Abstract
Advanced glycation end products (AGEs) can induce inflammatory signaling pathways through the receptor for AGEs (RAGE). Targeting RAGE could be a therapeutic strategy for treating chronic inflammation mediated by the AGE-RAGE axis. This study aimed to investigate the effects of Fimbristylis ovata and Artemisia vulgaris extracts on AGE-RAGE signaling and AGE-mediated oxidative stress and inflammation in THP-1 cells. F. ovata and A. vulgaris were extracted by a Soxhlet extraction, and antioxidant capacity was evaluated using DPPH and ABTS assays. The human monocytic cell line THP-1 was treated with AGE (600 µg/ml) with and without F. ovata and A. vulgaris extracts (100 µg/ml). The mitochondria-targeting antioxidant MitoQ (2 μg/ml) was used as a positive control. Cell viability, ROS generation, RAGE, AGE-RAGE signaling pathway components, and inflammatory cytokine levels were analyzed. F. ovata and A. vulgaris extracts showed antioxidative effects in non-cell-based assays. Treatment of THP-1 cells with AGE significantly increased the protein levels of RAGE and significantly increased the mRNA expression of cytokines, including TNF-α, IL-1β, and IL-6. AGEs induced the generation of ROS and levels of signaling molecules downstream of RAGE, including phosphorylated and total Erk1/2, JNK, and p38 MAPK, although not significantly. F. ovata and A. vulgaris extracts significantly decreased the protein levels of RAGE and significantly decreased the mRNA levels of cytokines. In conclusion, this study revealed that F. ovata and A. vulgaris extracts exert anti-inflammatory effects through the AGE-RAGE axis. However, details on this anti-inflammatory effect through AGE-RAGE signaling should be further investigated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Advanced glycation end products (AGEs) are heterogeneous molecules formed by nonenzymatic glycation and protein, lipid and nucleic acid oxidation. In biological systems, the process of endogenous AGE formation and accumulation in various tissues begins under diabetic hyperglycemia and oxidative stress conditions. In addition to endogenously produced AGEs, AGEs also exist in heat-processed foods and cigarette smoke [1,2,3,4]. AGE-induced inflammation has been recognized as a key mechanism underlying chronic diseases (e.g., atherosclerosis), as AGEs can activate several inflammatory signaling pathways by binding to their receptor, receptor for AGEs (RAGE), and regulate the release of inflammatory molecules through oxidative stress [5, 6]. A large number of studies have reported that the AGE-RAGE interaction leads to an increase in oxidative stress and to the activation of various cell signaling pathways, including mitogen-activated protein kinases (MAPKs), nuclear factor-kappa B (NF-κB), and phosphoinositide 3-kinase (PI3K)-Akt, which lead to the expression of inflammation-related genes and promote inflammation [7,8,9,10].
Increasing evidence has suggested that reactive oxygen species (ROS) affect the biosynthesis of inflammatory modulators at the transcriptional level by modulating redox-sensitive transcription factors, including NF-κB, Nrf2, and AP-1 [11]. ROS also play a role in promoting inflammation through MAPK signaling cascades, including extracellular signal-regulated protein kinase (ERK), p38MAPK, and c-Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK), which regulate the activity of downstream transcription factors (e.g., NF-κB, ATF-2, and AP-1) and lead to the increased production of numerous inflammatory mediators, growth factors, and proinflammatory cytokines, such as TNF-α, IL-1β, and IL-6 [12, 13].
A number of natural and synthetic antioxidative compounds have been mentioned as therapeutic strategies for the treatment of many pathophysiological conditions and oxidative stress-related inflammatory diseases [14]. Phytochemicals, a group of chemicals derived from many kinds of fruits and plants, have long been highlighted due to their benefits for human health and their pharmacological activities under several pathological conditions [15]. Numerous polyphenolic compounds and extracts of polyphenolic-rich plants possess antioxidant, anticancer, and anti-inflammatory properties [14]. An in vitro study has shown that flavonoids have therapeutic effects on complications of diabetes due to their antioxidant effects against oxidative stress mediated by AGEs [16]. It has been suggested that active flavonoid derivatives in herbs exhibit potent anti-inflammatory activity [17]. Traditional Thai herbal therapies may be an alternative treatment option for inflammation-related diseases, such as type 2 diabetes [18], cardiovascular diseases [19], rheumatoid arthritis [20], chronic inflammatory lung disease [21], asthma [22], and Alzheimer’s disease [23]. Fimbristylis ovata (Burm.f.) Kern (F. ovata) is a plant in the Cyperaceae family [24]. Previous studies reported that plants in the Cyperaceae family contain several antioxidant components and have anti-inflammatory properties [25,26,27], antipyretic effects, antinociceptive effects, and activity against Aedes mosquito species [28, 29]. F. ovata is traditionally used to treat various diseases [30, 31]. In our previous study, we have shown that F. ovata has antioxidant activity, anti-inflammatory properties, and neuroprotective potential. Moreover, chemical analysis of F. ovata extracts revealed potential active phytochemical compounds with neuroprotective substances [32, 33]. Artemisia vulgaris L. var. indica Maxim (A. vulgaris) belongs to the Compositae family. There is evidence that A. vulgaris contains several polyphenolic compounds [34]. Previous studies reported that A. vulgaris has therapeutic properties such as antimalarial, antioxidant, anti-inflammatory, and anticancer properties [35, 36]. However, no study has examined the antioxidant and anti-inflammatory effects of F. ovata and A. vulgaris extracts prepared with different conventional methods. Therefore, this study aimed to investigate whether F. ovata and A. vulgaris extracts can suppress AGE-RAGE signaling activation-induced inflammatory responses and oxidative stress in THP-1 monocytes. Discoveries from this study could help us to better understand the mechanisms of each extract separated by sequential extraction, which allows natural products to be divided according to their polarity in extraction solvents. We expect that these results will provide insights into the roles of these extracts in inflammatory conditions, particularly those caused by AGEs.
Materials and methods
Preparation of plant extracts
F. ovata and A. vulgaris were identified by Professor Kasin Suvatabhandhu Herbarium, Department of Botany, Faculty of Science, Chulalongkorn University, Thailand (voucher No. 013431 (BCU) and A015134 (BCU), respectively). The fresh plants were cleaned with distilled water and then oven-dried at 45 °C for 5 days. Dried plants were ground to powder and extracted with petroleum ether, dichloromethane, and methanol 1:10 (w/v) by a Soxhlet extractor. The extracts were filtered, and the solvent was evaporated. Dimethyl sulfoxide (DMSO) was used to dissolve the plant crude extract to establish the 100 mg/ml stock solution. Stock solutions were stored protected from light at − 20 °C.
DPPH assay
Antioxidant capacity was investigated by the 2,2-diphenyl-1-picrylhydrazyl (DPPH) method based on electron transfer between DPPH and the antioxidants in the plant extracts. Briefly, a calibration curve of ascorbic acid was prepared. Plant extracts (20 µl) were added to DPPH reagent (180 µl) in a 96-well plate and then incubated in the dark for 30 min. The absorbance at 517 nm was measured using a microplate reader (BioTek, VT, USA). The antioxidant activity was reported as mg vitamin C equivalent antioxidant capacity (VCEAC)/g of dried plant.
ABTS assay
Antioxidant activity was analyzed by the reaction between the plant extracts and 2,2’-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) cation radical (ABTS• +). Briefly, a fresh ABTS• + solution was prepared by reacting ABTS reagent with potassium persulfate. A calibration curve of ascorbic acid was prepared. The plant extracts (20 µl) were added to working reagent (180 µl) in a 96-well plate and then incubated in the dark for 45 min. The absorbance at 734 nm was measured. The antioxidant activity was reported as mg VCEAC/g of dried plant.
Cell culture
The human monocytic cell line THP-1 was grown in RPMI 1640 supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin and 100 µg/ml streptomycin in a humidified incubator with 5% CO2 at 37 °C.
MTS assay
Cell viability was investigated by a 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay, which measures the mitochondrial enzyme activity that reduces MTS to a formazan product that is soluble in the tissue culture medium. Briefly, THP-1 cells were seeded in a 96-well plate at a density of 5 × 105 cells/ml and differentiated into macrophages by stimulation with 10 ng/ml phorbol 12-myristate 13-acetate. Macrophages were then treated with either plant extracts or AGE-BSA for 24 h. Thereafter, MTS was added and incubated in a humidified incubator with 5% CO2 at 37 °C for 4 h. The absorbance at 490 nm was measured using a microplate reader. Cells without treatment were used as a negative control. Cell viability was calculated according to the following.
formula: % cell viability = [(absorbance of the treatment group – blank) × 100/(absorbance of the control group – blank)].
Determination of intracellular ROS generation
The percentage of cells undergoing oxidative stress based on the detection of intracellular superoxide radicals was analyzed using a Muse® Oxidative Stress kit (Merck, Darmstadt, Germany). The reagent is based on dihydroethidium (DHE), which is a cell-permeable fluorescent dye. Upon entering the cells, DHE and superoxide interact to form red fluorescent oxyethidium. The Muse® Cell Analyzer instrument uses microcapillary cytometry for single-cell analysis and laser-based fluorescence detection of each cell event. Briefly, THP-1 cells were seeded at a density of 5 × 105 cells/ml in 12-well plates and differentiated into macrophages by stimulation with 10 ng/ml PMA. Cells were exposed to 500 µM H2O2 or 600 µg/ml AGE-BSA alone or AGE-BSA in combination with 100 µg/ml plant extracts for 1 h. The mitochondria-targeted antioxidant mitoquinone mesylate (MitoQ, 2 μg/ml) was used with AGE-BSA as the positive control. MitoQ was chosen due to its protective effect against oxidative damage and inflammatory responses by inhibiting the RAGE signaling pathway [37,38,39,40,41,42,43,44]. Thereafter, the cells were incubated with working reagent for 30 min and were analyzed using the Muse® Cell Analyzer (Merck, Darmstadt, Germany).
Quantitative reverse transcription polymerase chain reaction (qRT-PCR)
THP-1 cells were seeded in 6-well plates at a density of 1 × 106 cells/ml and differentiated into macrophages by treatment with 10 ng/ml PMA. Then, the cells were exposed to 1 µg/ml LPS or 600 µg/ml AGE-BSA alone or AGE-BSA in combination with 100 µg/ml plant extracts or 2 μg/ml MitoQ for 24 h. Total RNA was extracted using TRIzol reagent (Invitrogen, MA, USA) following the manufacturer’s instructions. The RNA template was used for cDNA synthesis using the AccuPower® CycleScript RT PreMix Reverse Transcription System (Bioneer, Daejeon, Korea) and oligo(dT)18 primer. For the amplification reaction, qPCR was performed using the Exicycler™ 96 Real-Time Quantitative Thermal Block (Bioneer, Daejeon, Korea). mRNA expression was analyzed using the SYBR green primer pairs listed in Table 1. The specificity of the reaction products was assessed by performing melting curve analysis. The expression of each gene was normalized to the housekeeping gene β-actin. The fold change in expression was determined using the ΔΔCt method (2-ΔΔCt).
Western blotting analysis
THP-1 cells were seeded at a density of 1 × 106 cells/ml in 6-well plates and differentiated into macrophages by treatment with 10 ng/ml PMA. The cells were exposed to 1 µg/ml LPS or 600 µg/ml AGE-BSA alone or AGE-BSA in combination with 100 µg/ml plant extracts or 2 μg/ml MitoQ for 1 h or 24 h. Proteins were isolated from THP-1 cells using lysis buffer with phosphatase inhibitor. The protein concentration was measured by the Bradford protein assay (Bio-Rad, CA, USA). Proteins (15 µg) were separated by 10% SDS-PAGE and transferred to PVDF membranes. Unspecific protein-binding sites were blocked by incubating the membrane with TBS-T containing 5% nonfat dry milk for 1 h. The membranes were then incubated with primary antibodies (Table 2) overnight at 4 °C, followed by secondary antibodies (peroxidase-conjugated goat anti-mouse or anti-rabbit IgG, Cell Signaling Technology, MA, USA). The blots were incubated in ECL Select Western blotting Detection Reagent (GE Healthcare, IL, USA) and then visualized using high-performance chemiluminescence (GE Healthcare, IL, USA). The intensities of the protein bands were quantitated using ImageJ software (National Institute of Health, MD, USA), and β-actin was used as the housekeeping protein.
Statistical analysis
The results of 3 repeats are presented as the mean ± standard error of the mean (SEM) and were analyzed using one-way ANOVA with post hoc Bonferroni tests (Prism 7, GraphPad, CA, USA). A p value < 0.05 was considered statistically significant.
Results
Antioxidant capacity of F. ovata extracts
The results in Table 3 show that the antioxidant capacities of the dichloromethane and methanol extracts of F. ovata were similar and higher than that of the petroleum ether extract, as evidenced by both the ABTS and DPPH assays (p < 0.05).
Antioxidant capacity of A. vulgaris extracts
The results in Table 4 show that the antioxidant capacities of the dichloromethane and methanol extracts of A. vulgaris were significantly higher than that of the petroleum ether extract, as determined by the ABTS assay (p < 0.05). In the DPPH assay, the methanol extract of A. vulgaris showed the highest antioxidant capacity (p < 0.05 vs. dichloromethane and petroleum ether extracts), followed by the dichloromethane extract (p < 0.05 vs. petroleum ether), and the petroleum ether extract showed the lowest antioxidant capacity (Table 4).
Effect of F. ovata and A. vulgaris extracts, AGE-BSA, and BSA on cell viability
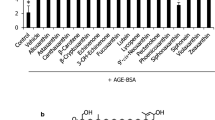
Upon incubation with various concentrations (0.78–100 µg/ml) of F. ovata and A. vulgaris extracts for 24 h, no significant change in the viability of THP-1 cells was observed (Fig. 1 a-b). Upon incubation with various concentrations of AGE-BSA and BSA (25–600 µg/ml) for 24 h, there was no significant change in cell viability (Fig. 1c).
Effect of F. ovata and A. vulgaris extracts on AGE-induced intracellular superoxide radical production
Treatment with H2O2 and AGE-BSA for 1 h increased intracellular superoxide radical production by 30% (Fig. 2). Treatment with 100 µg/ml petroleum ether, dichloromethane and methanol extracts of A. vulgaris marginally reduced superoxide radical production compared with the AGE-BSA treatment. The positive control MitoQ at a concentration of 2 μg/ml did not affect the viability of THP-1 cells (Supplementary Fig. S1). Treatment with MitoQ decreased superoxide radical production to the untreated level (Fig. 2).
Effect of F. ovata and A. vulgaris extracts on AGE-induced RAGE expression
Compared with the control condition, LPS treatment for 24 h increased RAGE protein expression by 50% without statistical significance (Fig. 3), and treatment with AGE-BSA for 24 h significantly increased RAGE protein expression (p < 0.05 vs. untreated; Fig. 3). In addition, treatment with the dichloromethane extracts of F. ovata and A. vulgaris and the methanol extract of A. vulgaris significantly attenuated RAGE protein expression compared with treatment with AGE-BSA (p < 0.05; Fig. 3). However, the petroleum ether extracts of F. ovata and A. vulgaris and the methanol extract of F. ovata did not affect RAGE protein levels. Treatment with MitoQ decreased RAGE protein expression compared with AGE-BSA treatment (p < 0.05; Fig. 3).
Protein expression of RAGE in THP-1 cells. Cells were exposed to AGE-BSA alone or AGE-BSA in combination with plant extracts or MitoQ for 24 h. MitoQ was used as a positive control. The results are expressed as the mean ± SE, n = 3. *p < 0.05 AGE-BSA vs. untreated, #p < 0.05, MitoQ and plant extracts vs. AGE-BSA
Effect of F. ovata and A. vulgaris extracts on MAPK signaling
Upon treatment with LPS and AGE-BSA for 1 h, the levels of phospho-Erk1/2 (Fig. 4a), total Erk1/2 (Fig. 4b), and phospho-JNK (Fig. 5a) were increased by 50%, although without statistical significance. The total JNK levels were increased marginally by LPS treatment and by 50% in the AGE-BSA treatment (Fig. 5b). Compared with the control condition, both the LPS and AGE-BSA treatments increased phospho-p38 MAPK levels by 70% but only marginally increased p38 MAPK levels without statistical significance (Fig. 6a, b). There was no change in the Erk1/2, JNK, and p38 MAPK protein levels between the AGE-BSA group and the plant extract groups (Figs. 4, 5, 6). MitoQ treatment marginally reduced phosphorylated and total Erk1/2, JNK, and p38 MAPK levels, although without statistical significance when compared with AGE-BSA treatment (Figs. 4, 5, 6). There was no change in phospho-Erk1/2/total Erk1/2, phospho-JNK/total JNK, or phospho-p38 MAPK/total p38 MAPK between all groups.
Phospho-p38 MAPK (a), total p38 MAPK (b), and phospho-p38 MAPK/total p38 MAPK (c) protein levels in THP-1 cells. Cells were exposed to AGE-BSA alone or AGE-BSA in combination with plant extracts for 1 h. MitoQ was used as a positive control. The results are expressed as the mean ± SE, n = 3. *p < 0.05 AGE-BSA vs. untreated
Effect of F. ovata and A. vulgaris extracts on NF-κB expression
The protein levels of the transcription factor NF-κB were increased by 40% and 60% by LPS and AGE-BSA treatments, respectively, compared with the control condition (Fig. 7). There was no difference in NF-κB levels between the AGE-BSA group and the plant extract groups, whereas MitoQ normalized NF-κB to the control level.
Effect of F. ovata and A. vulgaris extracts on AGE-induced inflammatory cytokine expression
Our data revealed that LPS and AGE-BSA significantly induced TNF-α, IL-1β, and IL-6 mRNA expression (p < 0.05 vs. untreated; Fig. 8a–c). Treatment with all extracts of F. ovata and A. vulgaris normalized TNF-α mRNA expression to the control level (p < 0.05 vs. AGE-BSA; Fig. 8a). IL-1β mRNA expression was significantly decreased (p < 0.05 vs. AGE-BSA; Fig. 8b) by treatment with the methanol extract of F. ovata and all extracts of A. vulgaris compared with treatment with AGE-BSA. Moreover, all the extracts of F. ovata and the petroleum ether extract of A. vulgaris normalized IL-6 mRNA expression to the control level (p < 0.05 vs. AGE-BSA; Fig. 8c). MitoQ treatment significantly inhibited TNF-α, IL-1β, and IL-6 mRNA expression compared with AGE-BSA treatment (p < 0.05; Fig. 8a–c).
Discussion
In this study, we assessed the antioxidant and anti-inflammatory capacity of F. ovata and A. vulgaris extracts. Different extraction methods were examined. The results from our study showed that the dichloromethane and methanol extracts of F. ovata had the same high antioxidant activity, while the methanol extract of A. vulgaris had the highest antioxidant activity. All extracts of F. ovata and A. vulgaris showed potent anti-inflammatory effects, which were associated with the inhibition of AGE-induced RAGE expression. The radical-scavenging capacity of plant extracts was dependent on solvent polarity, which is related to the polar nature of the active compounds in each plant. Among the major classes of phytochemicals, phenolic compounds are the most extensively studied, especially their health benefits due to potential protection against oxidative damage [45]. Polar solvents are efficiently used to recover phenolic compounds from plants [46]. Our results indicated that the natural antioxidants in F. ovata and A. vulgaris may mainly be preserved in the polar solvent extracts. Notably, there were some differences in the radical-scavenging capacity of the plant extracts detected by the DPPH and ABTS assays. The ABTS assay is superior to the DPPH assay and reveals antioxidant activity in a more sensitive manner since it has faster reaction kinetics. The ABTS assay is also useful for assessing the antioxidant capacity of samples extracted with acidic solvents and of samples containing hydrophilic, lipophilic, and pigment compounds [47, 48]. However, while none of the F. ovata and A. vulgaris extracts showed marked antioxidant activity in cells, the ROS production induced by AGE was inhibited by the known mitochondria-targeting antioxidant MitoQ. This may be due to the dose of F. ovata and A. vulgaris extracts used in this study. The inconsistency between our findings of antioxidative effects of plant extracts evaluated using non-cell-based assays and ROS generation in THP-1 cells may be due to the principle of oxidative stress kit in this study which is based on the detection of only superoxide radical production in cells. The production of other radicals, such as hydroxyl radicals, peroxyl radicals, and alkoxyl radicals, should be further investigated.
The AGE-RAGE interaction leads to an increase in oxidative stress and to the activation of various cell signaling pathways, including MAPKs, PI3K-Akt, and NF-κB, which causes the expression of a variety of inflammation-related genes and promotes inflammation [7, 8]. Increasing evidence suggests that the AGE-RAGE axis is a therapeutic target for chronic inflammation-related conditions. AGE inhibitors can prevent oxidative stress and have protective effects against inflammation [49]. In addition, clinical research revealed that sRAGE, acting as a RAGE competitor, could suppress vascular inflammation [50]. Moreover, knockout of the RAGE gene resulted in reduced atherosclerosis and vascular inflammation [51]. Therefore, targeting RAGE could be a therapeutic strategy for the treatment of conditions caused by AGE-RAGE axis-mediated oxidative stress and chronic inflammation.
In this study, AGEs induced the expression of RAGE, signaling molecules downstream of RAGE, including Erk1/2, JNK, p38 MAPK, and NF-κB, and inflammation-related genes, including TNF-α, IL-1β, and IL-6. Most F. ovata and A. vulgaris extracts suppressed RAGE expression and inflammatory cytokine production, except for the petroleum ether extracts. This anti-inflammatory effect was independent of their antioxidant capacity, at least at these experimental doses. The role of F. ovata and A. vulgaris extracts in the inhibition of AGEs is, at least, mediated by attenuating the increase in RAGE but without impacting Erk1/2, JNK, p38 MAPK, and NF-κB.
It has also been reported that AGE-mediated stimulation of Toll-like receptor-4 (TLR-4) signaling could induce the inflammatory cytokines IL-1β and IL-6 in THP-1 cells [52, 53]. RAGE and TLRs share common ligands and signaling pathways, suggesting a cooperative interaction in immune response stimulation. Incorporation of the RAGE signal into the TLR pathways markedly amplifies inflammatory signaling [54]. In this study, F. ovata and A. vulgaris extracts exerted a suppressive effect on RAGE and inflammatory cytokine levels but did not affect Erk1/2, JNK, p38 MAPK, or NF-κB; thus, AGE-mediated stimulation of TLRs should also be considered in our results. The effects of F. ovata and A. vulgaris extracts on the incorporation of RAGE and TLRs should be further investigated.
The PI3K/Akt [55, 56], protein kinase C [57], and JAK/STAT pathways [58] are among the known signaling cascades that can be activated by RAGE. Additional studies are needed to discover the alternative pathway that mediates the anti-inflammatory effects of F. ovata and A. vulgaris extracts. It would be interesting to further clarify the effect of F. ovata and A. vulgaris extracts on signaling cascades, including the PI3K/Akt, protein kinase C, and JAK/STAT pathways.
In addition to the transcription factor NF-κB, the AGE-RAGE axis could activate several proinflammatory transcription factors, including cAMP-response-element-binding protein (CREB) [59, 60], early growth response-1 (EGR-1) [61], and activator protein-1 (AP-1) [62]. We did not precisely determine the mechanisms of AGE-induced proinflammatory transcription factors; therefore, these issues should also be considered. In this study, AGEs induced the protein levels of NF-κB. However, F. ovata and A. vulgaris extracts did not affect NF-κB levels. Therefore, the effects of F. ovata and A. vulgaris extracts on NF-κB activities, such as the active form of NF-κB, IkappaB-alpha degradation, and NF-κB nuclear translocation, need to be further investigated.
In summary, F. ovata and A. vulgaris extracts have an inhibitory effect on AGE-mediated RAGE overexpression and inflammatory responses and showed antioxidative effects in non-cell-based assays. Therefore, F. ovata and A. vulgaris might be useful as alternative options to prevent AGE-RAGE signaling-mediated inflammatory conditions. Future studies are needed to elucidate the active compounds in various extracts and to confirm the effect of F. ovata and A. vulgaris extracts on more AGE-RAGE cascade components.
Abbreviations
- AGEs:
-
Advanced glycation end products
- A. vulgaris :
-
Artemisia vulgaris L. var. indica Maxim
- ERK1/2:
-
Extracellular signal-regulated protein kinases 1 and 2
- F. ovata :
-
Fimbristylis ovata (Burm.f.) Kern
- IL-1β:
-
Interleukin1 beta
- IL-6:
-
Interleukin6
- JNKs:
-
C-Jun N-terminal kinases
- MAPKs:
-
Mitogen-activated protein kinases
- NF-κB:
-
Nuclear factor kappa B
- PI3K:
-
Phosphoinositide 3-kinase
- RAGE:
-
Receptor for AGEs
- ROS:
-
Reactive oxygen species
- TNF-α:
-
Tumor Necrosis Factor alpha
References
Cerami C, Founds H, Nicholl I, Mitsuhashi T, Giordano D, Vanpatten S, Lee A, Al-Abed Y, Vlassara H, Bucala R, Cerami A (1997) Tobacco smoke is a source of toxic reactive glycation products. Proc Natl Acad Sci U S A 94:13915–13920. https://doi.org/10.1073/pnas.94.25.13915
Campos C, Guzmán R, López-Fernández E, Casado Á (2011) Urinary biomarkers of oxidative/nitrosative stress in healthy smokers. Inhal Toxicol 23:148–156. https://doi.org/10.3109/08958378.2011.554460
Prasad K, Dhar I, Caspar-Bell G (2015) Role of advanced glycation end products and its receptors in the pathogenesis of cigarette smoke-induced cardiovascular disease. Int J Angiol 24:75–80. https://doi.org/10.1055/s-0034-1396413
Goldberg T, Cai W, Peppa M, Dardaine V, Baliga BS, Uribarri J, Vlassara H (2004) Advanced glycoxidation end products in commonly consumed foods. J Am Diet Assoc 104:1287–1291. https://doi.org/10.1016/j.jada.2004.05.214
King GL, Brownlee M (1996) The cellular and molecular mechanisms of diabetic complications. Endocrinol Metab Clin North Am 25:255–270. https://doi.org/10.1016/s0889-8529(05)70324-8
Ramasamy R, Vannucci SJ, Yan SS, Herold K, Yan SF, Schmidt AM (2005) Advanced glycation end products and RAGE: a common thread in aging, diabetes, neurodegeneration, and inflammation. Glycobiology 15:16r–28r. https://doi.org/10.1093/glycob/cwi053
Lander HM, Tauras JM, Ogiste JS, Hori O, Moss RA, Schmidt AM (1997) Activation of the receptor for advanced glycation end products triggers a p21(ras)-dependent mitogen-activated protein kinase pathway regulated by oxidant stress. J Biol Chem 272:17810–17814. https://doi.org/10.1074/jbc.272.28.17810
Lin L, Park S, Lakatta EG (2009) RAGE signaling in inflammation and arterial aging. Front Biosci (Landmark Ed) 14:1403–1413. https://doi.org/10.2741/3315
Zhang SP, Wu YW, Wu ZZ, Liu HY, Nie JH, Tong J (2009) Up-regulation of RAGE and S100A6 in rats exposed to cigarette smoke. Environ Toxicol Pharmacol 28:259–264. https://doi.org/10.1016/j.etap.2009.04.013
Gassman JR, Lewis JB, Milner DC, Lewis AL, Bodine JS, Dunaway TM, Monson TD, Broberg DS, Arroyo JA, Reynolds PR (2016) Spatial expression of receptor for advanced glycation end-products (RAGE) in diverse tissue and organ systems differs following exposure to secondhand cigarette smoke. FASEB J. https://doi.org/10.1096/fasebj.30.1_supplement.lb741
Rahman I, Gilmour PS, Jimenez LA, MacNee W (2002) Oxidative stress and TNF-alpha induce histone acetylation and NF-kappaB/AP-1 activation in alveolar epithelial cells: potential mechanism in gene transcription in lung inflammation. Mol Cell Biochem 234–235:239–248. https://doi.org/10.1023/A:1015905010086
Whitmarsh AJ, Davis RJ (1996) Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J Mol Med (Berl) 74:589–607. https://doi.org/10.1007/s001090050063
Moens U, Kostenko S, Sveinbjørnsson B (2013) The role of mitogen-activated protein kinase-activated protein kinases (MAPKAPKs) in inflammation. Genes (Basel) 4:101–133. https://doi.org/10.3390/genes4020101
Udenigwe CC, Ramprasath VR, Aluko RE, Jones PJ (2008) Potential of resveratrol in anticancer and anti-inflammatory therapy. Nutr Rev 66:445–454. https://doi.org/10.1111/j.1753-4887.2008.00076.x
D’Amico R, Fusco R, Gugliandolo E, Cordaro M, Siracusa R, Impellizzeri D, Peritore AF, Crupi R, Cuzzocrea S, Di Paola R (2019) Effects of a new compound containing palmitoylethanolamide and baicalein in myocardial ischaemia/reperfusion injury in vivo. Phytomedicine 54:27–42. https://doi.org/10.1016/j.phymed.2018.09.191
Huang SM, Wu CH, Yen GC (2006) Effects of flavonoids on the expression of the pro-inflammatory response in human monocytes induced by ligation of the receptor for AGEs. Mol Nutr Food Res 50:1129–1139. https://doi.org/10.1002/mnfr.200600075
Tuchinda P, Reutrakul V, Claeson P, Pongprayoon U, Sematong T, Santisuk T, Taylor WC (2002) Anti-inflammatory cyclohexenyl chalcone derivatives in Boesenbergia pandurata. Phytochemistry 59:169–173. https://doi.org/10.1016/s0031-9422(01)00451-4
Pradhan A (2007) Obesity, metabolic syndrome, and type 2 diabetes: inflammatory basis of glucose metabolic disorders. Nutr Rev 65:S152-156. https://doi.org/10.1111/j.1753-4887.2007.tb00354.x
Golia E, Limongelli G, Natale F, Fimiani F, Maddaloni V, Pariggiano I, Bianchi R, Crisci M, D’Acierno L, Giordano R, Di Palma G, Conte M, Golino P, Russo MG, Calabro R, Calabro P (2014) Inflammation and cardiovascular disease: from pathogenesis to therapeutic target. Curr Atheroscler Rep 16:435. https://doi.org/10.1007/s11883-014-0435-z
McInnes IB, Schett G (2017) Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet 389:2328–2337. https://doi.org/10.1016/s0140-6736(17)31472-1
Chan SMH, Selemidis S, Bozinovski S, Vlahos R (2019) Pathobiological mechanisms underlying metabolic syndrome (MetS) in chronic obstructive pulmonary disease (COPD): clinical significance and therapeutic strategies. Pharmacol Ther 198:160–188. https://doi.org/10.1016/j.pharmthera.2019.02.013
Wieczfinska J, Sitarek P, Kowalczyk T, Skala E, Pawliczak R (2020) The anti-inflammatory potential of selected plant-derived compounds in respiratory diseases. Curr Pharm Des. https://doi.org/10.2174/1381612826666200406093257
Kinney JW, Bemiller SM, Murtishaw AS, Leisgang AM, Salazar AM, Lamb BT (2018) Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement (NY) 4:575–590. https://doi.org/10.1016/j.trci.2018.06.014
Santisuk T, Larsen K (1998) Flora of Thailand, vol 6/4. The Forest Herbarium, Royal Forest Department, Bangkok
Tungmunnithum D, Thongboonyou A, Pholboon A, Yangsabai A (2018) Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: an overview. Medicines (Basel). https://doi.org/10.3390/medicines5030093
Souto AL, Tavares JF, da Silva MS, Diniz Mde F, de Athayde-Filho PF, Barbosa Filho JM (2011) Anti-inflammatory activity of alkaloids: an update from 2000 to 2010. Molecules 16:8515–8534. https://doi.org/10.3390/molecules16108515
Guclu-Ustundag O, Mazza G (2007) Saponins: properties, applications and processing. Crit Rev Food Sci Nutr 47:231–258. https://doi.org/10.1080/10408390600698197
Roy R, Ud Daula A, Akter A, Sultana S, Barek MA, Liya IJ, Basher MA (2019) Antipyretic and anti-nociceptive effects of methanol extract of leaves of Fimbristylis miliacea in mice model. J Ethnopharmacol 243:112080. https://doi.org/10.1016/j.jep.2019.112080
Kamiabi F, Jaal Z, Keng CL (2013) Bioefficacy of crude extract of Cyperus aromaticus (Family: Cyperaceae) cultured cells, against Aedes aegypti and Aedes albopictus mosquitoes. Asian Pac J Trop Biomed 3:767–775. https://doi.org/10.1016/s2221-1691(13)60153-7
Khare C (2007) Indian medicinal plants an illustrated dictionary. Springer, USA
Burkill HM, Dalziel JM, Hutchinson J (1985) The useful plants of West tropical Africa, 2nd edn. Royal Botanic Gardens, Kew, UK
Sukjamnong S, Santiyanont R (2015) Effect of Fimbristylis ovata on receptor for advanced glycation end-products, proinflammatory cytokines, and cell adhesion molecule level and gene expression in U937 and bEnd.3 cell lines. Genet Mol Res 14:3984–3994. https://doi.org/10.4238/2015.April.27.13
Sirirattanakul S, Santiyanont R (2021) Fimbristylis ovata extract and its ability to encounter AGEs-induced neurotoxicity in SH-SY5Y. Toxicol Res 37:355–367. https://doi.org/10.1007/s43188-020-00072-z
Marina Radović J, Darko G, Jovana Tubić V, Aleksandra M, Milena M, Milan S, Nenad V, Milena V, Olivera M-D (2020) In vitro study of genotoxic and cytotoxic activities of methanol extracts of Artemisia vulgaris L. and Artemisia alba Turra. S Afr J Bot 132:117–126. https://doi.org/10.1016/j.sajb.2020.04.016
Abiri R, Silva ALM, de Mesquita LSS, de Mesquita JWC, Atabaki N, de Almeida EB, Shaharuddin NA, Malik S (2018) Towards a better understanding of artemisia vulgaris: botany, phytochemistry, pharmacological and biotechnological potential. Food Res Int 109:403–415. https://doi.org/10.1016/j.foodres.2018.03.072
Soon L, Ng PQ, Chellian J, Madheswaran T, Panneerselvam J, Gupta G, Nammi S, Hansbro NG, Hsu A, Dureja H, Mehta M, Satija S, Hansbro PM, Dua K, Collet T, Chellappan DK (2019) Therapeutic potential of artemisia vulgaris: an insight into underlying immunological mechanisms. J Environ Pathol Toxicol Oncol 38:205–216. https://doi.org/10.1615/JEnvironPatholToxicolOncol.2019029397
Manczak M, Mao P, Calkins MJ, Cornea A, Reddy AP, Murphy MP, Szeto HH, Park B, Reddy PH (2010) Mitochondria-targeted antioxidants protect against amyloid-beta toxicity in Alzheimer’s disease neurons. J Alzheimers Dis 20:S609-S631. https://doi.org/10.3233/jad-2010-100564
Gane EJ, Weilert F, Orr DW, Keogh GF, Gibson M, Lockhart MM, Frampton CM, Taylor KM, Smith RA, Murphy MP (2010) The mitochondria-targeted anti-oxidant mitoquinone decreases liver damage in a phase II study of hepatitis C patients. Liver Int 30:1019–1026. https://doi.org/10.1111/j.1478-3231.2010.02250.x
Dashdorj A, Jyothi KR, Lim S, Jo A, Nguyen MN, Ha J, Yoon KS, Kim HJ, Park JH, Murphy MP, Kim SS (2013) Mitochondria-targeted antioxidant MitoQ ameliorates experimental mouse colitis by suppressing NLRP3 inflammasome-mediated inflammatory cytokines. BMC Med 11:178. https://doi.org/10.1186/1741-7015-11-178
Xiao L, Xu X, Zhang F, Wang M, Xu Y, Tang D, Wang J, Qin Y, Liu Y, Tang C, He L, Greka A, Zhou Z, Liu F, Dong Z, Sun L (2017) The mitochondria-targeted antioxidant MitoQ ameliorated tubular injury mediated by mitophagy in diabetic kidney disease via Nrf2/PINK1. Redox Biol 11:297–311. https://doi.org/10.1016/j.redox.2016.12.022
Mao P, Manczak M, Shirendeb UP (2013) MitoQ, a mitochondria-targeted antioxidant, delays disease progression and alleviates pathogenesis in an experimental autoimmune encephalomyelitis mouse model of multiple sclerosis. Biochim Biophys Acta 1832 12:2322–2331. https://doi.org/10.1016/j.bbadis.2013.09.005
Li G, Chan YL, Sukjamnong S, Anwer AG, Vindin H, Padula M, Zakarya R, George J, Oliver BG, Saad S, Chen H (2019) A mitochondrial specific antioxidant reverses metabolic dysfunction and fatty liver induced by maternal cigarette smoke in mice. Nutrients. https://doi.org/10.3390/nu11071669
Sukjamnong S, Chan YL, Zakarya R, Saad S, Sharma P, Santiyanont R, Chen H, Oliver BG (2017) Effect of long-term maternal smoking on the offspring’s lung health. Am J Physiol Lung Cell Mol Physiol 313:L416-L423. https://doi.org/10.1152/ajplung.00134.2017
Sukjamnong S, Chan YL, Zakarya R, Nguyen LT, Anwer AG, Zaky AA, Santiyanont R, Oliver BG, Goldys E, Pollock CA, Chen H, Saad S (2018) MitoQ supplementation prevent long-term impact of maternal smoking on renal development, oxidative stress and mitochondrial density in male mice offspring. Sci Rep 8:6631. https://doi.org/10.1038/s41598-018-24949-0
Castañeda-Arriaga R, Pérez-González A, Reina M, Alvarez-Idaboy JR, Galano A (2018) Comprehensive investigation of the antioxidant and pro-oxidant effects of phenolic compounds: a double-edged sword in the context of oxidative stress? J Phys Chem B 122:6198–6214. https://doi.org/10.1021/acs.jpcb.8b03500
Baiano A, Del Nobile MA (2016) Antioxidant compounds from vegetable matrices: biosynthesis, occurrence, and extraction systems. Crit Rev Food Sci Nutr 56:2053–2068. https://doi.org/10.1080/10408398.2013.812059
Lee KJ, Oh YC, Cho WK, Ma JY (2015) Antioxidant and anti-inflammatory activity determination of one hundred kinds of pure chemical compounds using offline and online screening HPLC assay. Evid Based Complement Alternat Med 2015:165457. https://doi.org/10.1155/2015/165457
Dawidowicz AL, Olszowy M (2013) The importance of solvent type in estimating antioxidant properties of phenolic compounds by ABTS assay. Eur Food Res Technol 236:1099–1105. https://doi.org/10.1007/s00217-013-1982-1
Figarola JL, Shanmugam N, Natarajan R, Rahbar S (2007) Anti-inflammatory effects of the advanced glycation end product inhibitor LR-90 in human monocytes. Diabetes 56:647–655. https://doi.org/10.2337/db06-0936
Bucciarelli LG, Wendt T, Qu W, Lu Y, Lalla E, Rong LL, Goova MT, Moser B, Kislinger T, Lee DC, Kashyap Y, Stern DM, Schmidt AM (2002) RAGE blockade stabilizes established atherosclerosis in diabetic apolipoprotein E-null mice. Circulation 106:2827–2835. https://doi.org/10.1161/01.cir.0000039325.03698.36
Soro-Paavonen A, Watson AM, Li J, Paavonen K, Koitka A, Calkin AC, Barit D, Coughlan MT, Drew BG, Lancaster GI, Thomas M, Forbes JM, Nawroth PP, Bierhaus A, Cooper ME, Jandeleit-Dahm KA (2008) Receptor for advanced glycation end products (RAGE) deficiency attenuates the development of atherosclerosis in diabetes. Diabetes 57:2461–2469. https://doi.org/10.2337/db07-1808
Kanda A, Dong Y, Noda K, Saito W, Ishida S (2017) Advanced glycation endproducts link inflammatory cues to upregulation of galectin-1 in diabetic retinopathy. Sci Rep 7:16168. https://doi.org/10.1038/s41598-017-16499-8
Hodgkinson CP, Laxton RC, Patel K, Ye S (2008) Advanced glycation end-product of low density lipoprotein activates the toll-like 4 receptor pathway implications for diabetic atherosclerosis. Arterioscler Thromb Vasc Biol 28:2275–2281. https://doi.org/10.1161/atvbaha.108.175992
Gąsiorowski K, Brokos B, Echeverria V, Barreto GE, Leszek J (2018) RAGE-TLR crosstalk sustains chronic inflammation in neurodegeneration. Mol Neurobiol 55:1463–1476. https://doi.org/10.1007/s12035-017-0419-4
Xu H, He Y, Yang X, Liang L, Zhan Z, Ye Y, Yang X, Lian F, Sun L (2007) Anti-malarial agent artesunate inhibits TNF-alpha-induced production of proinflammatory cytokines via inhibition of NF-kappaB and PI3 kinase/Akt signal pathway in human rheumatoid arthritis fibroblast-like synoviocytes. Rheumatology (Oxford) 46:920–926. https://doi.org/10.1093/rheumatology/kem014
Lee C-W, Lin C-C, Lin W-N, Liang K-C, Luo S-F, Wu C-B, Wang S-W, Yang C-M (2007) TNF-alpha induces MMP-9 expression via activation of Src/EGFR, PDGFR/PI3K/Akt cascade and promotion of NF-kappaB/p300 binding in human tracheal smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 292:L799–L812. https://doi.org/10.1152/ajplung.00311.2006
Kontny E, Ziółkowska M, Ryzewska A, Maśliński W (1999) Protein kinase c-dependent pathway is critical for the production of pro-inflammatory cytokines (TNF-alpha, IL-1beta, IL-6). Cytokine 11:839–848. https://doi.org/10.1006/cyto.1998.0496
Huang JS, Guh JY, Chen HC, Hung WC, Lai YH, Chuang LY (2001) Role of receptor for advanced glycation end-product (RAGE) and the JAK/STAT-signaling pathway in AGE-induced collagen production in NRK-49F cells. J Cell Biochem 81:102–113. https://doi.org/10.1002/1097-4644(20010401)81:1%3c102::aid-jcb1027%3e3.0.co;2-y
Huttunen HJ, Kuja-Panula J, Rauvala H (2002) Receptor for advanced glycation end products (RAGE) signaling induces CREB-dependent chromogranin expression during neuronal differentiation. J Biol Chem 277:38635–38646. https://doi.org/10.1074/jbc.M202515200
Lee EJ, Kim JY, Oh SH (2016) Advanced glycation end products (AGEs) promote melanogenesis through receptor for AGEs. Sci Rep 6:27848. https://doi.org/10.1038/srep27848
Xu Y, Toure F, Qu W, Lin L, Song F, Shen X, Rosario R, Garcia J, Schmidt AM, Yan SF (2010) Advanced glycation end product (AGE)-receptor for AGE (RAGE) signaling and up-regulation of Egr-1 in hypoxic macrophages. J Biol Chem 285:23233–23240. https://doi.org/10.1074/jbc.M110.117457
Adamopoulos C, Piperi C, Gargalionis AN, Dalagiorgou G, Spilioti E, Korkolopoulou P, Diamanti-Kandarakis E, Papavassiliou AG (2016) Advanced glycation end products upregulate lysyl oxidase and endothelin-1 in human aortic endothelial cells via parallel activation of ERK1/2-NF-κB and JNK-AP-1 signaling pathways. Cell Mol Life Sci 73:1685–1698. https://doi.org/10.1007/s00018-015-2091-z
Acknowledgements
This work was financially supported by the 90th Anniversary of Chulalongkorn University Fund (Ratchadaphiseksomphot Endowment Fund) and National Research University Project, Office of Higher Education Commission (WCU-58-032-AS). MitoQ was provided by Greg Macpherson from MitoQ Limited, New Zealand. We thank the Center for Excellence in Omics-Nano Medical Technology Development Project Chulalongkorn University for allowing us to use various facilities.
Funding
Chulalongkorn University, the 90th Anniversary of Chulalongkorn University Fund, Suporn Sukjamnong, Office of the Higher Education Commission,WCU-58-032-AS,Rachana Santiyanont.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sukjamnong, S., Chen, H., Saad, S. et al. Fimbristylis ovata and Artemisia vulgaris extracts inhibited AGE-mediated RAGE expression, ROS generation, and inflammation in THP-1 cells. Toxicol Res. 38, 331–343 (2022). https://doi.org/10.1007/s43188-021-00114-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43188-021-00114-0