Abstract
Spinal scoliosis, a prevalent spinal deformity impacting both physical and mental well-being, has a significant genetic component, though the exact pathogenic mechanisms remain elusive. This review offers a comprehensive exploration of current research on embryonic spinal development, focusing on the genetic and biological intricacies governing axial elongation and straightening. Zebrafish, a vital model in developmental biology, takes a prominent role in understanding spinal scoliosis. Insights from zebrafish studies illustrate genetic and physiological aspects, including notochord development and cerebrospinal fluid dynamics, revealing the anomalies contributing to scoliosis. In this review, we acknowledge existing challenges, such as deciphering the unique dynamics of human spinal development, variations in physiological curvature, and disparities in cerebrospinal fluid circulation. Further, we emphasize the need for caution when extrapolating findings to humans and for future research to bridge current knowledge gaps. We hope that this review will be a beneficial frame of reference for the guidance of future studies on animal models and genetic research for spinal scoliosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spinal scoliosis is a common spinal deformity that hinders the physical and mental state of affected individuals. Research indicates a crucial role of genetic factors in the occurrence of spinal scoliosis. However, the precise mechanisms underlying its pathogenesis remain unclear. In this review, we discuss current studies on the embryonic development of the spine and factors contributing to deformities, elucidating the genetic and biological mechanisms mediating axial elongation and straightening during the developmental process.
Zebrafish, due to its particular morphology, structure and genetic characters, is a widely employed model for investigations in developmental biology, genetics, and clinical medicine for a variety of conditions such as spinal scoliosis. We summarize the latest findings from studies on spinal scoliosis, with a particular focus on zebrafish, exploring the genetic and physiological processes of notochord development and cerebrospinal fluid dynamics. In addition, we discuss how defects in these processes contribute to the development of spinal scoliosis. Moreover, we have highlighted aspects that remain unknown as well as the potential application of novel animal models and genetic research that should be considered to guide future studies in understanding the pathogenesis of spinal scoliosis.
Development of the spine (extension, straightening)
The developmental process of the spine is crucial for the normal growth of vertebrates. The notochord, originating from the mesoderm, exhibits a rod-like structural organization [1, 2]. During embryonic development, the notochord demonstrates diverse differentiation capabilities, giving rise to vital structures such as the neural tube, lungs, liver, pancreas, and gastrointestinal tract [3, 4]. Serving as a transient structure, the notochord gradually regresses and eventually disappears after embryonic development concludes [5].
During embryonic development, the vertebrate spine undergoes two key processes facilitating its elongation and straightening. First, notochordal cells undergo vacuolization. These cells, with a characteristic diameter of up to 50 μm, undergo continuous expansion, forming a bundle-like arrangement with fluid-filled interiors [6, 7]. The enlargement of these cell volumes establishes a statically stable framework capable of resisting mechanical stress, simultaneously maintaining the embryonic axis, and enabling the symmetric development of surrounding bone tissues [8, 9]. Moreover, these vacuolized cells undergo cavitation that divides the notochord into distinct functional differentiation units. Simultaneously, the surrounding sheath cell epithelium provides developmental guidance by inducing the migration of bone segments in the somite toward the midline and dorsal side. Ultimately, vertebral bodies and intervertebral fibrous rings form while muscle segments gradually construct the trunk muscles [10,11,12]. These two processes function in balance as some vacuolized notochordal cells accumulate below the ossification region and others fragment within the intervertebral disc tissue to form the nucleus pulpous [7, 13, 14].
In summary, the extension and straightening of the vertebrate embryonic axis involves interference with vacuolized notochordal cells and the sheath cell epithelium surrounding the notochord. Disturbances such as impaired vacuolization of notochordal cells, inadequate expansion of vacuolized cells, and fragmentation before maturation can lead to developmental abnormalities in the spine [8, 15, 16].
In addition, variations or deficiencies in the sheath cell epithelium and poor collagen secretion may render the notochordal sheath unable to effectively resist internal pressure, causing overexpression of ossification cells in certain regions, resulting in insufficient cartilage deposition, and ultimately triggering developmental abnormalities [17,18,19].
Spinal deformities in humans related to genetics typically include congenital scoliosis (CS), idiopathic scoliosis (IS), and neuromuscular or acquired scoliosis. Congenital scoliosis results from vertebral and rib malformations, occurring in approximately one in 1000 individuals [15]. Clinical and animal studies have shown that congenital scoliosis manifests as faulty vertebral segmentation, including hemivertebrae and wedge-shaped vertebrae [20, 21]. Current research indicates a strong correlation between congenital scoliosis and gene–environment interactions, with disruptions in the NOTCH signaling pathway playing a major role in vertebral segmentation loss under environmental stress [20, 22, 23].
In contrast, idiopathic scoliosis clinically presents as a three-dimensional rotational curvature of the spine without vertebral malformations, neurological-muscular abnormalities, or functional impairments. It affects about 4% of the population, representing over 80% of scoliosis cases, and is the most common spinal disorder in children and adolescents [24]. The term “idiopathic” signifies that the cause of this condition is not yet clear [25]. Several hypotheses related to the pathogenesis of idiopathic scoliosis have been proposed, including abnormalities in central nervous system development, abnormal spinal bone growth, bone metabolism, biomechanical environmental issues and hormonal imbalances [26,27,28,29,30,31]. Moreover, genetic factors play a crucial role as the probability of idiopathic scoliosis development is significantly higher in monozygotic twins compared to dizygotic twins (70% vs. 36%) [32]. In addition, there are genetic associations between congenital and idiopathic scoliosis [33,34,35].
To study human spinal deformities, researchers have conducted extensive studies by establishing animal models of spinal deformities. Early research generally focused on natural observation of small animals, such as the progression of thoracic scoliosis in 5–6-week-old chicks. It was found that the imbalance of paraspinal muscle development causes scoliosis. More studies have shown that differential expression of collagen is an important factor in causing scoliosis [36]. In addition, researchers selectively ablated the intercostal arteries of the thoracic vertebrae at four levels in rabbits through thoracotomy to create a rabbit model of scoliosis (Fig. 1). During the experiment, it was observed that some animals developed scoliosis after selective intercostal artery ablation. Histological examination revealed changes in the vertebral epiphyseal plate and the spinal cord, affecting the proprioceptive function of paraspinal muscles in tone control [37].
A series of studies have shown that melatonin plays a key role in the occurrence and development of scoliosis. Thus, a type of research in animal models uses pinealectomy (PINX) to create animal models of scoliosis (Fig. 2).
PINX chicken model
In 1959, Thillard completed the first pineal gland excision (PINX) model. Postoperative spinal curvature was observed in 65% of pinealectomized chicken [38]. Until 1983, Machida and Duboussett extended this idiopathic scoliosis model through morphological correlation mapping [39]. It has been confirmed that the neurotransmitter or neurohormone system in the pineal gland is the main factor contributing to this experimental type of scoliosis.
To prove the hypothesis that melatonin deficiency is related to spinal deformity, Machida et al. concluded that melatonin metabolism plays an important role in the occurrence and development of scoliosis, with the total incidence rate of scoliosis in PINX chickens ranging from 80 to 100% [39,40,41,42,43,44,45]. However, other researchers have reported significant differences in scoliosis incidence [46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65]. Bagnall et al. used 105 newly hatched Shan Hubbard chickens. After 5 weeks of PINX melatonin treatment, over 50% of the chickens still suffered from scoliosis. Therefore, the use of melatonin alone cannot effectively reduce the incidence of scoliosis in PINX chickens.
PINX fish (Atlantic salmon) model
To exclude the effects of body posture and gravity on spinal development and further demonstrate how melatonin deficiency alone promotes scoliosis, researchers evaluated the spinal development of PINX Atlantic salmon [66]. The spinal movement of salmon is mainly limited to lateral bending. The results of this study showed that 82% of pinealectomized fish exhibited abnormal spinal curvature. In addition, the calcium, phosphorus, and total mineral content in the vertebrae of PINX salmon were significantly reduced. It was concluded that melatonin may play a crucial role in spinal bone growth, bone mineralization, and the development of scoliosis.
Congenital melatonin deficiency rodent (mouse) model and PINX bipedal rodent (rat) model
To further demonstrate the relationship between melatonin and induced scoliosis, researchers established a mouse model of AA-NAT gene knock-out strain (C57BL/6 J) with congenital melatonin deficiency. Many studies have found that the bone density (BMD) of C57BL/6 J mice is significantly lower, indicating that this model may be ideal for evaluating the effects of melatonin on bones [67,68,69,70,71,72]. Machida et al. used PINX bipedal C57BL/6 J mice to evaluate the development of scoliosis. The results showed that the spinal deformity rate of bipedal mice with melatonin deficiency was 25%. They believe that treatment with exogenous melatonin can prevent the development of scoliosis in both models. The same team achieved a higher induction rate of scoliosis by amputating the forelimbs of C57BL/6 J mice without PINX. The incidence of scoliosis in mice with simple forelimb amputation is twofold lower than in bipedal C57BL/6 J mice. However, when PINX was administered in melatonin-rich C3H/HeJ mice, the incidence of scoliosis increased to 70%. This result is consistent with early reports that spinal deformities are more likely to occur in the presence of both melatonin deficiency and bipedal posture.
Many researchers have conducted similar studies in rats. O’Kelly et al. conducted PINX in rats to evaluate the incidence of scoliosis [54]. Compared with chicken models, PINX alone cannot induce spinal curvature in any rodent model [39, 40, 42, 44]. Rodents that walk on four legs are different from vertebrates that walk on two legs and are not affected by the crucial dynamic mechanical force of posture leading to the development of scoliosis. Therefore, researchers developed a bipedal rat model (with two forelimbs and tails removed from 3-week-old rats, food and water placed at a high position to gradually enhance the upright posture). The conclusion is that in the melatonin deficiency model after pinealectomy, any interference with balance and other postural mechanisms, especially bipedal posture, may promote the development of spinal deformities.
Non-human primate PINX (rhesus monkey) model
Due to the success of these experiments, researchers began to develop interest in non-human primates. In 2005, Cheung et al. conducted PINX in bipedal non-human primates [73]. Among 18 rhesus monkeys with PINX, 10 showed a significant decrease in melatonin secretion, but none of them showed the expected scoliosis after 29 months of postoperative follow-up. The limitation of this study is that monkeys are unable to move freely or stand upright in cages, which limits their natural posture [74]. Therefore, the spine is not affected by gravity, which is a crucial factor leading to the development of scoliosis. These findings further emphasize the importance of posture and posture-related mechanical forces, not just melatonin deficiency, which may be crucial for the development of scoliosis.
Recent studies have discovered spontaneous spinal deformities in wild populations of teleost fish, particularly zebrafish [75, 76]. Zebrafish are becoming increasingly popular among researchers for several reasons: (1) strong reproductive ability and large numbers; (2) similar spinal morphology and structure to humans, with many highly conserved genetic genes; (3) mechanical conditions of the spine during swimming in teleost fish are similar to human bipedalism; (4) transparent zebrafish embryos facilitate observation during external development; (5) comprehensive knowledge of the zebrafish genome allows for high-throughput bidirectional genetic regulation; and (6) the convenient size of the body for Micro-CT and scanning electron microscope analysis [77,78,79,80,81,82,83,84,85,86]. As a result, studies have been gravitating toward the use of zebrafish to establish models as well as to perform gene editing and sequencing to understand the pathogenesis of spinal deformities.
Zebrafish model of scoliosis and related genes
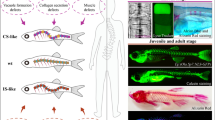
Recent years, many researchers have intervened in the embryonic and juvenile development of zebrafish specimens through gene knock-in or knock-out methods, successfully producing zebrafish variants with spinal deformity (Fig. 3).
The establishment of a model of congenital scoliosis is generally achieved through gene editing and interference with the development of early embryonic notochord structures. It is reported that regulation of the activity of bisserine/threonine and tyrosine protein kinase (Dstyk) to reduce the number of vacuolized cells, shorten the embryonic axis, and thus affect spinal cord development, ultimately leading to late onset spinal deformities due to vertebral growth defects [87, 88]. Other ones overexpressed the pkd1a/b and pkd2 genes by upregulating them, leading to an abnormal increase in collagen in the epithelium of sheath cells of the notochord, resulting in excessive curvature of the dorsal side of the notochord and the formation of spinal deformities [89, 90]. The Leviathan variant is one of the more distinctive types. Mutations in the col8a1a gene result in the loss of type VIII collagen in the extracellular matrix of notochord cells, further affecting the position of osteoblasts and leading to vertebral defects in later development, resulting in spinal deformities [91]. In addition to interfering with the notochord, some zebrafish variants that interfere with the development and segmentation of somites to create congenital spinal deformities have also been reported. Mutations in genes such as mine clock gene, myosin heavy chain, tbx6, her1−/−, her7−/− can all lead to developmental disorders of early embryonic somites, resulting in spinal deformities [92, 93].
Compared with congenital spinal deformities, idiopathic spinal deformities have more complex causes and a lack of clear etiology. At present, most of the reported interference is achieved through the function of specific structures during late embryonic development and juvenile development. The maturation, activity, and function of motile cilia are one of the main targets for the preparation of idiopathic scoliosis models. By editing genes such as ccdc151, ccdc40, dnaaf4, dnah10, and cfap298, the activity of motile cilia is interfered with, causing zebrafish to gradually form idiopathic scoliosis during juvenile development [94, 95]. Regarding the biogenesis of motile cilia, some scholars have used genetic variations such as kif6, kif7, and armc9 to interfere and obtain variants of zebrafish with idiopathic spinal deformities [96,97,98]. Meanwhile, some reports have edited genes such as sspo, uts2r3, and mapk7 to interfere with Reissner fiber, vasopressin receptor, and bone formation processes, thereby inducing zebrafish specimens to develop into idiopathic scoliosis variants [99,100,101,102].
Although zebrafish are similar to humans in terms of spinal morphology and development, they also have differences. However, due to their outstanding advantages as experimental specimens and the rapid development of gene editing and sequencing technology, zebrafish provides us with new perspectives and favorable tools for in-depth biological research on scoliosis.
The mechanical stress of the spine and the Piezo channel
After millions of years of evolution, humans have become bipedal vertebrates capable of upright walking, where the mechanical stress of gravity on the spine plays a crucial role. To maintain balance and achieve optimal range of motion in an upright posture, humans have evolved specific sagittal curvature of the spine and pelvis [103]. Importantly, it is the zebrafish that experience the same level of pressure exerted on the spine during swimming that recapitulate the effects of gravity on the human spine [76]. Recent studies have proposed sensory pathways capable of detecting mechanical stress and have identified the associated expression genes (Fig. 3). The Piezo pathway is widely present in the cell membranes of various human tissues, providing feedback for the conduction of mechanical stress. The Piezo genes include Piezo1 and Piezo2, with Piezo2 further divided into Piezo2a and Piezo2b [104]. Notably, Piezo1 gene is highly expressed in mesenchymal stem cells, osteoblasts, chondrocytes, and intervertebral disc nucleus pulposus [105]. Mutations or low expression of the Piezo1 gene can lead to reduced gene expression in chondrocytes and osteoblasts, resulting in wedge-shaped deformities of the vertebral bodies and thoracolumbar scoliosis [106,107,108]. Piezo2 gene plays a crucial role in proprioception and is mainly expressed in muscle spindles and tendon spindles. Mutations in this gene can cause joint deformities, vertebral fusion, spinal curvature, and hip joint malformation [109, 110]. When selectively knocking out the Piezo1 and Piezo2a genes in zebrafish, a model of spinal curvature is achievable in mature zebrafish [111]. The genes and pathways present in both the human body and teleost make the spinal deformity model more clinically meaningful. At the same time, the stage of action of this gene is more focused on the development direction of embryonic bone and cartilage, which is closer to the current understanding of the occurrence and development of spinal deformities. This achievement forms a robust foundation for future research in understanding the intricacies of spinal deformities.
Cerebrospinal fluid circulation stability, motile cilia, Reissner fiber, and tactile neurons
The stability of cerebrospinal fluid (CSF) circulation is paramount for the homeostasis of the central nervous system [112]. In vertebrates, CSF secreted by the choroid plexus of the brain ventricles serves not only as a medium for nutrient transport and metabolic waste removal but also as a source of various signaling molecules [113]. Disruption of normal CSF circulation can lead to severe neurological disorders [114]. In zebrafish larvae, motile cilia are widely distributed in various tissues, including liver macrophages, olfactory cells, pronephric ducts, choroid plexus epithelium, and sperm [115, 116]. The rhythmic beating of motile cilia on the surface of the choroid plexus epithelium ensures the stable circulation of CSF in the brain ventricles and the central canal of the spinal cord [117,118,119]. Therefore, studies have found a close association between choroid plexus epithelial cilia dysfunction, reduced CSF flow, increased neuroinflammation, and spinal deformities in genetically edited zebrafish, suggesting that treatments or interventions targeting related factors can improve spinal deformities. However, such mutants often struggle to survive to adulthood which makes it a difficult model to study in the long term [120, 121].
In contrast, the circulation of CSF in humans primarily relies on arterial pulsation and respiratory movements. In individuals with primary ciliary dyskinesia, a condition where cilia are immotile, hydrocephalus is rarely observed, indicating that the function of motile cilia within the human spinal cord is not yet well understood. Further research is needed to comprehend the role of motile cilia in the development and homeostasis of the human ventricles [122,123,124].
Reissner fiber, initially described by Reissner in 1860 within the body of hagfish [125], is a highly conserved structure found widely in the spinal cords of vertebrates [126,127,128,129,130]. Although Reissner fiber was first observed in the human spinal cord in 1922 by Agduhr [131], subsequent studies using antibodies against glycoproteins specific to the connecting filum or Reissner fiber, derived from human or chimpanzee sources, failed to produce immune reactions [132,133,134]. As an extracellular glycoprotein, Reissner fiber extends linearly from the brain along the central canal of the spinal cord to its base [135]. In zebrafish, Reissner fiber aggregates in the cerebrospinal fluid within the brain ventricle through the secretion of the SCO-Spodin protein by the connecting filum, floating and traversing the central axis of the spinal cord [128]. Current research suggests that the absence of Reissner fiber does not affect ciliary movement and CSF flow, but the rhythmic beating of cilia plays a crucial role in the aggregation of Reissner fiber [128, 135]. However, the function of Reissner fiber remains unclear.
Tactile neurons (CSF-cNs) are GABAergic sensory neurons widely distributed on the ventral and dorsal sides of the central canal of the spinal cords in vertebrates [136]. Over a century ago, it was hypothesized that the connecting filum interacts with Reissner fiber to form a “sagittal position organ” with a mechanical sensory system that maintains the stability of the body axis [137]. Recent studies using zebrafish experiments have found direct contact between tactile neurons and Reissner fiber [138]. The absence of Reissner fiber renders tactile neurons incapable of responding to mechanical stimuli such as axial bending, indicating that the function of Reissner fiber is like that of a physical rope along the central axis, aiding tactile neurons in sensing changes in the body axis. Studies have conducted in-depth molecular studies on tactile neurons, indicating that the vasopressin neuropeptide expressed by tactile neurons, when activated by stimuli, causes the adjacent muscles to contract, maintaining the body axis and reducing bending. Abnormalities in the vasopressin signaling pathway can cause Tactile nerves (CSF-cNs) to be unable to sense the mechanical stress changes that occur in the Reissner fiber during trunk bending, making it difficult to adjust and control the axial development of the embryonic spine in time, ultimately resulting in spinal deformities [139,140,141].
Considerations
Despite the significant contributions of numerous studies towards a deeper understanding of embryonic axial elongation, straightening, spinal development, and the molecular biology mechanisms underlying deformities, several critical considerations must be acknowledged:
-
1)
The uniqueness of human spinal development: while extensive research has shed light on the molecular intricacies of axial elongation and spinal development in various vertebrates, the unique characteristics of human spinal development remain incompletely understood. As a bipedal species with distinct vertebral dynamics, the developmental processes in humans differ from those observed in other vertebrates [142, 143].
-
2)
Physiological curvature discrepancies: the presence of natural physiological curvatures in the human spine (cervical lordosis, thoracic kyphosis, lumbar lordosis) contrasts with the generally straight spine observed in our closest relatives, such as chimpanzees [144].
-
3)
Existing research results may not fully explain the mechanisms underlying the developmental changes in the human spine from embryonic to adult stages, including elongation, straightening, and physiological curvatures.
-
4)
Cerebrospinal fluid circulation disparities: unlike experimental animals commonly used in research, human cerebrospinal fluid circulation relies significantly on arterial pulsation and respiratory movements. Consequently, the functional implications and potential effects highlighted by studies on motile cilia may not necessarily translate directly to spinal development and deformities in humans [133, 134].
-
5)
Absence of tactile neurons and Reissner fiber in humans: the absence of conclusive evidence regarding tactile neurons and Reissner fiber (RF) in the human spinal cord raises serious doubts about the direct applicability of findings related to the interactions between motile cilia, tactile neurons, and RF observed in experimental animals [131,132,133].
In light of these considerations, it is imperative to approach the interpretation of research outcomes with caution, recognizing the unique aspects of human spinal development and the limitations in replicating experimental results directly to the human context. Future investigations are needed to address these specificities to advance our comprehension of the complexities associated with human spinal development and deformities.
Summary and future perspective
Critical role of the notochord in spinal development
The notochord emerges as a pivotal factor in the process of spinal development, playing essential roles in providing templates, organizing structures, and facilitating arrangements. The expansion, accumulation, and arrangement of notochordal vacuolated cells contribute significantly to the elongation of the spinal cord, maintaining axial alignment. However, the notochord’s involvement extends beyond spinal development, influencing the embryonic development of various vital organs. Precision and selectivity in intervening with upstream genes are crucial aspects to be addressed in future research, considering the potential impact on the overall development of embryos. Challenges arise, as interference may compromise the viability of experimental specimens up to maturity.
Distinctive dynamics of cerebrospinal fluid circulation in humans
The unique modality of cerebrospinal fluid (CSF) circulation in humans sets it apart from other species. The mechanism underlying the role of motile cilia on the ependymal cells of the spinal canal’s membrane warrants further exploration. The distinctive nature of CSF circulation in humans demands an in-depth investigation into the functioning of motile cilia on the ependymal cells of the spinal canal’s membrane.
Absence of evidence for Reissner fiber and tactile neurons in the human spinal cord
Currently, there is an absence of conclusive evidence supporting the existence of Reissner fiber and tactile neurons within the central canal of the human spinal cord. Consequently, caution must be exercised in extrapolating findings from animal experiments and genetic studies to the human context. As bipedal organisms, humans exhibit variations in spinal morphology throughout different growth stages. Understanding whether these variations correlate with microscopic structural changes in the spinal cord during fetal development, birth, and the transition to upright ambulation in adolescence is imperative. This evolution in spinal morphology in humans is likely influenced by a combination of external and intrinsic factors.
In summary, the intricacies of spinal development and related phenomena necessitate ongoing research that delves into the molecular and biomechanical intricacies. Rigorous exploration of these aspects will contribute not only to expanding our fundamental understanding of spinal deformities but also to the potential development of targeted interventions and therapeutic strategies in the future.
Data availability
Data availability is not applicable to this article as no new data were created or analyzed in this study.
References
Choi KS, Cohn MJ, Harfe BD (2008) Identification of nucleus pulposus precursor cells and notochordal remnants in the mouse: implications for disk degeneration and chordoma formation. Dev Dyn 237:3953–3958. https://doi.org/10.1002/dvdy.21805
Choi K-S, Harfe BD (2011) Hedgehog signaling is required for formation of the notochord sheath and patterning of nuclei pulposi within the intervertebral discs. Proc Natl Acad Sci USA 108:9484–9489. https://doi.org/10.1073/pnas.1007566108
Lumsden A, Graham A (1995) Neural patterning: a forward role for hedgehog. Curr Biol 5:1347–1350. https://doi.org/10.1016/S0960-9822(95)00266-1
Cleaver O, Krieg PA (2001) Notochord patterning of the endoderm. Dev Biol 234:1–12. https://doi.org/10.1006/dbio.2001.0214
Haga Y, Dominique VJ 3rd, Du SJ (2009) Analyzing notochord segmentation and intervertebral disc formation using the twhh:gfp transgenic zebrafish model. Transgenic Res 18:669–683. https://doi.org/10.1007/s11248-009-9259-y
Ellis K, Bagwell J, Bagnat M (2013) Notochord vacuoles are lysosomerelated organelles that function in axis and spine morphogenesis. J Cell Biol 200:667–679. https://doi.org/10.1083/jcb.201212095
Norman J, Sorrell EL, Hu Y, Siripurapu V, Garcia J, Bagwell J, Charbonneau P, Lubkin SR, Bagnat M (2018) Tissue self-organization underlies morphogenesis of the notochord. Philos Trans R Soc Lond B Biol Sci 373:20170320. https://doi.org/10.1098/rstb.2017.0320
Bagwell J, Norman J, Ellis KL, Peskin B, Hwang J, Ge X, Nguyen S, McMenamin SK, Stainier DY, Bagnat M (2020) Notochord vacuoles absorb compressive bone growth during zebrafish spine formation. eLife 9:e51221. https://doi.org/10.7554/eLife.51221
Bhattachan P, Rae J, Yu H, Jung W, Wei J, Parton RG, Dong B (2020) Ascidian caveolin induces membrane curvature and protects tissue integrity and morphology during embryogenesis. FASEB J 34:1345–1361. https://doi.org/10.1096/fj.201901281R
Wopat S, Bagwell J, Sumigray KD, Dickson AL, Huitema LFA, Poss KD, Schulte-Merker S, Bagnat M (2018) Spine patterning is guided by segmentation of the notochord sheath. Cell Rep 22:2026–2038. https://doi.org/10.1016/j.celrep.2018.01.084
Eckalbar WL, Fisher RE, Rawls A, Kusumi K (2012) Scoliosis and segmentation defects of the vertebrae. Wiley Interdiscip Rev Dev Biol 1:401–423. https://doi.org/10.1002/wdev.34
Peskin B, Henke K, Cumplido N, Treaster S, Harris MP, Bagnat M, Arratia G (2020) Notochordal signals establish phylogenetic Identity of the teleost spine. Curr Biol 30(P2805–2814):E3. https://doi.org/10.1016/j.cub.2020.05.037
Yamamoto M, Morita R, Mizoguchi T, Matsuo H, Isoda M, Ishitani T, Chitnis AB, Matsumoto K, Crump JG, Hozumi K et al (2010) Mib-Jag1-Notch signalling regulates patterning and structural roles of the notochord by controlling cell-fate decisions. Development 137:2527–2537. https://doi.org/10.1242/dev.051011
Dale RM, Topczewski J (2011) Identification of an evolutionarily conserved regulatory element of the zebrafish col2a1a gene. Dev Biol 357:518–531. https://doi.org/10.1016/j.ydbio.2011.06.020
Garcia J, Bagwell J, Njaine B, Norman J, Levic DS, Wopat S, Miller SE, Liu X, Locasale JW, Stainier DYR et al (2017) Sheath cell invasion and trans-differentiation repair mechanical damage caused by loss of caveolae in the zebrafish notochord. Curr Biol 27:1982-1989.e1983. https://doi.org/10.1016/j.cub.2017.05.035
Lim YW, Lo HP, Ferguson C, Martel N, Giacomotto J, Gomez GA, Yap AS, Hall TE, Parton RG (2017) Caveolae protect notochord cells against catastrophic mechanical failure during development. Curr Biol 27:1968-1981.e1967. https://doi.org/10.1016/j.cub.2017.05.067
Lleras Forero L, Narayanan R, Huitema LF, VanBergen M, Apschner A, Peterson-Maduro J, Logister I, Valentin G, Morelli LG, Oates AC et al (2018) Segmentation of the zebrafish axial skeleton relies on notochord sheath cells and not on the segmentation clock. eLife 7:e33843. https://doi.org/10.7554/eLife.33843
Wopat S, Bagwell J, Sumigray KD, Dickson AL, Huitema LFA, Poss KD, Schulte-Merker S, Bagnat M (2018) Spine patterning is guided by segmentation of the notochord sheath. Cell Rep 22:2026–2038. https://doi.org/10.1016/j.celrep.2018.01.084
Pogoda HM, Riedl-Quinkertz I, Lohr H, Waxman JS, Dale RM, Topczewski J, Schulte-Merker S, Hammerschmidt M (2018) Direct activation of chordoblasts by retinoic acid is required for segmented centra mineralization during zebrafish spine development. Development. 145:dev159418. https://doi.org/10.1242/dev.159418
Giampietro PF, Dunwoodie SL, Kusumi K, Pourquie O, Tassy O, Offiah AC, Cornier AS, Alman BA, Blank RD, Raggio CL, Glurich I, Turnpenny PD (2009) Progress in the understanding of the genetic etiology of vertebral segmentation disorders in humans. Ann NY Acad Sci 1151:38–67. https://doi.org/10.1111/j.1749-6632.2008.03452.x
Giampietro PF, Raggio CL, Blank RD, McCarty C, Broeckel U, Pickart MA (2013) Clinical, genetic and environmental factors associated with congenital vertebral malformations. Mol Syndromol 4:94–105. https://doi.org/10.1159/000345329
Venzin OF, Oates AC (2020) What are you synching about? Emerging complexity of notch signaling in the segmentation clock. Dev Biol 460:40–54. https://doi.org/10.1016/j.ydbio.2019.06.024
Sparrow DB, Chapman G, Smith AJ, Mattar MZ, Major JA, O’Reilly VC, Saga Y, Zackai EH, Dormans JP, Alman BA et al (2012) A mechanism for gene-environment interaction in the etiology of congenital scoliosis. Cell 149:295–306. https://doi.org/10.1016/j.cell.2012.02.054
Cheng JC, Castelein RM, Chu WC, Danielsson AJ, Dobbs MB, Grivas TB, Gurnett CA, Luk KD, Moreau A, Newton PO, Stokes IA, Weinstein SL, Burwell RG (2015) Adolescent idiopathic scoliosis. Nat Rev Dis Primers 1:15030. https://doi.org/10.1038/nrdp.2015.30
Hresko MT (2013) Clinical practice. Idiopathic scoliosis in adolescents. N Engl J Med 368:834–841. https://doi.org/10.1056/NEJMcp1209063
Sarwark JF, Castelein RM, Maqsood A, Aubin CE (2019) The biomechanics of induction in adolescent idiopathic scoliosis: theoretical factors. J Bone Joint Surg Am 101:e22. https://doi.org/10.2106/JBJS.18.00846
Wang WJ, Yeung HY, Chu WC, Tang NL, Lee KM, Qiu Y, Burwell RG, Cheng JC (2011) Top theories for the etiopathogenesis of adolescent idiopathic scoliosis. J Pediatr Orthop 31:S14-27. https://doi.org/10.1097/BPO.0b013e3181f73c12
Cheng JC, Tang SP, Guo X, Chan CW, Qin L (2001) Osteopeniain adolescent idiopathic scoliosis: a histomorphometric study. Spine 26:E19-23. https://doi.org/10.1097/00007632-200102010-00002
Diarbakerli E, Savvides P, Wihlborg A, Abbott A, Bergstrom I, Gerdhem P (2020) Bone health in adolescents with idiopathic scoliosis. Bone Joint J 102-B:268–272. https://doi.org/10.1302/0301-620X.102B2.BJJ-2019-1016.R1
Xie N, Li M, Wu T, Liu J, Wang B, Tang F (2015) Does elevated osteopontin level play an important role in the development of scoliosis in bipedal mice? Spine J 15:1660–1664. https://doi.org/10.1016/j.spinee.2015.03.014
Kulis A, Gozdzialska A, Drag J, Jaskiewicz J, Knapik-Czajka M, Lipik E, Zarzycki D (2015) Participation of sex hormones in multifactorial pathogenesis of adolescent idiopathic scoliosis. Int Orthop 39:1227–1236. https://doi.org/10.1007/s00264-015-2742-6
Kesling KL, Reinker KA (1997) Scoliosis in twins. A metaanalysis of the literature and report of six cases. Spine 22:2009–2014. https://doi.org/10.1097/00007632-199709010-00014
Purkiss SB, Driscoll B, Cole WG, Alman B (2002) Idiopathic scoliosis in families of children with congenital scoliosis. Clin Orthop Relat Res. https://doi.org/10.1097/00003086-200208000-00005
Hayes M, Gao X, Yu LX, Paria N, Henkelman RM, Wise CA, Ciruna B (2014) ptk7 mutant zebrafish models of congenital and idiopathic scoliosis implicate dysregulated Wnt signalling in disease. Nat Commun 5:4777. https://doi.org/10.1038/ncomms5777
HubaudPourquié AO (2014) Signalling dynamics in vertebrate segmentation. Nat Rev Mol Cell Biol 15:709–721. https://doi.org/10.1038/nrm3891
Riggins RS, Abbott UK, Ashmore CR, Rucker RB, McCarrey JR (1977) Scoliosis in chickens. J Bone Joint Surg Am 59(8):1020–1026
De Salis J, Beguiristain JL, Cañadell J (1980) The production of experimental scoliosis by selective arterial ablation. Int Orthop 3(4):311–315. https://doi.org/10.1007/BF00266027
Thillard MJ (1959) Vertebral column deformities following epiphysectomy in the chick. C R Hebd Seances Acad Sci 248:1238–1240
Machida M, Dubousset J, Imamura Y, Iwaya T, Yamada T, Kimura J (1993) An experimental study in chickens for the pathogenesis of idiopathic scoliosis. Spine 18:1609–1615. https://doi.org/10.1097/00007632-199309000-00007
Machida M, Dubousset J, Imamura Y, Iwaya T, Yamada T, Kimura J (1995) Role of melatonin deficiency in the development of scoliosis in pinealectomised chickens. J Bone Jt Surg Br 77:134–138
Kono H, Machida M, Saito M, Nishiwaki Y, Kato H, Hosogane N, Chiba K, Miyamoto T, Matsumoto M, Toyama Y (2011) Mechanism of osteoporosis in adolescent idiopathic scoliosis: experimental scoliosis in pinealectomized chickens. J Pineal Res 51:387–393. https://doi.org/10.1111/j.1600-079X.2011.00901.x
Machida M, Dubousset J, Imamura Y, Iwaya T, Yamada T, Kimura J, Toriyama S (1994) Pathogenesis of idiopathic scoliosis: SEPs in chicken with experimentally induced scoliosis and in patients with idiopathic scoliosis. J Pediatr Orthop 14:329–335. https://doi.org/10.1097/01241398-199405000-00010
Machida M, Dubousset J, Satoh T, Murai I, Wood KB, Yamada T, Ryu J (2001) Pathologic mechanism of experimental scoliosis in pinealectomized chickens. Spine 26:E385–E391. https://doi.org/10.1097/00007632-200109010-00006
Machida M, Miyashita Y, Murai I, Dubousset J, Yamada T, Kimura J (1997) Role of serotonin for scoliotic deformity in pinealectomized chicken. Spine 22:1297–1301. https://doi.org/10.1097/00007632-199706150-00004
Machida M, Yamada H, Yamada T, Kimura J, Saito M, Shibasaki K (2005) Rib length in experimental scoliosis induced by pinealectomy in chickens. Spine 30:E692–E696. https://doi.org/10.1097/01.brs.0000187874.38074.77
Kanemura T, Kawakami N, Deguchi M, Mimatsu K, Iwata H (1997) Natural course of experimental scoliosis in pinealectomized chickens. Spine 22:1563–1567. https://doi.org/10.1097/00007632-199707150-00006
Turgut M, Basaloglu HK, Yenisey C, Ozsunar Y (2006) Surgical pinealectomy accelerates intervertebral disc degeneration process in chicken. Eur Spine J 15:605–612. https://doi.org/10.1007/s00586-005-0972-9
Bagnall K, Raso VJ, Moreau M, Mahood J, Wang X, Zhao J (1999) The effects of melatonin therapy on the development of scoliosis after pinealectomy in the chicken. J Bone Jt Surg Am 81:191–199. https://doi.org/10.2106/00004623-199902000-00006
Bagnall KM, Beuerlein M, Johnson P, Wilson J, Raso VJ, Moreau M (2001) Pineal transplantation after pinealectomy in young chickens has no effect on the development of scoliosis. Spine 26:1022–1027. https://doi.org/10.1097/00007632-200105010-00007
Beuerlein M, Wang X, Moreau M, Raso J, Mahood J, Bagnall K (2001) Development of scoliosis following pinealectomy in young chickens is not the result of an artifact of the surgical procedure. Microsc Res Tech 53:81–86. https://doi.org/10.1002/jemt.1071
Beuerlein M, Wilson J, Moreau M, Raso VJ, Mahood J, Wang X, Greenhill B, Bagnall KM (2001) The critical stage of pinealectomy surgery after which scoliosis is produced in young chickens. Spine 26:237–240. https://doi.org/10.1097/00007632-200102010-00007
Inoh H, Kawakami N, Matsuyama Y, Aoki T, Kanemura T, Natsume N, Iwata H (2001) Correlation between the age of pinealectomy and the development of scoliosis in chickens. Spine 26:1014–1021. https://doi.org/10.1097/00007632-200105010-00006
Nette F, Dolynchuk K, Wang X, Daniel A, Demianczuk C, Moreau M, Raso J, Mahood J, Bagnall K (2002) The effects of exposure to intense, 24 h light on the development of scoliosis in young chickens. Stud Health Technol Inform 91:1–6
O’Kelly C, Wang X, Raso J, Moreau M, Mahood J, Zhao J, Bagnall K (1999) The production of scoliosis after pinealectomy in young chickens, rats, and hamsters. Spine 24:35–43. https://doi.org/10.1097/00007632-199901010-00009
Turgut M, Yenisey C, Uysal A, Bozkurt M, Yurtseven ME (2003) The effects of pineal gland transplantation on the production of spinal deformity and serum melatonin level following pinealectomy in the chicken. Eur Spine J 12:487–494. https://doi.org/10.1007/s00586-003-0528-9
Wang X, Jiang H, Raso J, Moreau M, Mahood J, Zhao J, Bagnall K (1997) Characterization of the scoliosis that develops after pinealectomy in the chicken and comparison with adolescent idiopathic scoliosis in humans. Spine 22:2626–2635. https://doi.org/10.1097/00007632-199711150-00010
Wang X, Moreau M, Raso VJ, Zhao J, Jiang H, Mahood J, Bagnall K (1998) Changes in serum melatonin levels in response to pinealectomy in the chicken and its correlation with development of scoliosis. Spine 23:2377–2381. https://doi.org/10.1097/00007632-199811150-00002
Cheung KM, Lu DS, Poon AM, Wang T, Luk KD, Leong JC (2003) Effect of melatonin suppression on scoliosis development in chickens by either constant light or surgical pinealectomy. Spine 28:1941–1944. https://doi.org/10.1097/01.BRS.0000083140.80750.93
Cheung KM, Wang T, Hu YG, Leong JC (2003) Primary thoracolumbar scoliosis in pinealectomized chickens. Spine 28:2499–2504. https://doi.org/10.1097/01.BRS.0000092366.30032.97
Poon AM, Cheung KM, Lu DS, Leong JC (2006) Changes in melatonin receptors in relation to the development of scoliosis in pinealectomized chickens. Spine 31:2043–2047. https://doi.org/10.1097/01.brs.0000231796.49827.39
Fagan AB, Kennaway DJ, Oakley AP (2009) Pinealectomy in the chicken: a good model of scoliosis? Eur Spine J 18:1154–1159. https://doi.org/10.1007/s00586-009-0927-7
Akel I, Kocak O, Bozkurt G, Alanay A, Marcucio R, Acaroglu E (2009) The effect of calmodulin antagonists on experimental scoliosis: a pinealectomized chicken model. Spine 34:533–538. https://doi.org/10.1097/BRS.0b013e31818be0b1
Turhan E, Acaroglu E, Bozkurt G, Alanay A, Yazici M, Surat A (2006) Unilateral enucleation affects the laterality but not the incidence of scoliosis in pinealectomized chicken. Spine 31:133–138. https://doi.org/10.1097/01.brs.0000194781.53260.dc
Fu G, Yoshihara H, Kawakami N, Goto M, Tsuji T, Ohara T, Imagama S (2011) Microcomputed tomographic evaluation of vertebral microarchitecture in pinealectomized scoliosis chickens. J Pediatr Orthop B 20:382–388. https://doi.org/10.1097/BPB.0b013e3283474c6e
Yoshihara H, Kawakami N, Matsuyama Y, Inoh H, Imagama S, Ishiguro N (2005) A histomorphologic study of scoliosis in pinealectomized chickens. Spine 30:2244–2251. https://doi.org/10.1097/01.brs.0000182095.00577.ee
Fjelldal PG, Grotmol S, Kryvi H, Gjerdet NR, Taranger GL, Hansen T, Porter MJ, Totland GK (2004) Pinealectomy induces malformation of the spine and reduces the mechanical strength of the vertebrae in Atlantic salmon. Salmo salar J Pineal Res 36:132–139. https://doi.org/10.1046/j.1600-079x.2003.00109.x
Ebihara S, Marks T, Hudson DJ, Menaker M (1986) Genetic control of melatonin synthesis in the pineal gland of the mouse. Science 231:491–493. https://doi.org/10.1126/science.3941912
Sheng MH, Baylink DJ, Beamer WG, Donahue LR, Rosen CJ, Lau KH, Wergedal JE (1999) Histomorphometric studies show that bone formation and bone mineral apposition rates are greater in C3H/HeJ (high-density) than C57BL/6J (low-density) mice during growth. Bone 25:421–429. https://doi.org/10.1016/s8756-3282(99)00184-2
Turner CH, Hsieh YF, Muller R, Bouxsein ML, Rosen CJ, McCrann ME, Donahue LR, Beamer WG (2001) Variation in bone biomechanical properties, microstructure, and density in BXH recombinant inbred mice. J Bone Miner Res 16:206–213. https://doi.org/10.1359/jbmr.2001.16.2.206
Chen C, Kalu DN (1999) Strain differences in bone density and calcium metabolism between C3H/HeJ and C57BL/6J mice. Bone 25:413–420. https://doi.org/10.1016/s8756-3282(99)00185-4
Dimai HP, Linkhart TA, Linkhart SG, Donahue LR, Beamer WG, Rosen CJ, Farley JR, Baylink DJ (1998) Alkaline phosphatase levels and osteoprogenitor cell numbers suggest bone formation may contribute to peak bone density differences between two inbred strains of mice. Bone 22:211–216. https://doi.org/10.1016/s8756-3282(97)00268-8
Richman C, Kutilek S, Miyakoshi N, Srivastava AK, Beamer WG, Donahue LR, Rosen CJ, Wergedal JE, Baylink DJ, Mohan S (2001) Postnatal and pubertal skeletal changes contribute predominantly to the differences in peak bone density between C3H/HeJ and C57BL/6J mice. J Bone Miner Res 16:386–397. https://doi.org/10.1359/jbmr.2001.16.2.386
Cheung KM, Wang T, Poon AM, Carl A, Tranmer B, Hu Y, Luk KD, Leong JC (2005) The effect of pinealectomy on scoliosis development in young nonhuman primates. Spine 30:2009–2013. https://doi.org/10.1097/01.brs.0000179087.38730.5d
Reiter RJ, Tan DX, Manchester LC, Pilar Terron M, Flores LJ, Koppisepi S (2007) Medical implications of melatonin: Receptor-mediated and receptor-independent actions. Adv Med Sci 52:11–28
Gorman KF, Breden F (2007) Teleosts as models for human vertebral stability and deformity. Comp Biochem Physiol C Toxicol Pharmacol 145:28–38. https://doi.org/10.1016/j.cbpc.2006.10.004
Gorman KF, Breden F (2009) Idiopathic-type scoliosis is not exclusive to bipedalism. Med Hypotheses 72:348–352. https://doi.org/10.1016/j.mehy.2008.09.052
Bagnat M, Gray RS (2020) Development of a straight vertebrate body axis. Development. https://doi.org/10.1242/dev.175794
Boswell CW, Ciruna B (2017) Understanding idiopathic scoliosis: a new zebrafish school of thought. Trends Genet 33:183–196. https://doi.org/10.1016/j.tig.2017.01.001
Bird NC, Mabee PM (2003) Developmental morphology of the axial skeleton of the zebrafish, Danio rerio (Ostariophysi: Cyprinidae). Dev Dyn 228:337–357. https://doi.org/10.1002/dvdy.10387
Dietrich K, Fiedler IA, Kurzyukova A, Lopez-Delgado AC, McGowan LM, Geurtzen K, Hammond CL, Busse B, Knopf F (2021) Skeletal biology and disease modeling in zebrafish. J Bone Miner Res 36:436–458. https://doi.org/10.1002/jbmr.4256
Gorman KF, Breden F (2009) Idiopathic-type scoliosis is not exclusive to bipedalism. Med Hypotheses 72:348–352. https://doi.org/10.1016/j.mehy.2008.09.05
Gray RS, Gonzalez R, Ackerman SD, Minowa R, Griest JF, Bayrak MN, Troutwine B, Canter S, Monk KR, Sepich DS, SolnicaKrezel L (2021) Postembryonic screen for mutations affecting spine development in zebrafish. Dev Biol 471:18–33. https://doi.org/10.1016/j.ydbio.2020.11.009
Hoshijima K, Jurynec MJ, Grunwald DJ (2016) Precise editing of the zebrafish genome made simple and efficient. Dev Cell 36:654–667. https://doi.org/10.1016/j.devcel.2016.02.015
Mullins MC, Hammerschmidt M, Haffter P, Nusslein-Volhard C (1994) Large-scale mutagenesis in the zebrafish: in search of genes controlling development in a vertebrate. Curr Biol 4:189–202. https://doi.org/10.1016/s0960-9822(00)00048-8
Wu N, Liu B, Du H, Zhao S, Li Y, Cheng X, Wang S, Lin J, Zhou J, Qiu G, Wu Z, Zhang J (2019) The progress of CRISPR/Cas9-mediated gene editing in generating mouse/zebrafish models of human skeletal diseases. Comput Struct Biotechnol J 17:954–962. https://doi.org/10.1016/j.csbj.2019.06.006
Bergen DJM, Kague E, Hammond CL (2019) Zebrafish as an emerging model for osteoporosis: a primary testing platform for screening new osteo-active compounds. Front Endocrinol 10:6. https://doi.org/10.3389/fendo.2019.00006
Sun X, Zhou Y, Zhang R, Wang Z, Xu M, Zhang D, Huang J, LuoF LF, Ni Z, Zhou S, Chen H, Chen S, Chen L, Du X, Chen B, Huang H, Liu P, Yin L, Qiu J, Chen D, Deng C, Xie Y, Luo L, Chen L (2020) Dstyk mutation leads to congenital scoliosis-likevertebral malformations in zebrafish via dysregulated mTORC1/TFEB pathway. Nat Commun 11:479. https://doi.org/10.1038/s41467-019-14169-z
Bagwell J, Norman J, Ellis K, Peskin B, Hwang J, Ge X, NguyenSV MSK, Stainier DY, Bagnat M (2020) Notochordvacuoles absorb compressive bone growth during zebrafish spineformation. Elife. https://doi.org/10.7554/eLife.51221
Mangos S, Lam PY, Zhao A, Liu Y, Mudumana S, Vasilyev A, Liu A, Drummond IA (2010) The ADPKD genes pkd1a/b and pkd2 regulate extracellular matrix formation. Dis Model Mech 3:354–365. https://doi.org/10.1242/dmm.003194
Le Corre S, Eyre D, Drummond IA (2014) Modulation of the secretory pathway rescues zebrafish polycystic kidney disease pathology. J Am Soc Nephrol 25:1749–1759. https://doi.org/10.1681/ASN.2013101060
Gray RS, Wilm TP, Smith J, Bagnat M, Dale RM, Topczewski J, Johnson SL, Solnica-Krezel L (2014) Loss of col8a1a function during zebrafish embryogenesis results in congenital vertebral malformations. Dev Biol 386:72–85. https://doi.org/10.1016/j.ydbio.2013.11.028
Lleras-Forero L, Newham E, Teufel S, Kawakami K, Hartmann C, Hammond CL, Knight RD, Schulte-Merker S (2020) Muscle defects due to perturbed somite segmentation contribute to late adult scoliosis. Aging 12:18603–18621. https://doi.org/10.18632/aging.103856
Whittle J, Antunes L, Harris M, Upshaw Z, Sepich DS, Johnson AN, Mokalled M, Solnica-Krezel L, Dobbs MB, Gurnett CA (2020) MYH3-associated distal arthrogryposis zebrafish model is normalized with para-aminoblebbistatin. EMBO Mol Med 12:e12356. https://doi.org/10.15252/emmm.202012356
Grimes DT, Boswell CW, Morante NF, Henkelman RM, Burdine RD, Ciruna B (2016) Zebrafish models of idiopathic scoliosis link cerebrospinal fluid flow defects to spine curvature. Science 352:1341–1344. https://doi.org/10.1126/science.aaf6419
Wang Y, Troutwine BR, Zhang H, Gray RS (2022) The axonemal dynein heavy chain 10 gene is essential for monocilia motility and spine alignment in zebrafish. Dev Biol 482:82–90
Baker K, Beales PL (2009) Making sense of cilia in disease: the human ciliopathies. Am J Med Genet C Semin Med Genet 151C:281–295. https://doi.org/10.1002/ajmg.c.30231
Van De Weghe JC, Rusterholz TDS, Latour B, Grout ME, Aldinger KA, Shaheen R, Dempsey JC, Maddirevula S, Cheng YH, Phelps IG, Gesemann M, Goel H, Birk OS, Alanzi T, Rawashdeh R, Khan AO, Bamshad MJ, Nickerson DA, Neuhauss SCF, Dobyns WB, Alkuraya FS, Roepman R, Bachmann-Gagescu R, Doherty D (2017) Mutations in ARMC9, which Encodes a basal body protein, cause joubert syndrome in humans and ciliopathy phenotypes in zebrafish. Am J Hum Genet 101:23–36. https://doi.org/10.1016/j.ajhg.2017.05.010
Terhune EA, Cuevas MT, Monley AM, Wethey CI, Chen X, Cattell MV, Bayrak MN, Bland MR, Sutphin B, Trahan GD, Taylor MRG, Niswander LA, Jones KL, Baschal EE, Antunes L, Dobbs M, Gurnett C, Appel B, Gray R, Hadley Miller N (2021) Mutations in KIF7 implicated in idiopathic scoliosis in humans and axial curvatures in zebrafish. Hum Mutat 42:392–407. https://doi.org/10.1002/humu.24162
Gao W, Chen C, Zhou T, Yang S, Gao B, Zhou H, Lian C, Wu Z, Qiu X, Yang X, Alattar E, Liu W, Su D, Sun S, Chen Y, Cheung KMC, Song Y, Luk KKD, Chan D, Sham PC, Xing C, Khor CC, Liu G, Yang J, Deng Y, Hao D, Huang D, Li QZ, Xu C, Su P (2017) Rare coding variants in MAPK7 predispose to adolescent idiopathic scoliosis. Hum Mutat 38:1500–1510. https://doi.org/10.1002/humu.23296
Zhang X, Jia S, Chen Z, Chong YL, Xie H, Feng D, Wu X, Song DZ, Roy S, Zhao C (2018) Cilia-driven cerebrospinal fluid flow directs expression of urotensin neuropeptides to straighten the vertebrate body axis. Nat Genet 50:1666–1673. https://doi.org/10.1038/s41588-018-0260-3
Zhou T, Chen C, Xu C, Zhou H, Gao B, Su D, Liao Z, Li Y, Yang S, Su P (2018) Mutant MAPK7-induced idiopathic scoliosis is linked to impaired osteogenesis. Cell Physiol Biochem 48:880–890. https://doi.org/10.1159/000491956
Rose CD, Pompili D, Henke K, Van Gennip JLM, Meyer-Miner A, Rana R, Gobron S, Harris MP, Nitz M, Ciruna B (2020) SCO-Spondin defects and neuroinflammation are conserved mechanisms driving spinal deformity across genetic models of idiopathic scoliosis. Curr Biol 30(2363–2373):e6. https://doi.org/10.1016/j.cub.2020.04.020
Tardieu C, Hasegawa K, Haeusler M (2017) How did the pelvis and vertebral column become a functional unit during the transition from occasionalto permanent bipedalism? Anat Rec 300:912–931
Murthy SE, Dubin AE, Patapoutian A (2017) Piezos thrive under pressure: mechanically activated ion channels in health and disease. Nat Rev Mol Cell Biol 18:771–783. https://doi.org/10.1038/nrm.2017.92
Zhu D, Zhang G, Guo X, Wang Y, Liu M, Kang X (2021) A new hope in spinal degenerative diseases: piezo1. Biomed Res Int 2021:6645193. https://doi.org/10.1155/2021/6645193
Sun W, Chi S, Li Y, Ling S, Tan Y, Xu Y et al (2019) The mechanosensitive Piezo1 channel is required for bone formation. Elife 8:e47454. https://doi.org/10.7554/eLife.47454
Lee S, Park S, Kim HY, Chae JH, Ko JM (2021) Extended phenotypes of PIEZO1-related lymphatic dysplasia caused by two novel compound heterozygous variants. Eur J Med Genet 64:104295. https://doi.org/10.1016/j.ejmg.2021.104295
Chen F, Sun M, Peng F, Lai Y, Jiang Z, Zhang W et al (2023) Compressive stress induces spinal vertebral growth plate chondrocytes apoptosis via Piezo1. J Orthop Res 41:1792–1802. https://doi.org/10.1002/jor.25527
Haliloglu G, Becker K, Temucin C, Talim B, Küçükşahin N, Pergande M et al (2017) Recessive PIEZO2 stop mutation causes distal arthrogryposis with distal muscle weakness, scoliosis and proprioception defects. J Hum Genet 62:497–501. https://doi.org/10.1038/jhg.2016.153
Uehara M, Kosho T, Takano K, Inaba Y, Kuraishi S, Ikegami S et al (2020) Proximal junctional kyphosis after posterior spinal fusion for severe kyphoscoliosis in a patient with PIEZO2-deficient arthrogryposis syndrome. Spine 45:E600–E604. https://doi.org/10.1097/BRS.0000000000003347
Ramli AT, Watanabe M, Kondo S (2024) Piezo1 mutant zebrafish as a model of idiopathic scoliosis. Front Genet 14:1321379. https://doi.org/10.3389/fgene.2023.1321379
Spassky N, Meunier A (2017) The development and functions of multiciliated epithelia. Nat Rev Mol Cell Biol 18:423–436. https://doi.org/10.1038/nrm.2017.21
Petrik D, Myoga MH, Grade S, Gerkau NJ, Pusch M, Rose CR, Grothe B, Gotz M (2018) Epithelial sodium channel regulates adult neural stem cellproliferation in a flow-dependent manner. Cell Stem Cell 22:865-878.e868. https://doi.org/10.1016/j.stem.2018.04.016
Simon MJ, Iliff JJ (2016) Regulation of cerebrospinal fluid (CSF) flow in neurodegenerative, neurovascular and neuroinflammatory disease. Biochim Biophys Acta 1862:442–451. https://doi.org/10.1016/j.bbadis.2015.10.014
Song Z, Zhang X, Jia S, Yelick PC, Zhao C (2016) Zebrafish as a model for human ciliopathies. J Genet Genomics 43:107–120. https://doi.org/10.1016/j.jgg.2016.02.001
Zhao L, Gao F, Gao S, Liang Y, Long H, Lv Z, Su Y, Ye N, Zhang L, Zhao C, Wang X, Song W, Zhang S, Dong B (2021) Biodiversity-based development and evolution: the emerging research systems in model and non-model organisms. Sci China Life Sci 64:1236–1280. https://doi.org/10.1007/s11427-020-1915-y
Olstad EW, Ringers C, Hansen JN, Wens A, Brandt C, Wachten D, Yaksi E, Jurisch-Yaksi N (2019) Ciliary beating compartmentalizes cerebrospinal fluid flow in the brain and regulates ventricular development. Curr Biol 29(229–241):e6. https://doi.org/10.1016/j.cub.2018.11.059
Sternberg JR, Prendergast AE, Brosse L, Cantaut-Belarif Y, Thouvenin O, Orts-Del’Immagine A, Castillo L, Djenoune L, Kurisu S, McDearmid JR, Bardet PL, Boccara C, Okamoto H, Delmas P, Wyart C (2018) Pkd2l1 is required for mechanoception in cerebrospinal fluid-contacting neurons and maintenance of spine curvature. Nat Commun 9:3804. https://doi.org/10.1038/s41467-018-06225-x
Thouvenin O, Keiser L, Cantaut-Belarif Y, Carbo-Tano M, Verweij F, Jurisch-Yaksi N, Bardet PL, van Niel G, Gallaire F, Wyart C (2020) Origin and role of the cerebrospinal fluid bidirectional flow in the central canal. Elife. https://doi.org/10.7554/eLife.47699
Sun Z, Amsterdam A, Pazour GJ, Cole DG, Miller MS, Hopkins N (2004) A genetic screen in zebrafish identifies cilia genes as a principal cause of cystic kidney. Development 131:4085–4093. https://doi.org/10.1242/dev.01240
Zhao C, Malicki J (2007) Genetic defects of pronephric cilia in zebrafish. Mech Dev 124:605–616. https://doi.org/10.1016/j.mod.2007.04.004
Vieira JP, Lopes P, Silva R (2012) Primary ciliary dyskinesia and hydrocephalus with aqueductal stenosis. J Child Neurol 27:938–941. https://doi.org/10.1177/0883073811429856
Wallmeier J, Frank D, Shoemark A, Nö the-Menchen T, Cindric S, Olbrich H, Loges NT, Aprea I, Dougherty GW, Pennekamp P et al (2019) De novo mutations in FOXJ1 result in a motile ciliopathy with hydrocephalus and randomization of left/right body asymmetry. Am J Hum Genet 105:1030–1039. https://doi.org/10.1016/j.ajhg.2019.09.022
Morimoto Y, Yoshida S, Kinoshita A, Satoh C, Mishima H, Yamaguchi N, Matsuda K, Sakaguchi M, Tanaka T, Komohara Y et al (2019) Nonsense mutation in CFAP43 causes normal-pressure hydrocephalus with ciliary abnormalities. Neurology 92:e2364–e2374. https://doi.org/10.1212/WNL.0000000000007505
Reissner E (1860) Beiträ ge zur Kenntnis vom Bau des Rü ckenmarkes von Petromyzon fluviatilis L. Arch Anat Physiol Wiss Med 77:545–588
Olsson R (1972) Reissner’s fiber in ascidian tadpole larvae. Acta Zool 53:17–21. https://doi.org/10.1111/j.1463-6395.1972.tb00568.x
Gobron S, Creveaux I, Meiniel R, Didier R, Dastugue B, Meiniel A (1999) SCO-spondin is evolutionarily conserved in the central nervous system of the chordate phylum. Neuroscience 88:655–664. https://doi.org/10.1016/S0306-4522(98)00252-8
Cantaut-Belarif Y, Sternberg JR, Thouvenin O, Wyart C, Bardet PL (2018) The Reissner fiber in the cerebrospinal fluid controls morphogenesis of the body axis. Curr Biol 28:2479-2486.e2474. https://doi.org/10.1016/j.cub.2018.05.079
Vio K, Rodriguez S, Yulis CR, Oliver C, Rodriguez EM (2008) The subcommissural organ of the rat secretes Reissner’s fiber glycoproteins and CSFsoluble proteins reaching the internal and external CSF compartments. Cerebrospinal Fluid Res 5:3. https://doi.org/10.1186/1743-8454-5-3
Castenholz A, Zoltzer H (1980) Formation and morphology of Reissner’s fibre in primates. A scanning electron microscopic study. Cell Tissue Res 207:43–53
Agduhr E (1922) Über ein zentrales Sinnesorgan bei den Vertebraten. Z Anat Entwickl Gesch 66:223–360. https://doi.org/10.1007/BF02593586
Rodrıguez EM, Garrido O, Oksche A (1990) Lectin histochemistry of the human fetal subcommissural organ. Cell Tissue Res 262:105–113. https://doi.org/10.1007/BF00327751
Rodrıguez EM, Jara P, Richter H, Montecinos H, Flández B, Wiegand R, Oksche A (1993) Evidence for the release of CSF-soluble secretory material from the subcommissural organ, with particular reference to the situation in the human. Springer, Heidelberg, pp 121–131
Rodrıguez EM, Oksche A, Montecinos H (2001) Human subcommissural organ, with particular emphasis on its secretory activity during the fetal life. Microsc Res Tech 52:573–590. https://doi.org/10.1002/1097-0029(20010301)
Troutwine BR, Gontarz P, Konjikusic MJ, Minowa R, Monstad-Rios A, Sepich DS, Kwon RY, Solnica-Krezel L, Gray RS (2020) The Reissner fiber is highly dynamic in vivo and controls morphogenesis of the spine. Curr Biol 30:2353-2362.e3. https://doi.org/10.1016/j.cub.2020.04.015
Djenoune L, Wyart C (2017) Light on a sensory interface linking the cerebrospinal fluid to motor circuits in vertebrates. J Neurogenet 31:113–127. https://doi.org/10.1080/01677063.2017.1359833
Kolmer W (1921) Das “Sagittalorgan” der Wirbeltiere. Z Anat Entwicklungsgesch 60:652–717. https://doi.org/10.1007/BF02593657
Orts-Del’Immagine A, Cantaut-Belarif Y, Thouvenin O, Roussel J, Baskaran A, Langui D, Koeth F, Bivas P, Lejeune FX, Bardet PL et al (2020) Sensory neurons contacting the cerebrospinal fluid require the Reissner fiber to detect spinal curvature in vivo. Curr Biol 30(P827–839):E4. https://doi.org/10.1016/j.cub.2019.12.071
Quan FB, Dubessy C, Galant S, Kenigfest NB, Djenoune L, Leprince J, Wyart C, Lihrmann I, Tostivint H (2015) Comparative distribution and in vitro activities of the urotensin II-related peptides URP1 and URP2 in zebrafish: evidence for their colocalization in spinal cerebrospinal fluid-contacting neurons. PLoS One 10:e0119290. https://doi.org/10.1371/journal.pone.0119290
Zhang X, Jia S, Chen Z, Chong YL, Xie H, Feng D, Wu X, Song DZ, Roy S, Zhao C (2018) Cilia-driven cerebrospinal fluid flow directs expression of urotensin neuropeptides to straighten the vertebrate body axis. Nat Genet 50:1666–1673. https://doi.org/10.1038/s41588-018-0260-3
Lu H, Shagirova A, Goggi JL, Yeo HL, Roy S (2020) Reissner fibre-induced urotensin signalling from cerebrospinal fluid-contacting neurons prevents scoliosis of the vertebrate spine. Biol Open 9:bio052027. https://doi.org/10.1242/bio.052027
Tardieu C, Bonneau N, Hecquet J, Boulay C, Marty C, LegayeDuval-Beaupère JG (2013) How is sagittal balance acquired during bipedal gait acquisition? Comparison of neonatal and adult pelves in three dimensions. Evolutionary Implications. J Hum Evol 65:209–222
Meyer MR, Haeusler M (2015) Spinal cord evolution in early Homo. J Hum Evol 88:43–53
Tardieu C, Hecquet J, Barrau A, Loridon P, Boulay C, Legaye J, Carlier R, Marty C, Duval-Beaupère G (2006) Le bassin, interface articulaire entre rachis et membres inferieurs: analyse par le logiciel DE-VISU. C R Palevol 5:583–595
Funding
No funding was received for this work.
Author information
Authors and Affiliations
Contributions
CY, GJ, and LL: made substantial contributions to the conception or design of the work; drafted the work or revised it critically for important intellectual content; approved the version to be published; agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yan, C., Jin, G. & Li, L. Spinal scoliosis: insights into developmental mechanisms and animal models. Spine Deform (2024). https://doi.org/10.1007/s43390-024-00941-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43390-024-00941-9