Abstract
With the increasing application of steroid drugs as therapeutics, the demand for steroid drugs is increasing. In recent years, biological synthesis has become the standard approach to produce steroid intermediates, while this method still faces some problems such as unclear metabolic pathway and low yield. Mycobacterium sp. LY-1 can convert phytosterols into 9α-hydroxyandrost-4-ene-3,17-dione (9α-OH-AD) which is a key intermediate for the synthesis of steroid drugs with long effective time and significant pharmacological activity. In this work, the whole-genome sequence of the Mycobacterium sp. LY-1 was analyzed, and the side-chain degradation pathway of phytosterols in Mycobacterium sp. LY-1 was proposed. Meanwhile, the related key enzymes of phytosterol metabolism were identified through qRT-PCR. Through overexpressing the key enzymes including KshA2, KshB, and HsdB, the yield of 9α-OH-AD increased by 12.7% compared to that of the control. Furthermore, by optimizing the medium and culture conditions, the yield of 9α-OH-AD reached 50.4%. The maximum yield was 30.7% higher than that of the original strain. The results are of significance for the industrial production of 9α-OH-AD using metabolic engineering methods.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Steroid drugs are the second largest class of drugs in the world after antibiotics [1, 2]. In recent years, the clinical application of steroid drugs has shown an upward trend [3]. They are widely used to treat tumors [4], arthritis, asthma, and regulate hormone levels in the body [5, 6]. The oxidation state of the steroid nucleus and the attached functional groups determine the specific biological characteristics of each steroid compound [7]. 9α-Hydroxyandrost-4-ene-3,17-dione (9α-OH-AD) is an important steroidal drug intermediate which is difficult to obtain by the conventional chemical synthesis [8], with a special hydroxyl group at the C9 position of its structure. The C9 hydroxyl group can be used for the synthesis of corticosteroids after being chemically substituted by halogen atoms [9]. The corticosteroids produced by 9α-OH-AD have a long action time and significant pharmacological functions [10, 11]. In the mid-1970s, due to the gradual increase in the price of diosgenin, some pharmaceutical companies and research institutes turned to research new raw materials such as phytosterols. Phytosterols are natural sterols extracted from by-products of oil crops [12]. Using them as precursors, steroidal drug intermediates can be prepared by biotransformation of Mycobacterium [13]. Mycobacterium have been used to produce steroid drug intermediates on an industrial scale. For example, Mycobacterium smegmatis was engineered to produce AD and ADD [14], while Mycobacterium neoaurum ATCC 25795 was engineered to produce 9α-OH-AD [15]. The microbial transformation method has the advantages of less environmental pollution and fewer reaction steps, and thus, the current production of 9α-OH-AD mainly depends on the microbial transformation method [16, 17].
Previously, Shtratnikova et al. [18] reported the genome of a Mycobacterium sp. that could transform phytosterols to produce 9α-OH-AD for the first time. In 2016, Luthra et al. [19] improved the transformation efficiency of 9α-OH-AD by optimizing the medium resulting in a maximum titer of 9α-OH-AD of 9.10 mg/g. Mycobacterium sp. LY-1 that can transform phytosterols to produce 9α-OH-AD was isolated in our laboratory. Through phylogenetic tree analysis, Mycobacterium sp. LY-1 is the most closely related to M. neoaurum VKM Ac-1817D, which was reported to be a non-pathogenic mycobacteria. Zhou et al. [20] improved the solubility of the substrate phytosterols using the nonionic surfactant TX-40 and the yield of 9α-OH-AD reached 42.5% in 2019. Although methods such as media optimization and substrate solubilization can increase the yield of 9α-OH-AD, there are still problems such as low conversion efficiency and low substrate utilization.
Metabolic engineering [21, 22] or using resting cell catalysis [23, 24] are methods that have been used to resolve some of these problems; however, the microbial degradation pathways of phytosterols in some strains such as Mycobacterium sp. LY-1 are not clear, and the function of key enzymes has yet to be elucidated [25]. Therefore, it is particularly important to analyze the sterol metabolism in this strain, identify the key enzymes that affect the synthesis of the target product, and further improve the production efficiency of the target product through metabolic engineering methods. In the present study, to improve the ability of the strain to produce 9α-OH-AD, the whole genome of Mycobacterium sp. LY-1 was analyzed and the genes related to phytosterols metabolic pathway were identified. Thus, the putative sterol metabolic pathway of Mycobacterium sp. LY-1 was predicted. Differential transcription-level analysis was used to study the changes of metabolic pathway-related enzymes under different conditions with or without soya bean oil, and the key enzymes affecting the synthesis of 9α-OH-AD were determined. Furthermore, metabolic engineering methods were used to enhance the expression of single or multiple enzyme genes to construct genetically engineered recombinant strain with high yield of 9α-OH-AD. Combined with the optimization of the transformation process, the yield of 9α-OH-AD was further increased.
Materials and methods
Strains and plasmids
All strains and plasmids used in this study are shown in Table 1. The microorganism used is Mycobacterium sp. LY-1. This strain was stored in the China General Microbiological Culture Collection Center with the depositing No. 13031. Escherichia coli JM109 was used for plasmid construction. Plasmid pMV261 was used to overexpress in Mycobacterium sp. LY-1.
Medium and growth conditions
Phytosterols (β-sitosterol 47.0%, campesterol 24.6%, stigmasterol 15.5%, and brassicasterol 3.4%) was purchased from Hubei Jusheng Technology Co., Ltd., China; 9α-OH-AD was purchased from Shanghai Hanxiang Biotechnology (98.0% of purity), China; Soybean oil was purchased from Shanghai Kerry Food Industries Co., Ltd, China; Corn steep liquor was purchased from Anhui Huaheng Biotechnology Co., Ltd., China [20].
The (NH4)2HPO4 (0.6 g/L), NaNO3 (5.4 g/L), yeast extract (15 g/L), and glycerin (2%) medium was the seed medium. Mycobacterium sp. LY-1 was cultivated at 30 °C and 120 rpm for 3–5 days. Then, 1% seed culture was transferred into 50 mL of fermentation medium (NaNO3 5.4 g/L, (NH4)2HPO4 0.6 g/L, corn steep liquor 20.0 g/L, phytosterols 15 g/L) in 250-mL shake flasks and cultivated at 120 rpm and 30 °C for 168 h.
High-throughput sequencing and accession numbers
Genomic DNA was sheared, and then, 20 Kb double-stranded DNA fragments were selected. DNA fragments were end repaired and ligated with universal hairpin adapters. Subsequent steps were followed as per the manufacturer’s instruction to prepare SMRTbell library. The library was sequenced in PacBio RSII SMRT instrument, and then, the PacBio reads were assembled using PBcR of WGS-Assembler 8.2. The Glimmer gene-finding software has been used for finding coding genes in bacteria. Transfer RNAs (tRNAs) were detected in the genome using the program tRNAscan-SE with default parameter settings. rRNA were identified using RNAmmer. The coding genes were annotated with National Center for Biotechnology Information (NCBI) nr database by BLAST. Then, the functions of genes were annotated by GO (Gene Ontology) database, and the pathways were annotated using KEGG (Kyoto Encyclopedia of Genes and Genomes) database. The proteins encoded by genes were classificated on a phylogenetic classification by the database of COG (Clusters of Orthologous Groups).
The whole-genome sequence data reported in this paper have been deposited in the Genome Warehouse in National Genomics Data Center, Beijing Institute of Genomics, Chinese Academy of Sciences/China National Center for Bioinformation, under accession number GWHBHSJ00000000 that is publicly accessible at https://ngdc.cncb.ac.cn/gwh.
RNA extraction and quantitative real-time PCR analysis
For RNA extraction, we centrifuged the fermentation broth samples at different fermentation periods at 8000 rpm for 10 min, removed the supernatant, and washed the sample 2–3 times with PBS (pH = 7.0), and stored it at −80 °C. The frozen bacteria were then poured into a pre-cooled mortar and grind, where they were grinded with the addition of liquid nitrogen. Afterward, 1 mL of Trizol was added and the liquid was transferred to a 2 mL sterile EP tube. Total RNA was purified using a MasterPure™ RNA purification kit (Shanghai sangon, China). The first-strand cDNAs were synthesized by HisScript R II Q RT SuperMix (Takara, China) using total RNA (200 ng). For RT-qPCR, the 16S rRNA was chosen as the endogenous control. The reaction mixture was prepared in a qPCR tube and consisted of 10 μL of 2 × Power qPCR PreMix, 4 μL of forward and reverse primers (0.2 mM) respectively, 2 μL of cDNA, 0.4 μL 50 × Rox Reference Dye, and 3.6 μL of ddH2O. The RT-qPCR was performed on a Bio-Rad CFX96 Manager PCR system (Bio-Rad, United States) using the following parameters: 95 °C for 10 min followed by 40 cycles of denaturation at 95 °C for 10 s and 55 °C for 20 s and extension at 72 °C for 15 s. The 2−△△Ct method was applied to analyze the data, which were normalized to the transcription level of 16S rRNA.
Plasmid construction and electroporation
Genes were cloned from the genome of Mycobacterium sp. LY-1 using f1&r1, PrimeSTAR enzyme for PCR amplification. The PCR products were run on an agarose gel and purified according to a kit instructions (Generay, China). Purified fragments were digested with enzymes Hind III–BamH I and ligated into plasmid pMV261at the Hind III–BamH I site. The ligation product was transformed into E. coli JM109 competent cells. As for polygenic overexpression, the one Step Cloning Kit (Vazyme, China) was used to construct the plasmids.
For electroporation, 2 μg of the expression plasmid was added to Mycobacterium sp. LY-1 competent cells and left at 4 °C for 20–30 min. We used 2.5 kV as electroporation voltage and electric shock frequency of 5 ms; two electric shocks were applied followed by the addition of fresh seed medium and incubation at 30 °C for 3–4 h. We then collected the bacteria and spread it on resistant solid medium and cultured at 30 °C in a constant temperature incubator for 5–7 days until a clearly visible yellow colony was formed.
Analytical methods
During fermentation, we pipetted 1 mL of fermentation broth into a sterile 2 mL EP tube every 12 h, followed by centrifugation at 12,000 rpm for 10 min. We then added 1 mL of ethyl acetate, incubated with shaking at 1500 rpm for 30 min, and then centrifuged for 15 min. After repeating the above operation twice, we dried the EP tube to constant weight, and calculated the difference after weighing to obtain the biomass.
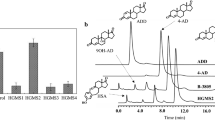
For high-performance liquid chromatography (HPLC), samples were dissolved in 2 mL ethyl acetate and were transferred into a clean tube. The extraction operation previously described was repeated five times, and the ethyl acetate extracts were combined. We concentrated the resulting extract with a nitrogen blower at room temperature and then added 4 mL of acetonitrile to re-dissolve it, followed by filtration through 0.22 μm of microporous membrane. HPLC was performed on an Agilent TC-C18 column (4.6 × 250 mm, 30 °C) using acetonitrile/water (7:3, v/v) as the mobile phase at a flow rate of 0.5 mL/min with ultraviolet detection at 254 nm and an injection volume of 10 μL. Peaks were compared to an internal standard of 9α-OH-AD to determine the exact amount of product during the fermentation [20].
The product yield was calculated using the following formula:
C1 standard concentration (g/L); Cd phytosterols concentration (g/L); Cc product concentration (g/L); P1 the peak area of standard analyzed by HPLC; Pc the peak area of product analyzed by HPLC; D sample dilution multiple; Mc product molar mass; Md phytosterols molar mass [20].
Results and discussion
Genome sequence analysis of Mycobacterium sp. LY-1
Based on comparative genomic analysis, preliminary genome sequence analysis of Mycobacterium sp. LY-1 was conducted (Table 2). The genome size of Mycobacterium sp. LY-1 was 6,320,069 bp. Through gene prediction analysis, the total length of Mycobacterium sp. LY-1 coding gene sequence was 5,877,051 bp, which contained 6129 genes, 54 tRNAs, 25 rRNAs, and 33 other RNAs. The coding sequence accounted for 93.0% of the entire genome, the average length of the gene was 959 bp, and the GC content was 66.5%.
Figure 1 is a phylogenetic analysis of Mycobacterium sp. LY-1. Nine Mycobacterium sp. genome sequences published on National Coalition Building Institute (NCBI) were selected to construct a phylogenetic tree based on the whole-genome sequence. The phylogenetic tree indicated that the genome features of Mycobacterium sp. LY-1 were close to two reference strains. One was M. neoaurum VKM Ac-1817D which had been reported to transform phytosterols to 9α-OH-AD and the other was M. tuberculosis H37Rv [26] which had been extensively studied.
Protein functions and sequences encoded by Mycobacterium sp. LY-1 genes were annotated based on the Cluster of Orthologous Groups (COG) database (Fig. 2). All open-reading frames in Mycobacterium sp. LY-1 could be divided into 1 unknown functional group and 20 functional groups, resulting in a total of 21 groups. The results indicated that the group with the most protein was Function unknown S (Function unknown), which accounted for 19.0% (Fig. S1). This result also illustrated the exploitability of this strain. In addition, the comprehensive analysis showed that the number of Mycobacterium sp. LY-1 in the Q, M, K, C, and E categories was more than the number in the A, B, W, and Z categories. The gene sequence prediction and functional annotation indicated that the functional proteins of Mycobacterium sp. LY-1 were mainly distributed in functional categories such as [Q] (secondary metabolism synthesis and metabolism), [C] (energy production and transport), and [K] (transcription). This result may indicate that strong secondary metabolism was accompanied by a large amount of energy production. This is consistent with the observation that there is a large amount of ATP during the transformation of phytosterols by mycobacteria [27]. At the same time, the functional proteins of Mycobacterium sp. LY-1 also accounted for a large proportion of [E] (amino acid transport and metabolism) and [K] (transcription). This may illustrate that Mycobacterium sp. LY-1 is a suitable host for expressing protein.
The side-chain degradation pathway of phytosterols of in Mycobacterium sp. LY-1
The protein sequences of Mycobacterium sp. LY-1 were compared and analyzed with the gene clusters of the sterol metabolic pathway in M. neoaurum VKM Ac-1817D and M. tuberculosis H37Rv (Table S2). According to the four stages of sterol metabolism, multiple sterol metabolism-related enzyme genes were found in Mycobacterium sp. LY-1, including one cholesterol oxidase (Cho) and three 3-hydroxyacyl-CoA dehydrogenases (Hsd) which were responsible for preliminary oxidation of sterols, one enoyl CoA thiolase (FadA5) and five acyl-CoA dehydrogenases (FadE26/27/28/29/30) which were responsible for side-chain degradation of sterols, one 2-Enoyl acyl-CoA hydratase (HsdB), five 3-sterone-9α-hydroxyoxygenase (KshA), one 3-sterone-9α-hydroxyreductase (KshB), and five 3-steroid Ketone-Δ1-dehydrogenases/3-Oxosteroid Δ1-dehydrogenases (KstD1/2/3/4/5) which were responsible for nucleus oxidation of sterols. Compared with M. tuberculosis H37Rv, Mycobacterium sp. LY-1 had more homologues of the genes responsible for nucleus oxidation of sterols, especially more copies of KshA. That may be the reason why Mycobacterium sp. LY-1 accumulated 9α-OH-AD. In addition, five 2,3-Dihydroxybiphenyl/1,2-Dioxygenases (HsaC1/2/3/4/5) which were responsible for nucleus cleavage of sterols were also identified (Table 3).
Based on the above results, combined with the previous research work, a putative phytosterol metabolic pathway in the Mycobacterium sp. LY-1 was predicted (Fig. 3). The results showed that phytosterols first underwent oxidative dehydrogenation by cholesterol oxidase, and then entered the complex sterol degradation pathway, and finally resulted in the target product 9α-OH-AD. Through the identification of the product composition and structure, we found that most products (> 98.8%) were C9 hydroxylation ones, which demonstrated that Mycobacterium sp. LY-1 had a strong C9 hydroxylation ability for sterols (Fig.S2). According to the previous reports, the enzyme for sterol C9 hydroxylation is 3-ketosteroid-9α-hydroxylase, and the process of hydroxylation requires KshA and KshB to act synergistically [28]. On the basis of the genome sequence analysis, there were five kshA genes and one kshB gene of 9 hydroxylation oxygen isoenzymes in Mycobacterium sp. LY-1. Therefore, we speculated that there may be a special 9α-OH-AD accumulation pathway. During the sterol metabolism of Mycobacterium sp. LY-1 (Fig. 3, in blue), after the oxidation of phytosterols, the cholestenone obtained is first hydroxylated by KshA and KshB, introducing a high metabolic flux into this pathway. 9α-OH-cholestenone was obtained by KshA and KshB co-catalysis, and then, 9α-OH-AD was directly obtained by side-chain degradation. Even if a small amount of metabolic flux was introduced into the production pathway of AD and ADD, they will be metabolized by the more active KshA and KshB into the corresponding 9-position hydroxylated products. Therefore, it may be one of the reasons why Mycobacterium sp. LY-1 accumulated a large amount of 9α-OH-AD.
Identification of the related key enzymes in sterol metabolic pathway of Mycobacterium sp. LY-1
In the conversion process of Mycobacterium sp. LY-1, adding soybean oil could improve the solubility of sterols and the vitality of the bacteria [29, 30]. Our previous studies indicated that in the presence of soybean oil, the ability of Mycobacterium sp. LY-1 to transform phytosterols was significantly improved. Figure 4 shows the fermentation curve of the strain with and without soybean oil. The maximum yield of 9α-OH-AD was increased from 10.03% to 35.42% and the biomass was also improved. To explore whether this result was related to the difference in the expression of partial enzyme genes in the sterol metabolic pathway, the difference in transcription level of sterol metabolism-related enzyme genes with and without oil were analyzed. 16S rDNA was used as the housekeeping gene, and the primers are shown in Table S1. cDNA at three time points, 60, 84, and 168 h during the transformation process was used as a template for qRT-PCR experiments.
The results indicated that at the three time points with oil conditions, gene expression levels were basically up-regulated (red) (Fig. 5). Among them, in the early stage of transformation (60 h), the nine enzyme genes whose expression levels were down-regulated were kshA1, kshA5, rdc1, kstd1, kstd5, hsdC, hsd2, hsd3, and hasC2, and the rest were all up-regulated; in the middle stage of transformation (84 h) and the later stage of transformation (168 h), all enzyme genes were up-regulated, and nine of them (cho, fadA5, fadE30, hsdB, kshA2, kshB, hsd1, kstD2, and kstD3) were up-regulated significantly. Among these nine up-regulated genes, there were seven genes (cho, fadA5, fadE30, hsdB, kshA2, kshB, and hsd1) that are associated with 9α-OH-AD synthesis and two genes (kstD2 and kstD3) associated with 9α-OH-AD degradation. The result indicated that as the fermentation process progressed, the expression level of enzymes related to the biotransformation of 9α-OH-AD increased, especially the above-mentioned up-regulated seven genes. Based on the analysis of the differential transcription levels of the enzymes related to the sterol metabolic pathway under the above-mentioned different culture conditions, seven key enzymes for the synthesis of 9α-OH-AD were mainly involved in the accumulation of 9α-OH-AD including Cho, Hsd1, FadA5, FadE30, HsdB, KshA2, and KshB. To verify the above hypothesis, single overexpression and combined overexpression of the proposed 9α-OH-AD synthetic key enzymes were conducted in the following experiments.
Overexpression of key enzymes in Mycobacterium sp. LY-1 to increased 9α-OH-AD production
Single overexpressing and co-overexpressing the key genes with significant up-regulation in the sterol metabolism was an effective strategy to improve 9α-OH-AD production [21, 28]. Seven key genes (cho, fadA5, fadE30, hsdB, kshA2, kshB, and hsd1) related to 9α-OH-AD synthesis were overexpressed separately to up-regulate the sterol metabolism (Fig. 6). Compared with the control, the yield of 9α-OH-AD in all recombinant strains showed a slight increase. Among them, the 9α-OH-AD production of the LY-1-kshA2 was the highest, and the yield of 9α-OH-AD was increased to 40.8%. At the same time, the biomass of these recombinant strains decreased significantly. These results showed that although the single overexpression of key enzyme genes could promote the accumulation of 9α-OH-AD to some extent, the degree of improvement was relatively weak (within 10%). Therefore, co-expression of key enzyme genes involved in sterol metabolism was further investigated.
α-OH-AD yield of Mycobacterium sp. LY-1 control strain and single overexpressing strains in shake flask culture. The Mycobacterium sp. LY-1 of the electroporated plasmid pMV261 was used as a control, the culture conditions were the same, the substrate concentration was 15 g/L, and the fermentation time was 7 days. All data represent the mean values from three independent experiments
Based on single overexpression responses, genes of four enzymes including kshA2, kshB, hsdB, and hsd1 which enabled a higher increase in the 9α-OH-AD production were selected to co-express in Mycobacterium sp. LY-1. To reduce the impact of multiple plasmids on the growth of Mycobacterium sp. LY-1, a single plasmid was used to overexpress all genes. As shown in Fig. 7, the yield of 9α-OH-AD of co-expressed strains further improved compared with the single overexpression recombinant strains, while the biomass also increased. The 9α-OH-AD yield of LY-1-kshA2/hsdB reached 42.07%, an increase of 9.0% from the wild Mycobacterium sp. LY-1 (38.58%).
9α-OH-AD yield of Mycobacterium sp. LY-1 control strain and co-expressing strains in shake flask culture. A Schematic of the co-overexpression in Mycobacterium sp. LY-1. B 9α-OH-AD production by the control strain and co-expressing strains at the 168th hours’ fermentation. The culture conditions were the same, and the substrate concentration was 15 g/L. All data represent the mean values from three independent experiments
The metabolic pathway from AD to 9α-OH-AD involved the key enzyme 3-ketosteroid-9α-dehydrogenase (Ksh). The Ksh activity consisted of two components: terminal oxygenase (KshA) and ferrooxidase (KshB) [31] and the existence of these two components at the same time could help the biotransformation of 9α-OH-AD. To better promote the C9 hydroxylation, LY-1-kshA2/kshB/hsdB was constructed based on LY-1-kshA2/hsdB overexpressing the third gene of kshB. The yield of 9α-OH-AD could reach 43.4% (Fig. 7) and the 9α-OH-AD yield of the LY-1-kshA2/kshB/hsdB increased by 12.7% compared with the wild Mycobacterium sp. LY-1. The results showed that the yield of 9α-OH-AD could be increased by co-overexpressing endogenous genes. In the future, the catalytic properties of the enzymes should be specifically investigated to further improve the yield of 9α-OH-AD by heterologous expression of the relevant key enzymes.
Optimization of biotransformation process of recombinant strain
In the microbial transformation process of phytosterols, the growth of microorganisms is closely related to their growth environment. Various components in the medium and culture conditions had great influences on the growth of strains and the expression of intracellular enzymes [32]. Therefore, the medium and culture conditions were further optimized based on the LY-1-kshA2/kshB/hsdB. The effects of carbon sources, nitrogen sources, phosphates, and metal ions in the culture medium on product yield were studied, and the optimum concentration of components in the medium was optimized through orthogonal experiment (Tables S3 and S4). Thus, the optimal medium was 30 g/L, KNO3 4 g/L, NaH2PO4 1.2 g/L, FeSO4 0.075 g/L, and CaCl2 0.1 g/L. After the optimization of the medium, the product yield of 9α-OH-AD reached 48.9% (Fig. 8). In addition, the culture conditions were optimized under the conditions of the above-mentioned optimal medium. Finally, it was determined that the optimal fermentation conditions were pH 8.0, inoculum amount 2%, and growth temperature of 30 °C (Fig. 9). Under the optimal conditions, the maximum concentration of 9α-OH-AD reached 5.59 g/L and 9α-OH-AD yield reached 50.4%.
Optimization of the medium. A Effects of different carbon sources on 9α-OH-AD yield. The control used corn steep liquor as the initial carbon source. B Effects of different nitrogen sources on 9α-OH-AD yield. The control used NaNO3 as the initial nitrogen source. C Effects of different phosphates on 9α-OH-AD yield. The control used (NH4)2HPO4 as the initial phosphates. D Effects of different metal ions on 9α-OH-AD yield. The control contained no added metal ions. The culture conditions were the same, and the substrate concentration was 15 g/L. All data represent the mean values from three independent experiments
Optimization of culture conditions. A Effects of cell concentration on 9α-OH-AD yield. B Effects of Initial medium pH on 9α-OH-AD yield. C Effects of temperature on 9α-OH-AD yield. The culture mediums were the same, and the substrate concentration was 15 g/L. All data represent the mean values from three independent experiments
Conclusions
In this study, the metabolic pathway of phytosterols was predicted through the analysis of the whole genome of Mycobacterium sp. LY-1. Our key observation that the yield of 9α-OH-AD can be significantly improved by adding soybean oil led to the identification of the key enzymes related to the synthesis of 9α-OH-AD in the sterol metabolism pathway. Strain LY-1-kshA2/kshB/hsdB with high yield of 9α-OH-AD was obtained by co-expressing three key enzymes. This metabolic engineering strategy when combined with the optimization of the transformation process resulted in further yield of 9α-OH-AD improvement, reaching 50.4% in the presence of 15 g/L phytosterols. This study provides a promising biotransformation method for 9α-OH-AD production in industrial application.
References
Donova MV. Steroid bioconversions. In: Barredo J-L, Herráiz I, editors. Microbial steroids. New York: Springer; 2017. p. 1–13.
Fernández-Cabezón L, Galán B, García JL. New insights on steroid biotechnology. Front Microbiol. 2018;9:958–73. https://doi.org/10.3389/fmicb.2018.00958.
Teixeira MP, Passos EF, Haddad NF, et al. In vitro antitumoral effects of the steroid ouabain on human thyroid papillary carcinoma cell lines. Environ Toxicol. 2021;36(7):1338–48. https://doi.org/10.1002/tox.23130.
Liu Z, Liu T, Li W, et al. Insights into the antitumor mechanism of ginsenosides Rg3. Mol Biol Rep. 2021;48(3):2639–52. https://doi.org/10.1007/s11033-021-06187-2.
Frye CA. Steroids, reproductive endocrine function, and affect. A review. Minerva Ginecol. 2009;61(6):541–62.
Tong WY, Dong X. Microbial biotransformation: recent developments on steroid drugs. Recent Pat Biotechnol. 2009;3(2):141–53. https://doi.org/10.2174/187220809788700157.
Lednicer D. Steroid chemistry at a glance. Chichester: John Wiley & Sons; 2011.
Javid M, Nickavar B, Vahidi H, et al. Baeyer-Villiger oxidation of progesterone by Aspergillus sojae PTCC 5196. Steroids. 2018;140:52–7. https://doi.org/10.1016/j.steroids.2018.07.008.
Herráiz I. Chemical pathways of corticosteroids, industrial synthesis from sapogenins. Methods Mol Biol. 2017;1645:15–27.
Capyk JK, Kalscheuer R, Stewart GR, et al. Mycobacterial cytochrome P450 125 (Cyp125) catalyzes the terminal hydroxylation of C27 Steroids. J Biol Chem. 2009;284(51):35534–42. https://doi.org/10.1074/jbc.M109.072132.
Karpova NV, Andryushina VA, Stytsenko TS, et al. A search for microscopic fungi with directed hydroxylase activity for the synthesis of steroid drugs. Appl Biochem Microbiol. 2016;52(3):316–23. https://doi.org/10.1134/S000368381603008X.
Vidal M, Becerra J, Mondaca M, et al. Selection of Mycobacterium sp. strains with capacity to biotransform high concentrations of beta-sitosterol. Appl Microbiol Biotechnol. 2001;57(3):385–9. https://doi.org/10.1007/s002530100693.
Sukhodolskaya GV, Nikolayeva VM, Khomutov SM, et al. Steroid-1-dehydrogenase of Mycobacterium sp. VKM Ac-1817D strain producing 9alpha-hydroxy-androst-4-ene-3,17-dione from sitosterol. Appl Microbiol Biotechnol. 2007;74(4):867–73. https://doi.org/10.1007/s00253-006-0728-4.
Galan B, Uhía I, García-Fernández E, et al. Mycobacterium smegmatis is a suitable cell factory for the production of steroidic synthons. Microb Biotechnol. 2017;10(1):138–50. https://doi.org/10.1111/1751-7915.12429.
Yao K, Xu LQ, Wang FQ, et al. Characterization and engineering of 3-ketosteroid-△ 1-dehydrogenase and 3-ketosteroid-9α-hydroxylase in Mycobacterium neoaurum ATCC 25795 to produce 9α-hydroxy-4-androstene-3, 17-dione through the catabolism of sterols. Metab Eng. 2014;24:181–91. https://doi.org/10.1016/j.ymben.2014.05.005.
Shao M, Zhang X, Rao Z, et al. Identification of steroid C27 monooxygenase isoenzymes involved in sterol catabolism and stepwise pathway engineering of Mycobacterium neoaurum for improved androst-1,4-diene-3,17-dione production. J Ind Microbiol Biotechnol. 2019;46(5):635–47. https://doi.org/10.1007/s10295-018-02135-5.
Szentirmai A. Microbial physiology of sidechain degradation of sterols. J Ind Microbiol Biotechnol. 1990;6(2):101–15. https://doi.org/10.1007/BF01576429.
Shtratnikova VY, Schelkunov MI, Dovbnya DV, et al. Complete genome sequence of Mycobacterium sp. strain VKM Ac-1817D, capable of producing 9α-Hydroxy-androst-4-ene-3,17-dione from phytosterol. Genome Announcements. 2015;3(1):e01447-14. https://doi.org/10.1128/genomeA.01447-14.
Luthra U, Bhosle V, Singh NK, et al. Media Optimization for 9α-hydroxyandrost-4-ene-3,17-dione Production by Mycobacterium spp. using Stat Des. 2016.
Zhou L, Li H, Xu Y, et al. Effects of a nonionic surfactant TX-40 on 9α-hydroxyandrost-4-ene-3,17-dione biosynthesis and physiological properties of Mycobacterium sp. LY-1. Process Biochem. 2019;87:89–94. https://doi.org/10.1016/j.procbio.2019.09.018.
Sun H, Yang J, He K, et al. Enhancing production of 9α-hydroxy-androst-4-ene-3,17-dione (9-OHAD) from phytosterols by metabolic pathway engineering of mycobacteria. Chem Eng Sci. 2021;230(47): 116195. https://doi.org/10.1016/j.ces.2020.116195.
Chang H, Zhang H, Zhu L, et al. A combined strategy of metabolic pathway regulation and two-step bioprocess for improved 4-androstene-3,17-dione production with an engineered Mycobacterium neoaurum - ScienceDirect. Biochem Eng J. 2020;164: 107789. https://doi.org/10.1016/j.bej.2020.107789.
Gao XQ, Feng JX, Wang XD, et al. Enhanced steroid metabolites production by resting cell phytosterol bioconversion. Chem Biochem Eng Quart. 2015;29(4):567–73. https://doi.org/10.15255/CABEQ.2014.2098.
Gao X, Feng J, Hua Q, et al. Investigation of factors affecting biotransformation of phytosterols to 9-hydroxyandrost-4-ene-3,-17-dione based on the HP-β-CD-resting cells reaction system. Biocatal Biotransform. 2014;32(5–6):343–7. https://doi.org/10.3109/10242422.2014.976633.
Malaviya A, Gomes J. Androstenedione production by biotransformation of phytosterols. Biores Technol. 2008;99(15):6725–37. https://doi.org/10.1016/j.biortech.2008.01.039.
O’Toole RF, Gautam SS. Limitations of the Mycobacterium tuberculosis reference genome H37Rv in the detection of virulence-related loci. Genomics. 2017;109(5–6):471–4. https://doi.org/10.1016/j.ygeno.2017.07.004.
Zhou X, Zhang Y, Shen Y, et al. Efficient repeated batch production of androstenedione using untreated cane molasses by Mycobacterium neoaurum driven by ATP futile cycle. Bioresource Technol. 2020;309:123307. https://doi.org/10.1016/j.biortech.2020.123307.
Liu HH, Xu LQ, Yao K, et al. Engineered 3-ketosteroid 9α-hydroxylases in Mycobacterium neoaurum: an efficient platform for production of steroid drugs. Appl Environ Microbiol. 2018;84(14):e02777-e2817. https://doi.org/10.1128/AEM.02777-17.
Ceen EG, Herrmann JPR, Dunnill P. Solvent damage during immobilised cell catalysis and its avoidance: studies of 11α-hydroxylation of progesterone by Aspergillus ochraceus. Enzyme Microbial Technol. 1987;25(6):491–4. https://doi.org/10.1016/0141-0229(87)90061-5.
Phase N, Patil S. Natural oils are better than organic solvents for the conversion of soybean sterols to 17-ketosteroids by Mycobacterium fortuitum. World J Microbiol Biotechnol. 1994;10(2):228–9. https://doi.org/10.1007/BF00360894.
Van der Geize R, Yam K, Heuser T, et al. A gene cluster encoding cholesterol catabolism in a soil actinomycete provides insight into Mycobacterium tuberculosis survival in macrophages. Proc Natl Acad Sci. 2007;104(6):1947–52. https://doi.org/10.1073/pnas.0605728104.
Yin Y. Effects of different carbon sources on growth, membrane permeability, β-sitosterol consumption, androstadienedione and androstenedione production by Mycobacterium neoaurum. Interdisc Sci Comput Life Sci. 2016;8(1):102–7. https://doi.org/10.1007/s12539-015-0116-9.
Acknowledgements
This work was supported by the National Key R & D Program of China (No. 2019YFA0905300), Tianjin Synthetic Biotechnology Innovation Capacity Improvement Project (No. TSBICIP-KJGG-001-14), the National Natural Science Foundation of China (No. 22078126), Qing Lan Project in Jiangsu Province and Fundamental Research Funds for the Central Universities (JUSRP221025).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, W., Li, H., Zhang, J.X. et al. Whole-genome analyses and metabolic modification of Mycobacterium sp. LY-1 to enhance yield of 9α-OH-AD. Syst Microbiol and Biomanuf 4, 127–137 (2024). https://doi.org/10.1007/s43393-022-00103-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43393-022-00103-w