Abstract
Study design
Retrospective case series.
Objectives
The objective was to assess the long-term outcomes on scoliosis following Chiari-I (CM-I) decompression in patients with CM-I and syringomyelia (SM). A secondary objective was to identify risk factors of scoliosis progression.
Background
The association between CM-I with SM and scoliosis is recognized, but it remains unclear if CM-I decompression alters the long-term evolution of scoliosis in patients with associated syringomyelia.
Methods
A retrospective review of children with scoliosis, CM-I, and SM during 1997–2015 was performed. Congenital, syndromic, and neuromuscular scoliosis were excluded. Clinical and radiographic characteristics were recorded at presentation, pre-decompression, after 1-year, and latest follow-up. A scale to measure syringomyelia area on MRI was used to evaluate SM changes post-decompression.
Results
65 children with CM-I, SM, and scoliosis and a mean age of 8.9 years (range 0.7–15.8) were identified. Mean follow-up was 6.9 years (range 2.0–20.4). Atypical curves were present in 28 (43%) children. Thirty-eight patients (58%) underwent decompression before 10 years. Syringomyelia size reduced a mean of 70% after decompression (p < 0.001). Scoliosis improved in 26 (40%), stabilized in 17 (26%), and progressed in 22 (34%) cases. Early spinal fusion was required in 7 (11%) patients after a mean of 0.5 ± 0.37 years and delayed fusion in 16 (25%) patients after 6.0 ± 3.24 years. The remaining 42 (65%) patients were followed for a median of 6.1 years (range 2.0–12.3) without spine instrumentation or fusion. Fusion patients experienced less improvement in curve magnitude 1-year post-decompression (p < 0.001) and had larger curves at presentation (43° vs. 34°; p = 0.004).
Conclusions
Syringomyelia size decreased by 70% after CM-I decompression and scoliosis stabilized or improved in two-thirds of patients. Greater curve improvement within the first year post-decompression and smaller curves at presentation decreased the risk of spinal fusion. Neurosurgical decompression is recommended in children with CM-I, SM, and scoliosis with the potential to treat all three conditions.
Level of evidence
Level IV.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intraspinal pathology (ISP) must be ruled out in certain patients with idiopathic scoliosis. ISP is frequently associated with scoliosis, but less is documented about the management and evolution of scoliosis in these patients [1,2,3,4,5,6]. Chairi-1 malformations (CM-I) are common neuraxis anomalies found in nearly 4% of children under 18 years, with concomitant syringomyelia (SM) and scoliosis found in 88% and 20%, respectively [5, 7, 8].
Syringomyelia in patients with CM-I can be associated with scoliosis [9,10,11,12,13,14]. Two theories for SM formation exist: (1) water-hammer theory and (2) one-way valve theory. The water-hammer theory proposes that arterial pulses from the choroid plexus transmit cerebrospinal fluid down an abnormal fourth ventricle and mediate SM formation [9]. The one-way valve theory postulates that unequal pressures generated through the Valsalva maneuver causes increased pressure in the spinal cord resulting in SM development [9]. Neither is proven; however, MRI studies have found aberrant communications between the ventricles in CM-I patients [9, 11]. Biochemical and histologic data suggest that enlarging SM causes asymmetrical injury to the anterior horn of the spinal cord that may potentiate scoliosis through denervation and weakness of paraspinal musculature [10, 13, 14].
The relationship between CM-I, SM, and scoliosis is adequately documented, but neurosurgical decompression of CM-I on scoliosis outcome is unknown and mixed results are reported [7, 15,16,17,18,19,20]. These studies are often limited to small heterogeneous case series with short follow-up and some believe the benefits of decompression are temporary [14, 16,17,18, 20,21,22,23,24]. A recent series of 23 patients by Ravindra et al. reported poor durability of decompression, with 30% experiencing late curve progression requiring fusion after 5 years [20]. To the authors’ knowledge, this is the largest study to date evaluating the long-term outcomes of scoliosis after neurosurgical decompression in children with CM-I and SM.
The main objectives of this study were: (1) to describe the presentation of scoliosis in children with CM-I and SM; and (2) to identify risk factors of scoliosis progression and spinal fusion after decompression.
Methods
IRB approval (IRB-P00023640) was obtained and electronic medical records were reviewed for children with scoliosis, CM-I, and SM during 1997–2015. Neuromuscular, congenital, and syndromic scoliosis and prior spinal deformity surgery were excluded. Scoliosis was defined using the Cobb method: coronal curve ≥ 10°. Demographics, symptoms, neurologic abnormalities, bracing characteristics, and surgical details were recorded. Neurosurgical posterior fossa decompression (PFD) consisted of primary sub-occipital decompression with C1 laminectomy and Y-shaped duraplasty in all cases. A fourth ventricle-to-subarachnoid space stent was placed during PFD in a minority of cases when severe arachnoid scarring limited fourth ventricle outflow.
Scoliosis evaluation
Direction, magnitude, and location of major curves were evaluated pre-decompression, 1 year, and latest follow-up. The Spiegel et al. classification was used to define atypical curves that included: left thoracic, double thoracic, triple, and long thoracic curves [25]. Factors associated with scoliosis progression were examined trichotomously. Post-decompression groups were created according to the SOSORT criteria: Cobb improvement > 5°, stabilization or ≤ 5° change in magnitude, and progression > 5°.
Non-operative treatment
With the exception of PFD, indications for bracing or casting were similar to the management of idiopathic scoliosis. Children < 2 years with curves ≥ 25° were treated with elongation, derotation, and lateral flexion casting (n = 1) until bracing was initiated. A Boston-style thoracolumbar sacral orthosis (≥ 18 h/day) or Charleston-type night brace (12 h/night) was prescribed in children ≥ 2 years with residual curves > 25°. Bracing was continued until curves stabilized < 25°, skeletal maturity was reached (determined by Risser 4 or 5), or surgical intervention was required in progressive curves ≥ 45°. Due to the long-term follow-up of this study, objective brace-wear parameters were not available and compliance was based on patient and surgeon report. Patients were considered compliant if braces were worn ≥ 2/3rds the prescribed duration. Skeletally immature patients with stable curves or residual curves < 25° were observed and followed up every 6 months to detect late curve progression.
Chiari I and syringomyelia evaluation
MRI’s were performed in cases of early onset scoliosis, atypical curves, or neurologic symptoms and confirmed the diagnosis of CM-I and SM. Chiari I malformations were defined as a caudal descent of the cerebellar tonsils ≥ 5 mm below the foramen magnum. A line was drawn between the inner margins of the foramen magnum, from basion to opisthion, and the distance from this line to the inferior cerebellar tonsils determined CM-I size. A central cyst on T2 MRI confirmed a syringomyelia. Syringomyelia length (vertebral levels spanned) and width (maximum diameter in millimeters) were measured [12]. Scaled SM size was calculated by multiplying length and width (represented as scaled SM units). Serial MRI’s were performed to evaluate SM area pre-and post-decompression.
Statistical analysis
Patient and condition characteristics were summarized using SAS V.9.4 (SAS Inc; Cary, NC, USA). Continuous characteristics were summarized by mean and SD, mean and range, or median and interquartile range (IQR 25th–75th percentile) and categorical characteristics by frequency and percent. Bivariate comparisons were conducted for patient, curve, and treatment characteristics using SOSORT outcome groups described above. Comparisons were conducted using analysis of variance or Chi squared test based on variable type.
Continuous changes in Cobb and SM size were calculated through the difference of pre-and post-decompression measurements: (+) change indicated an increase in size (progression); and (−) changes a decrease (improvement). Multivariable linear modeling was used to analyze the effects of age, sex, Cobb, curve type, CM-I size, SM size, and bracing on change in Cobb. Model selection procedures were implemented to find the most parsimonious model to estimate the effects of patient and curve characteristics on outcomes. Decisions were made using a combination of model fit (based on Akaike’s information criterion) and minimal change in effect estimates for significant effects. All tests were two-sided and p < 0.05 was considered significant.
Results
Patient demographics and presenting symptoms
Sixty-five patients (44 females) with a mean age of 8.9 ± 3.39 years (range 0.7–15.8) at scoliosis diagnosis were identified (Table 1). Mean follow-up was 6.9 years (range 2–20.4). 27 patients (42%) were adolescent-onset (≥ 10 years) and 38 (58%) were early onset scoliosis (< 10 years). 58 patients (89%) presented with scoliosis as the chief complaint, with CM-I and SM identified through MRI evaluation. Five (8%) were diagnosed incidentally. Two (3%) presented with neurologic symptoms: one clonus and another chronic headaches. Of the patients presenting with scoliosis, neurologic history revealed chronic headaches in eight (12%) and upper extremity sensory disturbances in three (5%). Neurologic symptoms were found in a total of 13 patients (20%).
Major and atypical curve patterns
Major curve patterns included: 19 (29%) right thoracolumbar, 19 (29%) left thoracolumbar, 13 (20%) right thoracic, 11 (17%) left thoracic, 2 (3%) left lumbar, and 1 (2%) right lumbar (Table 1). 28 patients (43%) displayed atypical curves defined by Spiegel et al. [25]. 51 (80%) single-major, 12 (18%) double major, and 2 (3%) triple-major curves were seen. Mean major Cobb angle at presentation was 36° ± 10.4° (range 14°–60°) and 20 patients (31%) had curves ≥ 40°. Of the curves > 40°, the mean Cobb was 49° ± 5.2 (range 43°–60°). No correlation was found between Cobb and SM size (r = 0.10; 95% CI − 0.15 to 0.34; p = 0.41) or CM-I size at presentation (r = 0.14; 95% CI − 0.11 to 0.38; p = 0.26).
Chiari I malformation
Chiari I malformations were a mean of 11.9 ± 4.2 mm (range 5.0–21.3 mm) below the foramen magnum (Table 1). No correlation was found between Chiari size and SM size (r = 0.20; 95% CI − 0.05 to 0.42; p = 0.12). Neurosurgical decompression was performed at a mean of 9.1 ± 3.5 years, with 38 (58%) < 10 years. A fourth ventricle to subarachnoid space stent was placed during PFD in six patients (9%).
Syringomyelia
Syringomyelia scaled size decreased a mean of 70% after PFD, from 104.8 to 27.0 units (Table 1, Figs. 1, 2). Mean SM width and vertebral levels decreased from 8.1 to 2.7 mm (p < 0.001) and from 12 to 8 levels (p < 0.001), respectively. Five patients (7%) did not have an appreciable decrease in SM size and required a second PFD. Of these, two PFD and three PFD with fourth ventricle to subarachnoid shunting were performed. All patients experienced SM improvement following secondary decompression.
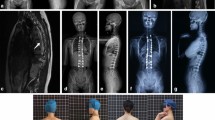
Case 1: 5.4-year-old girl that presented with scoliosis as the chief complaint. An MRI was performed due to the patient’s young age and curve severity. Pre-decompression sagittal T2 MRI demonstrating a Chiari I malformation 11.3 mm below the foramen magnum and a large septated syringomyelia that extends distally 14 levels from the craniocervical junction to below T7. Decompression was performed 2 months after the initial presentation
Scoliosis outcome
Scoliosis improved in 26 (40%) patients, 17 (26%) stabilized, and 22 (34%) progressed (Table 2, Figs. 3, 4). Differences were detected in SOSORT outcome groups with respect to age at PFD (p = 0.005), curve type (p < 0.001), curve shape (p = 0.02), syrinx width (p = 0.03), and brace compliance (p < 0.001) (Table 2). Children were compared based on < 10 or ≥ 10 years. A higher incidence of curve progression was seen in PFD in children ≥ 10 years (55% vs. 27%; p = 0.005). Outcomes also differed based on curve type. Both (100%) triple-major and 8/12 (67%) double major curves progressed compared to 23/51 (45%) single-major curves (p = 0.02).
Of these potential risk factors, multivariable analysis determined that change in SM scaled size and change in Cobb within the first year following PFD were the only factors significantly associated with long-term scoliosis outcome (Table 3, Fig. 5). Each additional ten unit decrease in SM scaled size resulted in a 0.8° reduction in curve after PFD (p = 0.03). Furthermore, after correcting for age and presenting Cobb, each additional 10° improvement in Cobb in the first year post-decompression resulted in a total of 9° reduction in Cobb at latest follow-up (p < 0.001) (Table 3).
Residual curves ≥ 25° seen post-decompression were braced until curve stabilization, progression ≥ 45° requiring spinal fusion, or skeletal maturity was reached. A total of 44 patients (68%) were braced. Of these, 41 patients (93%) were prescribed a Boston-style brace and 3 patients (7%) received a Charleston-type brace (Table 4). Twenty-one patients (32%) were not braced after decompression due to residual curves < 25°, progressive curves ≥ 45° requiring immediate spinal fusion, stable curves, or patients approaching skeletal maturity. Bracing details can be found in Table 4.
Spinal fusion
Spinal fusion was performed by the latest follow-up in 23 (35%) patients. Mean follow-up duration of the 42 (65%) non-fusion patients was 6.1 years (range 2.0–12.3). Fifty-two patients (80%) were followed up until skeletal maturity. Seven children (11%) underwent early fusion after PFD at a mean of 0.5 years (IQR 0.3–1.0) and with an average Cobb of 60° (range 41°–75°) (Table 4). The remaining 16 (25%) required delayed fusion after a mean of 6.0 years (IQR 3.5–8.4 years) and Cobb of 63° (range 35°–120°). In cases of late fusion, the Cobb progressed a mean of 22° (range − 4° to 63°) between decompression and fusion (Figs. 6, 7, 8, 9). Fusion patients had larger curves at presentation (43° vs. 34°; p = 0.004).
Case 2: 5.2-year-old girl that presented with scoliosis and back pain. Pre-decompression PA X-ray demonstrating a 36° left thoracolumbar curve. MRI revealed a Chiari I and syringomyelia of 10.8 mm and 188.7 SM scaled units, respectively, and decompression was performed 2 months after the initial visit
No difference in CM-I size (12.0 mm vs. 11.9 mm) was seen in cases with and without fusion. 11 of 20 (55%) patients presenting with curves ≥ 40° progressed after PFD and six required early fusion, but was not significant (p = 0.40). 8 of 12 (67%) patients with double-major curves received spinal fusion compared to 13/51 (25%) with single-major curves (p < 0.001). Both triple-major curves required fusion.
Discussion
Chiari-I malformations are the most common neuraxis anomalies in children [5]. A recent population based study of 14,118 patients found CM-I in 3.8% of children under 18 years [5]. CM-I is the most common cause of syringomyelia formation with reported incidences between 50 and 76% [26, 27]. The leading theory of scoliosis development postulates that SM expansion causes asymmetric injury to the anterior horn of the spinal cord [10, 13, 14]. This denervation and paraspinal muscle weakness can result in scoliosis. Biochemical and histologic studies support this theory and report improvement of paraspinal muscle innervation and spinal deformity following PFD [13].
Most authors recommend CM-I decompression in patients with SM to prevent or reverse neurological deterioration [28, 29]. However, the benefits of PFD on spinal deformity are less clear [7]. Prior studies report scoliosis improvement between 18%-38% [15, 16, 18, 20, 22, 30]. The follow-up in these series is limited and many question the long-term benefits of decompression [14, 16,17,18, 21,22,23,24].
While the relationship between SM and paraspinal muscle denervation has been studied in vitro, the association between SM and scoliosis remains unclear [10, 13, 14]. SM improvement following decompression is observed in 65%–93% of patients and is consistent with our results [15, 19, 30,31,32]. However, many studies report curve progression regardless of SM improvement [15, 16, 18, 19, 25, 30, 32,33,34]. These studies fail to identify an association between SM characteristics or initial curve magnitude and the risk of progression [16, 18, 30, 32,33,34]. In this study, a scale was used to quantify SM dimensions to analyze SM changes and scoliosis outcome. We found that SM reduction post-decompression was associated with scoliosis improvement or stabilization in two-thirds of patients. This effect was most pronounced immediately following decompression and patients with larger improvements in Cobb during the first year displayed a lower incidence of delayed spinal fusion.
Curve progression following decompression is not uncommon and prior studies report progression in 30%–89% [15, 16, 18, 20, 22, 30, 35]. In the current study, 22 (35%) progressed, 7 (11%) underwent early fusion, and 16 patients (25%) required delayed spinal fusion after long-term follow-up. This is similar to reports by Ravindra et al. who analyzed the long-term durability of decompression on scoliosis [20]. In that series of 23 patients, 7 (30%) required delayed spinal fusion after 5 years and the authors concluded poor durability of PFD on long-term curve control. The mean follow-up in the current series was 6.9 years. However, the IQR of delayed spinal fusion in the current series was 3.5–8.4 years after PFD and our results might underestimate the true incidence of late progression. Case 2 is an interesting example of delayed curve progression after significant initial curve improvement. This case demonstrates the importance of long-term follow-up in these patients, or until skeletal maturity is reached at a minimum.
Different curves are found in patients with CM-I and Spiegel et al. identified atypical curve patterns present in < 2% of idiopathic patients [25]. In this study, 28 (43%) patients presented with atypical curves suggesting an influence of ISP on curve formation [36, 37]. Prior studies also report atypical curves between 44 and 51% [12, 25]. However, most do not comment on scoliosis outcome [8, 16, 35, 38]. In this study, single-major curves improved more often compared to double-major curves and triple-major curves. Flynn et al. found that 8/9 double-major curves experienced progression following PFD [22]. Zhu also reported that double-major curves progressed in 47% compared to 11% without [39]. Senguta et al. observed increased improvement of left thoracic curves, with 75% avoiding spinal fusion [18].
Authors suggest increased progression or fusion rates in those presenting with curves ≥ 30°. Tubbs et al. saw no improvement in curves ≥ 40° [27]. Ghanem et al. also found that 5/5 patients with curves ≥ 40° required fusion [21]. Nagib complemented these studies and reported improvement in 6/6 patients with curves < 30° and stabilization in 4/4 ≥ 30° curves [40]. Other studies observed improvement ≥ 40° curves [7, 16]. This study found that 12/20 patients with curves ≥ 40° required fusion. Of these, six were early fusions and suggests that severe curves are more resilient to SM treatment. Fusion patients also had larger curves at presentation. We believe that decompression should be performed in all children prior to spinal fusion to reduce the risk of perioperative neurological deficits [7]. Furthermore, our results suggest that an increased benefit may be obtained by performing PFD at a younger age in an attempt to minimize initial curve progression.
Other studies also report increased benefits in younger patients. Muhonen et al. found scoliosis improvement in 3/3 patients < 10 years, despite one ≥ 40° curve [23]. Flynn et al. and Brockmeyer et al. found that 7/10 (70%) and 10/11 (91%) patients < 10, respectively, avoided fusion [16, 22]. This study also found decreased rates of progression and fusion in younger children. Few authors have evaluated the benefits of decompression in patients ≥ 10. However, our results are promising and decompression may also alter deformity progression in children ≥ 10.
The benefit of bracing has been observed in other studies. Zhu et al. found bracing a predictor of curve improvement in 54 patients [39]. Sha et al. reported that 8/33 (24%) of patients treated with bracing required spinal fusion compared to 13/21 (43%) without [13]. Objective brace compliance monitors were not available during the duration of this study and limits the conclusions that can be drawn from the effect of bracing. However, it is the senior authors’ belief that bracing is indicated in patients with residual curves > 25° following decompression and all patients should be followed up until skeletal maturity to detect late curve progression.
The retrospective nature is the largest limitation. Another limitation is the lack of follow-up until skeletal maturity in 13 patients. Late curve progression was seen after a mean of 6 years and studies with shorter follow-up are susceptible to underreporting the true incidence of curve progression and fusion. Another limitation difficult to overcome is the lack of a control group. It is the senior authors’ belief that all children should undergo decompression to prevent further progression and neurologic deterioration if an association is suspected between the CM-I and SM. A final limitation is that this study was conducted at a tertiary care center and may not be generalizable.
The biggest merit of this study is that it is the largest series of children with CM-I, SM, and scoliosis that evaluates the long-term outcomes of decompression on scoliosis. A SM scale allowed for a quantifiable method to analyze changes in SM size and correlate PFD to scoliosis outcomes. A reduction in syringomyelia following decompression is associated with improvement or stabilization of scoliosis in two-thirds of patients. The long-term prognosis is determined by the absolute reduction in SM size and the extent of curve improvement during the first year after decompression. Children that underwent decompression at a younger age experienced higher rates of curve improvement. However, scoliosis improvement can occur regardless of age and decompression is recommended in children of all ages.
Key points
-
A mean 70% decrease in syringomyelia scaled size was seen after neurosurgical decompression.
-
Scoliosis severity at presentation, smaller reductions in syringomyelia scaled size, less curve improvement in the first year following decompression, and double or triple major curves were risk factors for spinal fusion.
-
Spinal fusion was performed more frequently in children that underwent decompression at an older age.
-
Neurosurgical decompression is recommended in children of all ages and has potential to improve CM-I, SM, and scoliosis.
References
Arai S, Ohtsuka Y, Moriya H et al (1993) Scoliosis associated with syringomyelia. Spine (Phila Pa 1976) 18(12):1591–1592
Blake NS, Lynch AS, Dowling FE (1986) Spinal cord abnormalities in congenital scoliosis. Ann Radiol (Paris) 29(3–4):377–379
Bradford DS, Heithoff KB, Cohen M (1991) Intraspinal abnormalities and congenital spine deformities: a radiographic and MRI study. J Pediatr Orthop 11(1):36–41
Nokes SR, Murtagh FR, Jones JD 3rd et al (1987) Childhood scoliosis: MR imaging. Radiology 164(3):791–797. https://doi.org/10.1148/radiology.164.3.3615882
Strahle J, Smith BW, Martinez M et al (2015) The association between Chiari malformation Type I, spinal syrinx, and scoliosis. J Neurosurg Pediatr 15(6):607–611. https://doi.org/10.3171/2014.11.peds14135
Jankowski PP, Bastrom T, Ciacci JD et al (2016) Intraspinal pathology associated with pediatric scoliosis: a ten-year review analyzing the effect of neurosurgery on scoliosis curve progression. Spine (Phila Pa 1976) 41(20):1600–1605. https://doi.org/10.1097/brs.0000000000001559
Kelly MP, Guillaume TJ, Lenke LG (2015) Spinal deformity associated with chiari malformation. Neurosurg Clin N Am 26(4):579–585. https://doi.org/10.1016/j.nec.2015.06.005
Krieger MD, Falkinstein Y, Bowen IE et al (2011) Scoliosis and Chiari malformation Type I in children. J Neurosurg Pediatr 7(1):25–29. https://doi.org/10.3171/2010.10.peds10154
Eule JM, Erickson MA, O’Brien MF et al (2002) Chiari I malformation associated with syringomyelia and scoliosis: a twenty-year review of surgical and nonsurgical treatment in a pediatric population. Spine (Phila Pa 1976) 27(13):1451–1455
Huebert HT, MacKinnon WB (1969) Syringomyelia and scoliosis. J Bone Jt Surg Br 51(2):338–343
Oldfield EH, Muraszko K, Shawker TH et al (1994) Pathophysiology of syringomyelia associated with Chiari I malformation of the cerebellar tonsils. Implications for diagnosis and treatment. J Neurosurg 80(1):3–15. https://doi.org/10.3171/jns.1994.80.1.0003
Qiu Y, Zhu Z, Wang B et al (2008) Radiological presentations in relation to curve severity in scoliosis associated with syringomyelia. J Pediatr Orthop 28(1):128–133. https://doi.org/10.1097/bpo.0b013e31815ff371
Sha S, Li Y, Qiu Y et al (2017) Posterior fossa decompression in Chiari I improves denervation of the paraspinal muscles. J Neurol Neurosurg Psychiatry. https://doi.org/10.1136/jnnp-2016-315161
Zhu Z, Qiu Y, Wang B et al (2007) Abnormal spreading and subunit expression of junctional acetylcholine receptors of paraspinal muscles in scoliosis associated with syringomyelia. Spine (Phila Pa 1976) 32(22):2449–2454. https://doi.org/10.1097/brs.0b013e3181573d01
Attenello FJ, McGirt MJ, Atiba A et al (2008) Suboccipital decompression for Chiari malformation-associated scoliosis: risk factors and time course of deformity progression. J Neurosurg Pediatr 1(6):456–460. https://doi.org/10.3171/ped/2008/1/6/456
Brockmeyer D, Gollogly S, Smith JT (2003) Scoliosis associated with Chiari 1 malformations: the effect of suboccipital decompression on scoliosis curve progression: a preliminary study. Spine (Phila Pa 1976) 28(22):2505–2509. https://doi.org/10.1097/01.brs.0000092381.05229.87
Hwang SW, Samdani AF, Jea A et al (2012) Outcomes of Chiari I-associated scoliosis after intervention: a meta-analysis of the pediatric literature. Childs Nerv Syst 28(8):1213–1219. https://doi.org/10.1007/s00381-012-1739-3
Sengupta DK, Dorgan J, Findlay GF (2000) Can hindbrain decompression for syringomyelia lead to regression of scoliosis? Eur Spine J 9(3):198–201
Yeom JS, Lee CK, Park KW et al (2007) Scoliosis associated with syringomyelia: analysis of MRI and curve progression. Eur Spine J 16(10):1629–1635. https://doi.org/10.1007/s00586-007-0472-1
Ravindra VM, Onwuzulike K, Heller RS et al (2018) Chiari-related scoliosis: a single-center experience with long-term radiographic follow-up and relationship to deformity correction. J Neurosurg Pediatr 21(2):185–189. https://doi.org/10.3171/2017.8.PEDS17318
Ghanem IB, Londono C, Delalande O et al (1997) Chiari I malformation associated with syringomyelia and scoliosis. Spine (Phila Pa 1976) 22(12):1313–1317 (discussion 1318)
Flynn JM, Sodha S, Lou JE et al (2004) Predictors of progression of scoliosis after decompression of an Arnold Chiari I malformation. Spine (Phila Pa 1976) 29(3):286–292
Muhonen MG, Menezes AH, Sawin PD et al (1992) Scoliosis in pediatric Chiari malformations without myelodysplasia. J Neurosurg 77(1):69–77. https://doi.org/10.3171/jns.1992.77.1.0069
Ozerdemoglu RA, Transfeldt EE, Denis F (2003) Value of treating primary causes of syrinx in scoliosis associated with syringomyelia. Spine (Phila Pa 1976) 28(8):806–814
Spiegel DA, Flynn JM, Stasikelis PJ et al (2003) Scoliotic curve patterns in patients with Chiari I malformation and/or syringomyelia. Spine (Phila Pa 1976) 28(18):2139–2146. https://doi.org/10.1097/01.brs.0000084642.35146.ec
Kontio K, Davidson D, Letts M (2002) Management of scoliosis and syringomyelia in children. J Pediatr Orthop 22(6):771–779
Tubbs RS, Lyerly MJ, Loukas M et al (2007) The pediatric Chiari I malformation: a review. Childs Nerv Syst 23(11):1239–1250. https://doi.org/10.1007/s00381-007-0428-0
Alzate JC, Kothbauer KF, Jallo GI et al (2001) Treatment of Chiari I malformation in patients with and without syringomyelia: a consecutive series of 66 cases. Neurosurg Focus 11(1):E3
Schijman E, Steinbok P (2004) International survey on the management of Chiari I malformation and syringomyelia. Childs Nerv Syst 20(5):341–348. https://doi.org/10.1007/s00381-003-0882-2
Ozerdemoglu RA, Denis F, Transfeldt EE (2003) Scoliosis associated with syringomyelia: clinical and radiologic correlation. Spine (Phila Pa 1976) 28(13):1410–1417. https://doi.org/10.1097/01.brs.0000067117.07325.86
Navarro R, Olavarria G, Seshadri R et al (2004) Surgical results of posterior fossa decompression for patients with Chiari I malformation. Childs Nerv Syst 20(5):349–356. https://doi.org/10.1007/s00381-003-0883-1
Park JK, Gleason PL, Madsen JR et al (1997) Presentation and management of Chiari I malformation in children. Pediatr Neurosurg 26(4):190–196
Bhangoo R, Sgouros S (2006) Scoliosis in children with Chiari I-related syringomyelia. Childs Nerv Syst 22(9):1154–1157. https://doi.org/10.1007/s00381-006-0090-y
Charry O, Koop S, Winter R et al (1994) Syringomyelia and scoliosis: a review of twenty-five pediatric patients. J Pediatr Orthop 14(3):309–317
Farley FA, Puryear A, Hall JM et al (2002) Curve progression in scoliosis associated with Chiari I malformation following suboccipital decompression. J Spinal Disord Tech 15(5):410–414
Zhu Z, Wu T, Sha S et al (2013) Is curve direction correlated with the dominant side of tonsillar ectopia and side of syrinx deviation in patients with single thoracic scoliosis secondary to Chiari malformation and syringomyelia? Spine (Phila Pa 1976) 38(8):671–677. https://doi.org/10.1097/brs.0b013e3182796ec5
Zhu Z, Yan H, Han X et al (2016) Radiological features of scoliosis in Chiari I malformation without syringomyelia. Spine (Phila Pa 1976) 41(5):E276–E281. https://doi.org/10.1097/brs.0000000000001406
Mackel CE, Cahill PJ, Roguski M et al (2016) Factors associated with spinal fusion after posterior fossa decompression in pediatric patients with Chiari I malformation and scoliosis. J Neurosurg Pediatr 25(6):737–743. https://doi.org/10.3171/2016.5.peds16180
Zhu Z, Wu T, Zhou S et al (2013) Prediction of curve progression after posterior fossa decompression in pediatric patients with scoliosis secondary to Chiari malformation. Spine Deform 1(1):25–32. https://doi.org/10.1016/j.jspd.2012.07.005
Nagib MG (1994) An approach to symptomatic children (ages 4–14 years) with Chiari type I malformation. Pediatr Neurosurg 21(1):31–35
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Contributions
All authors: substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work, drafting the work, or revising it critically for important intellectual content and final approval of the version to be published.
Corresponding author
Ethics declarations
Ethical approval
IRB approval: R00023640-1.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Investigation performed at: Department of Orthopaedic Surgery, Boston Children’s Hospital (Harvard Teaching Hospital), Boston, MA, USA
Rights and permissions
About this article
Cite this article
Verhofste, B.P., Davis, E.A., Miller, P.E. et al. Chiari I malformations with syringomyelia: long-term results of neurosurgical decompression. Spine Deform 8, 233–243 (2020). https://doi.org/10.1007/s43390-019-00009-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43390-019-00009-z