Abstract
Microalgae production has been vastly exploited in the last decade by scientific institutions and private organizations. It is not only one of the most efficient photosynthetic organisms, but also requires reduced cultivation space when grown in photobioreactors, compared to crops. Light is one of the most important variables that can limit photosynthetic microalgae growth and biomass production. The objective of this text is to bring an overview on how light influences microalgae growth inside photobioreactors and to delineate the current state and progress of the latest research on the topic of light intensity and wavelength, and light control in microalgae production systems. We show that, despite the extensive number of studies focused on the effects of light wavelengths and intensity on the production of microalgae, very few of those were dedicated to explore and evaluate the control of light variables in photobioreactors, especially in an automated approach, making it an interesting topic to be further investigated and developed. Finally, this review contributes to the understanding of several aspects of algae growth that can be related with the light environment inside photobioreactors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microalgae are phototrophic microorganisms that convert light and carbon dioxide into biomass containing value-added products encompassing lipids, proteins, pigments, and bioactive compounds that find application in the food, chemical, and pharmaceutical industries, as well as in the energy sector (Brennan and Owende 2010). Recently, microalgae have been extensively studied due to their potential as feedstock for biodiesel production (Kim et al. 2022) and wastewaters remediation (Adbelfattah et al. 2023; Drira et al. 2016; Samal et al. 2020; Sathinathan et al. 2023). They are among the most efficient photosynthetic organisms, and some species have higher lipid yield than agricultural oleaginous crops, since their oil content may exceed 70% (w/w), compared with the maximum 5% of crops (Gris et al. 2014). Also, the low cultivation space requirement, since it can be produced in small photobioreactors, make microalgae even more attractive and promising for many biotechnology sectors.
The growth of photoautotrophic microalgae happens quite simply and naturally. It requires basically water containing salts such as nitrogen and phosphorus, CO2, and a light source. However, this growth can be optimized through six foundations, as shown in Fig. 1. A photobioreactor can manipulate the main variables that influence de metabolism and physiology of the species highlighted. All the variables must be controlled: temperature, pH, dissolved oxygen and carbon dioxide, nutrients and also the power of light and its wavelength. In general, bioreactors control all variables very well, except light intensity and quality. Hence, due to its crucial role on optimizing microalgae cultivation, before discussing photobioreactor variables and control strategies, it is important to understand what indeed is light and how it can be measured.
Visible light can be described as an electromagnetic wave, with a wavelength between 400 to 750 nm. This short interval corresponds to electromagnetic radiation that is sensitive to human eyes. The electromagnetic spectrum is divided into bands that have low wavelengths for gamma (lower than 0.01 nm), x-ray (from 0.01 to 10 nm) and ultraviolet rays (from 10 to 400 nm), and high values for infrared (from 750 to 106 nm) and radio waves (higher than 106 nm) (Carvalho et al. 2011).
The spectrum of emitted energy is not continuous, it is composed of discrete packages named quanta. This phenomenon was profoundly studied and discussed by Albert Einstein and many other notorious physicists in the early twentieth century. Quanta, in its turn, can be understood by a massless particles called photons, which carry the properties of electromagnetic radiation. Photons energy can also be calculated, being represented by the multiplication of its frequency by Planck’s constant. Thus, a photon of shorter wavelength (400 nm) has greater energy when compared to a photon of longer wavelength (700 nm) (Falkowski and Raven 2007). It is common to find the luminous flux expressed in lumens (lm), and the illumination intensity, lumens per area, in lux (lm.m−2). However, the photobiologists, prefer to measure the light radiation energy incident on a surface in units of power per area (W.m−2 or J.m−2.s−1). Therefore, once photosynthesis uses photons as the energy source that is captured by photosynthetic pigments, this irradiance can also be expressed by the number of photons that reach the incidence area for a certain time and is reported as μmolphotons.m−2.s−1 or μE.m− 2.s– 1 (Masojídek et al. 2013). The main source of light that feeds the planet earth comes from the sun, but artificial sources are also very important in the biotechnological field.
In this context, this review gathers information from the main publications available in the literature on the following questions: given that light interferes with metabolic processes, how has this relationship been measured and reported? How are biotechnological products from microalgae optimised? How has light intensity been controlled in these bioprocesses? What are the remaining bottlenecks in this field?
Light sources
The sun is a natural and renewable source of electromagnetic energy that radiates per year 1.17 × 1031 kJ (Spiro and Stiglian 2009), or 1373 W.m−2 (Angstrom 1965). Its emission covers the range of 300 to 4000 nm, thus reaching wavelengths from the ultraviolet to the infrared (Carvalho et al. 2011). However, the earth receives only a small portion of the energy emitted by the solar surface (Bhatia 2014; Rocha et al. 2009; Spiro and Stiglian 2009) and only 0.34% of the sunlight absorbed by the terrestrial surface is used by plants and algae in photosynthesis (Spiro and Stiglian 2009). This useful part of the light spectrum is called photosynthetically active radiation (PAR).
Photosynthetically active radiation is expressed as the number of photons in the 400–700 nm wave range per unit area and time (Jones 1992). Solar energy is still the most economical energy source for microalgae cultivation; however, it is not the most efficient when the interest is an optimized production, precisely because of the difficulty in controlling the intensity and quality of this emitting source.
Artificial light sources introduce a cost to the photoautotrophic microalgae production process. However, according to articles that discussed this specific topic, such as Glemser et al. (2016), there are notable benefits of using that type of technology. The addition of this value in the production process is feasible when the objective is high value-added products, such as food supplements or the ingestion of this biomass itself, targeting, for example, carotenoids and polyunsaturated fatty acids, or nutraceuticals. This unnatural energy source allows the manipulation of the photoperiod, the quality (wavelength) and the quantity (intensity) of the energy according to the interest of the process, resulting in gains in productivity and in the quality of biomass (Carvalho et al. 2011). However, the choice of artificial light source is not trivial as there is a range of options on the market, which must be evaluated according to their cost, quality and controllability.
There are several artificial light sources such as incandescent, fluorescent, halogen and light-emitting diodes (LEDs) lamps. The differences between these types are associated with characteristics such as: luminous efficiency (lumen released per electric power consumed), emitted spectrum and useful life. As can be seen in Fig. 2, in most cases, the LEDs have a luminous efficiency above the other types and still allow the spectrum emitted to be customized for each type of application, extremely important advantages for photosynthetic processes, insofar as energy expenditure can be concentrated on the wave frequency in fact absorbed by photosynthesis (Bantis et al. 2018).
Comparison of lamps luminous efficiency in relation to different types of artificial light sources. “A” represents the classic pear shape, followed by the number encoding the bulb size; “T” refers to the tubular shape, followed by the number that refers to the diameter size.
One of the most reported options encountered in works related to the production of microalgae are fluorescent lamps (Franco-Lara et al. 2006; Lee et al. 2006a, b; Lee et al. 2006b). Many studies, however, have described the use of light emitting diodes, LEDs, as a light source for photosynthetic processes (Schulze et al. 2014; Yan et al. 2016; Yanb et al. 2016; Baidya et al. 2021). The increasing use of the LEDs can be explained by its recent cheapening, its lifespan (~ 50,000 h), by the absence of mercury in its composition and also by emitting practically monochromatic light at various wavelengths (Olle and Viršile 2013), allowing full controllability, which can assist in obtaining microalgae biomasses with high-value and specific biochemical characteristics (Schulze et al. 2014).
LEDs are semiconductor devices consisting of a positive (P: electron acceptor) and a negative (N: electron donor) layer. When N and P are joined, excess carriers diffuse to the other side, resulting in a depletion region without free carriers. The application of an electric potential allows a recombination of the electrons from the N side and holes from the P side in the depletion region. This recombination can release a photon with the corresponding energy difference (energy gap between the bands). Simplistically, when an electric current is forced to overtake from one layer (at a certain energy level) to another layer (at another energy level), in a given semiconductor, its electrons need to dispense a photon matching to the energy difference between the layers (Schulze et al. 2014).
Different semiconductors and a combination of them result in a variety of wavelengths with diverse colors and energy. To obtain white LEDs it is possible to combine different color LED chips into one unit, cm-LEDs, or coat a single blue indium gallium nitride chip with a phosphorous layer, pc-LEDs. Recent technology advancement now provides LED wavelength peaks between 250 and 1100 nm, which can be obtained from various suppliers. When pc-LEDs harbor a predominantly red emission spectrum, they are commonly referred to as having a “warm-white” light color. By contrast, pc-LEDs harboring an enhanced blue emission spectrum are termed as having a “cold-white” light color (Glemser et al. 2016).
Does the photobioreactor affect microalgae photosynthesis?
Yes, it does. To support this answer, we need to understand the complexity of photosynthesis a little better. It is already extensively discussed in literature that microalgae, according to its evolutionary history, tend to have a preference for blue (λ ~ 420–470 nm) or red (λ ~ 660 nm) lights (Glemser et al. 2016; Keeling 2013; Wang et al. 2014). To understand this preference, it is important to analyze how the biological structures of the microalgae interact with the photons provided by the light source during the photosynthesis.
The process of photosynthesis starts with the photon’s emission by an energy source. Photons are the fundamental particle of light, the smallest discrete amount of electromagnetic radiation; they assume the dually wave/particle, a unique property in that they are both particle and wave, defined as quanta by Einstein (1905). However, despite being a particle, photons have no mass. They have some characteristics of particle like angular momentum, but their frequency is independent of the mass influence, and as a particle, light can collide with electrons. If each absorbed light energy quanta ionizes a molecule, then the relationship between the amount of light absorbed and the number of molecules it ionizes is directly proportional (Einstein 1905). When it ionizes, the electron absorbs the photon’s energy and gets excited. This collision of photons and electrons is the starting point for the complex reactions of photosynthesis, which is shown in a simplified way in Fig. 3.
A simplified visual diagram of the photosynthesis. (Based on Nelson and Cox 2008)
When a photon penetrates the chloroplast and hits a molecule of chlorophyll a (principal photoreceptor of most green plants and algae), an electron can absorb this energy and start the complex cycle of organic synthesis (Berg et al. 2002). For those who may be interested on studying the subject more deeply, details about light absorption by photosynthetic pigments can be found in Falkowski and Raven (2007).
Chlorophyll molecules are part of a complex of proteins, lipids and other molecules that, specifically arranged, form the Photosystem II (PSII). Light reactions occur in two photosynthetic units: Photosystem I (PSI) and Photosystem II (PSII) (Carvalho et al. 2011). According to Berg et al. (2002) PSI have more than 60 chlorophyll molecules, vitamin K1 among others. PSII is very complex to, including more than 30 chlorophyll molecules, among others molecules and ions. Photosystem I is activated by wavelengths about 700 nm, whereas PSII about 680 nm. More information about the architecture and functioning of PSI and PSII can be found in the detailed work of Caffarri et al. (2014).
However, it is not only the photosynthesizing molecules that interact with light, but also the walls of the photobioreactor. Both the material the bioreactor is made of and its shape interfere with the light transmitted to the cells. The shape of the illuminated surface directly affects the phenomenon of light reflection and refraction. The greater the curvature of this surface, the greater the reflection effect and therefore the lower the efficiency of light energy absorption, since the light coming from an external source is reflected back to where it came from and little of it is refracted into the interior of the photobioreactor.
One of the disadvantages of cylindrical photobioreactors, in contrast to their lower manufacturing costs, is their low light absorption efficiency due to the presence of highly reflective areas. Even so, the cylindrical shape is one of the most common options. There are several options when it comes to the materials from which closed vessels are made. Photobioreactors with translucent walls can be made of glass, borosilicate, acrylic or plastic. Opaque tanks with internal lighting can also be manufactured from stainless steel, PVC and other materials (Hincapie and Stuart 2014).
Each material has its own characteristics in terms of light absorption and must be carefully designed to meet the requirements of each micro-organism and product of interest. This is because photosynthesis is entirely dependent on the quality of the light that passes through the walls of the photobioreactor to excite the photoreceptors that initiate the autotrophic reactions.
Effects of light intensity on microalgae
After understanding the biological mechanisms of photosynthesis in microalgae, it is clear that light is one of the most important sources for this phenomenon to occur. Literature has shown that the intensity of light is an important factor that influences directly the growth and products of microalgae. Liu et al. (2012), for instance, have investigated the effects of three levels of light intensity on lipid production and fatty acid composition, which are fundamental parameters for the production of biodiesel. It was shown that carotenoid/chlorophyll ratio was higher when the microalga Scenedesmus sp. 11–1 grew under 250 and 400 μmolphotons.m−2.s−1 than under 50 μmolphotons.m−2.s−1, but protein content was lower. Highest biomass yield and lipid concentration were achieved at 400 μmolphotons.m−2.s−1 light intensity and the major fatty acids of Scenedesmus sp. were oleic acid (43–52%), palmitic acid (24–27%), and linoleic acid (7–11%). More recently, Lai et al. (2019) studied the growth of Dunaliella viridis in a photobioreactor with varied initial light intensities (100, 300, 400 and 600 μmolphotons.m−2.s−1) and nitrogen levels (9 and 60 g.m−3). They obtained that the maximum lipid production was in initial nitrogen concentration of 9 g.m−3 and 300 μmolphotons.m−2.s−1 light intensity. In addition, the study showed linear relationships between light intensity and biomass concentration, and also between light intensity and chlorophyll a. Furthermore, the authors showed that it is possible to use real time light data as method for quantification of biomass and chlorophyll a. Gris et al. (2014) studied Scenedesmus obliquus grown under different light intensity and light–dark cycles. They measured the microalga growth, productivity, photosynthesis, and biochemical composition. It was found that maximum growth rate was obtained at 150 μmolphotons.m−2.s−1, above this value, despite biomass accumulation, photoinhibition and oxidative stress decreased photosynthetic efficiency. Also, according to the authors, cultures exposed to pulsed light showed reduced growth compared to continuous light. Schulze et al. (2020), stated that flashing light does not improve photosynthetic performance and growth of green microalgae. They exposed cultures of Tetraselmis chui to several different light frequencies, light–dark cycles and light intensities, and the results showed that flashing light was not more efficient than continuous light. Likewise, batch cultures of Chlorella stigmatophora and T. chui did not have enhanced growth with flashing light either. Further information regarding effects of light intensity on microalgal growth can be found in the studies of Sforza et al. (2012); Iasimone et al. (2018); Huesemann et al. (2013); González-Camejo et al. (2018); Patel et al. (2019); Whitton et al. (2019) and Cuaresma et al. (2009). Below we present a table (Table 1) with results synopsis of the main recent articles on this topic.
Effects of different wavelengths on microalgae growth
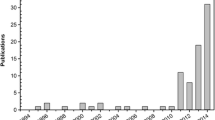
A significant number of studies analyzes the effects of different light wavelengths on microalgal growth. Figure 4 shows the number of publications over the years that focused on studying the effects of light wavelength on microalgae cultivation. This information was collected in survey carried out using the keywords “microalgae light” or “light quality microalgae” in Web of Science database. Besides the wavelength variation, the studies also encompass assorted types of reactors and cultivation methods. Next, it will be presented the majority of studies that have used wavelengths related to green, blue and red lights to grow microalgae. In total there were 119 publications that focused on the subject.
Considering the effects of using green light to grow microalgae, Mohsenpour and Willoughby (2013) disclosed that this wavelength promoted chlorophylls a and b production in a C. vulgaris strain. They also showed lowest biomass concentration for G. membranacea, when cultivating in luminescent acrylic photobioreactors. Das et al. (2010) obtained maximum fatty acid methyl esters yield for Nannochloropsis sp. when photo- and mixotrophic cultures were exposed to green LED. Baba et al. (2012), however, showed that when growing Botryococcus braunii, green light was less effective than blue and red for growth, photosynthetic CO2 fixation and hydrocarbon production. More recently, Jung et al. (2019) found that green LED promoted the highest lipid content, reaching ~ 61% (w/w) of the dry cell weight, for Phaeodactylum tricornutum, Dunaliella tertiolecta and Isochrysis galbana.
For blue light, Chen et al. (2010) studied Spirulina platensis in shaking flasks and observed that this wavelength yielded the best specific pigments production rates (chlorophyll a and phycocyanin) at high levels of light intensity, 3000 μmolphotons.m−2.s−1. Shu et al. (2012), and Pérez-Pazos and Fernández-Izquierdo (2011) cultivated Chlorella sp., the former in a bubble column reactor and the latter in a serpentine photobioreactor. Shu et al. (2012) results indicated that blue LED at 1000 lx was optimum for oil formation; Pérez-Pazos and Fernández-Izquierdo (2011) also showed that lipid production was higher under blue light. Izadpanah et al. (2017) worked with Chlorella sp. as well and investigated the role of light spectrum in isolation and growth of microalgae from urban wastewater. The largest cells, highest biomass and lipid densities were also found using blue light. Zhao et al. (2018) studied Chlamydomonas sp. and obtained optimal lutein content under blue light. Lutein is often used as food additive and plays a critical role in photosynthesis and photoprotection in microalgae. However, the best results of cell growth were found under white light. Fu et al. (2013) grew Dunaliella salina in a bubble column reactor under the combination of blue and red light and showed lutein and β-carotene accumulation. Han et al. (2019) had similar results cultivating the same organism and using a blue-red LED wavelength-shifting system: enhanced density and β-carotene productivity when compared to a single wavelength emitting diode. Bredda et al. (2020) also combined blue and red, and obtained the highest lipid productivity for Dunaliella salina, but optimal biomass productivity was reached under a mixture of 65% blue and 35% green lights. Beltran (2013), Katsuda et al. (2004) cultivated Haematococcus pluvialis in photobioreactors and glass vessels, respectively. The first discovered that for shorter wavelengths (blue light) the cell size, the quantity of red pigment and the growth kinetics were higher than the culture illuminated with green light. The second, in turn, showed that short wavelengths were found to induce astaxanthin accumulation and, also, that flashing light of blue LEDs is a promising illumination method for indoor algal cultivation. Marchetti et al. (2013) studied Isochrysis sp. and reported that relative carbohydrate content was lower under blue light than under white light, whereas chlorophyll a and photosynthesis activity were higher. In contrast, carbon quota was lower and protein content higher under blue light than under white light. Yoshioka et al. (2012) studied Isochrysis galbana, and according to their experiments cell density of the sample cultured under blue intermittent light was significantly higher than those of the other wavelengths. Das et al. (2010) and Kim et al. (2014) cultivated Nannochloropsis sp. The first achieved maximum specific growth rate (µ) with blue LEDs and the second had increase of photosynthetic oxygen evolution, carbon fixation and nutrient uptake using blue light.
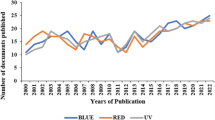
Regarding the effects of red light on microalgal growth, Kim et al. (2014) stated that more significant physiological changes were observed under red than under blue light for the cultivation of Nannochloropsis gaditana and, also, that red illumination may be useful for lipid production. Shu et al. (2012) achieved results that indicated that the optimal light wavelength and intensity for biomass formation of Chlorella sp. was red LED light at 1000 lx. Chen et al. (2010) also attested that red LED is the best for algae growth when cultivating Spirulina platensis. Rendón (2013), however, obtained the lowest values of biomass production for Chlorella vulgaris using red light, highest values were found when the culture was exposed to white light. Das et al. (2010) had also a minimum specific growth rate for red LEDs. More results about the use of green, blue and red light used to grow microalgae are available in Okumura et al. (2015); Koc and Anderson (2012); Kwon et al. (2013); Costa et al. (2013); Abiusi et al. (2014); Markou (2014); Kula et al. (2014); Atta et al. (2013); Teo et al. (2014); Xu et al. (2013); Vejrazka et al. (2012); Deniz et al. (2019); Bernstein et al. (2014) and Stevčić et al. (2019). Figure 5 shows the timeline for the studies about the effect of different wavelengths in microalgae growth previously discussed.
Light control in the production of microalgae
Control in processes or bioprocesses is a key part of increasing productivity and has become an overused tool since it optimizes operations. It is common to find control acting on most variables of different processes, such as: pH (Pataro et al. 2023; Capilla et al. 2021; Oflaz and Koku 2020), dissolved oxygen (Piotrowski et al. 2023; Mesquita et al. 2021; Huang et al. 2020; Gehan et al. 2019; Akisue et al. 2018), temperature (Li et al. 2023, 2021; Silva et al. 2021) and pressure (Hua et al. 2023; Fitzmaurice et al. 2021; Kumar and Mandal 2021).
The most-used techniques for bioprocess control are based on feedback systems. These systems manipulate the appropriate variables based on some logic and the measurement or estimation of the controlled variable. In this type of control, the measured variable is compared with the desired set point to generate a control response. There are several control algorithms available, from the simplest to the most complex, such as, On/Off, PID, Advanced Controls, Fuzzy Logic, Heuristics and Artificial Neural Networks (Castrucci et al. 2011; Ogata 2010). Thus, in this section the state of the art on light control in the production of microalgae will be provided, due to the importance previously discussed about this variable during the cultivation of microalgae.
The studies found that used light as a controlled variable can be classified into two groups: (i) manually manipulations, and (ii) automated control. This review initially presented the works found that controlled this variable in a non-automated way and later those that used automation to control light intensity.
Non-automated light control strategies
It is common to find works that control the luminous intensity using the pseudonym: lumostat, a term used in the literature to characterize a system that aims to prevent the photoinhibition of culture at low cell densities and also not to limit this process due to self-shading when high cell densities are reached (Eriksen et al. 1996). The works that mentioned this term are presented below.
Imaizumi et al. (2016) submitted a culture of Chlorella zofingiensis to lumostatic action, which implied an increase in the light intensity of the cultivation due to the increase in the microalgal biomass. The luminosity was measured with a quantum sensor (QSPL-2101, Biospherical Instruments, USA) and culture density was obtained by the dry biomass procedure, where the samples are filtered and its filter dried until reaching constant mass. This approach allowed for the highest cell density (13.5 ± 0.4 g.L−1) and volumetric biomass production rate (2.4 ± 0.1 g.L−1.day−1) ever reported in any previous photoautotrophic culture of this specie according to the authors.
Choi et al. (2003) also developed a lumostatic action on a bubble column photobioreactor for the cultivation of the specie Haematococcus pluvialis. The control parameter was the specific light capture rate (“qe”), which is the total amount of light energy absorbed by the culture divided by the total biomass present. The manipulation of luminous energy was done through the number of lamps and their distance from the photobioreactor. This light intensity was measured with a quantum sensor LI-COR (LI-190SA, LI-COR, Lincoln, NE, USA). The work demonstrated the superiority in relation to the cellular concentration that was increased by 200 and 250% (depending on the “qe”) in lumostatic action when compared to the results under constant supply of light energy.
The astaxanthin production is done in two stages, the first being the increase in cell concentration under lumostatic action, and the second which is the induction to pigment production in the cells. It is worth mentioning the study by Lee et al. (2016), who looked at the variable of control from another perspective and did not use the conventional measure of “qe”. The authors proposed variables derived from “qe” but that add details like the predominant pigment changes from chlorophyll to astaxanthin, as well as changes in cells concentration and size. The three new specific rates of light capture are based on characteristics of biomass, namely: number of cells, projection area and cell weight, which leads to unidimensional, bidimensional, and three-dimensional characteristics for the variables. The lumostatic action was then based on these up-to-date variables, but done in an already common way, where the light intensity was measured with a quantum sensor (LI-190SA, LI-COR, Lincoln, NE, USA) equipped with a data logger (LI-1400, LI-COR). Compact fluorescent lamps (Osram Korea, Ansan, Korea) were installed in a circular structure that involves the photobioreactor, but that allows the manipulation of the distance between the culture and the lamps. A maneuver was used to keep variables at the desired value. The authors also reported that the lumostatic operation was effective to increase the production of astaxanthin when compared to processes with constant light intensity.
A work like the previous one, developed a lumostat that was tested using the species Chlorella sp. The light was provided by two panels, each comprising 30 fluorescence lamps (Philips 8 W) where their intensity was varied by adjusting the number of lamps and the distance between the panels and the photobioreactor. The intensity of the incident light was measured on the inner surface of the photobioreactor in the absence of culture medium using a quantum sensor (LI-190SL from LI-COR Inc., USA). The specific average intensity of light, defined as the amount of luminous energy received per cell per unit of time in the photobioreactor, was selected as one of the parameters of light control in this study, which concluded that the lumostatic strategy improved the microalgae growth (Chen et al. 2011).
In the study of Lee et al. (2006a), the luminous intensity offered to the culture was given through fluorescent lamps (FL 18D, OSRAM, Korea) and the adjustment was also made by the number of lamps and the distance to the photobioreactor. The intensity was measured with a quantum sensor LI-COR (LI-190SA, LI-COR, Lincoln, NE, USA) and the parameter used in the lumostatic operation was the specific light capture rate (qe). The work concluded that lumostatic operations based on the specific light uptake rate of 3.5 × 10−8 μE.cell −1.s −1 was considered the best way to obtain cultures of H. rainfall with high cell density, also reporting that this strategy can be applied to other production systems in microalgae biotechnology.
Lumostatic operations using the specific light capture rate as a control parameter are not only an efficient way to obtain high cell densities in the crop, but are also an economical way to reduce cultivation time and save energy regardless of scale (Lee et al. 2006b). The authors demonstrated this in their work, where they evaluated different volumes of reactors (0.4, 2, 10 and 30 L) and in all cases presented higher results to cell concentrations in relation to cultures grown under constant light, reporting that the rate of specific light uptake is one of the best parameters for increasing the scale of photobioreactors. The experimental apparatus used by them was a quantum sensor LI-COR (LI-190SA, LI-COR, Lincoln, NE, USA) to measure the luminous intensity, which was given through a source built by fluorescent lamps (FL18D, OSRAM, Korea) distributed in circular panels positioned around the photobioreactors, the intensity was also adjusted by the number and distance of the lamps to the photobioreactor.
The study by Suh and Lee (2001) also addressed a lumostatic operation, in order to improve the luminosity profile offered for the culture of Synechococcus sp inside a tubular air-lift photobioreactor. The luminous energy needed for the lumostat was estimated using a model (Suh 2001) that calculates the light distribution profile and the average light intensity, that allowed the manipulation of the gradual activation of the number of light radiators. The drive increased as the average light intensity dropped from the desired value. The radiators were connected to a control station that had on / off switches. Each radiator had a fluorescent lamp 49 cm long, 1.25 cm in diameter, 18 W (DL18, BOAM USA Inc.). The density of the photon flow was measured using a DataLogger (LI-1000, LI-COR) equipped with a quantum sensor (LI-190SA, LI-COR) every 12 h of cultivation.
Yoon et al. (2008), in turn, used in their work to increase the light intensity during the cultivation of Anabaena variabilis a control parameter not yet mentioned in this review, the specific irradiation rate, which can be calculated from the product of the luminous intensity of perpendicular light and the surface area, divided by the product of cell concentration and the medium volume. The authors indicate this new parameter to be used in light controls in the microalgae production process, but assume that the specific rate of light capture (qe) is also a good strategy. The experimental apparatus used in the article ware: a quantum sensor (LI-COR, Model LI-190SA, Lambda Instrument Corp., USA) and a light meter (LI-COR, Model LI-250, Lambda Instrument Corp., USA); ten fluorescent lamps as the light source. The luminous intensity adjustment was controlled by the number of bulbs on and their distances to the photobioreactor.
The study by Hwang and Maier (2019) evaluated the quality of light including its wavelength and intensity on the lipid composition and growth of Neochloris oleoabundans. The article conducted the study using the correction of light intensity turning on and off lamps of different colors, so to increase the intensity of a chosen length they turned on more lamps of the same color, using a simple approach to solve the problem. The authors found that for this species, red light provides the highest growth rate, as well as favors the production of monounsaturated fatty acids according to the growth of light intensity, reporting that the strategy of controlling the wavelength can increase the production and improve the economic sustainability of microalgal crops.
Following this line of manipulating the distance between the lamps and the crop, Han et al. (2017) used the technique of polychromatic light control during cultivations of Nostoc flagelliforme. The measurement of light intensity was given by a LightScout Dual quantum sunlight sensor (Spectrum Technologies, USA). The results of the work allowed the authors to report a 66% increase in biomass and a 217% increase in the production of extracellular polysaccharides.
Next section will cover the few works found in the literature that manipulated the light intensity in a more refined way than those presented in this topic.
Automated light control strategies
The first authors to address automated light control were Eriksen et al. (1996), that manipulated the luminous intensity as a function of the pH, which in turn was controlled by pulses of CO2. In a simplified way, the light controller applies an increase or decrease in luminous power proportional to the addition of CO2. The step of increase or decrease was 25 μmolphotons.m−2.s−1, in a range from 240 to 65,025 μmolphotons.m−2.s−1. The culture of Synechoccus sp was used as case study and illuminated through cabinets coated with mirrors in order to exclude external light interference. Twelve fluorescent tube lamps (Philips TLD 18W/33) were controlled by voltage variation in the range of 30 V and 200 V by a dimming (EL1 × 36/2 × 18FD, Helvar, Finland) regulated by a digitally controlled potentiometer (X9MME, Xicor Inc., California). The luminous intensity of the lamps was measured by a Macam Q101 Quantum meter. The authors reported that the lumostatic action allowed the ideal use of light in photoautotrophic processes.
The second and last author to use an automated system was Ifrim et al. (2013), who implemented the feedback linearization control (FLC) to linearize a nonlinear model used to control the average light intensity (qi). The output of the controller was based on the ratio between the incident light flow and the biomass concentration. Its dynamics consisted of increasing the light intensity, in function of the gain in the biomass concentration, through manipulation of the power supply of the light-emitting diodes (LED) panel. The luminous intensity was measured by a quantum sensor LI-COR light meter LI-1400. It can be said that the controller behaves like a non-linear integrator, that is, at each interaction it increases, to the previous qi, a fraction proportional to the cellular concentration and to the error regulator. Biomass was calculated using a turbidity probe that was previously associated with biomass production. The validation of this system was done using the species Chlamydomonas reinahardtii and demonstrated through simulation that the control of incident light can produce at least 10% more biomass per mole of photon supplied to the culture when compared to systems with constant light intensity.
Control laws have also been reported by the work of Mairet et al. (2015), who proposed two non-linear approach that regulates the light attenuation factor in continuous microalgae culture, using the dilution rate as the manipulated variable in order to regulate the light attenuation factor, proposing two projects: a static controller and an adaptive controller. The first proposal considers the incident light constant and assumes that growth can be measured online or estimated indirectly, while the second proposal adds an adaptive gain to the feedback control law. Numerical simulations demonstrated that the adaptive controller presented satisfactory performance for regulation under constant light, pointing to an interesting mode of operation to optimize the production of biomass in external conditions.
The studies that investigated the feasibility of reducing light attenuation and thus optimizing lighting in photobioreactors can be divided into two broad categories, those that use manipulation in the light source and those that dilute the culture in order to increase its transparency for light diffusion. Using this concept the work of Liu et al. (2018) proposes an improvement in the microalgae production through the cultivation mode called continuous pre-harvest. This approach guaranteed an optimal light distribution for the cultivation of Spirulina platensis given in a closed system (photobioreactor). The continuous removal of biomass minimizes light attenuation and can maintain light conveyance at its optimum state. The authors did this using a culture overflow method during the exponential growth phase, where the amount overflowed was directed to a collector who separated the culture medium from the cells and sent it back to the photobioreactor via a peristaltic pump continuously. The light intensity was measured using a waterproof probe irradiatometer (FGH-1, Beijing Normal University Photoelectricity Instrument Company, China). This strategy achieved higher values of growth in the pre-harvest lots compared to the discontinuous ones.
From what it seems, the control of the luminous intensity reaching the cells is very important, and there are several interesting and more or less suitable techniques for each case. In general, it can be concluded that a combination of the techniques would be the most complete mode of operation: (i) implement an automatic light source power controller until reaching the operating limits and (ii) switch to an uninterrupted dilution mode, maintaining from this point thereafter, the constant cellular concentration, in a type of continuous operation, with or without recycling of filtered medium.
Controlling the intensity and quality of light could add a cost to the microalgae producing process, since most of the works that approach this tool use artificial sources of light energy. This is not difficult to agree, since controlling the costless solar radiation is not trivial, and not always feasible. However, this cost tends to minimize with the advancement of lighting technology, which already offers better alternatives than the fluorescent lamps used in most of the works mentioned in this review, such as, for example, light-emitting diodes (LEDs) that consume less energy and have a long lifespan, advantages that outweigh its value.
It is indeed important to emphasize that further research in this field of technology is still necessary in order to improve light control, since most of the existing works have carried out the manipulation of light by hand. When this variable is manually manipulated, there is naturally a longer response time than when the process is automated, and that delay leads to a non-optimal growth. Automated variation of the light intensity when the cell concentration increases, or when another parameter is varied in the process, generates an optimal absorption of that light. In this case, the culture will be offered what the cells, in fact, need at that moment without energy waste, thermal dissipation and illumination excess. Therefore, for the control to be carried out in full, as it is already used in several areas other than photo-bioprocess, sensors with readings in real time are also required. Indeed, the eyes of the supervisory system are the sensors, which sense the state of the controlled variable in real time and allow the controller to take actions to correct the effects of the process, what is currently being done with manual measurements of quantum sensors for detecting photosynthetically active radiation.
The control of light, including intensity and wavelength (white, red, green, blue), is promising for the cultivation of microalgae, as some works have already shown especially in the last decade of scientific research. Unfortunately, most of the works still have manual manipulation of light, but the technological trend points to increasing investments in automation and control of phototrophic bioprocesses, aiming at the optimization of biochemicals manufactures, further encouraged by the new environmentally committed industry.
Conclusion
As seen, the production optimization of photosynthesized compound’s demands: (1) the understanding of how light is biologically processed by the microorganism of interest; (2) the knowledge of the ideal light wavelength and intensity; (3) the maintenance, by a controller, of the optimum luminous profile during the process. Whether they are processes that aim to obtain biomass or those that seek to produce specific bioproducts, light quantity and quality are fundamental for microalgae. In this way, the present work went through all the concepts related to this important variable called light and also pointed out a gap that must be filled by science, the automatic control of light. This controller must regulate the amount of light absorbed at each wavelength and identify the saturation point of the light period to start the dark phase in an optimized and very specific way for each bioprocess.
Availability of data and materials
All data presented in this article are available from the corresponding author on reasonable request.
References
Abdelfattah A, Ali SS, Ramadan H, El-Aswar EI, Eltawab R, Ho SH, Elsamahy T, Li S, El-Sheekh MM, Schagerl M, Kornaros M, Sun J (2023) Microalgae-based wastewater treatment: mechanisms, challenges, recent advances, and future prospects. Environ Sci Ecotechnol. https://doi.org/10.1016/j.ese.2022.100205
Abiusi F, Sampietro G, Marturano G, Biondi N, Rodolfi L, D’Ottavio M, Tredici MR (2014) Growth, photosynthetic efficiency, and biochemical composition of Tetraselmis suecica FandM-M33 grown with LEDs of different colors. Biotechnol Bioeng 111(5):956–964. https://doi.org/10.1002/bit.25014
Angstrom A (1965) The solar constant and the temperature of the earth. Prog Oceanogr 3:1–5
Atta M, Idris A, Bukhari A, Wahidin S (2013) Intensity of blue LED light: a potential stimulus for biomass and lipid content in fresh water microalgae Chlorella vulgaris. Biores Technol 148:373–378. https://doi.org/10.1016/j.biortech.2013.08.162
Baba M, Kikuta F, Suzuki I, Watanabe MM, Shiraiwa Y (2012) Wavelength specificity of growth, photosynthesis, and hydrocarbon production in the oil-producing green alga Botryococcus braunii. Biores Technol 109:266–270. https://doi.org/10.1016/J.BIORTECH.2011.05.059
Baidya A, Akter T, Islam MR, Shah AKMA, Hossain MA, Salam MA, Paul SI (2021) Effect of different wavelengths of LED light on the growth, chlorophyll, β-carotene content and proximate composition of Chlorella ellipsoidea. Heliyon 7(12):e08525. https://doi.org/10.1016/j.heliyon.2021.e08525
Bantis F, Karamanoli K, Ainalidou A, Radoglou K, Constantinidou HIA (2018) Light emitting diodes (LEDs) affect morphological, physiological and phytochemical characteristics of pomegranate seedlings. Sci Hortic 234(14):267–274. https://doi.org/10.1016/j.scienta.2018.02.065
Beltran LM (2013) Web control and monitoring system: experimentation with haematococcus pluvialis. Int J Eng. https://doi.org/10.5829/idosi.ije.2013.26.03c.01
Berg JM, Tymoczko JL, Stryer L (2002) Biochemistry. W. H. Freeman Publishing, New York
Bernstein HC, Konopka A, Melnicki MR, Hill EA, Kucek LA, Zhang S, Shen G, Bryant DA, Beliaev AS (2014) Effect of mono- and dichromatic light quality on growth rates and photosynthetic performance of Synechococcus sp. PCC 7002. Front Microbiol. https://doi.org/10.3389/fmicb.2014.00488
Bhatia SC (2014) Advanced renewable energy systems. Woodhead Publishing India. https://www.sciencedirect.com/book/9781782422693/advanced-renewable-energy-systems
Bredda EH, Da Silva AF, Silva MB, Da Rós PCM (2020) Mixture design as a potential tool in modeling the effect of light wavelength on Dunaliella salina cultivation: an alternative solution to increase microalgae lipid productivity for biodiesel production. Prep Biochem Biotechnol 50(4):379–389. https://doi.org/10.1080/10826068.2019.1697936
Brennan L, Owende P (2010) Biofuels from microalgae-A review of technologies for production, processing, and extractions of biofuels and co-products. Renew Sustain Energy Rev 14(2):557–577. https://doi.org/10.1016/j.rser.2009.10.009
Caffarri S, Tibiletti T, Jennings R, Santabarbara S (2014) A comparison between plant photosystem I and photosystem II architecture and functioning. Curr Protein Pept Sci 15(4):296–331. https://doi.org/10.2174/1389203715666140327102218
Capilla M, San-Valero P, Izquierdo M, Penya-roja JM, Gabaldón C (2021) The combined effect on initial glucose concentration and pH control strategies for acetone-butanol-ethanol (ABE) fermentation by Clostridium acetobutylicum DSM 792. Biochem Eng J 167:107910. https://doi.org/10.1016/j.bej.2020.107910
Carvalho AP, Silva SO, Baptista JM, Malcata FX (2011) Light requirements in microalgal photobioreactors: an overview of biophotonic aspects. Appl Microbiol Biotechnol 89(5):1275–1288. https://doi.org/10.1007/s00253-010-3047-8
Castrucci PL, Bittar A, Sales R (2011) Controle Automático. Editora GEN/LTC
Chen H-B, Wu J-Y, Wang C-F, Fu C-C, Shieh C-J, Chen C-I, Wang C-Y, Liu Y-C (2010) Modeling on chlorophyll a and phycocyanin production by Spirulina platensis under various light-emitting diodes. Biochem Eng J 53:52–56. https://doi.org/10.1016/j.bej.2010.09.004
Chen X, Goh QY, Tan W, Hossain I, Chen WN, Lau R (2011) Lumostatic strategy for microalgae cultivation utilizing image analysis and chlorophyll a content as design parameters. Biores Technol 102(10):6005–6012. https://doi.org/10.1016/j.biortech.2011.02.061
Choi SL, Suh IS, Lee CG (2003) Lumostatic operation of bubble column photobioreactors for Haematococcus pluvialis cultures using a specific light uptake rate as a control parameter. Enzyme Microb Technol 33(4):403–409. https://doi.org/10.1016/S0141-0229(03)00137-6
Costa BS, Jungandreas A, Jakob T, Weisheit W, Mittag M, Wilhelm C (2013) Blue light is essential for high light acclimation and photoprotection in the diatom Phaeodactylum tricornutum. J Exp Bot 64(2):483–493. https://doi.org/10.1093/jxb/ers340
Cuaresma M, Janssen M, Vílchez C, Wijffels RH (2009) Productivity of Chlorella sorokiniana in a short light-path (SLP) panel photobioreactor under high irradiance. Biotechnol Bioeng 104(2):352–359. https://doi.org/10.1002/bit.22394
Das P, Lei W, Aziz S, Obbard JP (2010) Enhanced algae growth in both phototrophic and mixotrophic culture under blue light. Biores Technol. https://doi.org/10.1016/j.biortech.2010.11.102
Deniz I, Demirel Z, Imamoglu E, Dalay MC (2019) Enhanced microalgal lipid production in internally illuminated airlift photobioreactor. Mar Technol Soc J 53(2):38–45. https://doi.org/10.4031/MTSJ.53.2.4
Drira N, Piras A, Rosa A, Porcedda S, Shaouadi H (2016) Microalgae from domestic wastewater facility’s high rate algal pond: lipids extraction, characterization and biodiesel production. Biores Technol 206(2016):239–244
Einstein A (1905) Über einen die Erzeugung und Verwandlung des Lichtes betreffenden heuristischen Gesichtspunkt. Ann Phys 322(6):132–148. https://doi.org/10.1002/andp.19053220607
Eriksen NT, Geest T, Iversen JJL (1996) Phototrophic growth in the lumostat: a photo-bioreactor with on-line optimization of light intensity. J Appl Phycol 8(4–5):345–352. https://doi.org/10.1007/BF02178577
Falkowski PG, Raven JA (2007) Aquatic Photosynthesis, 2nd edn. Princeton Universtity Press, Princeton
Fitzmaurice BC, Appleby-Thomas GJ, Painter JD, Wood DC, Hazael R (2021) The effects of quasi-one-dimensional shock on Escherichia coli while controlling pressure and temperature. Icarus 359:114221. https://doi.org/10.1016/j.icarus.2020.114221
Franco-Lara E, Havel J, Peterat F, Weuster-Botz D (2006) Model-supported optimization of phototrophic growth in a stirred-tank photobioreactor. Biotechnol Bioeng 95(6):1177–1187. https://doi.org/10.1002/bit.21086
Fu W, Guomundsson Ó, Paglia G, Herjólfsson G, Andrésson ÓS, Palsson BO, Brynjólfsson S (2013) Enhancement of carotenoid biosynthesis in the green microalga Dunaliella salina with light-emitting diodes and adaptive laboratory evolution. Appl Microbiol Biotechnol 97(6):2395–2403. https://doi.org/10.1007/s00253-012-4502-5
Gehan O, Pigeon E, Menard T, Mosrati R, Pouliquen M, Fall LM, Reuter J (2019) Dissolved oxygen level output feedback control based on discrete-time measurements during a Pseudomonas putida mt-2 fermentation. J Process Control 79:29–40. https://doi.org/10.1016/j.jprocont.2018.10.004
Glemser M, Heining M, Schmidt J, Becker A, Garbe D, Buchholz R, Brück T (2016) Application of light-emitting diodes (LEDs) in cultivation of phototrophic microalgae: current state and perspectives. Appl Microbiol Biotechnol 100(3):1077–1088. https://doi.org/10.1007/s00253-015-7144-6
González-Camejo J, Barat R, Pachés M, Murgui M, Seco A, Ferrer J (2018) Wastewater nutrient removal in a mixed microalgae–bacteria culture: effect of light and temperature on the microalgae–bacteria competition. Environ Technol 39(4):503–515. https://doi.org/10.1080/09593330.2017.1305001
Gris B, Morosinotto T, Giacometti GM, Bertucco A, Sforza E (2014) Cultivation of Scenedesmus obliquus in photobioreactors: effects of light intensities and light-dark cycles on growth, productivity, and biochemical composition. Appl Biochem Biotechnol 172(5):2377–2389. https://doi.org/10.1007/S12010-013-0679-Z
Han PP, Shen SG, Wang HY, Yao SY, Tan ZL, Zhong C, Jia SR (2017) Applying the strategy of light environment control to improve the biomass and polysaccharide production of Nostoc flagelliforme. J Appl Phycol 29(1):55–65. https://doi.org/10.1007/s10811-016-0963-8
Han S-I, Kim S, Lee C, Choi Y-E (2019) Blue-Red LED wavelength shifting strategy for enhancing beta-carotene production from halotolerant microalga, Dunaliella salina. J Microbiol 57(2):101–106. https://doi.org/10.1007/s12275-019-8420-4
Hincapie E, Stuart BJ (2014) Design, construction, and validation of an internally lit air-lift photobioreactor for growing algae. Front Energy Res. https://doi.org/10.3389/fenrg.2014.00065
Hua X, Han J, Zhou X, Xu Y (2023) Gas pressure intensifying oxygen transfer to significantly improving the bio-oxidation productivity of whole-cell catalysis. AIChE J 69(3):e18005
Huang F, Shen W, Zhang X, Seferlis P (2020) Impacts of dissolved oxygen control on different greenhouse gas emission sources in wastewater treatment process. J Clean Prod 274:123233. https://doi.org/10.1016/j.jclepro.2020.123233
Huesemann MH, Van Wagenen J, Miller T, Chavis A, Hobbs S, Crowe B (2013) A screening model to predict microalgae biomass growth in photobioreactors and raceway ponds. Biotechnol Bioeng 110(6):1583–1594. https://doi.org/10.1002/bit.24814
Hwang JH, Maier N (2019) Effects of LED-controlled spatially-averaged light intensity and wavelength on Neochloris oleoabundans growth and lipid composition. Algal Res. https://doi.org/10.1016/j.algal.2019.101573
Iasimone F, Panico A, De Felice V, Fantasma F, Iorizzi M, Pirozzi F (2018) Effect of light intensity and nutrients supply on microalgae cultivated in urban wastewater: biomass production, lipids accumulation and settleability characteristics. J Environ Manag. https://doi.org/10.1016/j.jenvman.2018.07.024
Ifrim GA, Titica M, Boillereaux L, Caraman S (2013) Feedback linearizing control of light-to-microalgae ratio in artificially lighted photobioreactors. IFAC Proceedings Volumes (IFAC-PapersOnline), 12(PART 1), 169–174. https://doi.org/10.3182/20131216-3-IN-2044.00038
Imaizumi Y, Nagao N, Yusoff FM, Kurosawa N, Kawasaki N, Toda T (2016) Lumostatic operation controlled by the optimum light intensity per dry weight for the effective production of Chlorella zofingiensis in the high cell density continuous culture. Algal Res 20:110–117. https://doi.org/10.1016/j.algal.2016.09.015
Izadpanah M, Gheshlaghi R, Akhavan Mahdavi M, Elkamel A (2017) Effect of light spectrum on isolation of microalgae from urban wastewater and growth characteristics of subsequent cultivation of the isolated species. Algal Res 29:154–158. https://doi.org/10.1016/j.algal.2017.11.029
Jones HG (1992) Plants and microclimate: a quantitative approch to environmental physiology, 2nd edn. Cambridge University Press, Cambridge
Jung J-H, Sirisuk P, Ra CH, Kim J-M, Jeong G-T, Kim S-K (2019) Effects of green LED light and three stresses on biomass and lipid accumulation with two-phase culture of microalgae. Process Biochem 77:93–99. https://doi.org/10.1016/j.procbio.2018.11.014
Katsuda T, Lababpour A, Shimahara K, Katoh S (2004) Astaxanthin production by Haematococcus pluvialis under illumination with LEDs. Enzyme Microb Technol 35(1):81–86. https://doi.org/10.1016/j.enzmictec.2004.03.016
Keeling PJ (2013) The number, speed, and impact of plastid endosymbioses in eukaryotic evolution. Annu Rev Plant Biol 64(1):583–607. https://doi.org/10.1146/annurev-arplant-050312-120144
Kim CW, Sung M-G, Nam K, Moon M, Kwon J-H, Yang J-W (2014) Effect of monochromatic illumination on lipid accumulation of Nannochloropsis gaditana under continuous cultivation. Biores Technol 159:30–35. https://doi.org/10.1016/j.biortech.2014.02.024
Kim JY, Jung JM, Jung S, Park YK, Tsang YF, Lin KYA, Choi YE, Kwon EE (2022) Biodiesel from microalgae: recent progress and key challenges. Prog Energy Combust Sci. https://doi.org/10.1016/j.pecs.2022.101020
Koc C, Anderson G (2012) Use of red and blue light-emitting diodes (LED) and fluorescent lamps to grow microalgae in a photobioreactor. Israeli J Aquac. https://doi.org/10.13031/2013.36277
Kula M, Rys M, Możdżeń K, Skoczowski A (2014) Metabolic activity, the chemical composition of biomass and photosynthetic activity of Chlorella vulgaris under different light spectra in photobioreactors. Eng Life Sci 14(1):57–67. https://doi.org/10.1002/elsc.201200184
Kumar L, Mandal PN (2021) Pressure control of variable displacement radial piston pump with PID controller. Mater Today. https://doi.org/10.1016/j.matpr.2021.01.692
Kwon HK, Oh SJ, Yang HS, Kim DM, Kang IJ, Oshima Y (2013) Laboratory study for the phytoremediation of eutrophic coastal sediment using benthic microalgae and light emitting diode (LED). J Fac Agric Kyushu Univ 58:417–425
Lai Y-C, Karam AL, Sederoff HW, Ducoste JJ, De Los FL, Iii R (2019) Relating nitrogen concentration and light intensity to the growth and lipid accumulation of Dunaliella viridis in a photobioreactor. J Appl Phycol. https://doi.org/10.1007/s10811-019-01897-4
Lee H-S, Kim Z-H, Jung S-E, Kim J-D, Lee C-G (2006a) Specific light uptake rate can be served as a scale-up parameter in photobioreactor operations. J Microbiol Biotechnol 16:1890–1896
Lee H-S, Seo MW, Kim ZH, Lee CG (2006b) Determining the best specific light uptake rates for the lumostatic cultures in bubble column photobioreactors. Enzyme Microb Technol 39(3):447–452. https://doi.org/10.1016/j.enzmictec.2005.11.038
Li W, Zhang J, Zhao T, Ren J (2021) Experimental study of an indoor temperature fuzzy control method for thermal comfort and energy saving using wristband device. Build Environ 187:107432. https://doi.org/10.1016/j.buildenv.2020.107432
Li X, Dong X, Wang J, Tu X, Huang H, Cao Y, Wang C, Huang Y (2023) Fast and precise temperature control for axon stretch growth bioreactor based on fuzzy PID control. Appl Biochem Biotechnol. https://doi.org/10.1007/s12010-023-04449-2
Liu J, Yuan C, Hu G, Li F (2012) Effects of light intensity on the growth and lipid accumulation of microalga Scenedesmus sp. 11–1 under nitrogen limitation. Appl Biochem Biotechnol 166(8):2127–2137. https://doi.org/10.1007/s12010-012-9639-2
Liu H, Chen H, Wang S, Liu Q, Li S, Song X, Huang J, Wang X, Jia L (2018) Optimizing light distribution and controlling biomass concentration by continuously pre-harvesting Spirulina platensis for improving the microalgae production. Biores Technol 252:14–19. https://doi.org/10.1016/j.biortech.2017.12.046
Mairet F, Muñoz-Tamayo R, Bernard O (2015) Adaptive control of light attenuation for optimizing microalgae production. J Process Control 30:117–124. https://doi.org/10.1016/j.jprocont.2015.03.010
Marchetti J, Bougaran G, Jauffrais T, Lefebvre S, Rouxel C, Saint-Jean B, Lukomska E, Robert R, Cadoret JP (2013) Effects of blue light on the biochemical composition and photosynthetic activity of Isochrysis sp (T-iso). J Appl Phycol 25(1):109–119. https://doi.org/10.1007/s10811-012-9844-y
Markou G (2014) Effect of various colors of light-emitting diodes (LEDs) on the biomass composition of Arthrospira platensis cultivated in semi-continuous mode. Appl Biochem Biotechnol 172(5):2758–2768. https://doi.org/10.1007/s12010-014-0727-3
Masojídek J, Torzillo G, Koblízek M (2013) Photosynthesis in Microalgae. In: Richmond A, Hu Q (eds) Handbook of microalgal culture: applied phycology and biotechnology, 2nd edn. Blackwell Publishing Ltd., Hoboken
Mesquita TJ, Campani G, Giordano RC, Zangirolami TC (2021) Horta AC Machine learningapplied for metabolic flux-based control of micro-aerated fermentations in bioreactors. Biotechnol Bioeng 118:2076–2091. https://doi.org/10.1002/bit.27721
Mohsenpour SF, Willoughby N (2013) Luminescent photobioreactor design for improved algal growth and photosynthetic pigment production through spectral conversion of light. Biores Technol. https://doi.org/10.1016/j.biortech.2013.05.024
Nelson DL, Cox MM (2008) Lehninger principles of biochemistry, 5th edn. W.H. Freeman, New york
Oflaz FB, Koku H (2020) Pilot-scale outdoor photofermentative hydrogen production from molasses using pH control. Int J Hydrogen Energy. https://doi.org/10.1016/j.ijhydene.2020.10.086
Ogata K (2010). Engenharia de Controle Moderno (5°). Person
Okumura C, Saffreena N, Rahman MA, Hasegawa H, Miki O, Takimoto A (2015) Economic efficiency of different light wavelengths and intensities using LEDs for the cultivation of green microalga Botryococcus braunii (NIES-836) for biofuel production. Environ Prog Sustain Energy 34(1):269–275. https://doi.org/10.1002/ep.11951
Olle M, Viršile A (2013) The effects of light-emitting diode lighting on greenhouse plant growth and quality. Agric Food Sci 22(2):223–234. https://doi.org/10.23986/afsci.7897
Pataro IML, Gil JD, Guzmán JL, Berenguel M, Lemos JM (2023) A learning-based model predictive strategy for pH control in raceway photobioreactors with freshwater and wastewater cultivation media. Control Eng Pract 138:105619
Patel AK, Joun JM, Hong ME, Sim SJ (2019) Effect of light conditions on mixotrophic cultivation of green microalgae. Biores Technol 282:245–253. https://doi.org/10.1016/j.biortech.2019.03.024
Pérez-Pazos J-V, Fernández-Izquierdo P (2011) Synthesis of neural lipids in Chlorella sp. under different light and carbonate conditions. C.T.F Cienc Tecnol Futuro, 4
Piotrowski R, Wonia M, Wonia A (2023) Stochastic optimisation algorithm for optimisation of controller parameters for control of dissolved oxygen in wastewater treatment plant. J Water Process Eng 51:103357
Rendón (2013) Effect of carbon dioxide concentration on the growth response of chlorella vulgaris under four different led illumination. Int J Biotechnol Well Indus. https://doi.org/10.6000/1927-3037.2013.02.03.3
Rocha JC, Rosa AH, Cardoso AA (2009) Introdução a química ambiental. 2nd ed. Bookman
Samal DPK, Sukla LB, Pattanaik A, Pradhan D (2020) Role of microalgae in treatment of acid mine drainage and recovery of valuable metals. Mater Today. https://doi.org/10.1016/j.matpr.2020.02.165
Sathinathan P, Parab HM, Yusoff R, Ibrahim S, Vello V, Ngoh GC (2023) Photobioreactor design and parameters essential for algal cultivation using industrial wastewater: a review. Renew Sustain Energy Rev 173:113096
Schulze PSC, Barreira LA, Pereira HGC, Perales JA, Varela JCS (2014) Light emitting diodes (LEDs) applied to microalgal production. Trends Biotechnol 32(8):422–430. https://doi.org/10.1016/j.tibtech.2014.06.001
Schulze PSC, Brindley C, Fernández JM, Rautenberger R, Pereira H, Wijffels RH, Kiron V (2020) Flashing light does not improve photosynthetic performance and growth of green microalgae. Bioresour Technol Rep. https://doi.org/10.1016/j.biteb.2019.100367
Sforza E, Simionato D, Giacometti GM, Bertucco A, Morosinotto T (2012) Adjusted light and dark cycles can optimize photosynthetic efficiency in algae growing in photobioreactors. PLoS One 7(6):38975. https://doi.org/10.1371/journal.pone.0038975
Shu C-H, Tsai C-C, Liao W-H, Chen K-Y, Huang H-C (2012) Effects of light quality on the accumulation of oil in a mixed culture of Chlorella sp. and Saccharomyces cerevisiae. J Chem Technol Biotechnol 87(5):601–607. https://doi.org/10.1002/jctb.2750
Silva A, Silva FJG, Campilho RDSG, Neves PMPF (2021) A new approach to temperature control in the extrusion process of composite tire products. J Manuf Process 65:80–96. https://doi.org/10.1016/j.jmapro.2021.03.022
Spiro TG, Stiglian WM (2009) Química ambiental. Pearson Prentice Hall, Hoboken
Stevčić Č, Pulkkinen K, Pirhonen J (2019) Screening of microalgae and LED grow light spectra for effective removal of dissolved nutrients from cold-water recirculating aquaculture system (RAS) wastewater. Algal Res. https://doi.org/10.1016/j.algal.2019.101681
Suh I (2001) Light distribution model and lumostatic operation of a photobioreactor. Pohang University of Science and Technology, Pohang
Suh IS, Lee SB (2001) Cultivation of a cyanobacterium in an internally radiating air-lift photobioreactor. J Appl Phycol 13(4):381–388. https://doi.org/10.1023/A:1017979431852
Teo CL, Atta M, Bukhari A, Taisir M, Yusuf AM, Idris A, Bahru J (2014) Enhancing growth and lipid production of marine microalgae for biodiesel production via the use of different LED wavelengths. Biores Technol 162:38–44. https://doi.org/10.1016/j.biortech.2014.03.113
Vejrazka C, Janssen M, Streefland M, Wijffels RH (2012) Photosynthetic efficiency of Chlamydomonas reinhardtii in attenuated, flashing light. Biotechnol Bioeng 109(10):2567–2574. https://doi.org/10.1002/bit.24525
Wang SK, Stiles AR, Guo C, Liu CZ (2014) Microalgae cultivation in photobioreactors: an overview of light characteristics. Eng Life Sci 14(6):50–559. https://doi.org/10.1002/elsc.201300170
Whitton R, Ometto F, Villa R, Pidou M, Jefferson B (2019) Influence of light regime on the performance of an immobilised microalgae reactor for wastewater nutrient removal. Algal Res. https://doi.org/10.1016/j.algal.2019.101648
Xu B, Cheng P, Yan C, Pei H, Hu W (2013) The effect of varying LED light sources and influent carbon/nitrogen ratios on treatment of synthetic sanitary sewage using Chlorella vulgaris. World J Microbiol Biotechnol 29(7):1289–1300. https://doi.org/10.1007/s11274-013-1292-6
Yan C, Munoz R, Zhu L, Wang Y (2016) The effects of various LED (light emitting diode) lighting strategies on simultaneous biogas upgrading and biogas slurry nutrient reduction by using of microalgae Chlorella sp. Energy 106:554–561. https://doi.org/10.1016/j.energy.2016.03.033
Yanb C, Zhu L, Wang Y (2016) Photosynthetic CO2 uptake by microalgae for biogas upgrading and simultaneously biogas slurry decontamination by using of microalgae photobioreactor under various light wavelengths, light intensities, and photoperiods. Appl Energy 178:9–18. https://doi.org/10.1016/j.apenergy.2016.06.012
Yoon JH, Shin JH, Park TH (2008) Characterization of factors influencing the growth of Anabaena variabilis in a bubble column reactor. Biores Technol 99(5):1204–1210. https://doi.org/10.1016/j.biortech.2007.02.012
Yoshioka M, Yago T, Yoshie-Stark Y, Arakawa H, Morinaga T (2012) Effect of high frequency of intermittent light on the growth and fatty acid profile of Isochrysis galbana. Aquaculture 338–341:111–117. https://doi.org/10.1016/j.aquaculture.2012.01.005
Zhao X, Ma R, Liu X, Ho S-H, Xie Y, Chen J (2018) Strategies related to light quality and temperature to improve lutein production of marine microalga Chlamydomonas sp. Bioprocess Biosyst Eng 42:435–443. https://doi.org/10.1007/s00449-018-2047-4
Acknowledgements
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001. The authors also acknowledge grants #2017/23950-5 from São Paulo Research Foundation (FAPESP), and Brazilian National Council for Scientific and Technological Development (CNPq).
Funding
This study was financed in part by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES, Finance Code 001). The authors also acknowledge grants provided by the São Paulo State Research Foundation (FAPESP, #2018/07988–5 and #2017/23950–5).
Author information
Authors and Affiliations
Contributions
VCG and GMP conceived the research. ACLH supervised the study. VCG, GMP and ACLH wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guedes, V.C., Palma, G.M. & Horta, A.C.L. An evaluation of light wavelengths, intensity and control for the production of microalgae in photobioreactors: a review. Braz. J. Chem. Eng. (2023). https://doi.org/10.1007/s43153-023-00388-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43153-023-00388-x