Abstract
Study design
Retrospective descriptive, single-center study.
Objectives
To determine the effect of standardized intrawound vancomycin powder and betadine irrigation on surgical site infection (SSI) rates after posterior spinal fusion (PSF) in idiopathic scoliosis.
Summary of background data
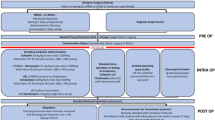
Since 2005, our pediatric spine center has implemented a series of changes to lower the risk of SSI. The most recent interventions—intrawound vancomycin powder and betadine irrigation—are applied just before closure, aiming to reduce the culture-positive bacterial contamination known to occur in many cases. We sought to determine the impact of these end-of-case measures on our center’s SSI rate.
Methods
We retrospectively reviewed patients who underwent PSF for idiopathic scoliosis at our institution from January 1, 2010, to June 30, 2018, identifying all cases that returned to the operating room for surgical debridement within 90 days of PSF. Cases were surgeon-audited to ensure inclusion of all infections that met Centers for Disease Control and Prevention (CDC) criteria for acute SSI. Vertical expandable prosthetic titanium ribs, growing rods, staged procedures, and nonidiopathic cases were excluded. Annual rates of SSIs were correlated with the initiation of each SSI prevention measure.
Results
Among 740 cases of PSF for idiopathic scoliosis from 2010 to 2018, the overall acute SSI rate by CDC criteria was 0.68%. The idiopathic SSI rate dropped significantly, from 1.70 to 0.20%, after the standardized introduction of intrawound vancomycin powder and betadine irrigation before closure (p < 0.04). The implementation of these end-of-case measures in 2012–2013 was soon followed by an institution best 3.5-year SSI-free period for idiopathic cases.
Conclusions
Since intrawound vancomycin powder and betadine irrigation were added to our SSI prevention bundle, we have seen a significantly lower SSI rate after PSFs for idiopathic scoliosis. These findings suggest that anti-SSI interventions to reduce wound contamination at the end of the case may have a particularly positive impact on SSI reduction.
Level of evidence
Level III, therapeutic.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Preventing surgical site infections (SSIs) has been an intense focus for those working to improve the quality, safety, and value in pediatric spinal deformity surgery. The costs of readmission and reoperation for SSI after posterior spinal fusion (PSF) are high, ranging from $26,977 to $961,722 [1]. Coupled with these high costs are well-established intraoperative contamination rates that make PSFs inherently high-risk procedures. Some studies have demonstrated that as many as 21% of idiopathic PSF cases are contaminated just before closure, whereas others have shown that nearly a third of PSF cases become contaminated at earlier stages of the procedure [2, 3]. As a result of these high rates of contamination, the risk of infection after PSF ranges from 0.5 to 1.6% in idiopathic scoliosis, and rates as high as 15.2% have been seen in series of neuromuscular scoliosis [4,5,6,7,8,9,10,11,12,13,14].
A wide variety of infection prevention strategies have been studied to address SSI after PSF, ranging from comprehensive antibiotic prophylaxis protocols to preoperative skin preparation to postoperative dressing changes [15,16,17,18]. Inconsistent results and practice variation across institutions have made it difficult to reach consensus on the most effective infection prevention strategies [18]. Consequently, Best Practice Guidelines (BPG) are often based on expert opinion rather than evidence-based research [19].
Beginning in 2005, our large pediatric spine center implemented an SSI Bundle to minimize SSI rates. The Bundle has been updated and improved periodically, based on new evidence. The Bundle includes measures before entering the operating room (OR; like chlorhexidine gluconate 4% [CHG] wipes), measures just before surgery (strict control of the timing of antibiotic administration), end-of-case measures to decrease wound contamination, and postoperative measures (antibiotics and wound care). We have closely tracked the impact of newly implemented changes to our Bundle over the past decade. The most recently implemented interventions, intrawound vancomycin powder and betadine irrigation, are applied just before closure. Both of these interventions were transitioned into our standard of care 2012–2013. The purpose of this study was to determine the effects of these end-of-case interventions on the infection rate after PSF for idiopathic scoliosis in a pediatric population and determine if it is the end that truly counts.
Materials and methods
We studied a consecutive series of patients who had a posterior spinal fusion between 2010 and June 2018 performed by one of the seven pediatric orthopaedic surgeons at a single tertiary care children’s hospital, and captured every acute SSI case. Cases of potential infection were surgeon-audited to identify all true infections that met the Centers for Disease Control and Prevention (CDC) definition of acute SSI—a postoperative wound infection within 90 days of the index procedure [20]. Juvenile and adolescent idiopathic scoliosis cases were included. Nonidiopathic (neuromuscular, syndromic, congenital) cases of scoliosis were excluded. Patients with previous spinal procedures including vertical expandable prosthetic titanium ribs, growing rods, and staged procedures were also excluded. Surgical techniques, including choice of implants, number of levels fused, osteotomies, and wound closure, were not standardized between surgeons or across the study period. Study approval was obtained from the institutional review board.
Annual surgical site infection rates were calculated for each year. Both intrawound vancomycin powder (500 mg) and betadine irrigation were transitioned into our standard of care during 2012–2013. January 1, 2013, was the start date for which both interventions were consistently used by all pediatric spine surgeons at our institution. These measures were added to our previously implemented infection prevention strategies—a Bundle in 2010 (intravenous cefazolin given within 60 min of incision, impervious dressing + CHG applied after wound closure) and distribution of CHG wipes for use at home preoperatively in 2011. We evaluated the effect of these end-of-case measures by comparing before and after intervention SSI rates. Statistical significance (two-tailed, p < 0.05) was tested using the χ2 and Fisher exact test, depending on the number of cases. All statistical analyses were conducted using SPSS version 23 (SPSS Inc., Armonk, NY).
Results
A consecutive series of 740 cases of PSF for idiopathic scoliosis from January 1, 2010, to June 30, 2018, were analyzed. Five cases met criteria for SSI, yielding an overall SSI rate of 0.68% during the 8.5-year study period. The mean time from surgery to reoperation for SSI was 27.8 days (range 14–69). Adolescent idiopathic scoliosis (AIS) was the most common etiology within the cohort (91.9%, n = 680), and all five infections occurred in patients with AIS. The remainder of the cohort consisted of patients with juvenile idiopathic scoliosis (JIS); no SSIs occurred in these patients.
Following the standardized introduction of intrawound vancomycin powder and betadine irrigation before closure, the SSI rate dropped significantly, from 1.70% (n = 4/235) to 0.20% (n = 1/505) (Fig. 1, p < 0.04). This corresponded to a number needed to treat (NNT) of 67 patients—to prevent one SSI within 90 days of surgery, these measures would have to be used in 67 cases. The implementation of these end-of-case measures in 2012–2013 was soon followed by an institution best 3.5-year SSI-free period for idiopathic cases. No significant changes in idiopathic SSI rates were seen after the standardized implementation of previous infection prevention measures, including the initial Bundle in 2010 and distribution of CHG wipes for preoperative use at home in 2011.
Discussion
Implementing effective infection prevention strategies in pediatric spinal deformity surgery has been a major quality focus for the past decade. Studies showing that nearly one-third of wounds become contaminated over the course of the procedure provide some insight regarding, where SSI prevention measures should focus [2, 3]. Shiono et al. studied 80 patients with cultures collected at multiple time points during the PSF procedure and demonstrated that although 25% of cases are contaminated after initial exposure, 32.5% are contaminated just before wound closure [3]. When considering only idiopathic pediatric cases of PSF, Nandyala et al. identified 11 of 52 (21%) cases that had positive microbial cultures from the paraspinal muscles just before wound closure [2]. Not every instance of contamination leads to infection, but even in idiopathic cases, nearly 2% of cases become infected in the immediate postoperative period [4,5,6,7,8].
The consequences of postoperative SSI are substantial, with high costs of management and the morbidity associated with removal of implants and potential progression of spinal deformity [1, 21]. There remains a substantial variation in infection prevention strategies between institutions and surgeons. A 2013 survey distributed by the Pediatric Orthopaedic Society of North America (POSNA) showed that among the 123 surgeons who responded, consensus (> 80% agreement) was reached for less than half of the 30 proposed SSI prevention measures [18]. Consequently, Best Practice Guidelines for infection prevention in pediatric spinal deformity surgery are largely based on expert consensus [15, 19].
Coordinating efforts with infectious disease experts, hospital administration and operating room leadership, our pediatric spine surgeons implemented a variety of potential SSI prevention measures, with continued OR surveillance and regular committee meetings to examine compliance and SSI rates. Although our infection prevention strategies were standardized, other aspects of surgical technique varied with surgeon preference and over time, particularly as new implants and techniques became available. Nonetheless, the overall SSI rate for our idiopathic cases (0.68%) is consistent with previous literature [4,5,6,7,8]. The standardized practice of the application of intrawound vancomycin powder and copious betadine irrigation just before wound closure led to a clinically and statistically significant decrease in SSI rates among patients undergoing PSF for idiopathic scoliosis (1.70% vs. 0.20%, NNT = 67).
The impact of betadine irrigation on SSI risk has been investigated in a variety of surgical fields [22,23,24,25]. Within pediatric orthopedics, betadine (povidone–iodine) irrigation has been shown to significantly decrease the risk of SSI among AIS patients compared with intraoperative gentamicin irrigation (26.7% vs. 7.0%) [26]. Despite this, less than half of surgeons routinely use betadine irrigation for infection prevention after PSF, with rates ranging from 9 to 39% [18, 19]. The efficacy of dilute betadine irrigation is more well-established in the adult spine literature [27,28,29,30]. Most notably, Tomov et al. demonstrated a 50% reduction in post-spinal surgery SSI rates in a cohort of more than 2400 adults after the standardized introduction of betadine irrigation and application of intrawound vancomycin powder [30]. Although some have proposed potentially adverse effects of betadine on wound healing through in vitro studies that demonstrated a reduction in fibroblast migration and proliferation, Chang et al. showed that diluted betadine solutions do not influence wound healing, bone union, or clinical outcome [27, 31]. Our clinical experiences with betadine irrigation align with their findings; we did not observe any change in rates of wound healing or fusion after the standardized implementation of betadine irrigation in 2012–2013.
Independently, the effectiveness of intrawound vancomycin powder at preventing SSI after PSF is less clear. Recent literature suggests that its widespread usage may increase the risk of Gram-negative and polymicrobial SSIs [32, 33]. Though in vitro studies have demonstrated its potentially harmful effects on bone healing and cellular viability and morphology, these adverse effects have not been found in clinical practice [34,35,36]. Similar to betadine irrigation, the role of intrawound vancomycin powder has been better evaluated in the adult spine literature. Some authors have contended that the usage of intrawound vancomycin powder improves infection rates both on its own and when combined with intravenous antibiotic prophylaxis [37,38,39,40,41]. Alternatively, others have shown that infection rates are similar, even after the implementation of intrawound vancomycin powder (5.3% vs. 5.1%), though the authors acknowledged that they planned to continue using the technique [41]. In one published pediatric study, van Herwijnen et al. showed that its usage in addition to betadine irrigation did not further reduce AIS SSI rates compared with betadine alone [26]. It is important to note, however, that their definition of SSI differed from ours and included superficial infections and a 1-year follow-up period.
Part of the difficulty in understanding the impact of intrawound vancomycin powder on pediatric SSI rates is the variability in microbe susceptibility, based on underlying etiology. Neuromuscular patients are more vulnerable to Gram-negative infections, which are not covered by prophylactic vancomycin [4, 6,7,8, 15, 42, 43]. Some have recommended that vancomycin be added to the wounds of these “high-risk” patients [19]. A post hoc analysis of our 197 neuromuscular PSF cases from 2010–2018 demonstrated that end-of-case measures did not improve the SSI rate (6.5% vs. 5.3%, p = 0.75), suggesting that intrawound vancomycin may play less of a preventive role in nonidiopathic cases, given their known risk of Gram-negative infections and fecal postoperative wound contamination (Table 1).
When taking a closer look at our data, there is a slight trend toward improving SSI rate from 2010 to 2012, before the implementation of end-of-case measures, but after the implementation of bundles and preoperative CHG wipes. These earlier interventions were previously shown to not significantly decrease SSI rates at our institution. Additionally, given what is known about progressively increased wound contamination, strategies applied before surgery, such as preoperative CHG wipes, are less likely to have an impact.
This study has several limitations. First, it is a single-center, retrospective study. The strength of this methodology is that our institution can serve as its own internal control—the OR environment, local biome, and surgeons’ habits are similar over time, and we can monitor compliance to ensure that interventions are being applied appropriately. The drawback is that this may limit its generalizability to other institutions that have different practice patterns, biomes and resources. Additionally, this study design and the lack of standardization of surgical technique over time limit our ability to control for other factors that could impact risk of postoperative surgical site infection, including BMI, type of implant, surgical techniques (i.e., osteotomies), surgical time, and number of levels fused. A randomized controlled trial would circumvent this limitation; however, the feasibility of such a study is low, in light of the established high rates of intraoperative contamination during PSF. Furthermore, the low incidence of SSI after PSF makes it difficult to demonstrate significant effects of each individual intervention. Though we cannot definitively distinguish the impact of individual measures vs. the cumulative effects of the changes implemented, our evidence now shows that the most recent end-of-case interventions are the first to significantly reduce SSI rates in our idiopathic scoliosis cohort.
In summary, a decade of practice changes, surveillance, and interventions at our institution has had varying impact on SSI rates after spinal fusion. Intrawound vancomycin powder and copious betadine irrigation at the end of the case are the first interventions to significantly lower the SSI rate after PSF for idiopathic scoliosis. These findings emphasize the importance of taking appropriate infection-preventing precautions just before closing the wound.
Key points
-
End-of-case measures, such as applying intrawound vancomycin powder and betadine irrigation, were particularly valuable in reducing SSI rates after PSF in patients with idiopathic scoliosis.
-
The standardized introduction of these two interventions led to an institution best 3.5-year SSI-free period in idiopathic cases.
-
These are the first in a series of anti-SSI interventions implemented at our institution to significantly reduce SSI rates after PSFs for idiopathic scoliosis.
References
Hedequist D, Haugen A, Hresko T et al (2009) Failure of attempted implant retention in spinal deformity delayed surgical site infections. Spine 34:60–64
Nandyala SV, Schwend RM (2013) Prevalence of intraoperative tissue bacterial contamination in posterior pediatric spinal deformity surgery. Spine 38:E482–E486
Shiono Y, Watanabe K, Hosogane N et al (2012) Sterility of posterior elements of the spine in posterior correction surgery. Spine 37:523–526
Cahill PJ, Warnick DE, Lee MJ et al (2010) Infection after spinal fusion for pediatric spinal deformity: 30 years of experience at a single institution. Spine 35:1211–1217
Ho C, Skaggs DL, Weiss JM et al (2007) Management of infection after instrumented posterior spine fusion in pediatric scoliosis. Spine 32:2739–2744
Labbe AC, Demers AM, Rodrigues R et al (2003) Surgical-site infection following spinal fusion: a case–control study in a children’s hospital. Infect Control Hosp Epidemiol 24:591–595
Linam WM, Margolis PA, Staat MA et al (2009) Risk factors associated with surgical site infection after pediatric posterior spinal fusion procedure. Infect Control Hosp Epidemiol 30:109–116
Mackenzie WGS, Matsumoto H, Williams BA et al (2013) Surgical site infection following spinal instrumentation for scoliosis: a multicenter analysis of rates, risk factors, and pathogens. J Bone Jt Surg Am 95:800–806
Mohamed Ali MH, Koutharawu DN, Miller F et al (2010) Operative and clinical markers of deep wound infection after spine fusion in children with cerebral palsy. J Pediatr Orthop 30:851–857
Sponseller PD, Shah SA, Abel MF et al (2010) Infection rate after spine surgery in cerebral palsy is high and impairs results: multicenter analysis of risk factors and treatment. Clin Orthop Relat Res 468:711–716
Teli MGA, Cinnella P, Vincitorio F et al (2006) Spinal fusion with Cotrel–Dubousset instrumentation for neuropathic scoliosis in patients with cerebral palsy. Spine 31:E441–E447
Tsirikos AI, Lipton G, Chang WN et al (2008) Surgical correction of scoliosis in pediatric patients with cerebral palsy using the unit rod instrumentation. Spine 33:1133–1140
Dias RC, Miller F, Dabney K et al (1996) Surgical correction of spinal deformity using a unit rod in children with cerebral palsy. J Pediatr Orthop 16:734–740
Borkhuu B, Borowski A, Shah SA et al (2008) Antibiotic-loaded allograft decreases the rate of acute deep wound infection after spinal fusion in cerebral palsy. Spine 33:2300–2304
Glotzbecker MP, Riedel MD, Vitale MG et al (2013) What’s the evidence? Systematic literature review of risk factors and preventive strategies for surgical site infection following pediatric spine surgery. J Pediatr Orthop 33:479–487
Kamath VHD, Cheung JPY, Mak KC et al (2016) Antimicrobial prophylaxis to prevent surgical site infection in adolescent idiopathic scoliosis patients undergoing posterior spinal fusion: 2 doses versus antibiotics till drain removal. Eur Spine J 25:3242–3248
Vandenberg C, Niswander C, Carry P et al (2018) Compliance with a comprehensive antibiotic protocol improves infection incidence in pediatric spine surgery. J Pediatr Orthop 38:287–292
Glotzbecker MP, Vitale MG, Shea KG et al (2013) Surgeon practices regarding infection prevention for pediatric spinal surgery. J Pediatr Orthop 33:694–699
Vitale MG, Riedel MD, Glotzbecker MP et al (2013) Building consensus: development of a best practice guideline (BPG) for surgical site infection (SSI) prevention in high-risk pediatric spine surgery. J Pediatr Orthop 33:471–478
Berríos-Torres SI, Umscheid CA, Bratzler DW et al (2017) Centers for disease control and prevention guideline for the prevention of surgical site infection, 2017. JAMA Surg 152:784–791
Harrod CC, Boykin RE, Hedequist DJ (2009) Complications of infection in pediatric spine surgery. Pediatr Health 3:579–592
Patel KS, Goldenberg B, Schwartz TH (2014) Betadine irrigation and post-craniotomy wound infection. Clin Neurol Neurosurg 118:49–52
Frisch NB, Kadri OM, Tenbrunsel T et al (2017) Intraoperative chlorhexidine irrigation to prevent infection in total hip and knee arthroplasty. Arthroplast Today 3:294–297
Mahomed K, Ibiebele I, Buchanan J (2016) The betadine trial—antiseptic wound irrigation prior to skin closure at caesarean section to prevent surgical site infection: a randomised controlled trial. Aust N Z J Obstet Gynaecol 56:301–306
Norman G, Atkinson RA, Smith TA et al (2017) Intracavity lavage and wound irrigation for prevention of surgical site infection. Cochrane Database Syst Rev 10:12234
van Herwijnen B, Evans NR, Dare CJ et al (2016) An intraoperative irrigation regimen to reduce the surgical site infection rate following adolescent idiopathic scoliosis surgery. Ann R Coll Surg Engl 98:320–323
Chang FY, Chang MC, Wang ST et al (2006) Can povidone-iodine solution be used safely in a spinal surgery? Eur Spine J 15:1005–1014
Cheng MT, Chang MC, Wang ST et al (2005) Efficacy of dilute betadine solution irrigation in the prevention of postoperative infection of spinal surgery. Spine 30:1689–1693
Chundamala J, Wright JG (2007) The efficacy and risks of using povidone-iodine irrigation to prevent surgical site infection: an evidence-based review. Can J Surg 50:473–481
Tomov M, Mitsunaga L, Durbin-Johnson B et al (2015) Reducing surgical site infection in spinal surgery with betadine irrigation and intrawound vancomycin powder. Spine 40:491–499
Thomas GW, Rael LT, Bar-Or R et al (2009) Mechanisms of delayed wound healing by commonly used antiseptics. J Trauma 66:82–90
Gans I, Dormans JP, Spiegel DA et al (2013) Adjunctive vancomycin powder in pediatric spine surgery is safe. Spine 38:1703–1707
Gande A, Rosinski A, Cunningham T et al (2019) Selection pressures of vancomycin powder use in spine surgery: a meta-analysis. Spine J 19:1076–1084
Eder C, Schenk S, Trifinopoulos J et al (2016) Does intrawound application of vancomycin influence bone healing in spinal surgery? Eur Spine J 25:1021–1028
Goldschimdt E, Rasmussen J, Chabot JD et al (2016) The effect of vancomycin powder on human dural fibroblast culture and its implications for dural repair during spine surgery. J Neurosurg Spine 25:665–670
Xie L, Zhu J, Luo S et al (2018) Do dose-dependent microbial changes occur during spine surgery as a result of applying intrawound vancomycin powder? A systematic literature review. Asian Spine J 12:62–70
Molinari RW, Khera OA, Molinari WJ (2012) Prophylactic intraoperative powdered vancomycin and postoperative deep spinal wound infection: 1,512 consecutive surgical cases over a 6-year period. Eur J Spine 21(suppl 4):S476–S482
Sweet FA, Roh M, Sliva C (2011) Intrawound application of vancomycin for prophylaxis in instrumented thoracolumbar fusions: efficacy, drug levels, and patient outcomes. Spine 36:2084–2088
Strom RG, Pacione D, Kalhorn SP et al (2013) Decreased risk of wound infection after posterior cervical fusion with routine local application of vancomycin powder. Spine 38:991–994
Caroom C, Tullar JM, Benton EGJ et al (2013) Intrawound vancomycin powder reduces surgical site infections in posterior cervical fusion. Spine 38:1183–1187
Martin JR, Adogwa O, Brown CR et al (2014) Experience with intrawound vancomycin powder for spinal deformity surgery. Spine 39:177–184
Floccari LV, Milbrandt TA (2016) Surgical site infections after pediatric spine surgery. Orthop Clin N Am 47:387–394
Aleissa S, Parsons D, Grant J et al (2011) Deep wound infection following pediatric scoliosis surgery: incidence and analysis of risk factors. Can J Surg 54:263–270
Funding
No funding was obtained for this study.
Author information
Authors and Affiliations
Contributions
BCM (none), DT (none), JMF (fees from Zimmer Biomet; personal fees from Wolters Kluwer Heath–Lippincott Williams & Wilkins, outside the submitted work; and board or committee member of the American Academy of Orthopaedic Surgeons, the American Board of Orthopaedic Surgery, Inc., Pediatric Orthopaedic Society of North America, and Scoliosis Research Society; Orthopedics Today, editorial or governing board).
Corresponding author
Ethics declarations
IRB approval
This study was approved by the Institutional Review Board.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Meza, B.C., Talwar, D. & Flynn, J.M. Measures to reduce end-of-case wound contamination: the impact of intra-wound vancomycin powder and betadine irrigation on surgical site infections in posterior spinal fusion. Spine Deform 8, 45–50 (2020). https://doi.org/10.1007/s43390-020-00033-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43390-020-00033-4