Abstract
Many heavy metals and metalloids (e.g., Pb, Cd, and Ni) can contaminate the environment and cause severe health problems. Through this study, investigated the possible corrective effects of Ficus carica extract (FCE) against nickel (Ni) induced stress response and damage on the liver of rats. Male Wistar rats were divided into four groups (8 rats per group) and co-treated with FCE (350 mg/kg) and exposed to Nickel chloride (10 mg/kg) for 4 weeks. The volatile compounds of FCE were characterized by solid phase micro-extraction (SPME) coupled with GC–MS, and the biochemical parameters of stress were determined. The SPME–GC/MS analysis of FCE indicated the presence of thirty (30) phyto-bioactive compounds including alcohols, aldehydes, organic acids, ketones, furans, terpenes, ester and others. The best capacity for scavenging DPPH free radicals and metal chelating were found with the IC50 values of 0.49 and 2.91 mg/mL, respectively. Ni induced damage to various macromolecules. Malondialdehyde, protein carbonyls, alanine aminotransferase and gamma glutamyl transferarse levels were significantly increased in Ni exposed group compared to control group and co-treatment with FCE reduced the levels of these parameters. In conclusion, current findings showed that Ni-induced oxidative damage and the administration of FCE can improve correct and restore the alteration in the rat liver.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Humans are exposed-daily to xenobiotics, drugs, and various toxic products [1]. Heavy metals mainly accumulate in the environment due to anthropogenic activities which may cause a number of disorders to both humans [2] and terrestrial biota [3]. Heavy metal pollution is becoming a potential harmful risk to human health. These xenobiotics induced the toxic effects and damage to the all organs and the liver is the most affected amongst them. Exposure to heavy metals causes lipid and protein oxidation, and oxidative DNA damage [2]. The metallic compounds of nickel (Ni) have many industrial applications because of their chemical and physical properties. Their concentrations have increased as a result of metal exploitation and industrial development [4].
Industrial waste is the main source of Ni contaminants in the environment. Various harmful effects of environmental Ni exposure and its compounds have been reported in humans [5], and considered as a type 1 carcinogen by the International Agency for Cancer Research [6]. Human exposure to nickel occurs primarily via inhalation and ingestion [7]. Drinking water and food are the two main sources of Ni exposure in humans and animals [8]. The environmental exposition of Ni induced the toxic effects in various organs [9, 10], such as immunotoxicity [11], and genotoxicity [12]. Ni intoxication results in loss of body weight and respiratory disorders, increased serum levels of kidney function biomarkers indicating the development of kidney failure [13]. Ni substitutes the metals (especially zinc) in the catalytic centers of enzymes, resulting alteration in the proteins function [14]. The liver is the primary organ managing homeostasis in the body, where metabolism and detoxification happen. It also plays a significant role in drug and heavy metals detoxification. Hepatotoxicity induced by Ni may be caused mainly by the oxidative stress reactions [15]. In vivo and in vitro studies revealed that Ni enhanced lipid peroxidationn, protein oxidation and DNA damage [9, 16].
In recent years, the genus Ficus has received more attention for their potential uses in the treatment and preventing diseases. These effects are linked to the antioxidant contents and various bioactive compounds. Ficus carica L. (Moraceae) is commonly known as figs. Leaves, fruits and latex of F. carica have been used as a source of food and health [17]. It’s reported that figs contain high amounts of fiber and polyphenols [18]. Its content can play a significant role in protecting the liver tissue from injury and considerably increase plasma antioxidant capacity [18], and considered as an excellent source of nutrients [19], traditionally used for their therapeutic effects as a laxative and anti-inflammatory remedies [20]. Hepatoprotective, hypoglycemic, antifungal, antioxidant and antimutagenic activities have been reported [17, 21,22,23,24]. The current study was established to evaluate the potential ameliorative effect of Figs against oxidative stress induced by Nickel through biochemical assay and histopathology study.
Materials and methods
Reagents and chemicals
Nickel chloride (NiCl2 98% purity) was purchased from Sigma–Aldrich Chemical Co (St. Louis, MO, USA). Folin-Ciocalteu reagent, gallic acid, methanol, hydrochloric acid (37%), sulphuric acid (H2SO4), sodium carbonate (Na2CO3), aluminium chloride (AlCl3), sodium hydroxide (NaOH), quercetin, polyvinyl polypyrolidone (PVPP), pyrogallol, and ferric chloride 6-hydrate were purchased from Merck (Darmstadt, Germany). 1,1-Diphenyl-2-picrylhydrazyl (DPPH, 98%), 3-(2-Pyridyl)-5,6-bis (4-phenyl-sulfonic acid)-1,2,4-triazine (Ferrozine), iron(II) chloride (FeCl2), ethylene-diamine-tetra acetic acid (EDTA), bovine serum albumin (BSA), thiobarbituric acid (TBA), butylated hydroxytoluene (BHT), trichloroacetic acid (TCA), and 2,4-dinitrophenylhydrazine (DNPH) were obtained from Sigma–Aldrich (Steinheim, Germany). All other chemicals used were analytical grade and were obtained from Merck, US.
Plant material and extract preparation
Fig (Ficus carica L.) variety “taamriout” was collected from Ain Karma (Oran, Algeria). They were identified taxonomically and authenticated by the Herbarium of Botany Directorate in Ahmed Ben-Bella Oran 1 University (voucher specimen No LB 0695). Preparation of fig fruit extract (FCE) was achieved by the method of Oliveira et al. [19]. Powder (50 g) was boiled for 15 min filtered and lyophilized.
Determination of total phenol, flavonoids and tannins content
The Folin-Ciocalteu colorimetric method was used to assay the total phenolic content as described by Singleton and Rossi [25], and expressed as gallic acid equivalent per g dry extract. Using the aluminum chloride colorimetric assay according to Kim et al. [26], flavonoid contents were measured against the blank at 510 nm and expressed as mg quercetin equivalent g−1 dry extract. The tannin content was determined according to the method described by Julkunen-Tiitto [27] and the results were expressed as mg catechin equivalent per g dry extract.
Determination of volatile compounds: SPME extraction and GC–MS analysis
The volatile compounds of Ficus carica extract (FCE) were characterized by solid phase micro-extraction (SPME) coupled with GC–MS according to the method of Arthur and Pawliszyn [28] with slight modifications. The SPME fiber used was divinylbenzene (DVB), carboxen (CAR), polydimethylsiloxane (PDMS) (50/30 μm DVB/CAR/PDMS) (Supelco, Bellefonte, USA). The filter was preliminary conditioned at 250 °C for 1 h before each use. After extraction, SPME fiber was desorbed in the GC apparatus. Analyses of volatile compounds were performed on an Agilent Technologies 7890A GC9 System, and compounds were separated on a VF-WAXms column (Agilent Technologies, USA; 30 m × 0.250 mm I.D, × 0.25 μm film thickness). The identification of the compounds was performed on the basis of chromatographic retention indices (RI) and by comparison of the recorded spectra with a Pal 600 K® mass spectral database. A sample was analysed in triplicate and expressed in terms of relative peak area.
In vitro activity
DPPH scavenging activity
Ficus carica extract (FCE) were tested for the scavenging effect on the DPPH radical according to Heimler et al. [29]. Briefly, 10 µL of the extract solutions was added to 1 mL of DPPH solution (4%), and incubated for 30 min. The absorbance was recorded at 515 nm. A control containing only DPPH solution is used as blank and the vitamin C as standard. The DPPH scavenging effect was calculated by the following formula:
Ferrous ion chelating capacity assay
The ferrous ion chelating of the extracts was evaluated by the method of Decker and Welch [30]. Briefly, 2 mL of extract (100 μg/mL) was added to 0.2 mL of 5 mM ferrozine and 0.1 mL of 2 mM FeCl2 solution. The absorbance was recorded at 562 nm. The percentage of inhibition of ferrozine-Fe2+ complex formation was calculated by the formula:
Where, ACT was the absorbance of control reaction and AFCE was the absorbance of extract. AS was the Absorbance of standard (100 µg EDTA/mL).
Animals and experimental design
A total of 32 healthy male Wistar rats (Rattus norvegicus), weighing 165 ± 5 g, were maintained under following conditions (12 h light/dark cycle, 23 ± 1 °C, relative humidity 50 ± 15%), food and water ad libitum. All experiments were conducted in accordance with the ethical principles and institutional guidelines of the National Institutes of Health Guide for the care and use of laboratory animals.
Animals were randomly divided into 4 groups (8 rats per lot): Group “C”: untreated control; Group “FC” treated with FCE (350 mg/kg BW) [21, 31] by gavage; Group “Ni”: intoxicated group received dose of 10 mg Nickel chloride /kg body weight (BW) by intraperitonial injection (i.p), the exposure dose was selected based on the work of Das and Buchner [32]; Group “Ni + FC”: Co-treatment with Ni and FCE for 4 weeks. At the end of the experiment, the rats were sacrificed and the liver was collected, rinsed and weighed. The samples and aliquots were stored at − 80 °C until analysis.
Serum transaminases and ALP activities assay
Serum liver biomarkers: aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP) and gamma glutamyl transferase (GGT) were estimated using diagnostic kits (ChronoLab, Spain) according to the manufacturer’s protocol.
Proteins measurement
Protein concentrations in homogenates were determined following the method of Bradford [33] with bovine serum albumin (BSA) as a standard.
Antioxidant enzymes assay
Determination of catalase activity
Catalase activity was estimated by the method of Aebi [34]. The absorption was monitoring at 240 nm. Specific enzyme activity was expressed in nmoles of H2O2 consumed/min/mg of protein.
Determination of superoxide dismutase (SOD) activity
SOD activity was determined by the pyrogallol assay according to the method of Marklund and Marklund [35]. One unit of (Cu–Zn) SOD activity was defined as the amount of the enzyme required causing 50% inhibition of pyrogallol autoxidation at 25 °C.
Measurement of malondialdehyde (MDA)
The MDA level was estimated by the method of Yagi [36] and Ohkawa et al. [37], and expressed as nmol per mg of protein.
Measurement of protein carbonyls
The oxidative proteins damage was determined by the method of Levine et al. [38]. The protein carbonyl content was expressed as nmol per mg of protein.
Histopathological studies
Fresh tissue pieces of liver were instantly immersed and fixed in 10% formalin, dehydrated through graded alcohol, embedded in paraffin, sliced and stained with haematoxylin and eosin.
Statistical analysis
The results obtained were analyzed by the one-way analysis of variance (ANOVA) followed by Tukey-multiple comparison test using SPSS program (version 23). A value of p < 0.05 was considered as statistically significant.
Results
Phenolic components and volatile compounds content
The results of total polyphenols and flavonoids in the FCE showed high amount estimated at 100.83 and 195.61 mg gallic acid equivalent per g dry extract, respectively. While the tannin dosage result showed a value of 0.75 mg of catechin equivalent per gram of dry extract (mg EQC/g of dry extract). The SPME/GC–MS analysis of the FCE indicated the presence of 30 different compounds, including alcohols, aldehydes, organic acids, ketones, furans, terpenes, ester and others. The identified compounds are represented in the Table 1.
Antioxidant capacity of Ficus carica extracts (FCE)
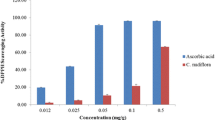
The antioxidant capacities of the tested concentrations (0.1–0.8 mg/mL) of FCE were evaluated by the most commonly used antioxidant assays: DPPH and Ferrous iron chelating methods. In the DPPH method, the results indicated that the FCE showed the inhibition values (except standard) ranged from 40.61 to 49.26%, with the IC50 values for scavenging activity of 495 µg/mL vs 142 µg/mL for ascorbic acid. The percentages of radical scavenging activities of the FCE at different concentrations within the range of 0.1–0.5 mg/mL are illustrated in Fig. 1. The chelating ability of ferrous ions of FCE was shown in Fig. 2. The absorbance of Fe2+—ferrozine complex was observed to be linearly decreased with the concentration of the extract and EDTA (from 0.2 to 0.8 mg/mL). The results indicated that the extract has a good chelating activity compared to the positive control “EDTA”. The percentages of the metal chelating assay at higher concentration of testing FCE and EDTA were found to be 76.54% and 91.09% respectively.
Effect of treatment on body weight and liver
The variations in the liver weight, body weight and liver body weight ratio of the rats subjected to different treatments are shown in Table 2. It observed that treatment with nickel chloride for one month induced a no significant variation in all this parameters compared to control group. But the FCE administrated rats showed progressive and significant (p < 0.001) increase in the body weight (+ 24%) and the body weight gain (+ 91%) in the Ni + FC group compared to the Ni group. The results showed also a significant (p< 0.01) increase of body weight gain (+ 52%) in FC group compared to untreated group. No significant difference in liver body weight ratio and liver weight was observed in rats of the different experimental groups.
Effect of treatment on biochemical analysis
Table 3 showed the mean values of the biochemical indicators of liver function of the control and experimental groups. The levels of GGT and ALT were significantly (p < 0.001) increased in Ni group compared to control. A significant diminution (p < 0.001) (− 25%) induced by FC administration was only noted in ALT level in Ni + FC group compared to Ni group.
Effect of treatment on the antioxidant enzyme activities
The activity of catalase was significantly (p < 0.05) increased by + 35% in the Ni group compared to the rats of the control group (Table 4). No significant variation in SOD activity was observed in this group. In contrast, administration of FCE was significantly (p < 0.001) increased SOD activity by + 85% in the Ni + FC group compared to the Ni group. A significant (p < 0.01) increase was also observed in the CAT and SOD activity in the FC group compared to the untreated group.
Effect of treatment on the lipid peroxidation and carbonyls levels
Changes in MDA and carbonyls levels were illustrated in Table 4. The result showed a significant (p < 0.001) increase in MDA level in Ni group compared to control. There was also a 4.92-fold increase in carbonyl levels in this group compared to the control rat. The administration of FCE induce an improvement very significantly (p < 0.001) in MDA and carbonyls levels.
Effect of treatment on histological changes
Microscopic examination of hepatic section (Fig. 3) revealed normal hepatic parenchyma architecture in the control and FC group (Table 5). However, in exposed group to Ni (Fig. 3, group “Ni”) was observed dilatation in the portal area, deformed cellular organization, hypertrophic cells, and inflammation accompanied with the widening of intracellular sinusoids. The administration of FC extract ameliorates liver histology (Fig. 3, group “Ni + FC”).
Effect of Nickel Chloride and Ficus carica aqueous extract (FCE) on the histological liver structure in control and experimental animals. BD bile duct; HPV, hepatic portal vein; HA, hepatic artery; CV central vein, H hepatocytes, S sinusoidal spaces, PA Portal artery, MC Mononuclear cells, N Necrosis, DR ductular reaction, SD sinusoidal dilatation, PAD portal area dilatation; BH Ballooned hepatocytes. Group “C”: control, untreated group; Group “FC” treated with FCE (350 mg/kg BW) by gavage; Group “Ni”: intoxicated lot with 10 mg Nickel chloride /kg body weight (BW) by intraperitonial injection (i.p); Group “Ni + FC”: Co-treatment with Ni and FCE for 4 weeks
Discussion
The fruits and vegetables, rich in antioxidants and different micronutrients, defend against varied types of xenobiotics induced hepatic injury and DNA damage [39]. Nickel is a potent toxicant with ability to disturb the cellular antioxidant defence system, induced damage to cell membranes [40]. In the present study we examined the possible protective effects of the FCE against Ni-induced hepatotoxicity in rats, and also we explored the antioxidant and the chelating activity of FCE in vitro using DPPH and ferrous ion-chelating assays.
The phytochemical composition reported by Debib et al. [41] indicates that figs were richer in polyphenols like gallic acid, chlorogenic acid and syringic acid. They also are abundant in flavonoids, specially -catechin, -epicatechin, and rutin [42], and anthocyanins [43]. It's known that polyphenols and flavonoids are able to reduce the hepatotoxicity induced by the xenobiotics [44]. Their positive effects can be linked to the inhibitory action on the free radical production [44]. The results of the phenolic components obtained are in agreement with those of Gilani et al. [45] who revealed that the aqueous extract of figs contains polyphenols, flavonoids, coumarins and terpenes. Similarly, the study of Veberic et al. [46] indicates that the figs harvested in the coastal zone to the north of the Mediterranean are very rich in flavonoid and phenolic acids. The flavonoids, a major constituent of our extract, have a wide spectrum of biological activities and chemical structure, including anti-free radical properties and contribute significantly to the taste, color, astringent flavors and aroma of figs [46]. Thirty (30) compounds have been identified by SPME GC–MS and distributed by distinct chemical classes (ketones, aldehydes, alcohols, furans acids, terpenes esters, and others). Our results are in accordance with those of Russo et al. [47] who identified 42 volatile compounds in white figs from southern Italy using the same technique compared to 55 compounds identified in Turkish figs; other studies have reported that figs contain between 46 and 59 volatile compounds [48]. Our data have shown that white figs of the “taamiriout” variety are distinguished by their abundance of aldehyde, among which are benzaldehyde (anti-carcinogenic and antimicrobial agent) and 2-methoxy-4-vinylphenol used as an antioxidant [49]. Vanillin is an aromatic aldehyde with antioxidant effect, followed by acid-type compounds, including palmitic acid and myristic acid. Our chromatogram also shows the presence of terpene compounds; limonene which is a powerful anti inflammatory agent with antioxidant and anticancer properties [50] and caryophyllene with analgesic and anti-inflammatory activity [51].
The FCE modulates the free radical DPPH compared with vitamin C. The content of FCE on the phenolic compounds may be related with their considerable activity of radical-scavenging. However, the structural variability of the phenolic compounds is extremely linked to the number and arrangements of hydroxyl groups [52]. Our results confirmed that FCE show the highest amount of antioxidant capacity. This ability is also seen in some other Ficus species like Ficus bengalensis and Ficus racemosa stem, bark, and fruit extracts. We have obtained that FCE has nearly the same chelating activity compared with EDTA. Viuda-Martosa et al. [53] reported that the Fig co-products obtained from peel showed higher ion-chelating activities at all concentrations and each cultivar studied. Concerning the in vivo analysis, our results don’t show any significant variation in the liver weight, body weight gain and liver body weight ratio in animals exposed to nickel (Ni group) compared to control. Several studies indicate that nickel induces a decrease in the body weight gain and liver weight [54, 55], and combine this reduction to a lower food consumption [29], hormonal imbalance, and a decrease in protein synthesis [56]. The FCE administration induced a significant gain in body weight gain which may be due to an increased appetite and promotion of protein synthesis. No significant improvement is observed for liver weight and liver body weight ration after administration of FCE. These results showed also that Ni pretreatment increased the serum transaminases (ALT) and GGT. Sindhu et al. [57] reported similar results, and Winter et al. [58] observed that high levels of liver enzymes show hepatocellular injury. After FCE administration, no significant improvement was showed in the markers of liver function except for the activity of ALT, which recorded a significant decrease in treated rats compared to pre-intoxicated and untreated rats.
A similar study proved that the administration of dried Fig supplements imparts protection against ethanol-induced oxidative injury [59]. We show also a significant increase in carbonyls and MDA levels in Ni- pre-intoxicated groups, which is the consequence of increased lipid peroxidation and proteins oxidation. Our results were in agreement with Pari and Amudha [60] who have shown that Ni-intoxication induces ROS formation leading to alterations in the redox status of several tissues in rats and mice. In the current study, a significant decrease in protein carbonyl and MDA level is shown after stopping metal intoxication and starting FCE administration. The antioxidant enzymes are the primary line of defence against oxidative stress that prevents bio-molecules from oxidative injury inside the cell. It’s proved that Ni disturbs significant the antioxidant enzyme activities. The results confirmed that nickel treatment induced a significant increase in CAT activity contrary to the study of Hfaïedh et al. [61] which indicates that nickel induces the decrease in the activity of antioxidant enzymes due to the inhibition of free radicals [44]. Conversely, the treatment with FCE had a potent protective effect against liver damage caused by nickel. These results were already found by Saoudi and El Feki [62] which confirms that FCE increased the SOD activity after a hepatic damage. These results indicated that FCE decreases oxidative stress and the toxic effect of Nickel in liver tissue.
The liver histological structure of the Ni-intoxicated group showed significant typical morphological changes to those reported in the literature such as the dilatation, cytoplasmic vacuolization, inflammatory cell infiltration, sinusoidal dilatation, cell necrosis, and cellular hypertrophy. This is possibly due to the formation of highly reactive radicals because of oxidative threat induced by nickel. Rao et al. [56] reported that Ni-induced several changes in the histological structure characterized by dilated sinusoids, vacuolization and distorted nuclei. However, the liver of the rats treated with FCE had architecture nearly comparable to the control group, except some cell infiltration limited in central vein. FCE administration ameliorates the histological structure of the liver. Aghel et al. [63] showed that treatment with the FCE resulted in the less pronounced destruction of the liver architecture. Our study demonstrates that the administration of FCE could correct and accelerate the capacity of liver rat to regenerate after Ni-induced oxidative stress [59].
In conclusion, the present study showed that Nickel chloride induced histological and biochemical liver damage and altered antioxidant defense system and the administration of FCE accelerate the improvement of the parameters and liver regeneration. FCE increase the activities of antioxidant enzymes and decrease oxidative stress. These results suggested that FCE has a protective effect on hepatotoxicity induced by nickel. This protection is obviously due to proactive molecules and antioxidants in the Fig fruits tested in vitro and represent promising natural tools against Nickel toxicity.
References
Lakshmi P, Tajdar HK, Tamanna J, Sarwat S (2006) Chemomodulatory effects of Terminalia chebula against nickel chloride induced oxidative stress and tumor promotion response in male Wistar rats. J Trace Elem Med Biol 20:233–239. https://doi.org/10.1016/j.jtemb.2006.07.003
Nemmiche S (2017) Oxidative signaling response to cadmium exposure. Toxicol Sci 156:4–10. https://doi.org/10.1093/toxsci/kfw222
Kumar A, Jigyasu DK, Kumar A, Subrahmanyam G et al (2021) Nickel in terrestrial biota: Comprehensive review on contamination, toxicity, tolerance and its remediation approaches. Chemosphere 275:129996. https://doi.org/10.1016/j.chemosphere.2021.129996
Nabinger DD, Altenhofen S, Rodrigues Bitencourt PE, Nery LR, Leite CE, Moreira R et al (2018) Nickel exposure alters behavioral parameters in larval and adult zebrafish. Sc Total Environ 624:1623–1633. https://doi.org/10.1016/j.scitotenv.2017.10.057
Song X, Fiati Kenstona SS, Kong L, Zhao J (2017) Molecular mechanisms of nickel induced neurotoxicity and chemoprevention. Toxicology 392:47–54. https://doi.org/10.1016/j.tox.2017.10.006
International Agency for Research on Cancer (2012) IARC monographs: arsenic, metals, Fibres, Dusts (100C)
Denkhaus E, Salnikow K (2002) Nickel essentiality, toxicity, and carcinogenicity. Crit Rev Oncol Hematol 42:35–56. https://doi.org/10.1016/S1040-8428(01)00214-1
Das KK, Chandramouli RR, Bagoji I, Das S et al (2018) Primary concept of nickel toxicity—an overview. J Basic Clin Physiol Pharmacol 30:141–152. https://doi.org/10.1515/jbcpp-2017-0171
Genchi G, Carocci A, Lauria G, Sinicropi MS, Catalano A (2020) Nickel: human health and environmental toxicology. Int J Environ Res Public Health 17:679. https://doi.org/10.3390/ijerph17030679
Filatova D, Cherpak C (2020) Mechanisms of nickel-induced cell damage in allergic contact dermatitis and nutritional intervention strategies. Endocr Metab Immune Disord Drug Targets 20:1010–1014. https://doi.org/10.2174/1871530320666200122155804
Singh A, Kumar M, Kumar V, Roy D et al (2019) Effects of nickel supplementation on antioxidant status, immune characteristics, and energy and lipid metabolism in growing cattle. Biol Trace Elem Res 190:65–75. https://doi.org/10.1007/s12011-018-1524-6
Guo H, Liu H, Wu H, Cui H et al (2019) Nickel carcinogenesis mechanism: DNA damage. Int J Mol Sci 20:4690. https://doi.org/10.3390/ijms20194690
Hfaïedh N, Allaqui MS, Croute F, Soleilhavoup JP, Jmmoussi K, Makniayadi F et al (2005) Interaction du jeûne intermittent sur les effets cytotoxiques rénaux du nickel chez le rat pubère. C R Biol 328:648–660. https://doi.org/10.1016/j.crvi.2005.03.001
Chen H, Giri NC, Zhang R, Yamane K, Zhang Y, Maroney M et al (2017) Nickel ions inhibit histone demethylase JMJD1A and DNA repair enzyme ABH2 by replacing the ferrous iron in the catalytic centers. J Biol Chem 292:10743. https://doi.org/10.1074/jbc.M109.058503
Renu K, Chakraborty R, Myakala H et al (2021) Molecular mechanism of heavy metals (lead, chromium, arsenic, mercury, nickel and cadmium)—induced hepatotoxicity—a review. Chemosphere 271:129735. https://doi.org/10.1016/j.chemosphere.2021.129735
Zambelli B, Uversky VN, Ciurli S (2016) Nickel impact on human health: an intrinsic disorder perspective. Biochim Biophys Acta Protein Proteonomics 1864:1714–1731. https://doi.org/10.1016/j.bbapap.2016.09.008
Barolo MI, Ruiz Mostacero N, López SN (2014) Ficus carica L. (Moraceae): an ancient source of food and health. Food Chem 164:119–127. https://doi.org/10.1016/j.foodchem.2014.04.112
Vinson JA, Zubik L, Bose P, Samman N, Proch J (2005) Dried fruits: excellent in vitro and in vivo antioxidants. J Am Coll Nutr 24:44–50. https://doi.org/10.1080/07315724.2005.10719442
Oliveira AP, Valentão P, José AP, Branca MS, Fernando T, Andrade PB (2009) Ficus carica L.: metabolic and biological screening. Food Chem Toxicol 47:2841–2846. https://doi.org/10.1016/j.fct.2009.09.004
Guarrera PM (2005) Traditional phytotherapy in Central Italy (Marche, Abruzzo, and Latium). Fitoterapia 76:1–25. https://doi.org/10.1016/j.fitote.2004.09.006
Hira S, Gulfraz M, Saqlan Naqvi SM, Qureshi RU, Hina G (2021) Protective effect of leaf extract of Ficus carica L. against carbon tetrachloride-induced hepatic toxicity in mice and HepG2 cell line. Trop J Pharm Res 20:113–119. https://doi.org/10.4314/tjpr.v20i1.17
Badgujar SB, Patel VV, Bandivdekar AH, Mahajan RT (2014) Traditional uses, phytochemistry and pharmacology of Ficus carica: a review. Pharm Biol 52:1487–1503. https://doi.org/10.3109/13880209.2014.892515
Lansky EP, Paavilainen HM, Pawlus AD, Newman RA (2008) Ficus spp. (fig): ethnobotany and potential as anticancer and antiinflammatory agents. J Ethnopharmacol 119:195–213. https://doi.org/10.1016/j.jep.2008.06.025
Yang XM, Yu W, Ou ZP, Ma HL, Liu WM, Ji XL (2009) Antioxidant and immunity activity of water extract and crude polysaccharide from Ficus carica L. fruit. Plant Foods Hum Nutr 64:167–173. https://doi.org/10.1007/s11130-009-0120-5
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16:144–158
Dae-Ok K, Ock Kyoung C, Young Jun K, Hae-Yeon M, Chang YL (2003) Quantification of polyphenolics and their antioxidant capacity in fresh plums. J Agric Food Chem 51:6509–6515. https://doi.org/10.1021/jf0343074
Julkunen-Tiitto R (1985) Phenolic constituents in the leaves of northern willows: methods for the analysis of certain phenolics. J Agric Food Chem 33:213–217. https://doi.org/10.1021/jf00062a013
Arthur Catherine L, Janusz P (1990) Solid phase microextraction with thermal desorption using fused silica optical fibers. Anal Chem 62:2145–2148. https://doi.org/10.1021/ac00218a019
Heimler D, Vignolini P, Dini MG, Romani A (2005) Rapid tests to assess the antioxidant activity of Phaseolus vulgaris L. dry beans. J Agric Food Chem 53:3053–3056. https://doi.org/10.1021/jf049001r
Decker EA, Welch B (1990) Role of ferritin as a lipid oxidation catalyst in muscle food. J Agric Food Chem 38:674–677. https://doi.org/10.1021/jf00093a019
Mohan GK, Pallavi E, Ravi Kumar B, Ramesh M, Venkatesh S (2007) Hepatoprotective activity of Ficus carica Linn. leaf extract against carbon tetrachloride-induced hepatotoxicity in rats. DARU J Pharm Sci 15:162–166
Das KK, Buchner V (2007) Effect of nickel exposure on peripheral tissues: role of oxidative stress in toxicity and possible protection by ascorbic acid. Rev Environ Health 22:157. https://doi.org/10.1515/reveh.2007.22.2.157
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Annal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126. https://doi.org/10.1016/s0076-6879(84)05016-3
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autooxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–474. https://doi.org/10.1111/j.1432-1033.1974.tb03714.x
Yagi K (1976) A simple fluorometric assay for lipoperoxide in blood plasma. Biochem Med 15:212–216. https://doi.org/10.1016/0006-2944(76)90049-1
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG et al (1990) Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 186:464–478. https://doi.org/10.1016/0076-6879(90)86141-h
Pérez-López FR, Chedraui P, Haya J, Cuadros JL (2009) Effects of the Mediterranean diet on longevity and age-related morbid conditions. Maturitas 64:67–79. https://doi.org/10.1016/j.maturitas.2009.07.013
Cempel M, Janicka K (2002) Distribution of nickel, zinc and copper in rat organs after oral administration of nickel (II) chloride. Biol Trace Elem Res 90:215–226. https://doi.org/10.1385/BTER:90:1-3:215
Debib A, Tir-Touil A, Mothana RA, Meddah B, Sonnet P (2014) Phenolic content, antioxidant and antimicrobial activities of two fruit varieties of Algerian Ficus carica L. J Food Biochem 38:207–215. https://doi.org/10.1111/jfbc.12039
Del Caro A, Piga A (2008) Polyphenol composition of peel and pulp of two Italian fresh fig fruits cultivars (Ficus carica L.). Eur Food Res Technol 226:715–719. https://doi.org/10.1007/s00217-007-0581-4
Duenas M, Perez-Alonso JJ, Santos-Buelga C, Escribano-Bailon T (2008) Anthocyanin composition in fig (Ficus carica L.). J Food Compos Anal 21:107–115. https://doi.org/10.1016/j.jfca.2007.09.002
Amamou F, Nemmiche S, Meziane RK, Didi A, Yazit SM, Chabane-Sari D (2015) Protective effect of olive oil and colocynth oil against cadmium-induced oxidative stress in the liver of Wistar rats. Food Chem Toxicol 78:177–184. https://doi.org/10.1016/j.fct.2015.01.001
Gilani AH, Malik HM, Khalid HJ, Arif-ullah K, Sheikh Arshad S (2008) Ethnopharmacological studies on antispasmodic and antiplatelet activities of Ficus carica. J Ethnopharmacol 119:1–5. https://doi.org/10.1016/j.jep.2008.05.040
Veberic R, Mateja C, Franci S (2008) Phenolic acids and flavonoids of fig fruit (Ficus carica L.) in the northern Mediterranean region. Food Chem 106:153–157. https://doi.org/10.1016/j.foodchem.2007.05.061
Russo F, Nicola C, Antonello P, Raffaele S (2017) Characterisation of volatile compounds in Cilento (Italy) figs (Ficus carica L.) cv. Dottato as affected by the drying process. Int J Food Prop 20:1366–1376. https://doi.org/10.1080/10942912.2017.1344991
Oliveira AP, Luís RS, Guedes de Pinho P, Gil-Izquierdo A, Valentão P, Branca MS, Pereira JA, Andrade PB (2010) Volatile profiling of Ficus carica varieties by HS-SPME and GC–IT-MS. Food Chem 123:548–557. https://doi.org/10.1016/j.foodchem.2010.04.064
Terpinc P, Tomaž P, Hanzlowsky NŠA, Ulrih NP, Abramovič H (2011) Antioxidant properties of 4-vinyl derivatives of hydroxycinnamic acids. Food Chem 128:62–69. https://doi.org/10.1016/j.foodchem.2011.02.077
Vuuren SFV, Viljoen AM (2007) Antimicrobial activity of limonene enantiomers and 1, 8-cineole alone and in combination. Flavour Fragr J 22:540–544. https://doi.org/10.1002/ffj.1843
Chavan MJ, Wakte PS, Shinde DB (2010) Analgesic and anti-inflammatory activity of caryophyllene oxide from Annona squamosa L. bark. Phytomedicine 17:149–151. https://doi.org/10.1016/j.phymed.2009.05.016
Hennia A, Nemmiche S, Dandlen S, Graça Miguel M (2019) Myrtus communis essential oils: insecticidal, antioxidant and antimicrobial activities: a review. J Essent Oil Res 31:487–545. https://doi.org/10.1080/10412905.2019.1611672
Viuda-Martosa X, Barberb J, Pérez-Álvareza A, Fernández-Lópeza J (2015) Assessment of chemical, physico-chemical, techno-functional and antioxidant properties of fig (Ficus carica L.). Ind Crops Prod 69:472–479. https://doi.org/10.1016/j.indcrop.2015.03.005
Gathwan KH, Al-Karkhi IHT, AL-Mulla EAJ (2013) Hepatic toxicity of nickel chloride in mice. Res Chem Intermed 39:2537–2542. https://doi.org/10.1007/s11164-012-0780-x
Samir D, Zine K (2013) Preventive effect of zinc on nickel-induced oxidative liver injury in rats. Afr J Biotech 12:7112–7119. https://doi.org/10.5897/AJB2013.12962
Rao MV, Chawla SL, Sharma SR (2009) Protective role of vitamin E on nickel and/or chromium induced oxidative stress in the mouse ovary. Food Chem Toxicol 47:1368–1371. https://doi.org/10.1016/j.fct.2009.03.018
Sindhu P, Garg ML, Morgenstern P, Vogt PJ, Butz T, Dhawan DK (2004) Role of zinc in regulating the levels of hepatic elements following nickel toxicity in rats. Biol Trace Elem Res 102:161–172. https://doi.org/10.1385/BTER:102:1-3:161
Winter MJ, Verweij F, Garofalo E, Ceradini S, Mckenzie EJ, Williams MA et al (2005) Tissue levels and biomarkers of organic contaminants in feral and caged chub (Leuciscus cephalus) from rivers in the West Midlands, UK. Aquat Toxicol 73:394–405. https://doi.org/10.1016/j.aquatox.2005.05.001
Turan A, Celik I (2016) Antioxidant and hepatoprotective properties of dried fig against oxidative stress and hepatotoxicity in rats. Int J Biol Macromol 91:554–559. https://doi.org/10.1016/j.ijbiomac.2016.06.009
Pari L, Amudha K (2011) Hepatoprotective rôle of naringin on nickel-induced toxicity in maie Wistar rats. Eur J Pharmacol 650:364–370. https://doi.org/10.1016/j.ejphar.2010.09.068
Hfaïedh N, Allaqui MS, Hfaïedh M, El Feki A, Zourgui L, Croute F (2008) Protective effect of cactus (Opuntia ficus indica) cladode extract upon nickel-induced toxicity in rats. Food Chem Toxicol 46:3759–3763. https://doi.org/10.1016/j.fct.2008.09.059
Saoudi M, El Feki A (2012) Protective role of Ficus carica stem extract against hepatic oxidative damage induced by methanol in male Wistar Rats. Evid Based Complement Alternat Med 2012:150458. https://doi.org/10.1155/2012/150458
Aghel N, Kalantari H, Rezazadeh S (2011) Hepatoprotective effect of Ficus carica leaf extract on mice intoxicated with carbon tetrachloride. Iran J Pharma Res 10:63–68. PMCID: PMC3869579
Funding
This project did not receive any specific grant from funding agencies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
All animal experiments were conducted in accordance with the ethical principles and institutional guidelines of the National Institutes of Health Guide for the care and use of laboratory animals.
Rights and permissions
About this article
Cite this article
Nemiche, S., Ait Hamadouche, N., Nemmiche, S. et al. Ameliorative or corrective effects of Fig “Ficus carica” extract on nickel-induced hepatotoxicity in Wistar rats. Toxicol Res. 38, 311–321 (2022). https://doi.org/10.1007/s43188-021-00118-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43188-021-00118-w