Abstract
Sulfuric acid was used in the pretreatment of corn stover to obtain xylose as a value-added by-product, and the pretreated corn stover (Pre-CS) was hydrolyzed to produce glucose for butanol fermentation. The aim of this work is to achieve high xylose accumulation and reduced wastewater by recycling the pretreatment solution. The pretreatment conditions were optimized as follows: 180 °C, 15 min, 1:7 solid–liquid ratio (w/w), 0.6% H2SO4 (w/w, first batch)/0.9% H2SO4 (w/w, second and third batches), in which pretreatment solution was recycled for three times. Under above conditions, pretreatment solution containing 56.3 g/L xylose and 4.5 g/L glucose was obtained. Pre-CS residue was further hydrolyzed by cellulase to achieve 35.7–39.9 g/L glucose. The condensed corn stover hydrolysate was subjected to simultaneous detoxification and sterilization using 1% (w/w) activated carbon and then applied in butanol fermentation. The highest butanol titer of 9.5 g/L was obtained in 72 h. The results provide a practical approach for coproducing xylose and biobutanol from corn stover.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Increasing environmental pollution and fluctuating petroleum prices have stimulated great interest in biofuels production. In the USA, 58.87 billion liters of ethanol was produced from corn in 2016 [1, 2]. Growing demand for “environmental-friendly” biofuels has driven increased food prices, even food shortage in many countries. Fermentation of lignocellulosic biomass is an attractive and feasible route to biofuels that supplements the fossil fuels [1, 3]. It has been estimated that 442 billion liters of bioethanol per year can be produced from lignocellulosic biomass all over the world [4]. Butanol has attracted increasing attentions as a biofuel, due to its excellent properties such as high energy density, low volatile and explosive, and miscible with gasoline in any proportion [5].
Corn stover is an abundant lignocellulosic biomass [6,7,8,9], with a global annual production of 0.95 billion tons [10]. However, pretreatment of corn stover is required to release sugars that can be utilized by microorganisms to produce biofuels [5, 8, 11]. Corn stover is composed of 37% cellulose and 19.8% xylan [12]. Various pretreatment methods have been reported, such as alkaline [8, 13, 14], acid [3, 15, 16], acid and alkaline [17], ethanol [18], glycerol [19], hydrogen peroxide [20, 21], alkaline hydrogen peroxide [22, 23], ionic liquids [24, 25] and deep eutectic solvents [26, 27].
Acid treatment is a well-known method for processing lignocellulosic biomass [28], especially in xylose, xylitol [10] and furfural production [29,30,31]. Acid pretreatment could increase the accessibility of enzymes to the cellulosic fractions by solubilizing the hemicellulosic fraction of the biomass [32]. Dilute H2SO4 (0.5–2%) is usually used for pretreatment because of the strong corrosivity of concentrated H2SO4 [28, 33]. Acid pretreatment could produce aromatic and toxic compounds such as furfural, hydroxyl methyl furfural (HMF), acetic acid and formic acid, which are inhibitory to microbial fermentation [1, 3]. Acid-pretreated solutions are highly toxic and usually need to be detoxified before subjected to hydrolysis and fermentation [7]. Xylose is an important component in acid-pretreated solutions, whereas the utilization of xylose is often inconvenient due to its low concentration. Corn stover is known to contain less xylan than corn cob, which contains about 33% of xylan and is commonly used for xylose and xylitol production [10]. Since the dry mass content is generally less than 13%, the xylose concentration after hydrolysis of corn stover was reported to be 16.4–20.5 g/L [10, 34]. However, a significantly higher substrate (xylose) concentration of about 200 g/L is required for xylitol production [35, 36]. Vacuum evaporation concentration is undesirable, due to its high energy consuming and generation of inhibitors such as furfural and hydroxyl methyl furfural (HMF).
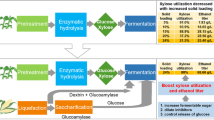
Herein, recycling of sulfuric acid pretreatment solution to accumulate xylose from corn stover was studied for the first time. Importantly, a high xylose concentration of 56.3 g/L was achieved as a by-product from recycled acid-pretreated solution. After pretreatment, the unwashed corn stover was subjected to hydrolyzation, and simultaneous detoxification and sterilization (SDS) fermentation, resulting in butanol titer of 9.5 g/L.
Materials and methods
Raw materials, strains and enzymes
Corn stover was kindly provided by Shandong Zesheng Bioengineering Technology. Corn stover was chopped and stored in a closed container at room temperature. Activated carbon was purchased from Sinopharm Chemical Reagent Co. Ltd.
Clostridium saccharobutylicum DSM 13864 was purchased from DSMZ and was manipulated as previously described [5, 7, 8, 11].
ACCELLERASE® 1500 cellulase was a generous gift from Genencor (Wuxi) Bio-Products Co.
Acid pretreatment of corn stover
One gram of untreated corn stover was filled into sealed stainless reactors with lining Teflon (200 mL) and soaked in 0–20% H2SO4 (w/w) at 170 °C or 180 °C for 0–1 h in an oil bath. After the pretreatment, the reactors were immersed in ice bath immediately to cool down. Addition of H2SO4 is based on the percent of liquid weight. Solid–liquid ratios (w/w) were 1:10, 1:8 and 1:7. Pretreated corn stover (Pre-CS) was filtered with a Buchner funnel. The pretreatment solution was collected and recycled for second batch of pretreatment. For unwashed group, filtered Pre-CS was soaked with water and adjusted pH to 4.8 with NaOH for the next enzymatic hydrolysis step. For washed group, filtered Pre-CS was washed with tap water until pH reached 4.8–6.0, dried at 65 °C for 24 h in an oven and stocked in sealed plastic bags for further use.
Recycled pretreatment solution for pretreating corn stover
For recycling pretreatment solution, 1 g of untreated corn stover was soaked in first and second batches of pretreatment solution (solid–liquid ratios (w/w) of 1:10), and 0%, 0.2%, 0.4%, 0.6%, 0.8%, 0.9%, 1.0% and 1.2% (w/w) H2SO4 were added to the pretreatment solution for second and third batches of pretreatment, respectively.
To optimize the solid–liquid ratio, 1 g of untreated corn stover was soaked in 10 g, 8 g and 7 g of first and second batches of pretreatment solution, and then 0.9% (w/w) H2SO4 was added to the pretreatment solution for second and third batches of pretreatment. Others conditions were the same as in “Acid pretreatment of corn stover”.
Enzymatic hydrolysis of pretreated corn stover
The washed Pre-CS and unwashed Pre-CS were soaked in citrate buffer (pH 4.8) in conical flasks with plugs. Then, 30 FPU/g (Pre-CS) cellulase was added to the mixture. Solid–liquid ratio was 1:10 to 1:7. The conical flasks were incubated in a water bath at 50 °C and 150 rpm for 48 h.
Enzymatic hydrolysates were condensed to 60– 65 g/L of total reducing sugar for butanol fermentation using a rotary evaporator under vacuum at 60 °C.
Samples were taken at 24 h and 48 h and then subjected to centrifugation at 12,000 rpm for 10 min. The glucose concentration was determined using HPLC analysis as described in “Analytical methods”.
Butanol fermentation
Butanol fermentation medium was composed of 55 g/L reducing sugar, 10 g/L corn steep liquor (CSL), 4 g/L CaCO3, 2 g/L (NH4)2SO4, 0.5 g/L K2HPO4 and 0.01 g/L MnSO4·H2O [5]. C. saccharobutylicum DSM 13864 cultured at 37 °C for 16–18 h was used as seed culture for butanol fermentation at an inoculum size of 10% (v/v). The butanol fermentation was conducted as follows.
Detoxification with separate sterilization (DSS) [7] about 50 mL of undetoxified and activated carbon detoxified corn stover hydrolysates was adjusted to pH 6.5 with 2 mol/L NaOH and then transferred into 100-mL anaerobic bottles for sterilization, respectively. Mixture of CSL and mineral salts [CaCO3, (NH4)2SO4, K2HPO4, MnSO4·H2O] was adjusted to pH 6.5 and sterilized. The sterilized CSL and salts mixture was then transferred into sterilized detoxified FRs hydrolysate to prepare fermentation medium as described above and then inoculated with 10% (v/v) seed culture for butanol fermentation.
Simultaneous detoxification and sterilization (SDS) [7] about 50 mL of un-detoxified corn stover hydrolysate was mixed with activated carbon, CSL and mineral salts [CaCO3, (NH4)2SO4, K2HPO4, MnSO4·H2O] and adjusted to pH 6.5 with 2 mol/L NaOH in a 100-mL anaerobic bottle for sterilization. Then, 10% (v/v) seed culture was inoculated for butanol fermentation.
Analytical methods
Corn stover hydrolysate was centrifuged (12,000 rpm, 5 min) and diluted for 10 or 20 folds. The total reducing sugar and monosaccharides in corn stover hydrolysate were measured as previous report [8].
Xylose, glucose and arabinose were analyzed by HPLC (Agilent 1260, Agilent Technologies Ltd., USA) using a refractive index detector (RID) and an Aminex HPX-87H column (300 mm × 7.8 mm, 9 μm; Bio-Rad, USA). The mobile phase was 5 mmol/L H2SO4 solution at a flow rate of 0.5 mL/min, and the column was operated at 55 °C.
Furfural was analyzed by HPLC using a UV detector at wavelength of 276 nm and a C18 column with 0.01% (v/v) H2SO4/CH3OH (95/5, v/v) as the mobile phase at a flow rate of 1 mL/min [37].
The concentrations of butanol, acetone, and ethanol were determined by GC (6890 N; Agilent Technologies, Wilmington, DE, USA) according to previously described method equipped with the flame ionization detector (FID) and an capillary column PEG-20M (30 m × 0.32 mm × 0.5 μm, JK, China) using nitrogen as the carrier gas [11, 38].
Results and discussion
Pretreatment of corn stover using sulfuric acid
Increased temperature, acid dose and pretreatment time could promote the formation of inhibitory compounds during biomass pretreatment [28, 39]. However, lower pretreatment temperature often requires higher dose of acids. Germec and Turhan reported the pretreatment of rice straw using 5% (w/w) H2SO4 at 126.5 °C [3]. In Cao and coworkers’ study, 0.5% (w/w) H2SO4 was applied in the pretreatment of poplar (solid loading 5% w/w) at 170 °C [40]. Pretreatment with higher acids concentration could result in higher ionic strength in hydrolysates, which is detrimental to further microbial fermentation [41]. Thus, 170 °C was chosen for H2SO4 pretreatment of corn stover.
Different doses of H2SO4 were tested in corn stover pretreatment at 170 °C (Fig. 1). Among xylan, dextran and araban, araban is regarded as the most stable polysaccharide under acidic conditions. At 10–20% (w/w) H2SO4, xylan and dextran were converted into furfural, 5-HMF, etc., [42]; araban, however, was hardly degraded. Under pretreatment conditions of 170 °C, 1 h and 10% (w/w) H2SO4, 0.8 g/L of glucose and 4.6 g/L of arabinose are detected in the enzymatic hydrolysate.
Using 0.6% (w/w) H2SO4, 17.2 g/L xylose and 22.8 g/L glucose were achieved in pretreatment solution and enzymatic hydrolysates, respectively. Although higher glucose (23.3 g/L) was reached at 0.8% (w/w) H2SO4 than that of 0.6% (w/w) H2SO4, lower xylose (15.5 g/L) was resulted at 0.8% (w/w) H2SO4 (Fig. 1). Thus, 0.6% (w/w) H2SO4 was used in further experiments.
Recycling of acid pretreatment solution
Because the price of xylose ($3500/ton) is significantly higher than that of glucose ($470/ton), it would be more practical to produce xylose (from hemicellulose) and glucose (from cellulose) separately in the pretreatment of lignocellulosic biomass. To achieve higher xylose and reduced waste liquid, the pretreatment solution was recycled in second batch of pretreatment (Fig. 2). Considering the reduced acidity after pretreatment [43], various amounts of sulfuric acid were added in the pretreatment solution. When 0.6% (w/w) of H2SO4 was added, the highest xylose concentration of 33.5 g/L was reached, along with 19.5 g/L glucose. At H2SO4 addition of 1.0% (w/w), the highest glucose level of 21.2 g/L was achieved, with reduced xylose of 29.2 g/L. Based on the above results, addition of 0.9% (w/w) H2SO4 was selected in second batch of pretreatment.
To further evaluate the accumulation of xylose in third batch of pretreatment, different concentrations of H2SO4 were added in the pretreatment solution of second batch (Fig. 3). The results show that xylose concentrations in third batch were not significantly increased. When 0.9% (w/w) of H2SO4 was added, xylose concentration reached the maximum level of 32.9 g/L, which was slightly decreased compared with second batch (33.5 g/L xylose). It has been reported that xylose is unstable at higher temperatures and can easily transformed into furfural and other compounds [44,45,46]. The concentration of glucose (18.7 g/L) in the hydrolysates was also lower than that in second batch, suggesting a further strengthened buffer capacity and weakened acidity of third batch of pretreatment solution. Addition of 0.9% (w/w) H2SO4 was used in second and third batches of pretreatment in further experiments.
Process optimization of recycled acid pretreatment solution over three batches
Higher temperature and shorter reaction time could result in higher xylose titer and furfural yield [47]. Considering the severe degradation of xylose in third batch of pretreatment at 170 °C for 1 h, shortened pretreatment at higher temperature (180 °C) and solid–liquid ratio (1:7) was attempted to reduce the degradation of xylose.
As shown in Fig. 4a, xylose concentration did not increase linearly with the increase of solid–liquid ratio (1:10, 1:8 and 1:7) at pretreatment temperature of 170 °C. Although xylose concentrations increased in recycled batches at all solid–liquid ratios, only 40.5 g/L of xylose was obtained in third batch under 170 °C-1H-1/7 (170 °C pretreatment for 1 h, solid–liquid ratio of 1:7).
Optimization of solid–liquid ratio, temperature and time for recycle of pretreatment solution. a Pretreatment at 170 °C for 1 h at various solid–liquid ratios; b pretreatment at 180 °C for 0 − 30 min at solid–liquid ratio of 1:7. 170 °C-1H-1/10: 170 °C pretreatment for 1 h, solid–liquid ratio of 1:10; 170 °C-1H-1/8: 170 °C pretreatment for 1 h, solid–liquid ratio of 1:8; 170 °C-1H-1/7: 170 °C pretreatment for 1 h, solid–liquid ratio of 1:7; 180 °C-0 M-1/7: 180 °C pretreatment for 0 min, solid–liquid ratio of 1:7; 180 °C-15 M-1/7: 180 °C pretreatment for 15 min, solid–liquid ratio of 1:7; 180 °C-30 M-1/7: 180 °C pretreatment for 30 min, solid–liquid ratio of 1:7. Addition of H2SO4: first: 0.6% H2SO4 (w/w); second: 0.9% H2SO4 (w/w); third: 0.9% H2SO4 (w/w)
To reduce the degradation of xylose, pretreatment at higher temperature and shorter time was attempted. As shown in Fig. 4b, the pretreatment condition was optimized to be 15 min and solid–liquid ratio 1:7 at 180 °C. In second batch, 41.3 g/L of xylose was accumulated, with only 2.5 g/L of glucose. In third batch, accumulation of xylose was further enhanced to 56.3 g/L, along with 4.5 g/L of glucose. For pretreatment at 170 °C, the highest xylose accumulation was merely 40 g/L, while glucose level reached 5 g/L (Fig. 4a). Consequently, higher temperature and shorter time could not only result in higher xylose, but also reduce the hydrolysis of cellulose. The recycled pretreatment conditions were optimized as follows: 180 °C, 15 min, 1:7 solid–liquid ratio (w/w), three batches of recycled pretreatment, 0.6% H2SO4 (w/w, first batch)/0.9% H2SO4 (w/w, second and third batches).
Enzymatic hydrolysis of acid-pretreated corn stover
To reduce the waste water generated in washing Pre-CS, unwashed Pre-CS was also subjected to enzymatic hydrolysis and further butanol fermentation. Washed Pre-CS and Unwashed Pre-CS in different batches were hydrolyzed, and the hydrolysates were analyzed (Table 1). Similar glucose concentrations of 39.96 g/L and 38.76 g/L were achieved from washed and unwashed Pre-CS in third batch, respectively. However, total reducing sugar of unwashed Pre-CS is 15.65 g/L higher than that of the washed Pre-CS in third batch, mainly due to the xylose accumulation in unwashed pretreatment solution. The highest xylose titer of 15.04 g/L was obtained from unwashed Pre-CS. This indicates that the pretreatment solution has little inhibitory effect on enzymatic hydrolysis. In further study, the potential application of hydrolysates of unwashed Pre-CS was evaluated in butanol fermentation.
Butanol fermentation utilizing SDS-detoxified hydrolysates
As shown in Fig. 5, butanol fermentation was carried out with enzymatic hydrolysates of acid Pre-CS washed (AW) and acid Pre-CS unwashed (AUW). The fermentation was performed following previously reported SDS fermentation [7]. Briefly, SDS is a process of detoxification and sterilization of the mixture containing hydrolysate, detoxification agents (resin or/and activated carbon), CSL and mineral salts in one pot at 121 °C for 20 min. SDS could not only reduce the toxicity of hydrolysates, but also remove toxic compounds generated during sterilization of hydrolysates (Maillard reaction). Clostridium saccharobutylicum DSM 13864 was used for biobutanol production, which has been successfully applied in the utilization of cane molasses, hydrolysates of rice straw and corn stover for producing biofuels [7, 8, 11, 26, 38]. When undetoxified medium was applied, cell growth was completely inhibited and no butanol was produced. The highest butanol titer of 9.5 g/L was obtained when 1% (w/w) activated carbon was added. Butanol titers of 9.5 g/L and 8.7 g/L were achieved at 72 h of fermentation from AUW and AW, respectively. When 2% of activated carbon was added, butanol concentration was significantly decreased, which could be attributed to the nonspecific adsorption of both toxins and nutrients in the medium by activated carbon [48,49,50]. In addition, higher butanol concentration was achieved with AUW than that of AW. It is speculated that trace amount of furfural remained in AUW might promote the cell growth and butanol production. Similar phenomenon was observed in previous study on butanol fermentation using furfural residues hydrolysates [7]. Low concentration of furfural (< 0.1 g/L) did not inhibit the cell growth; on the contrary, it promoted the cell growth and butanol production of C. saccharobutylicum (data not shown). The same observations have also been reported with Clostridium acetobutylicum ATCC 824 [51] and Clostridium beijerinckii NCIMB 8052 [52]. Low concentration of furfural could induce the expression of aldo/keto reductase and short-chain dehydrogenase/reductase, which have been identified to be involved in furfural detoxification and tolerance. It is speculated that these enzymes could not only reduce furfural and HMF, but also promote the metabolic pathway of butanol production.
Butanol fermentation using corn stover hydrolysates processed by simultaneous detoxification and sterilization (SDS). : enzymatic hydrolysate of acid Pre-CS washed (AW);
: enzymatic hydrolysate of acid Pre-CS washed (AW);  : enzymatic hydrolysate of acid Pre-CS unwashed (AUW); C1 (
: enzymatic hydrolysate of acid Pre-CS unwashed (AUW); C1 ( ): separately sterilized glucose as control 1; C2 (
): separately sterilized glucose as control 1; C2 ( ): corn stover hydrolysate processed by detoxification with separate sterilization (DSS) as control 2 [6]. SDS and DSS butanol fermentation were conducted in 100-mL anaerobic bottle at 37 °C for 72 h. Fermentation medium: 55 g/L reducing sugar, 10 g/L CSL, 4 g/L CaCO3, 2 g/L (NH4)2SO4, 0.5 g/L K2HPO4, 0.01 g/L MnSO4·H2O
): corn stover hydrolysate processed by detoxification with separate sterilization (DSS) as control 2 [6]. SDS and DSS butanol fermentation were conducted in 100-mL anaerobic bottle at 37 °C for 72 h. Fermentation medium: 55 g/L reducing sugar, 10 g/L CSL, 4 g/L CaCO3, 2 g/L (NH4)2SO4, 0.5 g/L K2HPO4, 0.01 g/L MnSO4·H2O
Using this approach, 82.7 ± 2.3 g of xylose and 61.0 ± 7.1 g of butanol could be produced from one kilogram of corn stover. As substitutes for sulfuric acid, nitric acid and phosphoric acid could potentially be applied in pretreatment. Nitrate and phosphate, formed upon being neutralized, could be used as nitrogen and phosphorus source for plants and microorganisms [53, 54] .
Conclusions
Sulfuric acid pretreatment of corn stover was optimized, and pretreatment solution containing 56.3 g/L xylose was obtained after recycling of pretreatment solution for 3 times. Pre-CS residue was enzymatically hydrolyzed to obtain 35.7–39.9 g/L glucose and 4.3–5.5 g/L arabinose. The concentrated hydrolysates were used for butanol fermentation following a SDS process. The highest butanol titer (9.5 g/L) was obtained at 72 h from hydrolysates of unwashed corn stover with 1% (w/w) activated carbon. This study provides a practical pretreatment approach for the utilization of corn stover for producing xylose and biobutanol.
References
Qureshi N, Saha BC, Klasson KT, Liu SQ. Butanol production from sweet sorghum bagasse with high solids content: part I-comparison of liquid hot water pretreatment with dilute sulfuric acid. Biotechnol Prog. 2018;34(4):960–6. https://doi.org/10.1002/btpr.2639.
Li H, Fan HC, Li YR, Shi GY, Ding ZY, Gu ZH, Zhang L. Construction and application of multi-host integrative vector system for xylose-fermenting yeast. FEMS Yeast Res. 2017;17(6):fox055. https://doi.org/10.1093/femsyr/fox055.
Germec M, Turhan I. Ethanol production from acid-pretreated and detoxified rice straw as sole renewable resource. Biomass Convers Biorefin. 2018;8(3):607–19. https://doi.org/10.1007/s13399-018-0310-1.
Kim S, Dale BE. Global potential bioethanol production from wasted crops and crop residues. Biomass Bioenergy. 2004;26(4):361–75. https://doi.org/10.1016/j.biombioe.2003.08.002.
Ding JC, Xu GC, Han RZ, Ni Y. Biobutanol production from corn stover hydrolysate pretreated with recycled ionic liquid by Clostridium saccharobutylicum DSM 13864. Bioresour Technol. 2016;199:228–34. https://doi.org/10.1016/j.biortech.2015.07.119.
Limayem A, Ricke SC. Lignocellulosic biomass for bioethanol production: Current perspectives, potential issues and future prospects. Prog Energy Combust Sci. 2012;38(4):449–67. https://doi.org/10.1016/j.pecs.2012.03.002.
Dong JJ, Han RZ, Xu GC, Gong L, Xing WR, Ni Y. Detoxification of furfural residues hydrolysate for butanol fermentation by Clostridium saccharobutylicum DSM 13864. Bioresour Technol. 2018;259:40–5. https://doi.org/10.1016/j.biortech.2018.02.098.
Dong JJ, Ding JC, Zhang Y, Ma L, Xu GC, Han RZ, Ni Y. Simultaneous saccharification and fermentation of dilute alkaline-pretreated corn stover for enhanced butanol production by Clostridium saccharobutylicum DSM 13864. FEMS Microbiol Lett. 2016;363(4):fnw003. https://doi.org/10.1093/femsle/fnw003.
Zheng P, Fang L, Xu Y, Dong JJ, Ni Y, Sun ZH. Succinic acid production from corn stover by simultaneous saccharification and fermentation using Actinobacillus succinogenes. Bioresour Technol. 2010;101(20):7889–944. https://doi.org/10.1016/j.biortech.2010.05.016.
Hong E, Kim J, Rhie S, Ha SJ, Kim J, Ryu Y. Optimization of dilute sulfuric acid pretreatment of corn stover for enhanced xylose recovery and xylitol production. Biotechnol Bioprocess Eng. 2016;21(5):612–9. https://doi.org/10.1007/s12257-016-0483-z.
Xu GC, Ding JC, Han RZ, Dong JJ, Ni Y. Enhancing cellulose accessibility of corn stover by deep eutectic solvent pretreatment for butanol fermentation. Bioresour Technol. 2016;203:364–9. https://doi.org/10.1016/j.biortech.2015.11.002.
Schell DJ, Farmer J, Newman M, Mcmillan JD. Dilute-sulfuric acid pretreatment of corn stover in pilot-scale reactor. In: Davison BH, Lee JW, Finkelstein M, McMillan JD (eds) Biotechnology for fuels and chemicals. Appl Biochem Biotechnol. Totowa: Humana Press; 2003. pp. 69–85. https://doi.org/10.1007/978-1-4612-0057-4_6.
Huang XJ, Ding Y, Liao XL, Peng B, He YC, Ma CL. Microbial lipid production from enzymatic hydrolysate of corn stover pretreated by combining with biological pretreatment and alkalic salt soaking. Ind Crops Prod. 2018;124:487–94. https://doi.org/10.1016/j.indcrop.2018.08.030.
He YC, Liu F, Di JH, Ding Y, Zhu ZZ, Wu YQ, Chen L, Wang C, Xue YF, Chong GG, Ma CL. Effective enzymatic saccharification of dilute NaOH extraction of chestnut shell pretreated by acidified aqueous ethylene glycol media. Ind Crops Prod. 2016;81:129–38. https://doi.org/10.1016/j.indcrop.2015.11.079.
Pielhop T, Reinhard C, Hecht C, Del Bene L, Studer MH, von Rohr PR. Application potential of a carbocation scavenger in autohydrolysis and dilute acid pretreatment to overcome high softwood recalcitrance. Biomass Bioenergy. 2017;105:164–73. https://doi.org/10.1016/j.biombioe.2017.07.005.
Qing Q, Huang M, He Y, Wang L, Zhang Y. Dilute oxalic acid pretreatment for high total sugar recovery in pretreatment and subsequent enzymatic hydrolysis. Appl Biochem Biotechnol. 2015;177(7):1493–507. https://doi.org/10.1007/s12010-015-1829-2.
Xiao M, Wang L, Wu YD, Cheng C, Chen LJ, Chen HZ, Xue C. Hybrid dilute sulfuric acid and aqueous ammonia pretreatment for improving butanol production from corn stover with reduced wastewater generation. Bioresour Technol. 2019;278:460–3. https://doi.org/10.1016/j.biortech.2019.01.079.
Huang CX, Ma JM, Liang C, Li X, Yong Q. Influence of sulfur dioxide-ethanol-water pretreatment on the physicochemical properties and enzymatic digestibility of bamboo residues. Bioresour Technol. 2018;263:17–24. https://doi.org/10.1016/j.biortech.2018.04.104.
Pascal K, Ren HY, Sun FF, Guo SX, Hu JG, He J. Mild acid-catalyzed atmospheric glycerol organosolv pretreatment effectively improves enzymatic hydrolyzability of lignocellulosic biomass. Acs Omega. 2019;4(22):20015–23. https://doi.org/10.1021/acsomega.9b02993.
Correia JAD, Marques JE, Goncalves LRB, Rocha MVP. Alkaline hydrogen peroxide pretreatment of cashew apple bagasse for ethanol production: study of parameters. Bioresour Technol. 2013;139:249–56. https://doi.org/10.1016/j.biortech.2013.03.153.
Zhao C, Shao QJ, Ma ZQ, Li B, Zhao XJ. Physical and chemical characterizations of corn stalk resulting from hydrogen peroxide presoaking prior to ammonia fiber expansion pretreatment. Ind Crops Prod. 2016;83:86–93. https://doi.org/10.1016/j.indcrop.2015.12.018.
Tareen AK, Punsuvon V, Parakulsuksatid P. Investigation of alkaline hydrogen peroxide pretreatment to enhance enzymatic hydrolysis and phenolic compounds of oil palm trunk. 3 Biotech. 2020;10:179. https://doi.org/10.1007/s13205-020-02169-6.
Li HL, Xiong L, Chen XD, Luo MT, Chen XF, Wang C, Huang C, Chen XD. Enhanced enzymatic hydrolysis of wheat straw via a combination of alkaline hydrogen peroxide and lithium chloride/N,N-dimethylacetamide pretreatment. Ind Crops Prod. 2019;137:332–8. https://doi.org/10.1016/j.indcrop.2019.05.027.
He YC, Liu F, Gong L, Di JH, Ding Y, Ma CL, Zhang DP, Tao ZC, Wang C, Yang B. Enzymatic in situ saccharification of chestnut shell with high ionic liquid-tolerant cellulases from Galactomyces sp. CCZU11–1 in a biocompatible ionic liquid-cellulase media. Bioresour Technol. 2016;201:133–9. https://doi.org/10.1016/j.biortech.2015.11.034.
Nasirpour N, Mousavi SM. RSM based optimization of PEG assisted ionic liquid pretreatment of sugarcane bagasse for enhanced bioethanol production: Effect of process parameters. Biomass Bioenergy. 2018;116:89–988. https://doi.org/10.1016/j.biombioe.2018.06.008.
Xing WR, Xu GC, Dong JJ, Han RZ, Ni Y. Novel dihydrogen-bonding deep eutectic solvents: pretreatment of rice straw for butanol fermentation featuring enzyme recycling and high solvent yield. Chem Eng J. 2018;333:712–20. https://doi.org/10.1016/j.cej.2017.09.176.
Tan YT, Ngoh GC, Chua ASM. Evaluation of fractionation and delignification efficiencies of deep eutectic solvents on oil palm empty fruit bunch. Ind Crops Prod. 2018;123:271–7. https://doi.org/10.1016/j.indcrop.2018.06.091.
Avci A, Saha BC, Kennedy GJ, Cotta MA. Dilute sulfuric acid pretreatment of corn stover for enzymatic hydrolysis and efficient ethanol production by recombinant Escherichia coli FBR5 without detoxification. Bioresour Technol. 2013;142:312–9. https://doi.org/10.1016/j.biortech.2013.05.002.
Chung NH, Phuong NTM, Dien LQ, Khanh NV, Hue NT. Saccharification of Acacia mangium wood sawdust with dilute sulfuric acid for furfural production. Vietnam J Chem. 2019;57(6):753–7. https://doi.org/10.1002/vjch.2019000117.
Zhu J, Chen L, Gleisner R, Zhu J. Co-production of bioethanol and furfural from poplar wood via low temperature (≤ 90 °C) acid hydrotropic fractionation (AHF). Fuel. 2019;254:115572. https://doi.org/10.1016/j.fuel.2019.05.155.
Bamufleh HS, Alhamed YA, Daous MA. Furfural from midribs of date-palm trees by sulfuric acid hydrolysis. Ind Crops Prod. 2013;42:421–8. https://doi.org/10.1016/j.indcrop.2012.06.008.
Singh J, Suhag M, Dhaka A. Augmented digestion of lignocellulose by steam explosion, acid and alkaline pretreatment methods: a review. Carbohydr Polym. 2015;117:624–31. https://doi.org/10.1016/j.carbpol.2014.10.012.
Mikulski D, Klosowski G. Efficiency of dilute sulfuric acid pretreatment of distillery stillage in the production of cellulosic ethanol. Bioresour Technol. 2018;268:424–33. https://doi.org/10.1016/j.biortech.2018.08.005.
Rodrigues RCLB, Kenealy WR, Jeffries TW. Xylitol production from DEO hydrolysate of corn stover by Pichia stipitis YS-30. J Ind Microbiol Biotechnol. 2011;38(10):1649–55. https://doi.org/10.1007/s10295-011-0953-4.
Baptista SL, Cunha JT, Romaní A, Domingues L. Xylitol production from lignocellulosic whole slurry corn cob by engineered industrial Saccharomyces cerevisiae PE-2. Bioresour Technol. 2018;267:481–91. https://doi.org/10.1016/j.biortech.2018.07.068.
Yuan XS, Tu S, Lin JP, Yang LR, Shen HH, Wu MB. Combination of the CRP mutation and ptsG deletion in Escherichia coli to efficiently synthesize xylitol from corncob hydrolysates. Appl Microbiol Biotechnol. 2020;104:2039–50. https://doi.org/10.1007/s00253-019-10324-0.
Dong BY, Chen YF, Zhao CC, Zhang SJ, Guo XW, Xiao DG. Simultaneous determination of furfural, acetic acid, and 5-hydroxymethylfurfural in corncob hydrolysates using liquid chromatography with ultraviolet detection. J AOAC Int. 2013;96(6):1239–44. https://doi.org/10.5740/jaoacint.12-290.
Ni Y, Xia ZY, Wang Y, Sun ZH. Continuous butanol fermentation from inexpensive sugar-based feedstocks by Clostridium saccharobutylicum DSM 13864. Bioresour Technol. 2013;129:680–5. https://doi.org/10.1016/j.biortech.2012.11.142.
Saha BC, Bothast RJ. Pretreatment and enzymatic saccharification of corn fiber. Appl Biochem Biotechnol. 1999;76(2):65–77. https://doi.org/10.1385/ABAB:76:2:65.
Cao S, Pu Y, Studer M, Wyman C, Ragauskas AJ. Chemical transformations of Populus trichocarpa during dilute acid pretreatment. RSC Adv. 2012;2(29):10925–36. https://doi.org/10.1039/C2RA22045H.
Yee N, Fein JB, Daughney CJ. Experimental study of the pH, ionic strength, and reversibility behavior of bacteria–mineral adsorption. Geochim Cosmochim Acta. 2000;64(4):609–17. https://doi.org/10.1016/S0016-7037(99)00342-7.
Hayes DJ, Fitzpatrick S, Hayes MHB, Ross JRH. The biofine process—production of levulinic acid, furfural, and formic acid from lignocellulosic feedstocks. In: Kamm B, Gruber PR, Kamm M (eds) Biorefineries-industrial processes and products: status quo and future directions. New Jersey: Wiley-Blackwell; 2008. pp. 139–164. https://doi.org/10.1002/9783527619849.ch7.
Chen WH, Ye SC, Sheen HK. Hydrolysis characteristics of sugarcane bagasse pretreated by dilute acid solution in a microwave irradiation environment. Appl Energy. 2012;93:237–44. https://doi.org/10.1016/j.apenergy.2011.12.014.
Vazquez M, Oliva M, Tellez-Luis SJ, Ramirez JA. Hydrolysis of sorghum straw using phosphoric acid: evaluation of furfural production. Bioresour Technol. 2007;98(16):3053–60. https://doi.org/10.1016/j.biortech.2006.10.017.
Rong CG, Ding XF, Zhu YC, Li Y, Wang LL, Qu YN, Ma XY, Wang ZC. Production of furfural from xylose at atmospheric pressure by dilute sulfuric acid and inorganic salts. Carbohydr Res. 2012;350:77–80. https://doi.org/10.1016/j.carres.2011.11.023.
Rivera OMP, Moldes AB, Torrado AM, Dominguez JM. Lactic acid and biosurfactants production from hydrolyzed distilled grape marc. Process Biochem. 2007;42(6):1010–20. https://doi.org/10.1016/j.procbio.2007.03.011.
Montané D, Salvadó J, Torras C, Farriol X. High-temperature dilute-acid hydrolysis of olive stones for furfural production. Biomass Bioenergy. 2002;22(4):295–304. https://doi.org/10.1016/S0961-9534(02)00007-7.
Aktas Ö, Cecen F. Bioregeneration of activated carbon: a review. Int Biodeterior Biodegrad. 2007;59(4):257–72. https://doi.org/10.1016/j.ibiod.2007.01.003.
Cheldelin VH, Williams RJ. Adsorption of organic compounds. I. Adsorption of ampholytes on an activated charcoal. J Am Chem Soc. 1942;64(7):1513–6. https://doi.org/10.1021/ja01259a008.
Yenisoy-Karakaş S, Aygün A, Güneş M, Tahtasakal E. Physical and chemical characteristics of polymer-based spherical activated carbon and its ability to adsorb organics. Carbon. 2004;42(3):477–84. https://doi.org/10.1016/j.carbon.2003.11.019.
Zhang Y, Han B, Ezeji TC. Biotransformation of furfural and 5-hydroxymethyl furfural (HMF) by Clostridium acetobutylicum ATCC 824 during butanol fermentation. New Biotechnol. 2012;29(3):345–51. https://doi.org/10.1016/j.nbt.2011.09.001.
Zhang Y, Ezeji TC. Transcriptional analysis of Clostridium beijerinckii NCIMB 8052 to elucidate role of furfural stress during acetone butanol ethanol fermentation. Biotechnol Biofuels. 2013;6(1):66–66. https://doi.org/10.1186/1754-6834-6-66.
Kim I, Lee B, Park J, Choi S, Han J. Effect of nitric acid on pretreatment and fermentation for enhancing ethanol production of rice straw. Carbohyd Polym. 2014;99:563–7. https://doi.org/10.1016/j.carbpol.2013.08.092.
Wu W, Rondon V, Weeks K, Pullammanappallil P, Ingram LO, Shanmugam KT. Phosphoric acid based pretreatment of switchgrass and fermentation of entire slurry to ethanol using a simplified process. Bioresour Technol. 2018;251:171–80. https://doi.org/10.1016/j.biortech.2017.12.041.
Acknowledgements
This work was supported by National Natural Science Foundation of China (31601463), National Key R&D Program of China (2018YFA0901700), National First-class Discipline Program of Light Industry Technology and Engineering (LITE2018-07), Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX19_1834) and Top-notch Academic Programs Project of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Dong, JJ., Ma, BJ., Liu, YM. et al. Coproduction of xylose and biobutanol from corn stover via recycling of sulfuric acid pretreatment solution. Syst Microbiol and Biomanuf 1, 200–207 (2021). https://doi.org/10.1007/s43393-020-00014-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43393-020-00014-8