Abstract
Purpose
Various strategies are utilised to reduce blood loss and allogenic blood transfusion for posterior instrumented correction of Adolescent Idiopathic Scoliosis (AIS). The aim of this study was to evaluate post-operative blood transfusion requirements to determine whether routine cross matching of blood is essential.
Methods
This is a prospective case series of 84 patients who underwent posterior correction of AIS between September 2016 and March 2018. We reviewed demographic, operative, radiological data and transfusion requirements. Results of transfusion requirements in 44 patients who underwent Ponte osteotomies (F:M = 36:8; mean age 14.8 years) were compared with 40 patients (F:M = 9:31; mean age 14.4 years) who did not and provided the control group. A transfusion trigger of 80 mg/dl with clinical caveats was utilised. Cross matching and procurement costs of allogenic blood/unit were ascertained.
Results
Five patients required postoperative blood transfusion on days 2 or 3. Anaesthetic time (p = 0.0003) and preoperative Cobb angle (p = 0.0166) were significant variables between both groups and post-operative Hb (p = 0.0084) and number of levels fused (p = 0.0312) being significant in patients requiring transfusion. Unutilised units on the day of the operation incurred £30,030 (£380/patient or £154/unit) in operational costs.
Conclusion
Our audit demonstrates that transfusion on the day of the operation was not required. We recommend that routine crossmatching is not essential for primary posterior correction for AIS with blood conservation techniques. Blood grouping with availability of urgent blood is sufficient at the onset of operation. This has financial implications and cost savings.
Levels of evidence
III.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intraoperative blood loss is an important consideration in surgical management of Adolescent Idiopathic Scoliosis (AIS). The blood loss is closely linked to the curve magnitude, duration of surgery and the number of levels instrumented [1, 2].
Several strategies have been reported to reduce the blood loss and reduce the need for allogenic blood transfusion (ABT). These include autologous blood donation, haemodilution, the use of Tranexamic acid, hypotensive anaesthesia, platelet and fibrinogen replacement and the use of cell salvage [3,4,5,6,7,8,9,10].
Strategies for reducing the blood loss in patients undergoing spinal deformity correction surgery at our institution, involve a combination of Tranexamic acid, controlled intraoperative hypotension and cell salvage. Our routine practice involves cross matching of two units of blood for the day of the operation. The aim of this audit was to document the utilisation of cross-matched blood for transfusion during the operation and in the post-operative period, and secondarily to explore the factors associated with the blood loss and the costs associated with our blood cross matching policy.
Patients and methods
We report a prospective series of patients undergoing primary posterior correction of AIS between September 2016 and March 2018. We excluded AIS patients that underwent additional anterior releases or revisional procedures. The study was registered by the audit department of our institution (No. 19–034).
The hospital records were reviewed for patient demographics, operative data such as pre and postoperative haemoglobin (Hb), curve magnitude (pre and post-operative Cobb angles), number of Ponte osteotomies, the number of levels instrumented, Estimated blood loss (EBL) and total anaesthetic time. Patients undergoing osteotomies (OG) were compared with a cohort of patients that did not require any osteotomies (NO). These were compared as a control group to be able to analyse any differences in ABT requirements. Patients requiring transfusion (TG) were then analysed with those not requiring transfusion (NTG) to assess any significant differences.
The standard anaesthetic protocol was TCI (Target Controlled Infusion) as TIVA (total intravenous anaesthesia) using Propofol and Remifentanil and a BIS monitor along with spinal cord monitoring as standard. Standard invasive pressure monitoring was also used to monitor arterial blood pressure (ABP). Tranexamic acid (10 mg/kg) was administered as a bolus and maintenance dose [11]. All patients were treated in the High Dependency Unit for 24–48 h and transferred to the wards after.
The current hospital guidelines require that all patients undergoing scoliosis correction surgery have two units of blood cross matched prior to the surgical procedure and are made available on the day of the operation. Cross matched blood has undergone blood group (Rhesus) and antibody testing to ensure compatibility with the patient and is then transported and stored at our local blood bank awaiting possible transfusion. Unused blood is returned to the central blood bank to be utilised as required. The cost analysis was based on the total expense incurred from obtaining the blood to the point of returning unutilised blood. The calculation of the intraoperative blood loss was based on the cell salvage system.
The cost of obtaining a unit of blood was ascertained to be £138 with £60/unit being the cost of processing, testing and storage. Transportation costs were based on annual inter-departmental agreement and were separate to the blood costs. The unutilised blood was transported back to the central unit if the cold chain was unbroken and discarded if so and other reasons such as being issued multiple times and being deemed unusable and expiry due to time constraints.
The procedure was performed by two fellowship trained consultant deformity surgeons from our dedicated spinal team. Patients were placed prone on the Jackson table with Allen frame. A standard midline approach with subfascial dissection was carried out with meticulous attention to haemostasis. Based on the stiffness and magnitude of the curve, an intra-operative decision for the need of Ponte osteotomies was made. Pedicle screws were placed by the free-hand technique and checked on 2D C-arm fluoroscopy. Following the correction and fusion, the surgical wound was closed in layers with elimination of dead spaces. Drains were not used routinely.
Statistical analysis was performed with Graphpad InStat software (Graphpad Software, La Jolla, California) [12]. An unpaired Student’s t test was used for continuous data. A p value < 0.05 was considered to be statistically significant.
Results
Eighty four patients (F, 67; M, 17) were included in our study with a mean age of 14.6 years (range 10–18 years) and mean Body Mass Index (BMI) of 20.7 (range 13–36).
Forty patients (F:M = 9:31) with a mean age of 14.4 years (SD ± 2.3) (range 10–18 years) treated with posterior correction and fusion without osteotomies (NO) were compared with forty-four patients (F:M = 36:8) with a mean age of 14.8 years (SD ± 1.5) (range 12–18 years) that underwent osteotomies (OG) at a mean of 3.6 levels (range 2–7) (Table 1).
Data analysis (unpaired t test) revealed no statistical difference for the BMI, preoperative Hb, EBL(including % Estimated Blood Volume-(EBV), replaced blood, postoperative Hb, number of levels fused and postoperative Cobb angle between the Osteotomy and Non-Osteotomy groups. However anaesthetic time (p = 0.0003—two tailed unpaired t test) [NO- 301.5 min (range 180–480) vs. OG—357.7 min (range 207–504)] and preoperative Cobb angle (p = 0.0166) (two tailed unpaired t test) [NO—54° (range 32°–92°) vs. OG—60° (30°–87°) were found to be significant variables in our analysis. The cell salvage resulted in a mean intra-operative replacement of 422.8 cc.
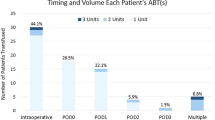
Five patients required postoperative ABT. None of the patients required intra-operative ABT. The transfusions were required on day 2 or 3 based on either transfusion trigger (80 mg/dl) or a clinical trigger (tachycardia/hypotension etc.). Data was then analysed between the transfused group (TG) (n = 5) and nontransfused group (NTG) (n = 79) with only postoperative Hb[TG-84.4 mg/dl (80–89) Vs NTG-99.7 (75–126)] (p = 0.0084—unpaired t test) and the number of levels fused [TG 13.2 (range 12–16) Vs. NTG 11.6 (range 8–16)] (p = 0.0312—two tailed unpaired t test) being significant (Table 2).
195 units of cross-matched blood were not utilised in our study resulting in a loss of £30,030 (£380/patient and £154/unit) in operating costs.
Discussion
Idiopathic scoliosis surgery can be associated with significant blood loss. Complexity of the operation with regards to the number of levels instrumented and the surgical approach can lead to an increased blood transfusion requirement in upto 30 percent of the patients [13, 14]. Reducing the need for transfusion is desirable as it reduces the possible adverse effects such as haemolytic and anaphylactic reactions and long-term immunosuppressive effects [15, 16]. Although the incidence of disease transmission and transfusion associated acute lung injury (TRALI) is diminishing, human errors continue to contribute to transfusion associated morbidity [17]. However post-operative anaemia if not treated can lead to Acute Kidney Injury (AKI), delirium, wound healing issues and increase the length of stay [18,19,20,21].
With our primary aim of assessing the transfusion requirements, we noted that none of our patients required transfusions intraoperatively or on the day of the operation. 5/84 (5.7%) needed a transfusion on postoperative days 2 or 3 post-operatively. These patients had sustained a higher blood loss, had a longer anaesthetic time and had a greater number of levels fused, that possibly explained the lower post-operative Hb level. These results indicate that cross matching of blood could have been safely performed as per clinical need postoperatively without the need for the routinely crossmatched two units.
Several studies have explored ways of mitigating allogenic transfusion such as the use of pre-donation of blood and the use of cell salvage [2, 22,23,24]. Cell salvage has been shown to be useful if anticipated blood loss is > 1000 cc or 20% of blood volume [25]. It has also positively reduced ABT, postoperative infection rates and length of stay [26]. In our study, we intraoperatively re-transfused an average of 423 cc from cell salvage. Forty-four patients in our study underwent osteotomies but only one patient required ABT which is contrary to the available evidence which suggests that an osteotomy increases the need for ABT [14, 27].
195 units of cross-matched blood not utilised resulted in a direct financial loss of £30,030. This study demonstrates that cross-matching blood can be safely deferred in uncomplicated AIS surgery until required. We concur with the recommendation of Popta et al. that preoperative cross matching should be performed only if there is a genuine anticipation of blood transfusion requirements [28]. Almanda et al. in their retrospective cohort study investigating the cost implications suggested that perioperative blood conservation strategies can be applied to minimise preoperative cross-matching [29].
The limitations of our study include small numbers, a single institution/spinal team protocol and results. While our findings support our conclusion that cross-matching blood for straightforward AIS surgery may not be required, further validation studies at external centres with a larger cohort of patients would be required to confirm our results.
Conclusion
Our study shows that routine crossmatching does not compromise patient safety for the standard AIS correction procedure. Our anaesthetic and blood conservation protocols obviated the need for transfusion on the day of the operation. This may have positive implications with avoiding transfusion complications and cost savings. Validation studies with larger cohorts to confirm our conclusions are required.
Data availability
Not applicable.
Code availability
Not applicable.
References
Shapiro F, Sethna N (2004) Blood loss in pediatric spine surgery. Eur Spine J 13:6–17. https://doi.org/10.1007/s00586-004-0760-y
Bowen RE, Gardner S, Scaduto AA et al (2010) Efficacy of intraoperative cell salvage systems in pediatric idiopathic scoliosis patients undergoing posterior spinal fusion with segmental spinal instrumentation. Spine (Phila Pa 1976) 35:246–251. https://doi.org/10.1097/BRS.0b013e3181bdf22a
Feldman JM, Roth JV, Bjoraker DG (1995) Maximum blood savings by acute normovolemic hemodilution. Anesth Analg 80:108–113. https://doi.org/10.1097/00000539-199501000-00019
Copley LAB, Richards BS, Safavi FZ et al (1999) Hemodilution as a method to reduce transfusion requirements in adolescent spine fusion surgery. Spine (Phila Pa 1976) 24:219–222. https://doi.org/10.1097/00007632-199902010-00005
Carey PA, Schoenfeld AJ, Cordill RD et al (2015) A comparison of cell salvage strategies in posterior spinal fusion for adolescent idiopathic scoliosis. J Spinal Disord Tech 28:1–4. https://doi.org/10.1097/BSD.0b013e318284e0b3
Liang J, Shen J, Chua S et al (2014) Does intraoperative cell salvage system effectively decrease the need for allogeneic transfusions in scoliotic patients undergoing posterior spinal fusion? A prospective randomized study. Geochemistry Int 53:270–275. https://doi.org/10.1007/s00586-014-3282-2
Jones KE, Butler EK, Barrack T et al (2017) Tranexamic acid reduced the percent of total blood volume lost during adolescent idiopathic scoliosis surgery. Int J Spine Surg 11:212–217
Florentino-Pineda I, Thompson GH, Poe-Kochert C et al (2004) The effect of amicar on perioperative blood loss in idiopathic scoliosis: the results of a prospective, randomized double-blind study. Spine (Phila Pa 1976) 29:233–238. https://doi.org/10.1097/01.BRS.0000109883.18015.B9
Meert KL, Kannan S, Mooney JF (2002) Predictors of red cell transfusion in children and adolescents undergoing spinal fusion surgery. Spine (Phila Pa 1976) 27:2137–2142. https://doi.org/10.1097/00007632-200210010-00012
Tate DE, Friedman RJ (1992) Blood conservation in spinal surgery. Spine (Phila Pa) 17:1450–1456. https://doi.org/10.1097/00007632-199212000-00002
Haynes AB, Weiser TG, Berry WR, et al (2011) WHO surgical safety checklist resources. https://www.who.int/patientsafety/safesurgery/checklist/en/
Instat GraphPad (2003) Instat GraphPad version 3.00 for windows
Yoshihara H, Yoneoka D (2014) National trends in spinal fusion for pediatric patients with idiopathic scoliosis: demographics, blood transfusions, and in-hospital outcomes. Spine (Phila Pa 1976) 39:1144–1150. https://doi.org/10.1097/BRS.0000000000000354
Yoshihara H, Yoneoka D (2014) Predictors of allogeneic blood transfusion in spinal fusion for pediatric patients with idiopathic scoliosis in the united states, 2004–2009. Spine (Phila Pa 1976) 39:1860–1867. https://doi.org/10.1097/BRS.0000000000000530
Suddock J, Crookston K (2020) Transfusion reactions. In: StatPearls [Internet]. StatPearls Publishing
Youssef LA, Spitalnik SL (2017) Transfusion-related immunomodulation: a reappraisal. Curr Opin Hematol 24:551–557. https://doi.org/10.1097/MOH.0000000000000376
Bolton-Maggs PHB (2017) Serious hazards of transfusion – conference report: celebration of 20 years of UK haemovigilance. Transfus Med 27:393–400. https://doi.org/10.1111/tme.12502
Musallam KM, Tamim HM, Richards T et al (2011) Preoperative anaemia and postoperative outcomes in non-cardiac surgery: a retrospective cohort study. Lancet 378:1396–1407. https://doi.org/10.1016/S0140-6736(11)61381-0
Lasocki S, Krauspe R, Von Heymann C et al (2015) PREPARE: The prevalence of perioperative anaemia and need for patient blood management in elective orthopaedic surgery: a multicentre, observational study. Eur J Anaesthesiol 32:160–167. https://doi.org/10.1097/EJA.0000000000000202
Hobson C, Ozrazgat-Baslanti T, Kuxhausen A et al (2015) Cost and mortality associated with postoperative acute kidney injury. Ann Surg 261:1207–1214. https://doi.org/10.1097/SLA.0000000000000732
Maldonado JR (2013) Neuropathogenesis of delirium: review of current etiologic theories and common pathways. Am J Geriatr Psychiatry 21:1190–1222. https://doi.org/10.1016/j.jagp.2013.09.005
Anand N, Idio FG, Remer S, Hoppenfeld S (1998) The effects of perioperative blood salvage and autologous blood donation on transfusion requirements in scoliosis surgery. J Spinal Disord 11:532–534. https://doi.org/10.1097/00002517-199812000-00014
Katranitsa L, Gkantsinikoudis N, Kapetanakis S et al (2018) Perioperative blood management in posterior instrumented fusion for adolescent idiopathic scoliosis: original study and short review of the literature. Folia Med (Plovdiv) 60:200–207. https://doi.org/10.1515/folmed-2017-0100
Ersen O, Ekıncı S, Bılgıc S et al (2012) Posterior spinal fusion in adolescent idiopathic scoliosis with or without intraoperative cell salvage system: a retrospective comparison. Musculoskelet Surg 96:107–110. https://doi.org/10.1007/s12306-012-0203-6
Ashworth A, Klein AA (2010) Cell salvage as part of a blood conservation strategy in anaesthesia. Br J Anaesth 105:401–416. https://doi.org/10.1093/bja/aeq244
Duffy G, Tolley K (1997) Cost analysis of autologous blood transfusion, using cell salvage, compared with allogeneic blood transfusion. Transfus Med 7:189–196. https://doi.org/10.1046/j.1365-3148.1997.d01-25.x
Koerner JD, Patel A, Zhao C et al (2014) Blood loss during posterior spinal fusion for adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 39:1479–1487. https://doi.org/10.1097/BRS.0000000000000439
Van Popta D, Stephenson J, Patel D et al (2014) The pattern of blood loss in adolescent idiopathic scoliosis. Spine J 14:2938–2945. https://doi.org/10.1016/j.spinee.2014.05.022
Alamanda VK, Massengill DL, Rozario N et al (2018) Blood loss trends and financial implications in adolescent idiopathic scoliosis. Clin Spine Surg 31:E418–E421. https://doi.org/10.1097/BSD.0000000000000689
Funding
No funding was received in relation to this article.
Author information
Authors and Affiliations
Contributions
SH: Original concept, discussed framework, analysed data, wrote and critically reviewed article for final acceptance. RT: Discussed framework, collected and analysed data, wrote and critically reviewed article for final acceptance. NN: Discussed framework, collected data, critically reviewed article for final acceptance. DG: Treated patients, analysed data, critically reviewed article for final acceptance. CB: Treated patients, analysed data, critically reviewed article for final acceptance. JM: Original concept, treated patients, discussed framework, analysed data, wrote and critically reviewed article for final acceptance.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest in relation to this article.
IRB/Ethics statement
Local audit committee approval was obtained in the form of service evaluation (Project reference number – 19-034).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Haleem, S., Thimmaiah, R., Nagrath, N. et al. The impact of blood conservation techniques on transfusion requirements for posterior adolescent idiopathic scoliosis corrections: do we need a routine cross-match for the operation?. Spine Deform 10, 589–593 (2022). https://doi.org/10.1007/s43390-021-00454-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43390-021-00454-9