Abstract
Ethanol is one of the most important platform chemicals that can be produced in a continuous biofilm reactor. The continuous system can be easily adapted to a biofilm reactor, which is a useful tool for ethanol production by microorganisms. In this study, two different media (the first medium: acid-pretreated/detoxified and glucose-enriched rice husk hydrolysate; the second medium: acid-pretreated/detoxified and xylose-enriched rice husk hydrolysate) were used for ethanol production in a continuous biofilm reactor. Both medium (1.5 L) were supplemented with 1% (w/v) yeast extract and 2% (w/v) peptone. The dilution rate for the first medium was between 0.02 and 0.12 h−1, while for the second medium, it was between 0.01 and 0.05 h−1. When the first medium was used for ethanol fermentation in a continuous system, maximum ethanol productivity of 0.418 g/L/h and maximum biomass productivity of 0.196 g/L/h were yielded at dilution rates of 0.08 and 0.10 h−1, respectively. As for the second medium for ethanol fermentation in a continuous system, their values were 0.083 and 0.079 g/L/h at dilution rates of 0.03 and 0.04 h−1, respectively. Additionally, the yield factors for biomass and ethanol (Y0X/S and Y0P/S) were also found to be 0.642 g X/g S and 0.49 g P/g S for the first medium and 0.254 g X/g S and 0.27 g P/g S for the second medium, respectively. In addition, although cost-effective ethanol production regarding energy cost and recovery time is desired, the use of the non-enriched sterile and enriched non-sterile media in a repeated-batch biofilm reactor caused low fermentation kinetics. Consequently, ethanol production was successfully performed by using Scheffersomyces stipitis in a continuous PCS-biofilm reactor including acid-pretreated/detoxified and glucose- or xylose-enriched rice husk hydrolysate, which gave higher ethanol concentration compared with subsequent ethanol fermentation in a repeated-batch biofilm reactor.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The growing population of the world is based on traditional non-renewable fossil fuels such as oil, wood mass, natural gas, and coal to meet the growing energy demands that cannot be met in the near future due to the ongoing depletion of these reserves. The sudden increase in crude oil prices and the recent uncertainties regarding its availability have been identified as the main drivers of alternative clean and cost-effective sustainable energy. In the face of international, a move away from traditional fuels and towards a greener future, many experiment laboratories around the world are rushing to find the next great breakthrough in the development of natural resources, particularly through highly effective ways of discovering biomass as a sustainable alternative raw material. There are a variety of promising renewable and cost-effective energy sources such as methane, hydrogen, ethanol, butanol, and methanol [1]. Agricultural lignocellulosic biomass such as tea processing waste, rice straw, spent tea waste, wheat straw, barley husk, oat husk, corn stover, sugarcane bagasse, and rye straw is the major source of renewable and cost-effective energy sources. Lignocellulosic biomass, composed of complex polysaccharides such as cellulose, hemicellulose, pectin, and non-carbohydrate lignin, is the most abundant potential material for the production of biofuels in the world [1,2,3,4,5,6,7]. Rice husk is also a lignocellulosic biomass, which contains 37.07% cellulose, 31.15% hemicellulose, 22.70% lignin, 9.08% extractive compounds, and 4.58% moisture [4]. Worldwide, according to data in 2017 of the Food and Agricultural Organization, rice production was about 770 million tons, 20% of which is generated as rice husk. Therefore, rice husk production on earth was about 154 million tons in 2017. This information indicates that rice husk is an important by-product to evaluate in the production of value-added products through biotechnological processes due to its high carbohydrate composition (68.22% cellulose + hemicellulose) [4].
Pretreatment is an important process in the efficient breakdown of lignin barriers to extract cellulosic contents fed to the hydrolysis step to convert them to simple monosaccharides such as glucose, xylose, mannose, galactose, and arabinose [1, 7]. To make the process more effective, pretreatments must prevent degradation or depletion of carbohydrates, improve sugar production in the direct or subsequent hydrolysis step, avoid the formation of compounds that inhibit hydrolysis and fermentation processes, limit energy demand, and minimize costs. Pretreatment techniques may include physical (chopping, grinding, irradiation), physicochemical (hot water, steam burst), chemical (acid and alkali), biological (lignin and manganese peroxidases) pretreatments, or combinations thereof [8]. Among them, in acid pretreatment, polysaccharides are severely broken down to monosaccharides by dilute acids such as HCl, HNO3, H3PO4, or H2SO4. The aim of this method is to maximize the conversion of polysaccharides to soluble sugars, to increase the biomass porosity, and to increase the hydrolysis of cellulosic fractions to glucose in the next enzymatic process [8, 9]. Dilute sulfuric acid pretreatment is widely used in order to obtain fermentable sugars from renewable resources, as a higher yield of hydrolysis from lignocellulosic biomass with H2SO4, compared with other acids are achieved [9, 10]. In addition to fermentable sugars, inhibitors such as organic acids, furans, and phenolic substances, which are known to inhibit the growth of microorganisms, are also produced during the pretreatment process. This is the main disadvantage of the corresponding acid pretreatment process. Therefore, before fermentation, the acid-pretreated hydrolysate should be detoxified in order to effectually convert the fermentable sugars into ethanol [9, 11]. Physical (vacuum evaporation), chemical (active charcoal), biological methods (peroxidase), or combinations thereof are used to remove the inhibitors in the hydrolysate during the detoxification process. The efficiency of the detoxification method is based on the microorganism and hydrolysis method due to the different degrees of tolerance of the microorganisms to toxic substances and different degrees of hydrolysate toxicity. Nevertheless, the raw material and its hydrolysate are also important factors in selecting the method to be used in detoxification of hydrolysate [12, 13]. Recently, the active charcoal detoxification method, which is a chemical process, has widely been used to remove inhibitors from the hydrolysates of renewable resources. It was reported that the inhibitors, especially phenolic substances and lactic acid, were effectively removed from the hydrolysate of the acid-pretreated renewable resource by active charcoal treatment [4]. In another study, it was noted that 5-hydroxymethylfurfural (HMF) and furfural were also effectively removed by this detoxification procedure [5, 6]. Thus, the active charcoal detoxification method was employed to remove the inhibitors from the acid-pretreated rice husk hydrolysate (RHH) in this study.
Because of the presence of C5 (mainly xylose) and C6 (mainly glucose) in the hydrolysate of the pretreated renewable resource, value-added products can be produced by fermentation. Ethanol is one of the value-added products that can be produced from renewable resources, which is called the second-generation biofuel [14]. Ethanol is employed as raw material, solvent, and fuel for a great variety of implementation containing beverages, pharmaceuticals, cosmetics, biofuel, chemicals, dye, etc. [14, 15]. Globally, a great majority of ethanol (90–95%) is produced through microbial fermentation technology. In the production of ethanol, naturally occurring yeasts Scheffersomyces stipitis, Candida shehatae, and Pachysolen tannophilus in nature can be used to more efficiently convert C5 and C6 into ethanol [14, 15]. Among them, Scheffersomyces stipitis (S. stipitis) is a xylose-fermenting yeast that promising for large-scale industrial ethanol production with high yield (35–44%), because this microorganism has the ability to convert fermentable sugars in the lignocellulosic hydrolysate into ethanol, resistance to contamination and thick cell wall [4,5,6, 16, 17]. Nevertheless, since it is a Crabtree-negative yeast, the pentose sugars can be converted into ethanol with high yield at a low oxygen transfer rate [18]. In addition, S. stipitis can be also fermented the pentose sugars into ethanol under anaerobic conditions, as it has both nicotinamidadenindinukleotid and nicotinamidadenindinukleotidphosphat-specific xylose reductase cofactor. Therefore, S. stipitis is a gene source for xylose-nonfermenting microorganisms in genetic engineering [18, 19].
On the other hand, biofilms are natural forms of cell immobilization and can be implemented in bioreactors. Accordingly, the biofilm reactors can be called the reactors in which microbial cells are attached on the support materials to form the biofilms. Biofilm reactors have been applied for various objectives containing the removal of toxic components, bioremediation, wastewater treatment, and water purification [20, 21]. Nonetheless, in recent times, high value-added products such as ethanol [22,23,24], β-mannanase [25], bacterial cellulose [26], human lysozyme [27], nisin [28], pullulan [29], succinic acid [30], menaquinone-7 [31], lactic acid [32], and kojic acid [33] have been manufactured in the biofilm reactors. Additionally, a continuous system can be also easily adapted to plastic composite support (PCS)-biofilm reactors. Continuous fermentation ensures various advantages compared with batch fermentation such as constant environmental conditions for biological systems, the elimination of substrate inhibition, and the reduction of toxicity. Besides, the microbial growth rate is equal to the dilution rate. Higher biomass and product productivity can be yielded. However, contamination and cell washout risk are the main problems for suspended-cell continuous bioreactors at high dilution rates [29, 32]. In the literature, there is no study related to ethanol production from acid-pretreated/detoxified and glucose- or xylose-enriched RHH in a continuous PCS-biofilm reactor and the effect of sterilization and enrichment on cost-effective ethanol production in the repeated-batch PCS-biofilm reactor containing the best selected medium as a result of the continuous fermentation. On the other hand, the kinetic parameters related to both continuous fermentation and repeated-batch fermentation were also calculated and interpreted in detail. Therefore, this study will fill the gap in the literature.
In the present study, there are two purposes. The first goal was to produce ethanol from acid-pretreated/detoxified and glucose- or xylose-enriched RHH by S. stipitis in a continuous PCS-biofilm reactor. For this purpose, two different media determined in our other study (submitted to another journal) were employed. The first medium contained 50% (v/v) RHH-based medium and 50% (v/v) glucose-based medium. The second medium also included 75% (v/v) RHH-based medium and 25% (v/v) xylose-based medium (see Section 2.4). Both media were also supplemented with 1% (w/v) yeast extract and 2% (w/v) peptone. Besides, to produce low-cost ethanol regarding energy cost and recovery time, the second goal was to investigate the effect of medium enrichment and sterilization on ethanol fermentation in a repeated-batch PCS-biofilm reactor containing the best selected medium among first and second media according to the results of the continuous fermentation.
2 Materials and methods
2.1 Raw materials
Rice husk was obtained from a local rice producer in Osmancık (a district of Çorum), Turkey. For dilute sulfuric acid pretreatment, rice husk was grounded by using a grinder (Tefal, Model GT110838, 180 W, Istanbul, Turkey) to increase the liberation of the reducing sugars into the extract. The particle size distribution of the raw materials was not characterized. Rice husk and its grounded form were stored at room temperature until used for pretreatment [4].
2.2 Dilute acid pretreatment
An autoclave (Hirayama HG-50, Saitama, Japan) was used for dilute sulfuric acid pretreatment of the raw materials. The pretreatment conditions were 127.14 °C of temperature, 1:10.44 (w/v) of the solid-to-liquid ratio, 2.52% (v/v) of dilute sulfuric acid ratio, and 22.01 min of implementation time. The reaction mixture was then cooled to room temperature and filtered through roughing filter paper to remove solid particles. After pretreatment, the hydrolysate obtained was stored at + 4 °C until used for active charcoal detoxification and fermentation [4].
2.3 Active charcoal detoxification
A shaking incubator (CERTOMAT® IS, Goettingen, Germany) was used for active charcoal detoxification of the hydrolysate. The detoxification process was performed at 150 rpm of agitation speed and 30 °C of temperature with 2% (w/v) of active charcoal for 30 min of implementation time. Subsequently, the active charcoal used was separated with the aid of a centrifuge (VWR Mega Star 3.0R, Osterode am Harz, Germany) at 4000 rpm of rotation speed and 20 °C of temperature for 30 min of application time. Then, the supernatant collected for use in ethanol production in a continuous PCS-biofilm reactor. Afterward, the pH of the acid-pretreated/detoxified and glucose- or xylose-enriched RHH was adjusted to 5.6 by the addition of 8 N NaOH and 4 N HCI [4,5,6].
2.4 Microorganism and medium
The microaerophilic yeast S. stipitis (ATCC 58785), which was obtained from the American Type Culture Collection (Manassas, VA, USA), was used for ethanol production. The cultivation medium contained 2% (w/v) glucose, 1% (w/v) yeast extract, and 2% (w/v) peptone in deionized water, and the conditions were 30 °C for 24 h. Before sterilization, the pH of the culture medium was adjusted to 5.6 by the addition of 4 N NaOH and HCI. The culture was stored at + 4 °C and renewed bi-monthly in order to maintain cell viability and productivity. For long-term storage, the stock culture was kept at − 80 °C in 20% glycerol. For inoculation, S. stipitis was cultivated in 250-mL flasks including 100 mL of the cultivation medium at 30 °C of temperature and 150 rpm of agitation speed for 24 h of incubation time [4].
In this study, two different media that determine through the repeated-batch fermentations (submitted to another journal) were used for ethanol production in the continuous PCS-biofilm reactor. The first medium contained 50% (v/v) detoxified RHH, 50% (v/v) glucose, 1% (w/v) yeast extract, and 2% (w/v) peptone. The second medium included 75% (v/v) detoxified RHH, 25% (v/v) xylose, 1% (w/v) yeast extract, and 2% (w/v) peptone (submitted to another journal). To clarify how the first medium composition was prepared, for instance, 50% (v/v) RHH-based medium contained 750 mL of acid-pretreated and detoxified RHH including 1% (w/v) yeast extract and 2% (w/v) peptone, and 50% (v/v) glucose-based medium contained 750 mL of glucose solution (15 g of glucose, 2% w/v) including 1% (w/v) yeast extract and 2% (w/v) peptone. Therefore, 1.5 L of the first medium included 50% (v/v) RHH, 50% (v/v) glucose, 1% (w/v) yeast extract, and 2% (w/v) peptone. The second medium was also prepared in this manner.
2.5 PCS material
The PCS material used in biofilm reactor was generated at Iowa State University (Ames, IA) by high-temperature extrusion in a co-rotating twin-screw extruder (Brabender PL2000, Model CTSE-V, CW Brabender Instruments, Inc., South Hackensack, NJ, USA) as defined by Ho et al. [34]. The composition of PCS material contained polypropylene (PP) (5%, w/w), ground vacuum-dried soybean hulls (35%, w/v), defatted soybean flour (5%, w/v), yeast extract (5%, w/v), dried bovine albumin (5%, w/v), and salts (2 g/kg of sodium acetate, 1.2 g/kg of MgSO4·7H2O, and 0.06 g/kg of MnSO4·7H2O).
2.6 Biofilm reactor configuration
A Sartorius Biostad B Plus stirred tank bioreactor with twin configuration (Goettingen, Germany) equipped with a 2-L vessel (1.5-L working volume) was employed to construct a biofilm reactor by attaching 10 pieces of PCS material (10.5 mm width and 65 mm long) to the agitator shaft in a grid-like fashion, with five rows of two parallel tubes (Fig. 1). The reactor vessel containing PCS materials was autoclaved at 121.1 °C for 60 min in deionized water. After the water was discharged, it was aseptically filled with 1.5 L of the culture medium. The bioreactor was then incubated at 30 °C and 150 rpm for 24 h to check sterility. The initial leachate solution was replaced with a fresh sterile medium. For biofilm formation, a 24-h grown culture of S. stipitis (ATCC 58785) was used for inoculation at a 1% (v/v) ratio. The fermentation conditions were set to 30 °C, 0.05 gas volume flow per unit of liquid volume per minute (vvm) (if necessary), pH 5.6, and 150 rpm (submitted to another journal). The pH was controlled by the automatic addition of 4 N NaOH and HCI. Seven repeated-batch fermentations were performed by changing the medium every 24 h to form biofilms on the PCS material [24].
2.7 Continuous ethanol fermentation
After the best nutritional compositions (see Section 2.4) were determined through the repeated-batch fermentation in a PCS-biofilm reactor (submitted to another journal), continuous ethanol fermentations in the same PCS-biofilm reactor were performed at 0.05 vvm (if necessary), 30 °C, pH 5.6, and 150 rpm. The first medium and then the second medium were evaluated for continuous ethanol fermentation in the PCS-biofilm reactor. The fermentation medium was continuously added into the reactor and the fermented broth was continuously collected from the fermenter at the same rate. When the first and second media were evaluated for continuous ethanol production, dilution rates were set from 0.02 to 0.12 h−1 and from 0.01 to 0.05 h−1, respectively. The dilution rates were assessed to determine optimum biomass and ethanol productivity (DX and DP, g/L/h, respectively) in a continuous PCS-biofilm reactor. Fermentations in the first and second media were started as batch fermentations until late-log phase, and then, the systems were switched to continuous fermentation by opening the inlet and outlet pumps at the specified dilution rates. Samples were taken every 24 h at steady-state conditions and analyzed for biomass, ethanol, and residual sugar concentrations. Based on the results of the continuous fermentations performed in two different media, the best medium (first medium) was selected and it was used for subsequent fermentations in the PCS-biofilm reactor. As a continuation of this study in the same PCS-biofilm reactor, the effects of sterile non-enriched medium (SNEM) and non-sterile-enriched medium (NSEM) on ethanol fermentation were also investigated in the repeated-batch PCS-biofilm reactor, for low-cost ethanol production regarding energy cost and recovery time. During the repeated-batch fermentation, the samples were collected every 2 or 4 h for the first 12 h and every 6 and 12 h for remaining times of fermentation. The collected samples were stored at 4 °C in a refrigerator.
2.8 Calculation of kinetics in continuous fermentation
The experimental data to be fit to proper mathematical equations in continuous fermentation were measured in a steady state. YX/S and YP/S were estimated by using Eqs. (1) and (2), respectively [35].
When the values of 1/YX/S or 1/YP/S were plotted versus 1/D values, Y0X/S and Y0P/S, and m were calculated by using flowing formula (Eqs. (3) and (4)) [35].
Since Y0X/S, Y0P/S, mX, and mP are known, (kd)X and (kd)P were calculated by using Eqs. (5) and (6) [35]. mX and mP are maintenance coefficients for cell growth and product formation, respectively.
Monod kinetic parameters, μmax and KS, can be calculated by using Lineweaver-Burk plot (Eq. (7)) [35]. When 1/(D+(kd)X) was plotted versus 1/S, the values of μmax and KS can be calculated [35].
In a similar manner, Eq. (8) was used for ethanol formation. When P was divided by X, Eq. (9) was obtained. If P/X was plotted versus 1/D, the values of a and β can be easily calculated [35].
On the other hand, DOptimum (Eq. (10)) and SOptimum (Eq. (11)) were also calculated as follows:
where X is the biomass concentration (g/L), P is the ethanol concentration (g/L), S0 is the initial sugar concentration (g/L), S is the residual substrate concentration at constant dilution rate and fixed conditions in bioreactor, YX/S is the biomass yield (g biomass/g substrate), YP/S is the ethanol yield (g ethanol/g substrate), Y0X/S is the yield factor for biomass (g biomass/g substrate), Y0P/S is the yield factor for ethanol (g ethanol/g substrate), m is the maintenance energy coefficient, D is the dilution rate (h−1), kd is the death constant of microorganisms or the lost constant of product (h−1), KS is the saturation constant (g/L), μmax is the maximum specific growth or production rate (h−1), a is the Luedeking Piret constant (g ethanol/g biomass), β is the Luedeking Piret constant (g ethanol/g biomass h), DOptimum is the optimum dilution rate (h−1), and SOptimum is the optimum substrate concentration (g/L).
2.9 Calculation of kinetics in repeated-batch fermentation
The following kinetics [5, 6, 36] was calculated using the experimental data of the repeated-batch fermentation performed to examine the effect of medium enrichment and sterilization on ethanol production (Eqs. (12)–(23)):
where ΔX is the biomass production (g/L); ΔP is the ethanol production (g/L); ΔS is the sugar consumption (g/L), Xmin, Pmin, and Smin are minimum biomass, ethanol, and sugar concentrations (g/L), respectively; Xmax, Pmax, and Smax are maximum biomass, ethanol, and sugar concentrations (g/L), respectively; YX/S is the biomass yield (%); YP/S is the ethanol yield (%); YP/X is the ethanol production per biomass (g ethanol/g biomass); QX is the maximum growth rate (g/L/h); QP is the maximum production rate (g/L/h); QS is the maximum consumption rate (g/L/h); SUY is the sugar utilization yield (%); td is the doubling time of the yeast (h); μx is the maximum specific growth rate (h−1); and η is the theoretical ethanol yield (%).
2.10 Analysis of fermentation broth
2.10.1 Biomass
The optical cell density was measured using a spectrophotometer (ThermoScientific Evolution 201 UV-Vis, Shanghai, China) at 600 nm. The un-inoculated fermentation broth was used as a blank. Absorbance (Abs) values measured at 600 nm were converted to cell concentration by using specific standard curve, which is y = 0.2639 × Abs600 + 0.0282. Here, y is the cell concentration (g/L) [37].
2.10.2 Ethanol
The ethanol concentration was determined by using high-performance liquid chromatography (HPLC) (ThermoScientific™, Waltham, Massachusetts, USA) equipped with a RefractoMax 520 refractive-index detector. The separation was performed using a Transgenomic COREGel 87P column (Transgenomic, Omaha, NE, USA, 300 × 7.8 mm2) at 70 °C column temperature and a flow rate of 0.5 mL/min and a sample volume of 20 μL. 0.01 N H2SO4 was used as a mobile phase. Before analysis, all samples were diluted 10-fold with ultrapure water (higher than 18.2 MΩ) and centrifuged (Eppendorf AG 5418, Hamburg, Germany) at 16,873g for 5 min and then filtered through 0.45-μm membrane filters (Macharey-Nagel, Düren, Germany) to remove the solid particles [3].
2.10.3 Residual sugar
The residual sugar concentration was determined with the 3,5-dinitrosalicylic acid method [38]. The measurement of absorbance at 575 nm was recorded. A calibration curve for the spectrophotometric measurements (ThermoScientific Evolution 201 UV-Vis, Shanghai, China) was formed using a glucose solution. Deionized water was used as a blank. Absorbance values were converted to residual sugar concentration by using a standard curve, which is y = 60.401 × Abs575 + 0.5751. Here, y is the glucose concentration (g/L) [3].
2.11 Statistical analysis
The data were assessed by using the SAS statistical program (SAS Institute, Cary, NC, USA). Duncan’s multiple comparison test was employed at a 95% confidence level (p = 0.05). Values were the average of two replicates (n = 2).
3 Results and discussion
Before this study, the best plastic composite support (PCS) material for the yeasts S. stipitis (ATCC 58784 and 58785) was determined; a biofilm reactor was established with the selected PCS material (Fig. 1), and biofilm formation was individually carried out for both strains. Additionally, different medium compositions including RHH and glucose or xylose at certain ratios were evaluated for ethanol production in the constructed PCS-biofilm reactor via repeated-batch fermentation. After determining the best yeast (S. stipitis ATCC 58785) and carbon source composition in terms of ethanol production, the effect of nitrogen sources (peptone, beef extract, and ammonium nitrate) on ethanol fermentation was examined via repeated-batch fermentations. Thus, the best medium composition was determined in terms of nitrogen source as well (submitted to another journal). Subsequently, using the same biofilm reactor and the yeast S. stipitis ATCC 58785, continuous ethanol fermentations were performed in the best determined medium compositions. After the continuous fermentations, effects of SNEM and NSEM on ethanol fermentation to determine whether it is cost-effective fermentation were investigated via repeated-batch PCS-biofilm reactor. The results are presented as follows:
3.1 Continuous ethanol fermentation in the PCS-biofilm reactor
Different dilution rates were assessed for ethanol production from acid-pretreated/detoxified and glucose- or xylose-enriched RHH in a continuous biofilm reactor and the optimum dilution rate was determined. Continuous fermentation was performed using both the first medium and second medium to see the effect of carbon source composition on ethanol production in a continuous PCS-biofilm reactor. For the sake of procedure, the dilution rate should be set to ensure microorganisms enough time to grow and generate the product at higher rates in continuous fermentation. At higher dilution rates, the cells can be washed out of the fermenter and as a result of this, the productivity may reduce [27].
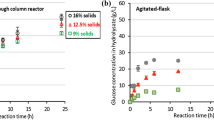
When the first medium was used in continuous PCS-biofilm reactor, ethanol and biomass concentrations stayed at the same around 2.43 g/L and 5.26 g/L at dilution rates of 0.02 and 0.08 h−1, respectively (Fig. 2). But, due to the washout effect, ethanol concentration decreased from 5.22 to 1.13 g/L (Fig. 2 and Table 1) and residual sugar concentration increased from 5.70 to 15.42 g/L when dilution rate increased from 0.08 to 0.12 h−1 (Table 1). On the other hand, the biomass productivity (DX) was maximum (0.196 g/L/h) at a dilution rate of 0.10 h−1 (Fig. 2 and Table 1). After this point, DX declined to 0.185 h−1. Besides, the ethanol productivity (DP) was maximum (0.418 g/L/h) at a dilution rate of 0.08 h−1. Subsequently, DP decreased to 0.204 and 0.136 g/L/h at dilution rates of 0.10 and 0.12 h−1, respectively (Fig. 2 and Table 1). Based on the results, the optimum dilution rate was 0.08 h−1, which ensured 0.186 and 0.418 g/L/h of DX and DP, respectively. On the other hand, while the highest YX/S (0.824 g X/g S) and YP/S (0.604 g P/g S) were obtained at a dilution rate of 0.12 h−1, their lowest values (0.20 g X/g S and 0.419 g P/g S) were determined at dilution rates of 0.08 and 0.10 h−1, respectively (Table 1). In batch fermentation, specific growth rate (μ = D), maximum growth rate (QX), and maximum production rate (QP) were calculated to be 0.08 h−1, 0.13 g/L/h, and 0.24 g/L/h, respectively (submitted to another journal). In continuous fermentation, the calculated DX and DP were higher than QX and QP. The results showed that productivity in continuous fermentation was greater than that of batch fermentation.
After, the second medium was evaluated for continuous ethanol production in a PCS-biofilm reactor. Figure 3 indicates the ethanol production at different dilution rates in a continuous PCS-biofilm reactor. Ethanol concentration remained around 2.88 g/L until a dilution rate of 0.03 h−1 and declined up to 1.60 g/L at higher dilution rates. The maximum DP was computed to be 0.083 g/L/h at a dilution rate of 0.03 h−1 (Fig. 3 and Table 1). Besides, the biomass concentration stayed around 2.95 g/L at dilution rates of 0.01–0.04 h−1. However, due to the washout effect, its value decreased to 1.54 g/L at a dilution rate of 0.05 h−1. It was determined that the highest DX was 0.079 g/L/h at a dilution rate of 0.04 h−1 (Fig. 3 and Table 1). On the other hand, the residual sugar concentration relatively stable until a dilution rate of 0.03 h−1. After that point, its level increased from 14.81 to 15.99 g/L (Table 1). The results indicated that the optimum dilution rate was 0.04 h−1. At this point, DX and DP were 0.079 and 0.082 g/L/h, respectively (Fig. 3 and Table 1). Additionally, the yield parameters were also calculated (Table 1). The maximum YX/S (0.254 g X/g S) achieved at a dilution rate of 0.04 h−1 while its minimum level of 0.202 g X/g S obtained at a dilution rate of 0.05 h−1 (Table 1). Similarly, the lowest level of YP/S (0.21 g P/g S) was obtained at a dilution rate of 0.05 h−1 while its highest value of 0.33 g P/g S was calculated at a dilution rate of 0.02 h−1 (Table 1). The best selected fermentation strategy should be compared in the case of a steady state (D = μ = 0.04 h−1) for batch and continuous fermentation parameters. In batch fermentation, QX and QP were determined to be 0.05 and 0.07 g/L/h, respectively (submitted to another journal). In a continuous system, they were calculated to be 0.079 and 0.082 g/L/h, respectively (Table 1). The results showed that the fermentation efficiency in the continuous system was better than the batch system. As a continuation of continuous fermentation, since higher results were achieved by the usage of the first medium compared with the second medium, the first medium was evaluated for subsequent repeated-batch fermentations. Namely, SNEM and NSEM of acid-pretreated/detoxified and glucose-enriched RHH were evaluated for ethanol production in the repeated-batch PCS-biofilm reactor.
Table 2 shows the kinetics calculated from the data in Table 1, which represents interesting results. When the first and second media were evaluated in continuous fermentation, the values of Y0X/S, mX, Y0P/S, and mP can be used to calculate YX/S and YP/S in continuous fermentation depending on the change at dilution rate (Eqs. (24)–(27)) (Table 2).
For the first medium
For the second medium
On the other hand, (kd)X and (kd)p were also specified as a function of the yield factor and maintenance energy coefficient, which were introduced into the Lineweaver-Burg plot. Therefore, when the values of 1/(D+(kd)X) were plotted versus 1/S, μmax, and KS were calculated to be 0.772 h−1 and 61.31 g/L for the first medium and 0.00228 h−1 and 16.73 g/L for the second medium (Table 2). When these values were placed into Eq. (7), the following equations were obtained (Eqs. (28) and (29)).
For the first medium
For the second medium
Additionally, as the values of a and β were estimated (Table 2), Eqs. (30) and (31) were suggested for product formation in the first and second media as follows.
For the first medium
For the second medium
So what do the equations do (Eqs. (24)–(31))? Namely, Eqs. (24)–(27) can be used in the estimation of YX/S and YP/S in a continuous PCS-biofilm reactor at a certain dilution rate. For instance, by using Eq. (24), the values of YX/S can be calculated as 0.35 and 0.45 g X/g S at dilution rates of 0.05 and 0.1 h−1 when using the first medium for fermentation, respectively. Therefore, to obtain more information about the kinetic-metabolic nature of continuous fermentation, these equations can be used. Similar calculations can be also made for Eqs. (30) and (31). On the other hand, Eqs. (28) and (29) can be employed to calculate the residual substrate concentration in a continuous PCS-biofilm reactor at a given dilution rate. For instance, the residual substrate concentrations can be estimated to be 8.24 and 13.76 g/L at dilution rates of 0.05 and 0.1 h−1 when the first medium was used for continuous fermentation, respectively. Thus, an idea of how the residual substrate concentration is in continuous fermentation in a PCS-biofilm reactor can be obtained. Therefore, it can be understood at which dilution rate the substrate is not consumed in continuous fermentation.
In addition, the values of DOptimum and SOptimum were also estimated by using Eqs. (10) and (11), respectively (Table 2). For the first medium, these values are normally acceptable since they are in the dilution range used in continuous fermentation and are compatible with the residual sugar concentration in continuous fermentation. However, for the second medium, they are not compatible with the residual sugar concentration and the dilution range used in the continuous fermentation. Such that, this result suggests that continuous fermentation should be performed on the second medium at a very low dilution rate.
This was the first report on continuous ethanol production from acid-pretreated/detoxified and glucose- or xylose-enriched RHH by the yeast S. stipitis in the PCS-biofilm reactor. However, similar studies are available in the literature. Kunduru and Pometto [39] produced ethanol by using Z. mobilis and S. cerevisiae in the continuous packed-bed biofilm reactor with PP or PCSs for 60 days. Maximum ethanol productivities of 536 g/L/h (39% yield) and 499 g/L/h (37% yield) were achieved with Z. mobilis on PP and PCSs of soybean hull-zein, respectively. For Z. mobilis, 50% ethanol yield and 96 g/L/h ethanol productivity were obtained at a dilution rate of 1.92 h−1 for soybean hull-zein PCSs. However, ethanol yield and productivity stayed at 32% and 76 g/L/h when PPs were used as support materials, respectively. For S. cerevisiae, the ethanol productivity and yield were 76 g/L/h and 45% on PCS at a dilution rate of 2.88 h−1, respectively. PP support biofilm reactors were discontinued because of the bioreactor plugging by the cell mass accumulation. It was also specified that the shape of the used support material was responsible for bioreactor plugging due to extensive biofilm development on the PCSs. Maximum productivities of 5 g/L/h in the suspended cell fermentation were yielded with a yield of 24% and 25% with S. cerevisiae and Z. mobilis at a dilution rate of 0.5 h−1. The authors also reported that washout performed in the suspended cell continuous fermentation at a dilution rate of 1.0 h−1. To sum up, they stated that biofilm reactors outperformed the suspended cell reactors with 15- to 100-fold higher productivities and with higher percentage yields for S. cerevisiae and Z. mobilis in continuous ethanol fermentation, respectively [39]. Demirci et al. [40] produced ethanol by using S. cerevisiae in a stirred tank biofilm reactor. The support material made of 40% soybean hulls, 5% soybean flour, 5% yeast extract-salt, and 50% PP mixture was selected and used in continuous and repeated-batch fermentation in various media containing lowered nitrogen concentration with selected PCS. During continuous fermentation, S. cerevisiae gave 2–10-fold higher ethanol production in the PCS-biofilm reactor than the PP control. The yeast used generated 30 g/L ethanol on PCS with ammonium sulfate medium in repeated-batch fermentation, whereas PP control generated 5 g/L ethanol. Accordingly, increased productivity in a cost-effective medium yielded beyond conventional fermentation using the PCS-biofilm reactor design. On the other hand, when the nitrogen-free medium was used in the PPS-control bioreactor, S. cerevisiae cells washed out of the fermenter, whereas ethanol production in the PCS-biofilm reactor was 3 g/L with a productivity of 1.44 g/L/h at a dilution rate of 0.48 h−1. When the ammonium sulfate medium was used in the PPS-control reactor, no ethanol was produced by the yeast; however, the ethanol production was 9.1 g/L with a productivity of 4.37 g/L/h in the continuous PCS-biofilm reactor at a dilution rate of 0.48 h−1 [40]. Similarly, the free and immobilized S. cerevisiae cells were used for continuous ethanol production from pineapple cannery waste and reported that the maximum productivity of 42.8 g/L/h was achieved at a dilution rate of 1.5 h−1 with the immobilized cells, while it was 3.75 g/L/h at a dilution rate of 0.15 h−1 with the free cells. Thus, the volumetric ethanol productivity of the immobilized cells was 11.4-fold higher than the free cells [41, 42]. In another study, the acid-pretreated and detoxified bamboo was used to produce ethanol in the continuous system by S. cerevisiae KF-7. The fermentation was performed at 33 °C, pH 4, and a dilution rate of 0.3 h−1 for 18 days. It was found that the ethanol production, the fermentation yield, and the productivity were 27.2 g/L, 47%, and 8.2 g/L/h, respectively. It was reported that the process is effective for production of fuel ethanol from bamboo [43]. Purwadi and Taherzadeh [44] investigated the performance of serial bioreactors in rapid continuous ethanol production from acid-pretreated spruce hydrolysate using immobilized S. cerevisiae cells at different dilution rates of 0.22, 0.43, 065, and 0.86 h−1. It was reported that the immobilized cells in a continuous stirred tank bioreactor consumed 75% and 54% of the initial fermentable sugars at dilution rates of 0.43 and 0.86 h−1. It was determined that the ethanol yields from the hydrolysate ranged from 0.41 and 0.48 g P/g S. It was also found that the specific and volumetric ethanol productivities were 1.13 g/g/h and 5.98 g/L/h for the single fermenter and 0.98 g/g/h and 5.98 g/L/h for the serial fermenter at a dilution rate of 0.86 h−1 [44]. Microorganisms play an important role in the production of ethanol from lignocellulosic material. A compelling problem in rice bran bioconversion is the presence of toxic inhibitors in the lignocellulosic acid hydrolysate. In a study, various Z. mobilis (ZM4, TISTR 405, 548, 550, and 551) grown under biofilm or planktonic modes were employed to investigate the bioconversion of rice bran hydrolysate and ethanol production efficiencies. Z. mobilis strains attached on the plastic surfaces and microscopic analysis showed that Z. mobilis ZM4 rapidly improved homogeneous biofilm structures within 24 h, while other Z. mobilis strains improved heterogeneous biofilm structures. The metabolic activity of Z. mobilis ZM4 grown as a biofilm was also greater than the same strain grown planktonically, as measured by ethanol production from rice bran hydrolysate (13.40 g/L vs. 0.43 g/L, respectively). The potential to increase ethanol production by using bacterial biofilms in the bioconversion of a readily available and normally unusable low-value by-product was indicated with this study [45]. On the other hand, the lab-scale packed-bed biofilm reactor was designed to ferment rice straw with a multi-stage continuous process [46]. Z. mobilis ZM4 and TISTR 551 were analyzed under continuous processes with optimal dilution rates of 37.8 and 13.5 h−1 for ethanol production efficiencies in biofilm reactors, respectively. It was reported that, under steady-state condition, YP/S of ZM4 biofilms was 0.17–0.19 g P/g S (33.33–37.25% theoretical yield), whereas TISTR 551 biofilm reactor yielded 0.35–0.47 g P/g S (68.63–92.16% theoretical yield). Based on the results, it was stated that biofilm reactors are ideal for large-scale production applications [46]. Besides, high value-added products such as pullulan [29], lactic acid [32], succinic acid [30], human lysozyme [27], butanol [47], and ethanol [39, 40] were also successfully produced in continuous biofilm reactors. The common characteristics of all of them were obtained higher yield values than control fermentation by using a biofilm reactor. Consequently, a continuous PCS-biofilm reactor was successfully used for ethanol production from acid-pretreated/detoxified and glucose- or xylose-enriched RHH by the yeast S. stipitis (ATCC 58785), especially with glucose enriched.
3.2 Effect of enrichment and sterilization on ethanol fermentation in the PCS-biofilm reactor
The first medium gave better results in the continuous PCS-biofilm reactor compared with the second medium. Therefore, the effect of enrichment and sterilization on ethanol production in the repeated-batch PCS-biofilm reactor was examined in the first medium. Namely, for cost-effective ethanol production regarding energy cost and recovery time, SNEM and NSEM were assessed, respectively. The kinetics and fermentation curves were given in Table 3 and Fig. 4a and b, respectively. When the results in Table 3 were examined, SNEM generally gave higher results than NSEM. Such that, the values of ΔS, ΔP, YP/S, YP/X, QS, QP, μX, and SUY calculated from the fermentation of SNEM were 3.32-, 7.50-, 2.45-, 12.88-, 6.0-, 2.25-, 1.88-, and 3.50-fold higher than NSEM (Table 3). Although the microbial cell concentration was 1.68-fold more than SNEM, SUY of the sugars in NSEM stayed at 8.08%, which means that 91.92% of sugars in the medium were not consumed by the yeast. Therefore, the values of ΔP, YP/S, YP/X, QS, QP, and μX were considerably lower than those of SNEM (Table 3). In conclusion, even if the fermentation medium is enriched, sterilization is essential for being an efficient fermentation process of this study. Therefore, SNEM was more successful than NSEM. However, it concluded that both SNEM and NSEM are unsuitable for low-cost ethanol production through repeated-batch fermentation in a PCS-biofilm reactor owing to the recovery time and low energy requirement.
Similarly, the ethanol was produced from non-sterile carob extract by immobilized S. cerevisiae in batch and fed-batch fermentation. It was reported that, in fed-batch system, both suspended and immobilized cells yielded the same maximum ethanol concentration of 62 g/L at an initial substrate concentration of 300 g/L and feeding rate of 167 mL/h. The maximum ethanol productivity of 4.4 g/L/h was achieved both suspended and immobilized cells at a substrate concentration of 300 g/L and feeding rate of 334 mL/h. In repeated-batch fermentation, it was reported that the immobilized cells gave greater ethanol concentration than the suspended cells [48]. In another study, non-sterile carob extract was used for ethanol production in a rotary shaker. It was found that maximum ethanol concentration and sugar utilization yield were 71 g/L and 95% at the 12th hour of the fermentation, respectively [49]. Clementz et al. [50] performed ethanol fermentation from non-sterile and non-enriched carrot must by using immobilized S. cerevisiae in batch fermentation. It was stated that the values of ethanol concentration, ethanol yield, and productivity were determined to be 29.9 g/L, 40.9%, and 7.45 g/L/h, respectively. It was reported that these values were similar to those registered when free cells were used. In a similar manner, Germec et al. [23] studied on the ethanol production from non-sterile-enriched and non-sterile non-enriched carob extract media by S. cerevisiae in PCS-biofilm reactor. It was found that YP/S, ΔP, and QP were calculated to be 33.76%, 18.46 g/L, and 1.56 g/L/h and 38.14%, 19.57 g/L, and 1.15 g/L/h, respectively. Thus, significant results were obtained from the non-sterile carob extract. On the other hand, the efficient ethanologenic microorganisms can be fully explained based on values for fermentation performance variables such as ethanol production and tolerance (> 40 g/L), genetic stability, inhibitor tolerance, growth rate, osmotic stress tolerance/more acidic pH/higher temperature values, productivity (> 1 g/L/h) and yield (> 90% theoretical), and specificity range [14]. Depending on this explanation, further research is needed to produce cost-effective ethanol from lignocellulosic hydrolysates, to minimize the effect of toxic inhibitors on fermentation and to maximize productivity. Consequently, however, as in similar studies in the literature and as in our study, the fermentation efficiency decreased in the non-sterile or non-enriched medium.
4 Conclusions and future directions
In the study, acid-pretreated/detoxified and glucose- or xylose-enriched RHH were evaluated for ethanol production in a continuous PCS-biofilm reactor and were assessed to examine the effect of medium enrichment and sterilization on cost-effective ethanol production in repeated-batch biofilm reactor. The use of a continuous PCS-biofilm reactor with first and second media showed a positive effect on ethanol productivity, biomass productivity, and yield factors. Dilution rates of 0.10 and 0.08 h−1 in the first medium resulted in the highest DX and DP (0.196 and 0.418 g/L/h, respectively) compared with batch fermentation (0.13 and 0.24 g/L/h, respectively (submitted to another journal)). Similarly, when the second medium was evaluated for ethanol production in the continuous system, maximum DX and DP (0.079 and 0.083 g/L/h), which were higher than those of batch fermentation (0.05 and 0.07 g/L/h (submitted to another journal)), were yielded at dilution rates of 0.04 and 0.03 h−1, respectively. Additionally, the yield factors (Y0X/S and Y0P/S) were 0.642 g X/g S and 0.49 g P/g S in the first medium and 0.254 g X/g S and 0.27 g P/g S in the second medium, respectively. In addition, the enrichment and sterilization of the medium played a significant role in the ethanol fermentation in the PCS-biofilm reactor, which resulted in highly lower kinetics. Consequently, it indicated that ethanol can be successfully produced from acid-pretreated/detoxified and glucose-enriched RHH in a continuous PCS-biofilm reactor. Further studies are required to improve the bioreactor design for large-scale industrial bio-productions. Besides, ethanol production from the hydrolysates (detoxified or non-detoxified) of the cheap and abundantly available renewable resources such as wheat straw, tea processing waste, spent tea waste, sugarcane bagasse, and corn stover would be also performed in a continuous biofilm reactor in the future. The conditions such as medium composition, initial substrate concentration, pH and pH shifting, temperature, agitation speed, oxygen supplementation, or aeration would be also optimized by using optimization methods such as Box-Behnken, Central Composite, Plackett-Burman, and Taguchi Designs. On the other hand, as the biofuel industry generates waste that potentially impacts the environment and human health, the environmental sustainability aspects of this industry should be carefully and seriously assessed. In line with this need, more advanced engineering methods and indicators are being developed and applied to decide on biofuels production and usage ways. Among these developed methods, emergy, life cycle assessment, energy, and exergy are the most common. These methods are promising for the quantitative and qualitative appraisement of biofuel production and consumption systems and will, therefore, aid the biofuels industry to further advance [51].
References
Machineni L (2019) Lignocellulosic biofuel production: review of alternatives. Biomass Conversion Biorefinery:1–13
Germec M, Bader N, Turhan I (2018) Dilute acid and alkaline pretreatment of spent tea leaves to determine the potential of carbon sources. Biomass Conversion Biorefinery 8(3):529–544
Germec M, Demirel F, Tas N, Ozcan A, Yilmazer C, Onuk Z, Turhan I (2017) Microwave-assisted dilute acid pretreatment of different agricultural bioresources for fermentable sugar production. Cellulose 24(10):4337–4353
Germec M, Kartal F, Bilgic M, Ilgin M, Ilhan E, Güldali H, Isci A, Turhan I (2016) Ethanol production from rice hull using Pichia stipitis and optimization of acid pretreatment and detoxification processes. Biotechnol Prog 32(4):872–882
Germec M, Turhan I (2018) Ethanol production from acid-pretreated and detoxified tea processing waste and its modeling. Fuel 231:101–109
Germec M, Turhan I (2018) Ethanol production from acid-pretreated and detoxified rice straw as sole renewable resource. Biomass Conversion Biorefinery 8(3):607–619
Karagoz P, Bill R, Ozkan M (2019) Lignocellulosic ethanol production: evaluation of new approaches, cell immobilization and reactor configurations. Renew Energy
Panahi H, Dehhaghi M, Aghbashlo M, Karimi K, Tabatabaei M (2019) Shifting fuel feedstock from oil wells to sea: Iran outlook and potential for biofuel production from brown macroalgae (Ochrophyta; Phaeophyceae). Renew Sust Energ Rev 112:626–642
Panahi H, Dehhaghi M, Aghbashlo M, Karimi K, Tabatabaei M (2020) Conversion of residues from agro-food industry into bioethanol in Iran: an under-valued biofuel additive to phase out MTBE in gasoline. Renew Energy 145:699–710
Menon V, Rao M (2012) Trends in bioconversion of lignocellulose: biofuels, platform chemicals & biorefinery concept. Prog Energy Combust Sci 38(4):522–550
Jönsson LJ, Martín C (2016) Pretreatment of lignocellulose: formation of inhibitory by-products and strategies for minimizing their effects. Bioresour Technol 199:103–112
Larsson S, Reimann A, Nilvebrant N, Jönsson L (1999) Comparison of different methods for the detoxification of lignocellulose hydrolyzates of spruce. Appl Biochem Biotechnol 77(1):91–103
Mussatto S, Roberto I (2004) Alternatives for detoxification of diluted-acid lignocellulosic hydrolyzates for use in fermentative processes: a review. Bioresour Technol 93(1):1–10
Kazemi Shariat Panahi H, Dehhaghi M, Kinder J, Ezeji T (2019) A review on green liquid fuels for the transportation sector: a prospect of microbial solutions to climate change. Biofuel Res J 6(3):995–1024
Sarris D, Papanikolaou S (2016) Biotechnological production of ethanol: biochemistry, processes and technologies. Eng Life Sci 16(4):307–329
Unrean P, Nguyen N (2012) Rational optimization of culture conditions for the most efficient ethanol production in Scheffersomyces stipitis using design of experiments. Biotechnol Prog 28(5):1119–1125
Yücel H, Aksu Z (2015) Ethanol fermentation characteristics of Pichia stipitis yeast from sugar beet pulp hydrolysate: use of new detoxification methods. Fuel 158:793–799
Papini M, Nookaew I, Uhlén M, Nielsen J (2012) Scheffersomyces stipitis: a comparative systems biology study with the Crabtree positive yeast Saccharomyces cerevisiae. Microb Cell Factories 11(1):136
Agbogbo F, Coward-Kelly G (2008) Cellulosic ethanol production using the naturally occurring xylose-fermenting yeast, Pichia stipitis. Biotechnol Lett 30(9):1515–1524
Cheng K, Demirci A, Catchmark J (2010) Advances in biofilm reactors for production of value-added products. Appl Microbiol Biotechnol 87(2):445–456
Ercan D, Demirci A (2015) Current and future trends for biofilm reactors for fermentation processes. Crit Rev Biotechnol 35(1):1–14
Germec M, Karhan M, Demirci A, Turhan I (2018) Ethanol production in a biofilm reactor with non-sterile carob extract media and its modeling. Energy Sources Part A 40(22):2726–2734
Germec M, Turhan I, Demirci A, Karhan M (2016) Effect of media sterilization and enrichment on ethanol production from carob extract in a biofilm reactor. Energy Sources Part A 38(21):3268–3272
Germec M, Turhan I, Karhan M, Demirci A (2015) Ethanol production via repeated-batch fermentation from carob pod extract by using Saccharomyces cerevisiae in biofilm reactor. Fuel 161:304–311
Germec M, Yatmaz E, Karahalil E, Turhan I (2017) Effect of different fermentation strategies on β-mannanase production in fed-batch bioreactor system. 3. Biotech 7(1):77
Cheng K, Catchmark J, Demirci A (2011) Effects of CMC addition on bacterial cellulose production in a biofilm reactor and its paper sheets analysis. Biomacromolecules 12(3):730–736
Ercan D, Demirci A (2015) Effects of fed-batch and continuous fermentations on human lysozyme production by Kluyveromyces lactis K7 in biofilm reactors. Bioprocess Biosyst Eng 38(12):2461–2468
Pongtharangku T, Demirci A (2007) Online recovery of nisin during fermentation and its effect on nisin production in biofilm reactor. Appl Microbiol Biotechnol 74(3):555–562
Cheng K, Demirci A, Catchmark J (2011) Continuous pullulan fermentation in a biofilm reactor. Appl Microbiol Biotechnol 90(3):921–927
Urbance S, Pometto A, DiSpirito A, Denli Y (2004) Evaluation of succinic acid continuous and repeat-batch biofilm fermentation by Actinobacillus succinogenes using plastic composite support bioreactors. Appl Microbiol Biotechnol 65(6):664–670
Mahdinia E, Demirci A, Berenjian A (2018) Optimization of Bacillus subtilis natto growth parameters in glycerol-based medium for vitamin K (Menaquinone-7) production in biofilm reactors. Bioprocess Biosyst Eng 41(2):195–204
Cotton J, Pometto A, Gvozdenovic-Jeremic J (2001) Continuous lactic acid fermentation using a plastic composite support biofilm reactor. Appl Microbiol Biotechnol 57(5–6):626–630
Liu J-M, Yu T-C, Lin S-P, Hsu R-J, Hsu K-D, Cheng K-C (2016) Evaluation of kojic acid production in a repeated-batch PCS biofilm reactor. J Biotechnol 218:41–48
Ho K, Pometto A, Hinz P, Dickson J, Demirci A (1997) Ingredient selection for plastic composite supports for L-(+)-lactic acid biofilm fermentation by Lactobacillus casei subsp. rhamnosus. Appl Environ Microbiol 63(7):2516–2523
Türker M (2005) Reaksiyon kinetiğinin modellenmesi. In: Türker M (ed) Biyoreaksiyon Mühendisliği, 1st edn. Su Vakfı Yayınları, Kocaeli, pp 241–299
Germec M, Ozcan A, Yilmazer C, Tas N, Onuk Z, Demirel F, Turhan I (2017) Ethanol fermentation from microwave-assisted acid pretreated raw materials by Scheffersomyces stipitis. AgroLife Sci J 6(1):112–118
Germec M, Kartal F, Guldali H, Bilgic M, Isci A, Turhan I (2016) Obtaining growth curves for Scheffersomyces stipitis strains and their modeling. Sci Bull Ser F Biotechnol 20:263–268
Miller G (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31(3):426–428
Kunduru M, Pometto A (1996) Continuous ethanol production by Zymomonas mobilis and Saccharomyces cerevisiae in biofilm reactors. J Ind Microbiol 16(4):249–256
Demirci A, Pometto A, Ho K (1997) Ethanol production by Saccharomyces cerevisiae in biofilm reactors. J Ind Microbiol Biotechnol 19(4):299–304
Nigam J (1999) Continuous ethanol production from pineapple cannery waste. J Biotechnol 72(3):197–202
Nigam J (2000) Continuous ethanol production from pineapple cannery waste using immobilized yeast cells. J Biotechnol 80(2):189–193
Sun Z, Tang Y, Iwanaga T, Sho T, Kida K (2011) Production of fuel ethanol from bamboo by concentrated sulfuric acid hydrolysis followed by continuous ethanol fermentation. Bioresour Technol 102(23):10929–10935
Purwadi R, Taherzadeh M (2008) The performance of serial bioreactors in rapid continuous production of ethanol from dilute-acid hydrolyzates using immobilized cells. Bioresour Technol 99(7):2226–2233
Todhanakasem T, Sangsutthiseree A, Areerat K, Young GM, Thanonkeo P (2014) Biofilm production by Zymomonas mobilis enhances ethanol production and tolerance to toxic inhibitors from rice bran hydrolysate. New Biotechnol 31(5):451–459
Todhanakasem T, Salangsing O, Koomphongse P, Kanokratana P, Champreda V (2019) Zymomonas mobilis biofilm reactor for ethanol production using rice straw hydrolysate under continuous and repeated batch processes. Front Microbiol 10:1777
Qureshi N, Lai L, Blaschek H (2004) Scale-up of a high productivity continuous biofilm reactor to produce butanol by adsorbed cells of Clostridium beijerinckii. Food Bioprod Process 82(2):164–173
Roukas T (1994) Ethanol production from nonsterilized carob pod extract by free and immobilized Saccharomyces cerevisiae cells using fed-batch culture. Biotechnol Bioeng 43(3):189–194
Roukas T (1994) Kinetics of ethanol production from carob pods extract by immobilized Saccharomyces cerevisiae cells. Appl Biochem Biotechnol 44(1):49–64
Clementz A, Aimaretti N, Manuale D, Codevilla A, Yori J (2015) Optimization of ethanol fermentation from discarded carrots using immobilized Saccharomyces cerevisiae. Int J Energy Environ Eng 6(2):129–135
Rosen M (2018) Environmental sustainability tools in the biofuel industry. Biofuel Res J 5(1):751–752
Funding
This study was supported by the Akdeniz University Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bader, N.B., Germec, M. & Turhan, I. Scheffersomyces stipitis biofilm reactor for ethanol production from acid-pretreated/detoxified and glucose- or xylose-enriched rice husk hydrolysate under a continuous process. Biomass Conv. Bioref. 11, 2909–2921 (2021). https://doi.org/10.1007/s13399-020-00611-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-00611-6