lee J-H, Lee D-E, Lee B-U, Kim H-S (2003) Global analyses of transcriptomes and proteomes of a parent strain and an l-threonine-overproducing mutant strain. J Bacteriol 185(18):5442–5451. https://doi.org/10.1128/JB.185.18.5442-5451.2003
Lee M-H, Lee H-W, Park J-H, Ahn J-O, Jung J-K, Hwang Y-I (2006) Improved l-threonine production of Escherichia coli mutant by optimization of culture conditions. J Biosci Bioeng 101(2):127–130. https://doi.org/10.1263/jbb.101.127
Lee KH, Park JH, Kim TY, Kim HU, Lee SY (2007) Systems metabolic engineering of Escherichia coli for L-threonine production. Mol Syst Biol 3. https://doi.org/10.1038/msb4100196
Lee JH, Sung BH, Kim MS, Blattner FR, Yoon BH, Kim JH, Kim SC (2009) Metabolic engineering of a reduced-genome strain of Escherichia coli for L-threonine production. Microb Cell Factories 8:2. https://doi.org/10.1186/1475-2859-8-2
Lee JH, Jung S-C, Bui LM, Kang KH, Song J-J, Kim SC (2013) Improved production of l-threonine in Escherichia coli by use of a DNA scaffold system. ApplEnviron Microbiol 79(3):774–782. https://doi.org/10.1128/AEM.02578-12
Leuchtenberger W, Huthmacher K, Drauz K (2005) Biotechnological production of amino acids and derivatives: current status and prospects. Appl Microbiol Biotechnol 69(1):1–8. https://doi.org/10.1007/s00253-005-0155-y
Li N, Zhang B, Chen T, Wang Z, Tang YJ, Zhao X (2013) Directed pathway evolution of the glyoxylate shunt in Escherichia coli for improved aerobic succinate production from glycerol. J Ind Microbiol Biotechnol 40(12):1461–1475. https://doi.org/10.1007/s10295-013-1342-y
Li N, Zhang B, Wang Z, Tang YJ, Chen T, Zhao X (2014) Engineering Escherichia coli for fumaric acid production from glycerol. Bioresour Technol 174:81–87. https://doi.org/10.1016/j.biortech.2014.09.147
Li Y, Huang B, Wu H, Li Z, Ye Q, Zhang YP (2016) Production of succinate from acetate by metabolically engineered Escherichia coli. ACS Synth Biol 5(11):1299–1307. https://doi.org/10.1021/acssynbio.6b00052
Lin F, Chen Y, Levine R, Lee K, Yuan Y, Lin XN (2013) Improving fatty acid availability for bio-hydrocarbon production in Escherichia coli by metabolic engineering. PLoS One 8(10):e78595. https://doi.org/10.1371/journal.pone.0078595
Liu F, Qimuge, Hao J, Yan H, Bach T, Fan L, Morigen (2014) AspC-mediated aspartate metabolism coordinates the Escherichia coli cell cycle. PLoS One 9(3):e92229. https://doi.org/10.1371/journal.pone.0092229
Liu Y, Li Q, Zheng P, Zhang Z, Liu Y, Sun C, Cao G, Zhou W, Wang X, Zhang D, Zhang T, Sun J, Ma Y (2015) Developing a high-throughput screening method for threonine overproduction based on an artificial promoter. Microb Cell Factories 14:121. https://doi.org/10.1186/s12934-015-0311-8
Liu M, Ding Y, Chen H, Zhao Z, Liu H, Xian M, Zhao G (2017) Improving the production of acetyl-CoA-derived chemicals in Escherichia coli BL21 (DE3) through iclR and arcA deletion. BMC Microbiol 17:10. https://doi.org/10.1186/s12866-016-0913-2
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Livshits VA, Zakataeva NP, Aleshin VV, Vitushkina MV (2003) Identification and characterization of the new gene rhtA involved in threonine and homoserine efflux in Escherichia coli. Res Microbiol 154(2):123–135. https://doi.org/10.1016/s0923-2508(03)00036-6
Ning Y, Wu X, Zhang C, Xu Q, Chen N, Xie X (2016) Pathway construction and metabolic engineering for fermentative production of ectoine in Escherichia coli. Metab Eng 36:10–18. https://doi.org/10.1016/j.ymben.2016.02.013
Nolden L, Farwick M, Krämer R, Burkovski A (2001) Glutamine synthetases of Corynebacterium glutamicum: transcriptional control and regulation of activity. FEMS Microbiol Lett 201(1):91–98. https://doi.org/10.1111/j.1574-6968.2001.tb10738.x
Sharan SK, Thomason LC, Kuznetsov SG, Court DL (2009) Recombineering: a homologous recombination-based method of genetic engineering. Nat Protoc 4(2):206–223. https://doi.org/10.1038/nprot.2008.227
Skorokhodova AY, Gulevich AY, Morzhakova AA, Shakulov RS, Debabov VG (2013) Comparison of different approaches to activate the glyoxylate bypass in Escherichia coli K-12 for succinate biosynthesis during dual-phase fermentation in minimal glucose media. Biotechnol Lett 35(4):577–583. https://doi.org/10.1007/s10529-012-1108-z
Song CW, Kim DI, Choi S, Jang JW, Lee SY (2013) Metabolic engineering of Escherichia coli for the production of fumaric acid. Biotechnol Bioeng 110(7):2025–2034. https://doi.org/10.1002/bit.24868
Song CW, Lee J, Ko YS, Lee SY (2015) Metabolic engineering of Escherichia coli for the production of 3-aminopropionic acid. Metab Eng 30:121–129. https://doi.org/10.1016/j.ymben.2015.05.005
Wang J, Cheng LK, Chen N (2014) High-level production of L-threonine by recombinant Escherichia coli with combined feeding strategies. Biotechnol BiotechnolEquip 28(3):495–501. https://doi.org/10.1080/13102818.2014.927682
Xie X, Liang Y, Liu H, Liu Y, Xu Q, Zhang C, Chen N (2014) Modification of glycolysis and its effect on the production of L-threonine in Escherichia coli. J Ind Microbiol Biotechnol 41(6):1007–1015. https://doi.org/10.1007/s10295-014-1436-1
Xu Y, Liu Y, Li F, Cao G, Zheng P, Sun J, Wen J, Zhang D (2017) Identification of a new gene yecC involved in threonine export in Escherichia coli. FEMS Microbiol Lett 364(17):fnx174. https://doi.org/10.1093/femsle/fnx174
Yamamoto K, Ishihama A (2003) Two different modes of transcription repression of the Escherichia coli acetate operon by IclR. Mol Microbiol 47(1):183–194. https://doi.org/10.1046/j.1365-2958.2003.03287.x
Yuzbashev TV, Vybornaya TV, Larina AS, Gvilava IT, Voyushina NE, Mokrova SS, Yuzbasheva EY, Manukhov IV, Sineoky SP, Debabov VG (2013) Directed modification of Escherichia coli metabolism for the design of threonine-producing strains. Appl Biochem Microbiol 49(9):723–742. https://doi.org/10.1134/s0003683813090056
Zakataeva NP, Aleshin VV, Tokmakova IL, Troshin PV, Livshits VA (1999) The novel transmembrane Escherichia coli proteins involved in the amino acid efflux. FEBS Lett 452(3):228–232. https://doi.org/10.1016/S0014-5793(99)00625-0
Zhang Z, Gosset G, Barabote R, Gonzalez CS, Cuevas WA, Saier MH Jr (2005) Functional interactions between the carbon and iron utilization regulators, Crp and Fur, in Escherichia coli. J Bacteriol 187(3):980–990. https://doi.org/10.1128/JB.187.3.980-990.2005
Zhang Y, Meng Q, Ma H, Liu Y, Cao G, Zhang X, Zheng P, Sun J, Zhang D, Jiang W, Ma Y (2015a) Determination of key enzymes for threonine synthesis through in vitro metabolic pathway analysis. Microb Cell Factories 14:86. https://doi.org/10.1186/s12934-015-0275-8
Zhang T, Wang Z, Deng L, Tan T, Wang F, Yan Y (2015b) Pull-in urea cycle for the production of fumaric acid in Escherichia coli. Appl Microbiol Biotechnol 99(12):5033–5044. https://doi.org/10.1007/s00253-015-6556-7
Zheng J, Yates SP, Jia Z (2012) Structural and mechanistic insights into the bifunctional enzyme isocitrate dehydrogenase kinase/phosphatase AceK. Philos Trans R Soc Lond B Biol Sci 367(1602):2656–2668. https://doi.org/10.1098/rstb.2011.0426
Zhu L, Lin J, Ma J, Cronan JE, Wang H (2010) Triclosan resistance of Pseudomonas aeruginosa PAO1 is due to FabV, a triclosan-resistant enoyl-acyl carrier protein reductase. Antimicrob Agents Chemother 54(2):689–698. https://doi.org/10.1128/AAC.01152-09
Funding
This study was supported by the Collaborative Innovation Center of Jiangsu Modern Industrial Fermentation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Compliance with ethics requirements
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Zhao, H., Fang, Y., Wang, X. et al. Increasing l-threonine production in Escherichia coli by engineering the glyoxylate shunt and the l-threonine biosynthesis pathway. Appl Microbiol Biotechnol 102, 5505–5518 (2018). https://doi.org/10.1007/s00253-018-9024-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-9024-3