Abstract
Succinic acid is one of the most useful intermediate chemicals that can be produced in a biorefinery approach. In this study, Actinobacillus succinogenes was immobilized to produce succinic acid using non-detoxified corn fiber hydrolysate (CFH) and a control mimicking the sugars in CFH. Tests were carried out in a hollow fiber membrane packed-bed biofilm reactor (HFM–PBR) operated in a continuous mode. Under steady-state conditions, the bioconversion process was characterized in terms of sugar consumption, succinic acid and other organic acid production. Steady states were obtained at dilution rates of 0.025, 0.05, 0.075, 0.1, 0.2, and 0.3 h−1. The optimal results were achieved at the dilution rate of 0.05 h−1 and recirculation rate of 50 ml/min with a maximum succinic acid concentration, yield and productivity of 31.1 g/L, 0.61 g/g and 1.56 g/L h, respectively, when control was used. Succinic acid concentration, yield and productivity of 23.4 g/L, 0.51 g/g and 1.17 g/L h, respectively, were obtained when CFH was used. Productivity in the HFM–PBR was between 1.3 and 1.9 times higher than productivities for succinic acid production from CFH stated in the literature. The results demonstrated that immobilized A. succinogenes has the potential for effective conversion of an inexpensive biomass feedstock to succinic acid. Furthermore, the process has the potential to serve as a means for value-added chemical biomanufacturing in an integrated corn biorefinery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Growing concerns over the contribution of fossil fuels-based processes to global warming and the strong demand for environmentally friendly energy sources have inspired a deep interest in developing more sustainable processes with lower cost and energy consumption that affords the same product using renewable biomass [1, 2]. Succinic acid, an important building-block chemical, was featured as one of the top value-added chemicals from biomass by the US Department of Energy [3]. The development of bio-based succinic acid processes has gained traction due to the environmental impact of fossil-fuel dependent processes, which has been the traditional method to produce succinic acid [4]. Another major driver is succinic acid’s key role in the synthesis of many large-volume chemicals and many consumer products such as 1,4 butanediol [5, 6].
Succinic acid can be obtained by microbial fermentation of sugars by Actinobacillus succinogenes, a native high-succinic acid producer that possesses the ability to use a wide variety of sugars and biomass hydrolysates for succinic acid production [7,8,9,10]. Moreover, the process captures the greenhouse gas CO2, which is a substrate in succinic acid production [11,12,13]. Significant steps for succinic acid production by A. succinogenes, such as the metabolic flux of carbon and the activity of phosphoenolpyruvate (PEP) carboxykinase, are regulated by pH and dissolved CO2 concentration in fermentation media. When gaseous CO2 is supplied during fermentation, it possesses poor solubility in media at 1 atm, negatively affecting succinic acid production. In addition, due to the low affinity of the enzymes responsible for CO2 fixation, high CO2 partial pressures may be required succinic acid fermentations [14]. To further enhance CO2 concentration in media, different strategies such as the addition of many kinds of carbonate salts as indirect supplementation of CO2, high-pressure fermenters, and conventional bioreactors with sparging and intense agitation have been utilized [15,16,17]. Nevertheless, supplying high concentrations of carbonate salts is not economically feasible from an industrial point of view, and safety precautions during full scale operation of high-pressure fermenters requires further attention [18]. Also, due to both the damage to cells and high power consumption caused by excessive agitation speed or gas lift mechanisms, conventional stirred tank bioreactors need reconsideration. Another strategy could be the use of hollow fiber membranes (HFMs) to enhance the dissolved CO2 concentration in succinic acid fermentations. HFMs diffuse gases through their micropores without forming bubbles yielding a large surface area for both gas–liquid transfer and, therefore, enhancing the amount of dissolved gas in fermentation broth [19]. HFMs have been employed successfully to enhance mass transfer of gases in wastewater and water treatments and ethanol and syngas fermentations [19,20,21].
Most A. succinogenes fermentations have been performed in batch and fed-batch mode. However, A. succinogenes’ growth is inhibited by acids produced during fermentation [22, 23], which reduces volumetric productivity of batch processes [24]. Continuous fermentations offer an improvement in product production rates and productivity. However, biofilm formation is prevalent A. succinogenes fermentations [25, 26], which makes establishing steady-state conditions in a continuous stirred tank reactor very difficult due to cells attaching to internal surfaces within the reactor [25].
Actinobacillus succinogenes’ proclivity for biofilm formation can be taken advantage of by allowing the biofilm to attach to support in a continuous packed bed reactor (PBR) [24,25,26,27]. Ferrone et al. [24] used a mixture of glucose, xylose, and arabinose that mimicked lignocellulosic biomass hydrolysate to produce succinic acid in a 166 mL PBR operated in continuous mode for five months. They achieved a succinic acid concentration of 43.0 g/L, a glucose conversion of 88%, and a volumetric productivity of 22 g/L h at the dilution rate 0.5 h−1. Maharaj et al. [27] reported a continuous fermentation by A. succinogenes in a PBR with Poraver® beads using pure glucose. The authors reported a succinic acid volumetric productivity of 10.8 g/L h at a dilution rate = 0.7 h−1.
In view of the above context, it is valid to explore processes that use renewable materials that have lower costs than pure sugar feedstocks, which is one of the major bottlenecks to establishing a successful industrial succinic acid bioconversion [28]. To the best of the authors’ knowledge, there are no published studies investigating continuous succinic acid production from renewable feedstocks using a PBR and a HFM for supplying CO2 to the fermentation media. Corn fiber, an inexpensive source of sugars, has been used previously in batch fermentations to achieve succinic acid yields similar to yields obtained from a pure sugar mixture [29]. The bioconversion process could be integrated into a corn bioethanol facility as corn fiber and gaseous CO2 are low-value coproducts generated in these facilities. Using such coproducts at the point of production eliminates logistical costs of raw material supply. In this study, continuous succinic acid production in a PBR coupled with a HFM was used to produce succinic acid yield from corn fiber hydrolysate (CFH). This work presents important insights into the operation of a hollow fiber membrane packed bed reactor (HFM–PBR) and the effect of dilution rate (D) and recirculation flow rate on CFH sugars consumption and succinic yield and productivity.
Materials and methods
Microorganism, inoculum, and medium
Actinobacillus succinogenes 130Z was obtained from the American Type Culture Collection (ATCC) (Manassas, VA, USA) and was used to produce succinic acid from CFH. The culture in the form of freeze-dried pellets was reactivated according to the procedure suggested by ATCC and stored following the recommendations of Long et al. [30]. Stock cell tubes were preserved at − 80 °C in 5% DMSO in 1.5 mL culture tubes and used for the inoculation. A. succinogenes cells were inoculated in anaerobic culture tubes containing seed medium (30 g tryptic soy broth/L and 15 g glucose/L) and incubated in a shaker at 37 °C, 250 rpm for 14–16 h. The culture was washed with sterile 0.89% sodium chloride solution and resuspended with fermentation medium and inoculated to the reactor. The growth medium based on Maharaj et al. [27], with some modifications, and had the following composition (per L): 16.0 g yeast extract, 1.0 g NaCl, 1.36 g NaC2H3O2, 0.20 g MgCl2·6H2O, and 0.20 g CaCl2·2H2O. The carbon sources were a control that was a sugar solution mimicking the sugars contained in CFH, and CFH, a sugar solution obtained from corn fiber as explained in the next section. All media were sterilized at 121 °C for 20 min before use.
Preparation of CFH
The preparation of CFH was according to Vallecilla-Yepez et al. [29] and proceeded as follows: ground corn fiber provided by E-Energy Adams, LLC, (Adams, NE, USA) was subjected to liquid hot water pretreatment in a 7.5 L Parr reactor as described below (Parr Reactor Model 4552, Parr Instrument Co., Moline, IL, USA). Samples of 0.9 kg ground corn fiber (dry basis) were mixed with 5.1 kg water to achieve 15% solids loading. The mixture was agitated at 300 rpm, heated to 180 °C, and held at 180 °C for 10 min. After the pretreatment, pretreated corn fiber was separated from the liquid by filtration and washed with 9 kg of water. Enzymatic hydrolysis of the pretreated corn fiber was done by with Ctec2 enzyme (Novozymes, Inc., Franklinton, NC, USA) in the ratio of 20 FPU/g glucan (Filter Paper Units enzyme/g glucan) and water to achieve 10% solids loading. The hydrolysis was carried out in a 42 L Techfors-S bioreactor (INFORS HT, Basel, Switzerland) at a mixing rate of 1000 rpm and at a temperature of 50 °C for 72 h. The pH was controlled at 5.0 with 5 M NaOH and 5 M H2SO4 solutions using the Techfors-S and Eve control software (INFORS HT). After hydrolysis, solid residues were separated by filtration and the filtrate was sterilized by passing the resultant solution through a 0.22 μm bottle top filter (Nalgene™ Rapid-Flow™ Sterile Single Use Bottle Top Filters, Thermo Fisher Scientific, USA). The filtered solution containing the sugars was designated as CFH, and it did not undergo detoxification procedures. CFH was kept at 4 °C until further use for fermentation.
pH control and buffer selection
pH is a key parameter in the succinic acid bioconversion process because both intracellular enzymatic activities and cellular maintenance in A. succinogenes are strictly pH dependent. The optimal pH range for succinic acid productivity was found to be between 6.0 and 7.2 with a maximum production of succinic acid achieved at 6.7 [15]. In addition, for succinic acid producing bacteria, the optimal pH for the PEP–carboxykinase activity and a higher effect of CO2 on succinic acid yield have been observed at pH 6.5 [31]. Tests have been reported elsewhere in the literature to assess the best pH regulation strategy in succinic acid fermentation in attempts to improve its feasibility at industrial scale [16, 32]. In this study, a mixture of Mg(OH)2 (5 M) and NaOH (5 M) in a mass ratio of 1:1 was used to control pH at 6.6, which is between the enzymes' optimal pH for succinate formation and for maximum CO2 fixation.
Biofilm carriers
Two biofilm carriers were used during this study. Preliminary tests were performed with carrier 1, which was 238 g of packing material comprised of 568 HDPE plastic rings (model BCN 030, GEA 2H Water Technologies GmbH, Germany, Europe) used in a previous study that used a PBR to produce aryl alcohol oxidase with filamentous fungi [33]. The length and diameter of the rings were equal to 30 mm and 30–36 mm, respectively, with a surface of 320 m2/m3 and a protected surface of 259 m2/m3. The rings occupied a volume of ± 3.3 L (approx. 45% of the column volume). The second carrier, carrier 2, consisted of a constant mass of 5.8 kg of solid soda lime spheres (Avogadro's Lab Supply, Inc., Shamong, NJ, USA) to ensure an approximately constant volume and surface area available for attachment. The diameter and density of the spheres were equal to 0.01 m and 2.49 kg/m3, respectively. The support beads occupied a volume of ± 6.7 L (approx. 91% of the column volume).
HFM–PBR design
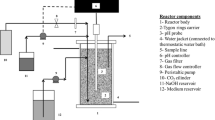
An HFM–PBR photographed in Fig. 1 and shown schematically in Fig. 2 was designed for succinic acid fermentation. The novel reactor consisted of a 0.71 m tall glass column (Ace Glass Incorporated, Vineland, NJ, USA) with an internal diameter of 0.08 m that was jacketed for heat exchange. The glass column was incompletely filled with the packing material to provide a head space that allowed a thermowell to be in contact with the fermentation medium. A perforated borosilicate glass support plate (3 mm rectangular slits) (Ace Glass Incorporated, Vineland, NJ, USA) was inserted below the packing to support it while allowing flow of liquid and gas. The system contained a polydimethylsiloxane hollow fiber membrane module (PermSelect®-Silicone Gas Exchange Membranes, Ann Arbor, MI, USA). The module was a polycarbonate shell which envelops a polyurethane potting with 2500 cm2 surface area, based on the outside area of the hollow fibers. Figure 3 shows the module connection to the HFM–PBR, which was configured to add gas to the liquid. In this type of configuration, the liquid (fermentation medium) flows in the shell side contacting with CO2 that flows on the lumen side (inside the hollow fibers).
The inlets for fresh and recycled CFH were placed on the top of the reactor, as well as a thermocouple to measure the column temperature, and a 169-kPa (5 psig) safety pressure release valve to protect the glass column. A bioreactor (BioFlo 115, New Brunswick Scientific Co., NJ, USA) equipped with a 1.3 L vessel containing a 6-Blade Rushton impeller was used as a mixing vessel and connected to the reactor. A sampling port at the bottom of the column allowed periodic sampling of the liquid broth. The inoculation port, pH probe, and a second thermocouple were located in the mixing vessel. The product line was connected at the bottom of the reactor and a sampling port in the product line allowed periodic sampling. Two peristaltic pumps (MasterFlex 7523-20, Barnant Co., Barrington, IL, USA) were used for media recirculation to provide mixing in the reactor. One pump took media out from the mixing vessel and into the HFM–PBR. The second pump took medium from the HFM–PBR to the mixing vessel at the same rate as the first pump. When the HFM–PBR operated in a continuous mode, a dual channel peristaltic pump (Masterflex® L/S® Digital Miniflex® Pump Systems, Barnant Co., Barrington, IL, USA) was used to add fresh medium into the system at the same rate that the product was collected. Filter-sterilized CO2 (0.2 mm PTFE filter, Pall Corporation, NY, USA) was sparged into the CFH through the HFM membrane. Temperature was controlled using column’s water jacket connected to a thermostatic water bath.
HFM–PBR fermentation
The HFM–PBR was sterilized by filling it with deionized water and autoclaving it at 121 °C for 45 min. The 1 L mixing vessel, tubing and fittings for the system were autoclaved separately and assembled later. After sterilization, the reactor was drained and refilled with sterile fermentation medium that was autoclaved separately. The working volume of the reactor (including the recirculation line) was 2.6 L. CO2 was fed into the reactor at 169 kPa (5 psig) through the HFM to maintain anaerobic conditions. Medium pH was monitored by a pH-mV controller (Metter Toledo 405-DPAS-SC-K8S/225) in the mixing vessel and maintained at 6.6 by automatic addition of the mixture of Mg(OH)2:NaOH described earlier. The stirring speed in the mixing vessel was set as 70 rpm, and mixing vessel temperature was controlled at 37 °C with a heating blanket. Once temperature and pH were stabilized in the system, inoculum (10% v/v based on the total HFM–PBR volume) was added into the mixing vessel. Unless otherwise indicated, the recirculation flow rate was kept constant at 50 mL min−1 in all fermentations to maintain similar shear conditions.
Design of experiments and data analysis
Preliminary experiments were carried out in the HFM–PBR using a sugar control solution to select a biofilm carrier to establish the best operation conditions and dilution rates for the process of succinic acid production from CFH. The HFM–PBR was operated in batch mode until all glucose and xylose were consumed and biofilm was observed on the carrier. When this occurred, continuous operation of the HFM–PBR started with varying dilution rate. Profiles of sugar consumption, succinic acid and other organic acids production and succinic acid yield were determined for each dilution rate. In this paper, only steady-state results are reported. Steady state was assumed when the absolute deviation of the succinic acid concentration, captured over a period of at least 12 h, did not exceed 10% of the mean value. All dilution rates except 0.2 and 0.3 h−1 were evaluated in duplicate.
Analytical methods
Concentrations of glucose, xylose, succinic acid, lactic acid, formic acid, acetic acid, and ethanol in the samples were monitored by high-performance liquid chromatography (HPLC). A 10 μL cell-free sample was injected into a BioRad chromatographic column (Aminex HPX-87H, 7.8 mm 300 mm, Biorad, Hercules, CA, USA), using 0.01 N H2SO4 as the mobile phase at a pump rate of 0.6 mL/min. The temperature of the refractive index detector, RI101 (Shodex, New York, NY, USA) and column were maintained at 50 and 65 °C, respectively.
Results and discussion
Biofilm start-up and selection of carrier for A. succinogenes immobilization
Nine separate fermentations were independently performed with the sugar control solution in the HFM–PBR. Three independent fermentations were performed with the biofilm carrier 1. Initially, the bioreactor was operated in batch mode to allow cells to grow and attach to the carrier; however, after 2 days, cell attachment to carrier 1 was not developed and continuous fermentation could not be started. Next, a series of repeated-batch succinic acid fermentations were performed over 4 days using carrier 1 to develop an A. succinogenes biofilm on the supports. Although succinic acid production was achieved (see Table 1), biofilm was not established on carrier 1 (see Fig. 4a).
A second support was then employed, and seven independent fermentations were performed with carrier 2. It took approximately 24 h in batch fermentation for carrier 2 to be covered with biofilm (see Fig. 4b). Once the biofilm was attached to carrier 2 and the sugars in the media were depleted, the operation of the reactor was switched from batch to continuous mode. It is worth noting that for carrier 2, succinic acid and other organic acids production and sugars consumption after 24 h were in the same range of the values obtained for carrier 1 after 96 h (Table 1). Although similar operation conditions were used with both packing materials, biofilm attachment only developed on carrier 2. Several factors can affect the performance of packing materials in cell attachment. Microorganisms naturally inhabit the outer and inner surfaces of gravel, sand, or stone [34] and this could be a plausible explanation of why A. succinogenes biofilm was established on glass beads (carrier 2) rather than on plastic rings. Moreover, some factors are also inherent to the biofilm carrier, such as surface area (a very large surface area for microbial growth is needed), pore size, porosity, surface roughness, orientation of the packing material, and appropriate contact between liquid and gas phases [35, 36]. In fixed-bed reactors, organic matter removal efficiency is directly related to the support material used for immobilization of anaerobic microorganisms [37]. Furthermore, common bioreactor operation factors, such as media recirculation rate, dilution rate, and pressure drop are important in cell attachment [38, 39]. Packing structure is also a critical characteristic in supporting microbes’ growth. For example, carrier 1 are packing rings that are less structured materials that result in higher pressure drops and therefore lower mass transfer efficiencies than structured packings, such as carrier 2, which makes carrier 2 more desirable for biofilm formers [35].
Continuous fermentations in the HFM–PBR using sugar control
The recirculation rate for the initial continuous operation of the HFM–PBR was set at 50 mL/min and was kept at that value during the experiment evaluating different dilution rates. For all steady states, multiple HPLC samples were taken and accordingly averaged. Steady-state data on succinic acid and other organic acids production and glucose and xylose consumption at dilution rates from 0.025 to 0.3 h−1 are shown in Fig. 5a, b. The reproducibility of the steady states was tested at all dilution rates in duplicate with four samples taken at each steady-state condition.
a Steady-state succinic acid (SA), glucose (Glu) and xylose (Xyl) concentration and b formic acid (FA), lactic acid (LA) and acetic acid (AA) concentrations at various dilution rates. Data are average values of duplicate experiments, and error bars represent compound standard deviation. Glu glucose, Xyl xylose, Ara arabinose, succinic acid succinic acid, LA lactic acid, FA formic acid, and AA acetic acid. Error bars are not shown for dilution rates of 0.2 and 0.3 h−1 where repeat runs were not performed
Succinic acid was the major product produced in fermentations using the control and lactic, formic and acetic acid were also produced. Metabolite concentrations increased with corresponding decreases in dilution rate. The initial operation of the continuous fermentations was performed at a low dilution rate (0.025 h−1) to allow the culture to adapt to the medium. After steady state at that D was achieved, D was subsequently increased to D = 0.05, 0.075, 0.1, 0.2, and 0.3 h−1. At dilution rate of 0.025 h−1, nearly all glucose and xylose were consumed, succinic acid concentration was 32.3 g/L (Fig. 5a) and lactic, formic and acetic acids concentrations were 3.9, 5.9, and 10.1 g/L, respectively (Fig. 5b). The % consumption of sugars at D = 0.025 h−1 was 100% of glucose and 98.6% of xylose.
The second highest succinic acid and organic acid concentrations were found at D = 0.05 h−1, where succinic acid concentration was 31.1 g/L (Fig. 5a) and lactic, formic and acetic acids concentrations were 2.2, 3.3, and 9.4 g/L, respectively (Fig. 5b). At this operation condition, 91.3% of glucose and 90% of xylose were consumed. The consumption of glucose and xylose decreased gradually with increasing dilution rate from 100% of glucose and 98.6% of xylose at D = 0.025 h−1 to 17.0% of glucose and 45.7% of xylose at D = 0.3 h−1 (Fig. 5a). An order of preference in sugar utilization by A. succinogenes was not observed as all sugars were consumed simultaneously suggesting the absence of carbon catabolite repression of fermentation of sugar control in the HFM–PBR, which agrees with previous research [40]. The achieved succinic acid yield for both dilution rates, 0.025 and 0.05 h−1, were 0.61 g/g, however, a higher productivity of 1.56 g/L h was observed at D = 0.05 h−1 compared to productivity of 0.81 g/L h at D = 0.025 h−1. Therefore, 0.05 h−1 was selected as the dilution rate to carry out succinic acid production from CFH. Moreover, the maximum succinic acid concentration in this study (32.3 g/L) was higher than that of our previous batch study (28.7 g/L) [29].
Effect of the mixing recirculation rate in succinic acid production
The effect of the mixing recirculation rate was studied in order to evaluate if changing the shear conditions affected biofilm attachment, succinic acid production, and substrate utilization by A. succinogenes in the HFM–PBR. The mixing recirculation rate varied from 25 to 75 mL/min at constant dilution rate of 0.05 h−1. The maximum rate allowed by the peristaltic pump for the tubing used in the recirculation line was 80 mL/min.
As can be seen in Fig. 6, production of succinic acid and other organic acids and consumption of sugars with variations in the mixing recirculation rate. The highest succinic acid concentration of 23.4 g/L and sugar utilization of 75.4% for glucose and 81.3% for xylose were achieved at the recirculation flow rate of 50 mL/min. At the highest recirculation rate (75 mL/min), it was observed that biofilm attachment in the packing media decreased and the succinic acid concentration was 21.1 g/L and sugar utilization was 62.0 and 67.7% for glucose and xylose, respectively. Previous investigations in biofilm reactors have increased the recirculation rates to scrub loose biofilm and remove cell segments from the packing material [28]. Also, gas–liquid mass transfer in reactors for syngas fermentation decreased with higher recirculation rates due to an increase in liquid hold-ups in a PBR [20]. It was expected that increasing the recirculation rate would decrease substrate utilization and succinic acid production due to decreased gas–liquid mass transfer and increased shear force preventing biofilm formation, and that is what was observed. At the lowest recirculation rate (25 mL/min), succinic acid concentration was 19.5 g/L and sugar utilization was 58.3% for glucose and 67.7% for xylose. Moreover, it was noticed that biofilm formation increased in the mixing vessel and the HFM rather than in the packing material. In general, media recirculation rate in processes that involve conversion of gas substrate by microorganisms can be crucial since mass transfer is limiting. Sugar utilization and succinic acid production decreased when recirculation rate was decreased from 50 to 25 mL/min while maintaining a fixed D = 0.05 h−1. It was expected that decreasing the recirculation rate would enhance substrate utilization and succinic acid production since the media broth spends a longer time in contact with the biofilm, thus enhancing mass transfer time, which improves diffusion of substrate into the A. succinogenes cells on the carrier. However, biofilm attached to the glass walls and internals of the mixing vessel and attachment to the column packing was reduced compared to what was observed in the dilution rate experiment (Fig. 7). Another observation was the lower succinic acid concentration and sugar utilization achieved during the recirculation rate experiment compared to the values obtained during the dilution rate experiment. At 25 mL/min, biofilm attachment occurred not only in the mixing vessel, but also in the HFM, affecting both gas–liquid transfer and the amount of dissolved gas in fermentation broth and therefore this could result in lower succinic acid yields. As recirculation rates were increased, some biofilm was removed from the HFM, but much still remained. After the recirculation rate experiment was completed, a new HFM was installed in the system. An experiment with the new HFM was conducted at D = 0.05 h−1 and 50 ml/min and succinic acid concentration and sugar utilization was similar to the dilution effect experiment were achieved at 50 ml/min (data not shown here).
During all the experiments, biofilm attachment in the HFM internals was observed (Fig. 8); however, the system tubing was not clogged and continuous fermentations were performed without any impediments. The highest succinic acid concentration and sugar utilization was found at 50 mL/min, suggesting that at this recirculation rate there are more suitable conditions such as residence time, homogeneous media distribution through the packing bed, and better gas–liquid transfer that allow a higher succinic acid production and biofilm attachment to the packing material. Once the recirculation rate for the highest succinic acid production was established, continuous succinic acid production in the HFM–PBR from CFH was carried out at D = 0.05 h−1 and 50 mL/min.
Continuous succinic acid production in the HFM–PBR using corn fiber hydrolysate
A fermentation was performed in the HFM–PBR using CFH. The HFM–PBR was able to operate continuously for 6 days without encountering any clogging or contamination problems. At 24 h of batch operation, a stable biofilm was developed in the packed column; however, HPLC analysis showed high formic acid and low succinic acid concentrations (2.8 and 11.0 g/L, respectively). Analysis of the CFH showed that while furfural and hydroxymethylfurfural compounds were not present in the hydrolysate, formic acid and acetic acid were detected at initial concentrations of 12.8, and 1.8 g/L, respectively. These compounds are known inhibitors of A. succinogenes [25]. Batch fermentation was continued until 36 h, after which biofilm formation was observed, the concentrations of succinic, lactic, formic, and acetic acids were 18.3, 2.7, 11.8, and 9.7 g/L, respectively, while sugars in the medium were depleted. A. succinogenes adapted to the CFH from 24 to 36 h. Once sugars were consumed at 36 h, the system was switched to continuous mode at a dilution rate of 0.05 h−1; however, after 12 h of continuous operation biofilm attachment on the packing material was not developed. The dilution rate was reduced to 0.025 h−1, which was a strategy used in a previous study of continuous succinic acid production from dilute acid pretreated corn stover hydrolysate [40]. After 12 h operation at D = 0.025 h−1, biofilm attachment was observed. Then, the operation was held at D = 0.025 h−1 for 12 more h to allow the culture to adapt to the CFH and develop further biofilm formation. After 24 h, dilution rate was increased to 0.05 h−1 and the performance of the system was assessed under steady-state conditions at D = 0.05 h−1. Figure 9a shows the biofilm development on the HFM–PBR around carrier 2 at D = 0.05 h−1 and 50 mL/min recirculation rate using the CFH. In Fig. 9b, biofilm formation can be observed in the HFM internals using the CFH, but it did not impede the recirculation of media through the system or normal operation of the HFM–PBR.
The maximum amount of succinic acid produced from CFH was 23.4 g/L, with a yield of 0.51 g/g and productivity of 1.17 g/L h. The productivity achieved in this study represents the highest succinic acid productivity achieved from CFH in literature (between 1.3 and 1.9 times) [8, 29, 41, 42]. Lactic, formic, and acetic acid concentrations were 4.1, 11.7, and 11.5 g/L, respectively, and this includes the acids that were initially in the CFH. In agreement with the results of continuous succinic acid fermentation using the control, the sugars were consumed simultaneously but at different rates with no utilization preferences as seen in the present study. Succinic acid production and the conversion of glucose (86.3%) and xylose (79.4%) at 0.05 h−1 in the CFH fermentation were lower than that of the sugar control fermentation at the same dilution rate. The concentration of produced succinic acid decreased by 24.7% with respect to the control, which could be attributed to the presence of inhibitors. As it was stated previously, formic acid was detected in the CFH at initial concentration of 12.8 g/L. Weak organic acids, such as formic acid, have inhibited the conversion efficiency of anaerobic bacteria used in succinic acid, acetone–butanol–ethanol, and H2 fermentation [25, 43, 44]. When microbial cells are exposed to fermentation media with formic acid produced during a physicochemical pretreatment of biomass, there is a higher maintenance cell requirement compared to media without formic acid. This occurs because the cells spend more energy (ATP) in translocating outside anions and protons across the plasma membrane, which results in a lower molar growth yield with respect to the carbon source [45]. However, succinic acid was the major compound produced. Moreover, it was found that A. succinogenes adapted to the hydrolysate, suggesting a strong evolutionary response to the toxic compounds in the CFH.
Other analysis
The total accumulative time span of HFM–PBR operation was 1200 h. This time includes the establishment of biofilm during start-up, steady state conditions and recovery from undesirable events such as reactor drainage, setting of the recirculation rate and constant media level in the mixing vessel, and adjusting flow lines in the system. Furthermore, high mixing and submerged spargers were not required to feed CO2 to the bacteria as was required in the two previous immobilized reactors for succinic production [28, 40]. Since HFMs yield a large surface area for both liquid and mass transfer without forming bubbles, no foaming was observed during the fermentations and therefore antifoaming agents were not necessary during the HFM–PBR operation. Moreover, strategies such as foam traps or CO2 feeding into recycle lines were not used in the configuration of the HFM–PBR.
Conclusions
In this study succinic acid was produced continuously by immobilized A. succinogenes in a novel HFM–PBR converting almost 100% of sugars present in a sugar control with a succinic acid concentration of 31.1 g/L, yield of 0.61 g/g, and productivity 1.56 g/L h. Succinic acid productivity of 1.17 g/L h, yield of 0.51 g/g, and concentration of 23.6 g/L were achieved when a non-detoxified CFH was the carbon substrate, which was a 24% reduction in succinic acid concentration compared to the control. Formic acid, a known inhibitor of A. succinogenes, in the CFH likely inhibited succinic acid production.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Clark S. Global bio succinic acid market (applications and geography)-size, share, trends, analysis, research, future demand, scope and forecast, 2013–2020. Allied Mark. Res; 2014.
Yim H, Haselbeck R, Niu W, Pujol-Baxley C, Burgard A, Boldt J, et al. Metabolic engineering of Escherichia coli for direct production of 1, 4-butanediol. Nat Chem Biol. 2011;7(7):445–52.
Werpy T, Petersen G. US Department of Energy; 2004. https://doi.org/10.2172/15008859.
Longanesi L, Frascari D, Spagni C, DeWever H, Pinelli D. Succinic acid production from cheese whey by biofilms of Actinobacillus succinogenes: packed bed bioreactor tests. J Chem Technol Biotechnol. 2018;93(1):246–56.
Ahn JH, Jang YS, Lee SY. Production of succinic acid by metabolically engineered microorganisms. Curr Opin Biotechnol. 2016;42:54–66.
Nghiem N, Kleff S, Schwegmann S. Succinic acid: technology development and commercialization. Fermentation. 2017;3(2).
Borges ER, Pereira N Jr. Succinic acid production from sugarcane bagasse hemicellulose hydrolysate by Actinobacillus succinogenes. J Indus Microbiol Biotechnol. 2011;38(8):1001–11.
Chen K, Jiang M, Wei P, Yao J, Wu H. Succinic acid production from acid hydrolysate of corn fiber by Actinobacillus succinogenes. Appl Biochem Biotechnol. 2010;160(2):477–85.
Wan C, Li Y, Shahbazi A, Xiu S. Succinic acid production from cheese whey using Actinobacillus succinogenes 130 Z. In: Adney WS, McMillan JD, Mielenz J, Klasson KT, editors. Biotechnology for fuels and chemicals. Totowa: Humana Press. p. 111–9. https://doi.org/10.1007/978-1-60327-526-2_1310.
Zheng P, Fang L, Xu Y, Dong J-J, Ni Y, Sun Z-H. Succinic acid production from corn stover by simultaneous saccharification and fermentation using Actinobacillus succinogenes. Bioresour Technol. 2010;101(20):7889–94.
Bechthold I, Bretz K, Kabasci S, Kopitzky R, Springer A. Succinic acid: a new platform chemical for biobased polymers from renewable resources. Chem Eng Technol. 2008;31(5):647–54.
Guettler MV, Rumler D, Jain MK. Actinobacillus succinogenes sp. nov., a novel succinic-acid-producing strain from the bovine rumen. Int J Syst Evol Microbiol. 1999;49(1):207–16.
Pateraki C, Patsalou M, Vlysidis A, Kopsahelis N, Webb C, Koutinas AA, et al. Actinobacillus succinogenes: advances on succinic acid production and prospects for development of integrated biorefineries. Biochem Eng J. 2016;112:285–303.
Samuelov N, Lamed R, Lowe S, Zeikus J. Influence of CO2- HCO3-levels and pH on growth, succinate production, and enzyme activities of Anaerobiospirillum succiniciproducens. Appl Environ Microbiol. 1991;57(10):3013–9.
Liu Y-P, Zheng P, Sun Z-H, Ni Y, Dong J-J, Wei P. Strategies of pH control and glucose-fed batch fermentation for production of succinic acid by Actinobacillus succinogenes CGMCC1593. J Chem Technol Biotechnol. 2008;83(5):722–9.
Wang CC, Zhu LW, Li HM, Tang YJ. Performance analyses of a neutralizing agent combination strategy for the production of succinic acid by Actinobacillus succinogenes ATCC 55618. Bioprocess Biosyst Eng. 2012;35(4):659–64.
Zou W, Zhu L-W, Li H-M, Tang Y-J. Significance of CO2 donor on the production of succinic acid by Actinobacillus succinogenes ATCC 55618. Microb Cell Fact. 2011;10(1):1–10.
Amulya K, Kopperi H, Mohan SV. Tunable production of succinic acid at elevated pressures of CO2 in a high pressure gas fermentation reactor. Bioresour Technol. 2020;309: 123327.
Shen Y, Brown R, Wen Z. Syngas fermentation of Clostridium carboxidivorans P7 in a hollow fiber membrane biofilm reactor: evaluating the mass transfer coefficient and ethanol production performance. Biochem Eng J. 2014;85:21–9.
Nerenberg R, Rittmann B. Hydrogen-based, hollow-fiber membrane biofilm reactor for reduction of perchlorate and other oxidized contaminants. Water Sci Technol. 2004;49(11–12):223–30.
Orgill JJ, Atiyeh HK, Devarapalli M, Phillips JR, Lewis RS, Huhnke RL. A comparison of mass transfer coefficients between trickle-bed, hollow fiber membrane and stirred tank reactors. Bioresour Technol. 2013;133:340–6.
Lin SKC, Du C, Koutinas A, Wang R, Webb C. Substrate and product inhibition kinetics in succinic acid production by Actinobacillus succinogenes. Biochem Eng J. 2008;41(2):128–35.
Ferone M, Raganati F, Olivieri G, Salatino P, Marzocchella A. Biosuccinic acid from lignocellulosic-based hexoses and pentoses by Actinobacillus succinogenes: characterization of the conversion process. Appl Biochem Biotechnol. 2017;183:1465–77. https://doi.org/10.1007/s12010-017-2514-4.
Ferone M, Raganati F, Ercole A, Olivieri G, Salatino P, Marzocchella A. Continuous succinic acid fermentation by Actinobacillus succinogenes in a packed-bed biofilm reactor. Biotechnol Biofuels. 2018;11:138.
Urbance SE, Pometto AL, DiSpirito AA, Denli Y. Evaluation of succinic acid continuous and repeat-batch biofilm fermentation by Actinobacillus succinogenes using plastic composite support bioreactors. Appl Microbiol Biotechnol. 2004;65(6):664–70. https://doi.org/10.1007/s00253-004-1634-2.
Van Heerden CD, Nicol W. Continuous succinic acid fermentation by Actinobacillus succinogenes. Biochem Eng J. 2013;73:5–11.
Maharaj K, Bradfield MF, Nicol W. Succinic acid-producing biofilms of Actinobacillus succinogenes: reproducibility, stability and productivity. Appl Microbiol Biotechnol. 2014;98(17):7379–86.
Kuenz A, Hoffmann L, Goy K, Bromann S, Prüße U. High-level production of succinic acid from crude glycerol by a wild type organism. Catalysts. 2020;10(5):470.
Vallecilla-Yepez L, Ramchandran D, Long D, Saha R, Wilkins MR. Corn fiber as a biomass feedstock for production of succinic acid. Bioresour Technol Rep. 2021;16: 100868.
Long DS, Immethun CM, Vallecilla-Yepez L, Wilkins MR, Saha R. One step forward, two steps back: transcriptional advancements and fermentation phenomena in Actinobacillus succinogenes 130Z. PLoS ONE. 2021;16(5): e0245407.
Lee PC, Lee WG, Kwon S, Lee SY, Chang HN. Succinic acid production by Anaerobiospirillum succiniciproducens: effects of the H2/CO2 supply and glucose concentration. Enzyme Microb Technol. 1999;24(8–9):549–54.
Li J, Zheng XY, Fang XJ, Liu SW, Chen KQ, Jiang M, et al. A complete industrial system for economical succinic acid production by Actinobacillus succinogenes. Bioresour Technol. 2011;102(10):6147–52.
Pardo-Planas O, Prade RA, Muller M, Atiyeh HK, Wilkins MR. Prevention of melanin formation during aryl alcohol oxidase production under growth-limited conditions using an Aspergillus nidulans cell factory. Bioresour Technol. 2017;243:874–82.
Singh S, Prerna P. Review of recent advances in anaerobic packed-bed biogas reactors. Renew Sustain Energy Rev. 2009;13(6–7):1569–75.
Seader J, Henley E. Separation process principles. Hoboken: Wiley; 2006.
Yang Y, Tada C, Miah MS, Tsukahara K, Yagishita T, Sawayama S. Influence of bed materials on methanogenic characteristics and immobilized microbes in anaerobic digester. Mater Sci Eng C. 2004;24(3):413–9.
Picanco A, Vallero M, Gianotti E, Zaiat M, Blundi C. Influence of porosity and composition of supports on the methanogenic biofilm characteristics developed in a fixed bed anaerobic reactor. Water Sci Technol. 2001;44(4):197–204.
Ranade VV, Chaudhari R, Gunjal PR. Trickle bed reactors: reactor engineering and applications. Amsterdam: Elsevier; 2011.
Tchobanoglous G, Burton FL, Stensel H. Wastewater engineering. Management. 1991;7(1):4.
Bradfield MF, Mohagheghi A, Salvachua D, Smith H, Black BA, Dowe N, et al. Continuous succinic acid production by Actinobacillus succinogenes on xylose-enriched hydrolysate. Biotechnol Biofuels. 2015;8:181.
Chen KQ, Li J, Ma JF, Jiang M, Wei P, Liu ZM, et al. Succinic acid production by Actinobacillus succinogenes using hydrolysates of spent yeast cells and corn fiber. Bioresour Technol. 2011;102(2):1704–8.
Guettler MV, Jain MK, Rumler D. Method for making succinic acid, bacterial variants for use in the process, and methods for obtaining variants. US Patent 5,573,931; 1996.
Kumar G, Cheon H-C, Kim S-H. Effects of 5-hydromethylfurfural, levulinic acid and formic acid, pretreatment byproducts of biomass, on fermentative H2 production from glucose and galactose. Int J Hydrog Energy. 2014;39(30):16885–90.
Liu H, Zhang J, Yuan J, Jiang X, Jiang L, Zhao G, et al. Omics-based analyses revealed metabolic responses of Clostridium acetobutylicum to lignocellulose-derived inhibitors furfural, formic acid and phenol stress for butanol fermentation. Biotechnol Biofuels. 2019;12(1):1–20.
Yuzbashev TV, Yuzbasheva EY, Laptev IA, Sobolevskaya TI, Vybornaya TV, Larina AS, et al. Is it possible to produce succinic acid at a low pH? Bioeng Bugs. 2011;2(2):115–9.
Acknowledgements
Not applicable.
Funding
The authors wish to acknowledge the Nebraska Corn Board for funding this work under award number 88-R-1718-01 and the financial support of the University of Nebraska-Lincoln Agricultural Research Division.
Author information
Authors and Affiliations
Contributions
Both authors contributed to the study conception and design. LVY was responsible for experiment execution, data collection and analysis, and writing the original draft of the article. MRW was responsible for securing funding, project administration, and review and editing of the article.
Corresponding author
Ethics declarations
Conflict of interest
Lisbeth Vallecilla-Yepez is now employed by Novozymes A/S, whose enzymes were used in this study.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Authors’ information
Mark R. Wilkins is now employed by Kansas State University and Lisbeth Vallecilla-Yepez is now employed by Novozymes A/S. The study described was performed at the University of Nebraska-Lincoln, thus the authors’ affiliations on the title page are with the University of Nebraska-Lincoln.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vallecilla-Yepez, L., Wilkins, M.R. Continuous succinic acid production from corn fiber hydrolysate by immobilized Actinobacillus succinogenes in a hollow fiber membrane packed‑bed biofilm reactor. Syst Microbiol and Biomanuf 3, 765–775 (2023). https://doi.org/10.1007/s43393-022-00149-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43393-022-00149-w