Abstract
Purpose
The aim of the current review is to summarize the current evidence on graft materials used in fusion procedures for spinal deformity corrections.
Methods
PubMed, Embase, and Cochrane Library were searched for relevant published observational studies and clinical trials using osteobiologics and biomaterials in spinal deformity surgery.
Results
The use of autograft in deformity correction surgeries has been reported in a limited number of studies, with the harvest sites including iliac crest, ribs, and local bone. Various allografts and biologics have been used in the treatment of spinal deformities including idiopathic and degenerative scoliosis, either as stand alone or in combination with autograft. Limited number of studies reported no differences in fusion rates or outcomes. Use of rh-BMP2 in anterior, posterior or front/back approaches showed higher fusion rates than other graft materials in patients with spinal deformities. Due to the limited number of quality studies included in the review, as well as alternative factors, such as costs, availability, and surgeon expertise/preference, no definitive conclusion or recommendations can be made as to the ideal graft choice in spinal deformity surgery.
Conclusions
Most commonly used grafts included autograft, allograft and rh-BMP2, with new biologics and biomaterials constantly emerging in the market. Limited number of high-quality comparative studies and heterogeneity in study design prevented direct comparisons that can lead to meaningful recommendations. Further studies are needed to prove superiority of any single graft material and/or biologic that is also cost-effective and safe.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There have been significant advancements in the field of spinal instrumentation and biologics to promote bony fusion in spinal deformity surgery [1, 2]. However, pseudarthrosis continues to be a problem and contributes to significant postoperative pain, implant failure, and increased risk of reoperation [3, 4]. Historical rate of pseudarthrosis quoted in the literature is 17% following adult deformity correction and 5–34% following fusion for degenerative conditions [4, 5]. Reasons for failure ranges from biomechanics and construct design, local biology, and patient-related comorbidities leading to poor bone quality and to compromised new bone formation [6].
Solid bony fusion requires an intricate interplay between three principles of bone graft material properties: osteogenesis, osteoinductivity, and osteoconductivity [7]. In principle, osteogenic stem cells must be stimulated to migrate to the site of fusion, while osteoinductive signals must support the cell population lineage, and a low-strain mechanical conductive environment must be maintained until fusion occurs [8]. Autograft and allograft may possess each of these properties to a varying degree and are placed within the surgical fusion bed to promote arthrodesis. Iliac crest bone graft (ICBG) has historically been used as the gold standard graft material, although risk of harvest-site morbidity and subsequent long-term functional impairment is significant [9]. In addition, the native levels of matrix factors in autografts may also vary greatly between individuals based on age, smoking status, and medical comorbidities. Consequently, there has been significant research interest and financial investment in designing novel graft materials and biologics that can reproduce high fusion rates without the risk of autologous bone harvest [10].
Ceramic-based substitutes including hydroxyapatite (HA) and tricalcium phosphate (TCP) have chemical similarities to bone and possess biocompatibility, osteoconductivity, and strong mechanical properties despite lacking osteogenic potential [7, 11]. Demineralized bone matrix (DBM) is a composite of collagen, noncollagenous proteins, and growth factures with increased osteoconductive and osteoinductive properties. It is also provided in many formulations, including a putty, which allows the DBM to be molded to the target lesion with greater ease of application [12,13,14]. Its ease of use has made it a popular option for surgeons and is commonly combined with local autograft for added osteogenic effect.
Bone-morphogenetic proteins (BMPs) represent a family of differentiation factors that promote bone formation and remodeling. In 2002, the Food and Drug Administration (FDA) approved rhBMP-2 with a collagen carrier as an iliac crest bone graft (ICBG) substitute for single level anterior lumbar interbody fusion (ALIF). Patients with rhBMP-2 had significantly better fusion rates (98% BMP vs. 76% ICBG), shorter length of surgery, decreased blood loss and shorter hospital stay [15]. Given its success in ALIFs, off-label use of rhBMP-2 has increased and is now used in approximately 25% of all fusion cases nationally including posterolateral fusions for deformity [15]. Complication rates following use of rhBMP-2 vary; however, for thoracic fusion cases, these are negligible if rhBMP-2 is used in appropriate amounts. Given the recent increase in the array of products and techniques used to enhance fusion rates, it is of paramount importance for surgeons and scientists to critically assess the efficacy, risks, and costs of these products compared to traditional autografts. As such, the aim of this review was to analyze the current literature regarding the use of biologics in thoracic/ thoracolumbar deformity surgeries.
Methods
To conduct this review, PubMed, Embase, and Cochrane Library were searched for relevant published observational studies and clinical trials using biologics and biomaterials in spine deformity surgery. To qualify for inclusion, studies had to focus on thoracic or thoracolumbar deformity patients who had deformity correction and spine fusion with a graft material. The included studies had to report on clinical or radiological outcomes, complications, or costs of the used graft material.
Search terms included “autograft”, “allograft”, “bone morphogenetic protein 2”, “stem cells”, “bone marrow aspirate”, “demineralized bone matrix”, “ceramics”, “peptide”, “synthetic bone graft”, “bone substitute”, OR “biosynthetic bone graft” AND “scoliosis” OR “spine deformity”, AND “spine fusion”, “scoliosis correction”, “spinal deformity surgery”. Studies were restricted to those published from 2000 to 2021 to reflect the current trends in practice. Only English articles with available full text were chosen. Identified articles were first screened by title and abstract, then full-text screening was conducted. Finally, the included studies were categorized based on the type of graft used, and each category was used for the relevant section of this review.
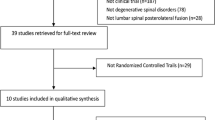
After title and abstract screening, the full texts of 110 articles were assessed. We found 57 articles that focused of biologics in spine deformity surgery and their outcomes, complications, or costs and were used for the main part of this review. The other 53 articles were used for the introduction and the introductory statement of each osteobiologics (Fig. 1).
Results
Iliac crest bone graft (ICBG) and local autografts
Autografts used in the correction of spinal deformities are harvested from the iliac crest, spinous process, transverse process, lamina, or facets (Tables 1, 2) [16,17,18,19,20]. Iliac crest bone graft (ICBG) is known as the gold standard for spinal fusion surgeries due to its osteogenic, osteoconductive, and osteoinductive properties [16, 21]. There are multiple methods suitable for the preparation of autogenous bone grafts for implantation, including forming struts or morselizing the bone. In comparing the use of rib strut grafts to morselized rib bone, Ouellet et al. concluded that there was no difference between the two in the maintenance of coronal or sagittal plane correction in scoliosis patients when using single solid rod instrumentation; however, there was a significant difference in pseudarthrosis rates between the two (Tables 1, 2) [22].
Fusion rates
Franzin et al. reported fusion rates of 95.4% and 100% for ICBG and local autograft, respectively, in posterior spinal fusion with pedicle screw instrumentation [17]. There were no statistically significant differences in fusion or pseudarthrosis rate, loss of correction over time, or quality of life in adolescent idiopathic scoliosis (AIS) patients who received ICBG vs. local autograft.
Iwai et al. showed radiographic evidence of complete fusion in all 10 AIS patients in their study using a fibular strut autograft (FSAG) with a rib strut graft in the space between the cut fragment and the remaining vertebral bodies, resembling a “hot dog” [25]. There was no evidence of postoperative erosion after a mean follow-up time of 9 years and 9 months. Likewise, Farshad et al. showed a 100% fusion rate across 20 patients with local autograft from the rib in anterior short fusion with pedicle screws [21]. Milinkovic et al. showed a 98% fusion rate across 188 patients with local autograft in posterior spinal fusion with dual-rod fixation [19]. Similarly, Presenti et al. showed a success rate of 97.7% with local autograft from the spinous processes in posterior spinal fusion with sublaminar bands in 44 patients [20].
Outcomes
Kager et al. have concluded that ICGB should remain the gold standard for anterior or posterior instrumented fusion as there is minimal risk for postoperative harvest site pain, especially in adolescents [24]. They reported that 10% of patients experienced postoperative pain (NRS pain score of 3/10) at the harvest site. For those with a 1-year follow-up the pain dropped to 9% of patients with NRS scores of 1–2/10. None of these patients took pain medication, nor reported any reduction in activity. Postoperative pain caused by ICBG harvest can be effectively treated as well. Samartzis et al. conducted a pilot study on the effect of levobupivacaine infusion at the ICBG site compared to a control group receiving saline [23]. They found a twofold decrease in pain at the ICBG site in the experimental group, as well as a fourfold overall physical pain decrease in a group of Southern Chinese participants.
Complications
The use of autografts eliminates the risk of viral, bacterial, or prion disease transmission or the risk of immune response, which can occur when using allograft or bone substitutes [17, 19, 20]. However, ICBG is associated with postoperative donor site pain, numbness, and fracture, as well as infection, hematoma formation, arterial injury, nerve injury, increased blood loss, and increased operative time [16, 19, 20, 23]. It also requires a separate incision in the skin, unlike alternative graft options [20].
Pseudarthrosis occurs when solid intervertebral fusion fails to take place and can cause persistent back pain, which may indicate revision surgery [20]. Franzin et al. reported 4.6% pseudarthrosis (1/41) in a study comparing thoracic spine arthrodesis of AIS patients with pedicle screws with or without ICBG [17]. There was no significant difference in pseudarthrosis between the local autograft and ICBG groups. Milinkovic et al. saw 2% (4/188) pseudarthrosis with mild back pain in a retrospective case study of AIS patients who underwent posterior fusion [19]. The pseudarthrosis rate was 30% (15/50) for a retrospective study by Ouellet et al. comparing strut autografts and morselized autografts and was significantly different between graft types (p = 0.029) [22]. Pseudarthrosis occurred in 2 of 18 (11.1%) patients who were given strut grafts and 13 of 32 (40.6%) who were given morselized grafts. Three of these patients required revision surgery, two for pain and one for implant failure and loss of correction. Pesenti et al. saw a rate of 2.3% (1/44) for the local autograft group and 4.5% (2/44) for the control group with no graft with no significant difference between the groups [20]. One of the two patients in the control group had a rod breakage that caused loss of correction and significant back pain, resulting in reoperation. Iwai et al. reported 10% (1/10) pseudarthrosis using a “hot dog” application of rib strut grafts during anterior fusion of AIS patients. This patient required reoperation [25].
Additional complications reported include delayed deep infection in 2% (4/188) that was resolved after implant removal in all cases (Milinkovic et al.) [19], 8% (1/12) wound dehiscence (Samartzis et al.) [23], 20% (2/10) cerebral spinal fluid leakage, and 20% (2/10) extensive blood loss of more than 4000 mL during the operation (Iwai et al.) [25].
Cost
Autografts, including ICBG, are less costly than allografts or bone substitutes, because the graft comes from the patient themselves. Presenti et al. stated that bone substitutes account for 6% of the total cost of fusion surgery when employed [20]. However, one must consider that increased operative time for harvest also adds expense [24]. Kobayashi et al. analyzed the costs of autografting in Japan, which increased from $734 to $1,862 per person during the span of 2008 to 2017 [18]. The overall cost of scoliosis correction surgery has gone up in the same time period by about 1.6 times (from $9515 to $15,130), despite the slight national decrease in costs over time. Decreased costs include hospital fees per day and the substantial decrease in reimbursement prices for surgical instruments, which are a result of cost control policies by the Japanese government.
Allograft
Allograft is another commonly used biological material that has shown success in thoracic deformity procedures (Tables 3, 4). While autologous ICBG is typically considered the gold standard graft material in spine surgery, allograft has the benefit of avoiding donor site morbidity. In addition, particularly in the frail and/or pediatric population, harvesting a sufficient amount of ICBG for grafting can be challenging, and thus use of allograft can mitigate this issue [26]. Studies have shown that allograft can achieve high rates of fusion and minimal loss of curve, both alone and in combination with autograft [27,28,29], with minimal or no complications [26]. In addition, comparative studies have demonstrated that allograft produces clinical outcomes equal to [29,30,31] or better [27, 28] than autograft, providing evidence for its efficacy as a suitable alternative graft material.
Fusion/correction rates
A study by Johari et al. used irradiated femoral head and tibial slice allograft in pediatric patients with various forms of scoliosis, and 100% of patients showed union at the grafting site. No patients demonstrated evidence of infection, pseudarthrosis, fracture, or any other complication (Table 4) [26]. Another study in children under 18 with idiopathic scoliosis demonstrated a radiographic fusion rate of 92.7%, with the mean number of fused levels being 12.4 [27].
A study on thoracoscopic anterior scoliosis correction in pediatric patients (mean age 14.9 years) did not report a significant difference (p = 0.96) in Sucato fusion scores involving T5–L1 between allograft (fusion score = 2.22) vs. autograft groups (fusion score = 2.15) [29]. Similarly, Theologis et al. reported nearly equivocal fusion rates among allograft vs. autograft vs. bone substitute patients in treatment of adolescent idiopathic scoliosis (mean age 14.7) [30]. All three groups had a mean of 11.3–11.4 levels of fusion. A study by Weinzapfel et al. on thoracoscopic release for idiopathic scoliosis in teens compared anterior vertebral levels fused and curve correction between an allograft cohort vs. patients treated with a flexible demineralized bone matrix sheet (Grafton Flex DBM) [33]. At most recent follow-up, 60/73 (82%) levels in the allograft group and 100/109 (92%) levels in the DBM group were fused; this difference was not statistically significant (p = 0.088). Sinagra et al. investigated whether the volume of allograft per level fused or the addition of autograft to the volume of allograft impacted fusion rates in T1–T11 in idiopathic adolescent scoliosis (average age of patients was 16 years) [34]. Groups were given either 10 g or 15 g of allograft per level fused. They reported that neither the increased amount of allograft used nor the addition of autograft significantly improved rates of fusion (p = 0.3258). The average number of levels fused was 11 in all groups.
Complications
Efforts have been made to elucidate which biologic is responsible for the least postoperative morbidity or complications. A study by Betz et al. demonstrated that use of solely allograft for augmentation in the treatment of idiopathic scoliosis (mean age 14.5) resulted in pseudarthrosis in 1 of 37 (2.7%, Table 4) [35]. Another study again reported a pseudarthrosis rate of 3/111 in patients aged 12–14 for correction of idiopathic adolescent scoliosis (2.7%, Table 4) [36]. Pseudarthrosis rates have been also found to be lower in allograft patients (1/25; 4%) in comparison with autograft (1/16; 6.25%) [37].
In regards to risk of postoperative proximal junctional kyphosis in association with pseudarthrosis, one study found that the use of allogenic bone in adolescents (average age 15) has been reported to put patients at significantly lower odds (OR = 0.04) of developing proximal junctional kyphosis in comparison with patients receiving autograft bone (p = 0.001) [38]. However, another study in adolescents demonstrated no difference in risk of developing proximal junctional kyphosis in an allograft group compared with an allograft plus autograft supplement group (p = 0.6910) [34].
Lowe et al. that used a cortical allograft dowel to supplement autograft in T9–L4 resulted in no neurologic injuries, infections or reoperations [31]. A study comparing various autograft/allograft combinations in levels T10–L4 showed that use of posterior allograft had significantly fewer major medical complications (0%) in comparison with the posterior mixture of allograft and autograft (27%) (p = 0.01) and anterior strut allograft with posterior mixture of morselized allograft and autograft (25%) (p = 0.04) groups. However, the anterior autograft group had significantly lower complications (0%) in comparison with the mixed autograft/allograft groups (posterior mixture of morselized allograft and autograft: 25%, the posterior mixture of allograft and autograft: 27%) (p < 0.05) [39]. Nonetheless, this study ultimately determined that complication rates overall were not statistically significant based on type of bone graft used, but rather on surgical approach, with combined anteroposterior approaches carrying a greater likelihood of complication than isolated posterior or anterior approaches. Another study that reported that in posterior spinal fusions for idiopathic adolescent scoliosis, the allograft group had more than double the failure rate (28% vs. 12.5%) than the autograft group, which was defined by a loss of correction. The authors noted their use of strict criteria for failure, however, and postulated that if failure had instead been defined by need for repeat spinal instrumentation as a result of pseudarthrosis, the failure rate would have been higher in the autograft group (6.25%) than in the allograft group (4.0%) [37].
Cost
Cost can be a potentially limiting factor to the use of allograft. One study performed in a nonprofit community hospital reported an average cost of $1495 worth of bone graft per patient who underwent correction for AIS, which reportedly comprised 3.3% of the total hospital cost [41]. Another study conducted in a single tertiary center in Western Australia reported a cost of $4,650 AUD (~ $3505 USD) per 30–50 g of irradiated allograft. This study used 10–15 g of allograft per patient [34]. However, a multicenter retrospective study reported a much lower per-patient allograft cost range of $415–$830 (Table 4) [36].
Bone morphogenetic protein-2 (BMP-2)
The Food and Drug Administration (FDA) approved the use of recombinant human BMP-2 (rhBMP-2) for single-level anterior lumbar interbody fusion (ALIF) in 2002 as an alternative to iliac crest bone graft (ICBG) [42]. Since then, rhBMP-2 use has expanded as an off-label application to include several spinal procedures, including deformity (Tables 5, 6) [43].
In spine deformity correction surgery, it is challenging to provide a sufficient amount of autologous bone to satisfy the required long fusion, making the need to use a bone graft substitute, such as rhBMP-2, of paramount importance. The use of rhBMP-2 eliminates the morbidity related to harvesting bone autografts from the iliac crest, or ribs [44]. Ruofeng et al. reported steadily increasing use of rhBMP-2 for posterior long segment fusion from 2005 to 2011, with the exception of a dramatic drop in 2010 [45].
Fusion rates
Luhman et al. used rhBMP-2 to achieve anterior or posterior fusion in 70 patients with adult spine deformity (ASD). With a minimum follow-up of 1 year, the reported fusion rates were 93% in the posterior and 96% in the anterior group, and 100% in posterior compassionate-use patients [44].
Mulconrey et al. reported fusion rates of 91%, 97%, and 100% for anterior (10 mg/level), posterior (20 mg/level), and high-dose (40 mg/level) posterior compassionate-use fusions, respectively [46].
Maeda et al. reported a higher rate of solid fusion in the BMP group (22/23 patients; 95.7%) than the ICBG group (23/32 patients; 71.9%) [47]. Similarly, Kim et al. reported a higher fusion rate with BMP compared to ICBG, 93.5% vs. 71.9%, respectively [48].
Outcomes
Maeda et al. reported that the BMP group had a slightly better but statistically non-significant correction rate than the ICBG group (50.6% vs. 42.5%) [47]. Kim et al. reported that the BMP group had higher Scoliosis Research Society scores within pain, function, self-image, and domains [48]. Puvanesarajah et al. reported that elderly patients with ≥ 8 fused levels were significantly less likely to require revision surgery when BMP was used [49]. Safaee et al. reported an 11% absolute risk reduction of revision for pseudarthrosis when BMP was used [50].
Complications
Bess et al. evaluated the acute perioperative complications with or without rhBMP-2 use, with a mean follow-up of 34 months [43]. The rhBMP-2 group had a longer operative time, greater number of osteotomies per patient. Overall, rhBMP-2 patients had a significantly greater number of complications per patient (1.4 vs. 0.6). However, multivariate analysis found no significant correlation between rhBMP-2 use and neurological, wound, or superficial and deep infection complications.
Luhman et al. reported a low complication rate (3/70 patients), including superficial wound dehiscence (n = 1), deep wound infection (n = 1) and wound hematoma (n = 1) [44]. Mulconrey et al. reported a low pseudarthrosis of 5% and only one case of subfascial hematoma [46].
Costs
In a recent cost analysis multicenter study, Jain et al. reported that the mean total cost of index surgery was significantly higher in ASD surgery with rhBMP-2 use ($60,000 ± $17,000) than without rhBMP-2 ($41,000 ± $8900) and that the mean direct cost of using rhBMP-2 in ASD surgery was $14,000 ± $6400 [51]. Similarly, Puvanesarajah et al. and Safaee et al. reported that rhBMP-2 use led to a significant increase in primary surgery costs and hospital charges [49, 50].
Synthetics
Bioceramics are biodegradable synthetic calcium-based bone graft substitutes usually used in combination with autogenous bone or bone-marrow aspirate (BMA) [52]. The available ceramics usually contain substances with varying porosity, including β-tricalcium phosphate (β-TCP), hydroxyapatite (HA), calcium phosphate, or calcium sulfate [52]. Glass ceramics are bioactive due to their composition of SiO2, CaO, Na2O, and P2O5, which attract osteoblasts and osteoprogenitor cells and stimulate bone formation and integration [53]. Silicated calcium phosphate (Si–CaP) mimics the trabecular architecture of natural cancellous bone. By enhancing vascularity in the host bone, the silicate substitute significantly improves bone formation [54, 55].
Fusion rates
With a mean follow-up period of 34.7 months, Ameri et al. reported a 90% solid fusion rate using metal-derived bioactive glass in adolescent idiopathic scoliosis (AIS) surgery and 85% solid fusion rate using ICBG (Tables 5, 6) [53]. With a mean follow-up period of 2.94 years, Harshavardhana et al. reported a 100% fusion rate by 3 months postoperatively using Si–CaP ceramic mixed with locally harvested bone graft in AIS surgery [55]. Mashoof et al. reported 100% fusion rate using coralline hydroxyapatite ceramic mixed with ICBG [56]. Muschik et al. reported a 100% fusion rate using β-TCP mixed with autograft [57].
Outcomes
Delécrin et al. compared synthetic calcium phosphate ceramic graft and ICBG and reported lower blood loss in the ceramic group [58]. Successful integration of the ceramic blocks into the fusion mass was achieved within 12 months and both groups achieved a satisfactory maintained degree of deformity correction. Ilharreborde et al. reported that the use of bioactive glass compared to ICBG was associated with a significantly higher mean gain of frontal balance, 8.1 vs. 0.8 mm, as well as a significantly lower rate of loss of correction of the main thoracic curve, 11% vs. 15.5% [59]. Lerner et al. reported a lower degree of loss of curve correction in the β-TCP group compared to the ICBG group, 2.6 and 4.2, respectively [60]. Using Si–CaP enriched with BMA, Lerner et al. reported a significantly improved health-related quality of life and a 93% rate of patients' management satisfaction [61].
Complications
Harshavardhana et al. reported that two out of 35 patients had revision surgery for deep infection (n = 1) and implant failure (n = 1) [55]. In the ceramic group, Delécrin et al. reported one patient with superior grips dislodgement and had revision surgery [58]. Two other patients developed delayed-onset localized inflammatory reactions with prominent implants and had revisions. Ilharreborde et al. reported infection (2%), and mechanical failure requiring revision (2%) in the bioglass group [59]. The complications reported in Mashoof et al. study included superficial infection (n = 1), deep infection requiring debridement and implant removal (n = 1), and proximal hook dislodge (n = 1) [56]. Muschik et al. reported one case of deep infection [57].
Some studies report minimal differences in complication rates between various types of biologics and alternative graft materials. In a study by Smith (2014) on treatment of adjacent segment disease in older patients (mean age 54.8), they demonstrated no significant difference (p > 0.05) in the risk of rod fracture among adult spinal deformity patients with allograft vs. autograft vs. demineralized bone matrix vs. rhBMP-2 [40]. Another study reported no significant differences between curve type, number of levels fused, postoperative infections, pseudarthrosis, reoperations, or Scoliosis Research Scoiety-30 scores across each of the types of grafts used (allograft, autogenous iliac bone crest graft, or bone substitute) in the treatment of adolescent idiopathic scoliosis. Bone substitutes included DBM (DePuy Synthes), tricalcium phosphate (Depuy Synthes), coralline hydroxyapatite (Medtronic), and Cellect (DePuy Synthes—Selective Cell Retention technology with a combination of bone-marrow aspirate with matrix [30]. Moreover, a study with similar demographics comparing allograft and a strip of flexible demineralized bone matrix (Grafton DBM Flex) reported no pseudarthrosis, anterior overgrowth, or implant failure in either of the two groups [33].
To our knowledge, there are no randomized controlled studies comparing the efficacy of biologics to autograft alone in preventing pseudarthrosis following deformity correction surgery. Lower quality comparative studies have its flaws as difference in fusion technique and deformity correction principles add on to time-dependent biases when cohorts of different eras are compared.
Nanotechnology and osteobiologics
Nanotechnology has been used in various biomedical applications, and the newer addition of nanotechnology to synthetics can have promising results in enhancing spine fusion. Nanoparticles are particles with a size between 10 and 1000 nm [62].
Scaffold materials for rBMP2 have reduced affinity for it, leading to widespread release causing complications of hematomas and seromas, as well as exaggerated inflammatory responses [63]. The use of bioactive peptide amphiphile nanofiber scaffolds was reported by Lee et al. as an effective method for localized controlled delivery of rBMP2 to the site of fusion [64]. Moreover, the mineral structure of nanophase hydroxyapatite is nearly identical to bone with enhanced osteoblastic adhesion [65].
Bioactive glass nanofibres have a higher mesenchymal stem cell activity compared to conventional bioactive glass [66]. Further high-quality comparative studies are needed to better elucidate the clinical efficacy of these newer graft material.
Conclusions
Although there are several published studies looking at the use of individual graft material and biologics for spinal deformity surgery, there is a significant paucity of randomized comparative studies due to several limitations. The gold standard efficacy of ICBG and local autografts are so well established that subsequent materials such as allografts, bone matrixes, ceramics, biologics, and bio-materials have been compared to historical data rather than direct randomized comparisons. In addition, variability among surgeon technique/expertise and the lack of standardization of graft materials used for each case invites substantial outcome variable and makes direct comparison difficult. With increasing FDA approval for newer graft materials and more industry sponsored data emerging constantly, it is important for surgeons to understand these limitations and the quality of the data when deciphering the literature on graft materials. Based on all these issues, and the lack of consistent and comparable data, we are unable to consistently recommend any one specific biological material over another for the purpose of achieving reliable fusion for spinal deformity cases. Future high-quality comparative studies and/or continued collaborative registry data are needed to objectively compare outcomes.
Availability of data and materials
Not applicable.
Code availability
Not applicable.
References
Cahill KS, Chi JH, Day A et al (2009) Prevalence, complications, and hospital charges associated with use of bone-morphogenetic proteins in spinal fusion procedures. JAMA 302(1):58–66. https://doi.org/10.1001/jama.2009.956
Lipson SJ (2004) Spinal-fusion surgery—advances and concerns. N Engl J Med 350(7):643–644. https://doi.org/10.1056/NEJMp038162
Hofler RC, Swong K, Martin B et al (2018) Risk of pseudoarthrosis after spinal fusion: analysis from the healthcare cost and utilization project. World Neurosurg 120:e194–e202. https://doi.org/10.1016/j.wneu.2018.08.026
Reid JJ, Johnson JS, Wang JC (2011) Challenges to bone formation in spinal fusion. J Biomech 44(2):213–220. https://doi.org/10.1016/j.jbiomech.2010.10.021
Kim YJ, Bridwell KH, Lenke LG et al (2005) Pseudarthrosis in primary fusions for adult idiopathic scoliosis: incidence, risk factors, and outcome analysis. Spine 30(4):468–474. https://doi.org/10.1097/01.brs.0000153392.74639.ea
Wang MC, Chan L, Maiman DJ et al (2007) Complications and mortality associated with cervical spine surgery for degenerative disease in the United States. Spine 32(3):342–347. https://doi.org/10.1097/01.brs.0000254120.25411.ae
Campana V et al (2014) Bone substitutes in orthopaedic surgery: from basic science to clinical practice. J Mater Sci Mater Med 25(10):2445–2461. https://doi.org/10.1007/s10856-014-5240-2
Grabowski G, Cornett CA (2013) Bone graft and bone graft substitutes in spine surgery: current concepts and controversies. J Am Acad Orthop Surg 21(1):51–60. https://doi.org/10.5435/JAAOS-21-01-51
Dimitriou R, Mataliotakis GI, Angoules AG et al (2011) Complications following autologous bone graft harvesting from the iliac crest and using the RIA: a systematic review. Injury 42(2):S3-15. https://doi.org/10.1016/j.injury.2011.06.015
Duarte RM, Varanda P, Reis RL et al (2017) Biomaterials and bioactive agents in spinal fusion. Tissue Eng Part B Rev 23(6):540–551. https://doi.org/10.1089/ten.TEB.2017.0072
Gupta A, Kukkar N, Sharif K et al (2015) Bone graft substitutes for spine fusion: a brief review. World J Orthop 6(6):449–456. https://doi.org/10.5312/wjo.v6.i6.449
Peterson B, Whang PG, Iglesias R et al (2004) Osteoinductivity of commercially available demineralized bone matrix. Preparations in a spine fusion model. J Bone Joint Surg Am 86(10):2243–2250. https://doi.org/10.2106/00004623-200410000-00016
Tilkeridis K, Touzopoulos P, Ververidis A et al (2014) Use of demineralized bone matrix in spinal fusion,". World J Orthop 5(1):30–37. https://doi.org/10.5312/wjo.v5.i1.30
Aghdasi B, Montgomery SR, Daubs MD et al (2013) A review of demineralized bone matrices for spinal fusion: the evidence for efficacy. Surgeon 11(1):39–48. https://doi.org/10.1016/j.surge.2012.08.001
Mulconrey DS, Bridwell KH, Flynn J, Cronen GA, Rose PS (2008) “Bone morphogenetic protein (RhBMP-2) as a substitute for iliac crest bone graft in multilevel adult spinal deformity surgery: minimum two-year evaluation of fusion,” (in eng). Spine 33(20):2153–2159. https://doi.org/10.1097/BRS.0b013e31817bd91e
Choo QQ, Chiu CK, Lisitha KA et al (2018) Quantitative analysis of local bone graft harvested from the posterior elements during posterior spinal fusion in adolescent idiopathic scoliosis patients. J Orthop 16(1):74–79. https://doi.org/10.1016/j.jor.2018.12.004.PMID:30662243;PMCID:PMC6324765
Franzin FJ, Gotfryd AO, Neto NJ et al (2014) Radiographic and functional evaluation of the iliac bone graft in the treatment of adolescent idiopathic scoliosis. J Pediatr Orthop B 23(4):307–311. https://doi.org/10.1097/BPB.0000000000000037
Kobayashi K, Ando K, Machino M et al (2020) Trends in medical costs for adolescent idiopathic scoliosis surgery in Japan. Global Spine J 10(8):1040–1045. https://doi.org/10.1177/2192568219886265
Milinković ZB, Krneta O, Milicković S et al (2010) Are the additional grafts necessary? Acta Chir Iugosl 57(1):69–72. https://doi.org/10.2298/aci1001069m
Pesenti S, Ghailane S, Varghese JJ et al (2017) Bone substitutes in adolescent idiopathic scoliosis surgery using sublaminar bands: is it useful? A case-control study. Int Orthop 41(10):2083–2090. https://doi.org/10.1007/s00264-017-3512-4
Farshad M, Frey A, Jentzsch T et al (2021) Reducing the kyphosis effect of anterior short thoracolumbar/lumbar scoliosis correction with an autograft fulcrum effect. BMC Musculoskelet Disord 22(1):216. https://doi.org/10.1186/s12891-021-04083-1
Ouellet JA, Johnston CE 2nd (2002) Effect of grafting technique on the maintenance of coronal and sagittal correction in anterior treatment of scoliosis. Spine 27(19):2129–2135. https://doi.org/10.1097/00007632-200210010-00010 (Discussion 2135-6)
Samartzis D, Bow C, Cheung JP et al (2016) Efficacy of postoperative pain management using continuous local anesthetic infusion at the iliac crest bone graft site in patients with adolescent idiopathic scoliosis: a parallel, double-blinded, randomized controlled pilot trial. Global Spine J. 6(3):220–228. https://doi.org/10.1055/s-0035-1558656
Kager AN, Marks M, Bastrom T, Newton PO (2006) Morbidity of iliac crest bone graft harvesting in adolescent deformity surgery. J Pediatr Orthop 26(1):132–134. https://doi.org/10.1097/01.bpo.0000188996.36674.56
Iwai C, Taneichi H, Inami S et al (2013) Clinical outcomes of combined anterior and posterior spinal fusion for dystrophic thoracolumbar spinal deformities of neurofibromatosis-1: fate of nonvascularized anterior fibular strut grafts. Spine 38(1):44–50. https://doi.org/10.1097/BRS.0b013e318261ec74
Johari A, Shingade V, Gajiwala AL et al (2007) The use of irradiated allograft in a paediatric population: an Indian experience. Cell Tissue Bank 8(1):13–22. https://doi.org/10.1007/s10561-006-9001-4
Jones KC, Andrish J, Kuivila T et al (2002) Radiographic outcomes using freeze-dried cancellous allograft bone for posterior spinal fusion in pediatric idiopathic scoliosis. J Pediatr Orthop 22(3):285–289
Watkins RG, Hussain N, Freeman BJ et al (2006) Anterior instrumentation for thoracolumbar adolescent idiopathic scoliosis: do structural interbody grafts preserve sagittal alignment better than morselized rib autografts? Spine 31(20):2337–2342. https://doi.org/10.1097/01.brs.0000240201.14208.68
Izatt MT, Carstens A, Adam CJ et al (2015) Partial intervertebral fusion secures successful outcomes after thoracoscopic anterior scoliosis correction: a low-dose computed tomography study. Spine Deform 3(6):515–527. https://doi.org/10.1016/j.jspd.2015.04.007
Theologis AA, Tabaraee E, Lin T, Spinal Deformity Study Group et al (2015) Type of bone graft or substitute does not affect outcome of spine fusion with instrumentation for adolescent idiopathic scoliosis. Spine 40(17):1345–1351. https://doi.org/10.1097/BRS.0000000000001002
Lowe TG, Alongi PR, Smith DAB et al (2003) Anterior single rod instrumentation for thoracolumbar adolescent idiopathic scoliosis with and without the use of structural interbody support. Spine 28(19):2232–2241. https://doi.org/10.1097/01.BRS.0000085028.70985.39
Hostin R, O’Brien M, McCarthy I et al (2016) Retrospective study of anterior interbody fusion rates and patient outcomes of using mineralized collagen and bone marrow aspirate in multilevel adult spinal deformity surgery. Clin Spine Surg 29(8):E384-388. https://doi.org/10.1097/BSD.0b013e318292468f
Weinzapfel B, Son-Hing JP, Armstrong DG et al (2008) Fusion rates after thoracoscopic release and bone graft substitutes in idiopathic scoliosis. Spine 33(10):1079–1083. https://doi.org/10.1097/BRS.0b013e31816f69b3
Sinagra Z, Cunningham G, Dillon D et al (2020) Proximal junctional kyphosis and rates of fusion following posterior instrumentation and spinal fusion for adolescent idiopathic scoliosis. ANZ J Surg 90(4):597–601. https://doi.org/10.1111/ans.15706
Betz RR, Petrizzo AM, Kerner PJ et al (2006) Allograft versus no graft with a posterior multisegmented hook system for the treatment of idiopathic scoliosis. Spine 31(2):121–127. https://doi.org/10.1097/01.brs.0000194771.49774.77
Knapp DR, Jones ET, Blanco JS et al (2005) Allograft bone in spinal fusion for adolescent idiopathic scoliosis. J Spinal Disord Tech 18:S73-76. https://doi.org/10.1097/01.bsd.0000128694.21405.80
Price CT, Connolly JF, Carantzas AC et al (2003) Comparison of bone grafts for posterior spinal fusion in adolescent idiopathic scoliosis. Spine 28(8):793–798
Wang J, Zhao Y, Shen B et al (2010) Risk factor analysis of proximal junctional kyphosis after posterior fusion in patients with idiopathic scoliosis. Injury 41(4):415–420. https://doi.org/10.1016/j.injury.2010.01.001
Buttermann GR, Glazer PA, Hu SS et al (2001) Anterior and posterior allografts in symptomatic thoracolumbar deformity. J Spinal Disord 14(1):54–66. https://doi.org/10.1097/00002517-200102000-00009
Smith JS, Shaffrey E, Klineberg E et al (2014) Prospective multicenter assessment of risk factors for rod fracture following surgery for adult spinal deformity. J Neurosurg Spine 21(6):994–1003. https://doi.org/10.3171/2014.9.SPINE131176
Bozzio AE, Hu X, Lieberman IH (2019) Cost and clinical outcome of adolescent idiopathic scoliosis surgeries-experience from a nonprofit community hospital. Int J Spine Surg 13(5):474–478. https://doi.org/10.14444/6063
Ong KL, Villarraga ML, Lau E et al (2010) Off-label use of bone morphogenetic proteins in the United States using administrative data. Spine 35:1794–1800. https://doi.org/10.1097/BRS.0b013e3181ecf6e4
Bess S, Line BG, Lafage V et al (2014) Does recombinant human bone morphogenetic protein-2 use in adult spinal deformity increase complications and are complications associated with location of rhBMP-2 use? A prospective, multicenter study of 279 consecutive patients. Spine 39:233–242. https://doi.org/10.1097/BRS.0000000000000104
Luhmann SJ, Bridwell KH, Cheng I et al (2005) Use of bone morphogenetic protein-2 for adult spinal deformity. Spine 30:S110-117. https://doi.org/10.1097/01.brs.0000175184.27407.6a
Ruofeng Y, Cohen JR, Buser Z et al (2016) Trends of posterior long segment fusion with and without recombinant human bone morphogenetic protein 2 in patients with scoliosis. Global Spine J 6:422–431. https://doi.org/10.1055/s-0035-1564416
Mulconrey DS, Bridwell KH, Flynn J et al (2008) Bone morphogenetic protein (RhBMP-2) as a substitute for iliac crest bone graft in multilevel adult spinal deformity surgery: minimum two-year evaluation of fusion. Spine 33:2153–2159. https://doi.org/10.1097/BRS.0b013e31817bd91e
Maeda T, Buchowski JM, Kim YJ et al (2009) Long adult spinal deformity fusion to the sacrum using rhBMP-2 versus autogenous iliac crest bone graft. Spine 34:2205–2212. https://doi.org/10.1097/BRS.0b013e3181b0485c
Kim HJ, Buchowski JM, Zebala LP et al (2013) RhBMP-2 is superior to iliac crest bone graft for long fusions to the sacrum in adult spinal deformity: 4- to 14-year follow-up. Spine 38:1209–1215. https://doi.org/10.1097/BRS.0b013e31828b656d
Puvanesarajah V, Jain A, Cancienne JM et al (2017) BMP use and the risk of revision surgery after long posterolateral fusions in the elderly. Clin Spine Surg 30:E931–E937. https://doi.org/10.1097/BSD.0000000000000489
Safaee MM, Dalle Ore CL, Zygourakis CC et al (2019) Estimating a price point for cost-benefit of bone morphogenetic protein in pseudarthrosis prevention for adult spinal deformity surgery. J Neurosurg Spine. https://doi.org/10.3171/2018.12.SPINE18613
Jain A, Yeramaneni S, Kebaish KM et al (2020) Cost-utility analysis of rhBMP-2 use in adult spinal deformity surgery. Spine 45:1009–1015. https://doi.org/10.1097/BRS.0000000000003442
Yoo JS, Ahn J, Patel DS et al (2019) An evaluation of biomaterials and osteobiologics for arthrodesis achievement in spine surgery. Ann Transl Med 7:S168. https://doi.org/10.21037/atm.2019.06.80
Ameri E, Behtash H, Mobini B et al. Bioactive glass versus autogenous iliac crest bone graft in adolescent idiopathic scoliosis surgery. Acta Medica Iranica 2009: 41–45
Hing KA, Revell PA, Smith N et al (2006) Effect of silicon level on rate, quality and progression of bone healing within silicate-substituted porous hydroxyapatite scaffolds. Biomaterials 27:5014–5026. https://doi.org/10.1016/j.biomaterials.2006.05.039
Harshavardhana NS, Noordeen MH (2015) Surgical results with the use of silicated calcium phosphate (SiCaP) as bone graft substitute in posterior spinal fusion (PSF) for adolescent idiopathic scoliosis (AIS). Scoliosis 10:27. https://doi.org/10.1186/s13013-015-0051-x
Mashoof AA, Siddiqui SA, Otero M et al (2002) Supplementation of autogenous bone graft with coralline hydroxyapatite in posterior spine fusion for idiopathic adolescent scoliosis. Orthopedics 25:1073–1076
Muschik M, Ludwig R, Halbhubner S et al (2001) Beta-tricalcium phosphate as a bone substitute for dorsal spinal fusion in adolescent idiopathic scoliosis: preliminary results of a prospective clinical study. Eur Spine J 10(2):S178-184. https://doi.org/10.1007/s005860100271
Delecrin J, Takahashi S, Gouin F et al (2000) A synthetic porous ceramic as a bone graft substitute in the surgical management of scoliosis: a prospective, randomized study. Spine 25(5):563-9. https://doi.org/10.1097/00007632-200003010-00006
Ilharreborde B, Morel E, Fitoussi F et al (2008) Bioactive glass as a bone substitute for spinal fusion in adolescent idiopathic scoliosis: a comparative study with iliac crest autograft. J Pediatr Orthop 28:347–351. https://doi.org/10.1097/BPO.0b013e318168d1d4
Lerner T, Bullmann V, Schulte TL et al (2009) A level-1 pilot study to evaluate of ultraporous beta-tricalcium phosphate as a graft extender in the posterior correction of adolescent idiopathic scoliosis. Eur Spine J 18:170–179. https://doi.org/10.1007/s00586-008-0844-1
Lerner T, Liljenqvist U (2013) Silicate-substituted calcium phosphate as a bone graft substitute in surgery for adolescent idiopathic scoliosis. Eur Spine J 22(2):S185-194. https://doi.org/10.1007/s00586-012-2485-7
Bazylinska U, Lewinska A, Lamch L, Wilk KA (2014) Polymeric nanocapsules and nanospheres for encapsulation and long sustained release of hydrophobic cyanine-type photosensitizer. Colloids Surf A Physicochem Eng Asp 442:42–49. https://doi.org/10.1016/j.colsurfa.2013.02.023
Viswanathan VK, Rajaram Manoharan SR, Subramanian S et al (2019) Nanotechnology in spine surgery: a current update and critical review of the literature. World Neurosurg 123:142–155. https://doi.org/10.1016/j.wneu.2018.11.035
Lee SS, Hsu EL, Mendoza M et al (2015) Gel scaffolds of BMP-2-binding peptide amphiphile nanofibers for spinal arthrodesis. Adv Healthc Mater 4(1):131–141. https://doi.org/10.1002/adhm.201400129
Stylios G, Wan T, Giannoudis P (2007) Present status and future potential of enhancing bone healing using nanotechnology. Injury 38(1):S63-74. https://doi.org/10.1016/j.injury.2007.02.011
Brannigan K, Griffin M (2016) An update into the application of nanotechnology in bone healing. Open Orthop J 30(10):808–823. https://doi.org/10.2174/1874325001610010808
Funding
No funding was received for this review paper.
Author information
Authors and Affiliations
Contributions
KEC: contribution to the study design, drafted a section of the manuscript, approved final version, agree to be accountable for all aspects of the work. MKM: contribution to acquisition and analysis, drafted a section of the manuscript, approved final version, agree to be accountable for all aspects of the work. ZF: contribution to acquisition and analysis, drafted a section of the manuscript, approved final version, agree to be accountable for all aspects of the work. ES: contribution to acquisition and analysis, drafted a section of the manuscript, approved final version, agree to be accountable for all aspects of the work. ZB: contribution to study design, acquisition, drafted and critically revised the manuscript, approved final version, agree to be accountable for all aspects of the work. JCW: contribution to study design, critically revised the manuscript, approved final version, agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflicts of interest
There are no conflicts of interest in this study. Disclosures outside of submitted work: ZB-consultancy: Cerapedics (past), Xenco Medical (past), AO Spine (past); Research Support: SeaSpine (past, paid to the institution), Medical Metrics (past, paid directly to institution), Next Science (past, paid directly to institution); North American Spine Society: committee member; Lumbar Spine Society: Co-chair Education committee, AOSpine Knowledge Forum Degenerative: Associate member; AOSNA Research committee—committee member; JCW—Royalties—Biomet, Seaspine, Synthes; Investments/Options—Bone Biologics, Pearldiver, Electrocore, Surgitech; Board of Directors—AO Foundation, American Orthopaedic Association; Fellowship Funding (paid to institution): AO Foundation.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chang, KE., Mesregah, M.K., Fresquez, Z. et al. Use of graft materials and biologics in spine deformity surgery: a state-of-the-art review. Spine Deform 10, 1217–1231 (2022). https://doi.org/10.1007/s43390-022-00529-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43390-022-00529-1