Abstract
Fatty compounds have significant industrial applications as coatings, plastics, cleaning products, emulsifiers, structuring, and gelling agents. Due to these applications, it is important to understand their physical and chemical properties, such as melting behavior, crystallization processes, and phase transitions, all of them related to solid–liquid equilibrium (SLE). In this study, six binary mixtures composed of tristearin (SSS) plus fatty acids (capric acid, lauric acid, and myristic acid) and tristearin plus fatty alcohol compounds (1-decanol, 1-dodecanol, and 1-tetradecanol) were investigated using Differential Scanning Calorimetry (DSC) and optical microscopy analysis. The phase diagrams formed by tristearin + fatty acids presented a monotectic behavior and the phase diagrams formed by tristearin + fatty alcohol compounds, on the other hand, exhibited a solid solution formation on the entire composition range, although the liquidus line shape was very similar to that observed in the systems formed by fatty acids. The liquidus line of all systems were well-described by NRTL and three-suffix Margules models, and also by ideal assumption. The best result was observed for the three-suffix Margules model, with a root mean square deviation (RMSD) equal to 0.26.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The worldwide production and consumption of oils and fats are growing over the years, and they are a source of energy and play an important role in human nutrition. Oils and fats are a multicomponent system composed of triacylglycerols (TAGs) and their minor constituents as free fatty acids and fatty alcohol compounds that can affect physical properties, such as texture, and polymorphic forms. (Wright and Marangoni 2005; Maximo et al. 2013; Matos et al. 2015).
TAGs are formed by a chemical bond of three fatty acids to a molecule of glycerol. The type and positioning of fatty acids within a TAG determines their melting behavior, which is especially significant to food industries, since it can affect texture and spreadability, for example (Wright and Marangoni 2005).
Tristearin, the TAG used in this study, is one of the major constituents of vegetable oils, readily available on high purity. Its crystalline behavior is well known for presenting three major crystalline forms: α-crystallite, which is the most prominent and kinetically stable, β- and β’-crystallite, which are thermodynamically stable (Da Silva et al. 2016). Besides, this TAG is used as hardstock to produce trans-free fat bases for shortenings and bakery margarines (Kellens et al. 1991; Oh et al. 2002; Matovic et al. 2005; Webster et al. 2018).
Fatty acids are carboxylic acids with an alkyl chain ranging from four to 36 carbons (C4–C36), considered phase change materials due to their high-energy storage density (Wei et al. 2014; Zhao et al. 2014; Zhang et al. 2015). They can be used in food and cosmetic formulation and also to produce biodiesel and lubricants (Inoue et al. 2004a, b; Eckert et al. 2016). Fatty alcohol compounds are classified as long chain aliphatic alcohols, with six or more carbon atoms (Fisk et al. 2009). Concerning their use in industry, they are generally used to produce emulsifiers, emollients, and thickeners applied in food and cosmetic formulations (Johnson 1988; Kogan and Garti 2006; Pernetti et al. 2007).
The use of fat compounds in different fields requires the understanding of their phase and mixture phase behavior, which is possible by the study of solid–liquid equilibrium (SLE). SLE can provide information regarding the interaction between different acyl chains, helping to determine the physical properties and the phase behavior of complex lipids and their mixtures (Inoue et al. 2004a, b; Dorighello Carareto et al. 2015). Thus, SLE allows the development and validation of models for better understanding the crystallization, fractionation, melting, and stabilization processes of fatty mixtures (Tadie et al. 2013; Matos et al. 2015).
SLE phase diagrams of fatty mixtures can be experimentally determined by several techniques, such as differential scanning calorimetry (DSC), X-ray, and optical microscopy. DSC determines the phase transition temperatures, which enables the determination of eutectic and monotectic points in phase diagram construction (Wesdorp 1990; Maximo et al. 2014a, b; Matos et al. 2016; Pelaquim et al. 2019). In addition, DSC is a fast, accurate, and reliable technique, requiring small amounts of sample.
This work came to complement previous published research that studied the SLE phase diagrams of TAGs + (fatty acids or fatty alcohol compounds) (Maximo et al. 2013; Matos et al. 2015, 2016; Pelaquim et al. 2019). Thus, the SLE behavior of six different binary mixtures measured by DSC technique are presented. Some thermal transitions were evaluated with the aid of an optical microscope linked to a temperature controller. The components chosen were: tristearin (1) as TAG and capric acid (2), lauric acid (3), myristic acid (4), 1-decanol (5), 1-dodecanol (6), and 1-tetradecanol (7). The parameters of three-suffix Margules and NRTL models were adjusted to the experimental data to describe the liquidus lines of the studied systems.
Experimental methods
Materials
High pure components (Table 1) were used to prepare the binary mixtures without further purification. The DSC calibration was carried out considering onset temperatures, using a heating rate of 1 K min−1 and the following standards: indium (≥ 0.990 molar fraction, CAS number 7440-74-6) from TA Instruments; naphthalene (≥ 0.990 molar fraction, CAS number 91-20-3) from Fluka Analytical (Germany); and cyclohexane (≥ 0.990 molar fraction, CAS number 110-82-7) from Sigma-Aldrich (USA).
Apparatus and procedure
Binary mixture preparation
Binary mixtures were prepared by weighting known quantities of each compound using an analytical scale with a precision of ± 2 \(\times\) 10−4 (Shimadzu AUY220, Japan), totalizing 0.50 g of each mixture, to obtain the entire range of the phase diagram composition in increments of 0.1 or 0.2 tristearin molar fractions. A Thermoprep equipment (Metrohm, Herisau), the oven used for Karl Fischer titration, was used to melt the samples, and ensure their complete homogenization under a nitrogen atmosphere. After melting, the samples were cooled down to room temperature and kept under refrigeration until their use in the DSC equipment. The uncertainty obtained due to error propagation from the weighted masses was estimated as not higher than 6 \(\times\) 10−4 (in molar fraction).
Differential scanning calorimetry (DSC)
SLE experimental data (melting temperatures and enthalpies of fusion) of the binary mixtures were determined on a Differential Scanning Calorimetry equipment (MDSC 2920, TA Instruments, New Castle) equipped with a refrigerated cooling system operated in a temperature range from 248 to 360 K; high-purity nitrogen was used as purge gas at 50 mL/min. The melting temperature and heat flow were previously calibrated with the primary calibration standards: indium (certificated by TA Instruments), naphthalene (Fluka Analytical, Germany), and cyclohexane (Sigma Aldrich, USA), with purity greater than 0.99 mass fraction, at a heating rate of 1 K min−1 for at least three times with each calibration. The standard deviations of the measurements ranged from 0.07 to 0.1 K.
Each sample mixture was weighed (4–7 mg) using a microanalytical scale with 2 \(\times\) 10−6 accuracy (AD6, PerkinElmer, Waltham) and sealed in aluminum pans. The samples were submitted to a thermal treatment according to Costa et al. (2007) to erase previous thermal histories because of the fatty compound polymorphism effects. The thermal treatment consisted of a heating step at 5 K min−1 from room temperature until 15 K above the melting point of the fat compound with the highest melting point of the mixture, followed by an isothermal time of 20 min; a cooling run at 1 K min−1 up to 25 K below the smaller melting point of the fat compound with the lower melting point of the mixture and another isothermal step of 30 min. After this thermal treatment, the run for data acquisition was done at a heating rate of 1 K min−1.
Thermal event temperatures were analyzed using the TA Universal Analysis software. The transitions, eutectic, and melting temperatures was calculated through the peak temperature and the enthalpy was calculated from the area of the corresponding peak (Costa et al. 2007). Onset temperature is more recommended to find the melting temperature due to its low dependence on heating rate and sample parameters. However, the use of onset temperature to determine the melting peak may be inaccurate due to the influence of other peaks and overlapped peaks in the differential thermal curve that are generally observed in fatty compounds added to low values of heat transfer coefficient especially observed in mixtures of organic compounds (Höhne et al. 2003; Costa et al. 2007). Therefore, in this work we used peak temperature because of better repeatability and ease determination.
Triplicate runs were done for some selected compositions to verify the repeatability of the temperatures attributed to each thermal event and the reliability of the methodology and equipment. It was observed that all the uncertainties of fusion temperature for pure components and binary mixtures were below 0.30 K, indicating that the DSC equipment presents a good reproducibility. For a better visualization, the triplicate of SSS + C14OH at x1 = 0.40 are presented in the Supplementary Information, as well as the comparison between the peak temperature of each event (Figure S7 and Table S1). One can observe that the results of the temperatures determined for the endothermic and exothermic peaks remain very close.
Optical microscopy
An optical microscope (Leica DM 2700 M, China) with a temperature controller (Linkam LTS 420, United Kingdom) was used to evaluate the solid–liquid transitions of some selected binary mixtures. The sample amount used for this experiment was approximately 50 mg. A heating rate of 0.1 K min−1 was employed from 10 degrees under the eutectic temperature until reaching the sample melting temperature. Images were captured every 60 s with a magnification of 10 or 20 times.
SLE thermodynamic modelling
To describe the liquidus lines of the binary mixture, the activity coefficient was calculated by three-suffix Margules and NRTL models, considering the ideal behavior of the liquid phase. The models were used in Eq. 1 as discussed by Prausnitz et al. (1999), where the solid–solid transitions and molar heat capacity of pure components were not considered. Moreover, the activity coefficient of component i in the solid phase at equilibrium was replaced by the activity coefficient of the pure component (\({x}_{i}^{s}{\gamma }_{i}^{s}=1\)), representing the immiscibility of both components in that phase.
where \({x}_{i}^{L}\) is the molar fraction of component i in the liquid (L) phase; \({\gamma }_{i}^{L}\) is the activity coefficient of component i also in the liquid phase; \({\Delta }_{fus,i}H\) is the fusion enthalpy at the melting temperature of component i; \({T}_{m,i}\) is the melting temperature of component i; \(T\) is the calculated temperature; and \(R\) is the universal gas constant.
The parameters of three-suffix Margules and NRTL models were adjusted to find the activity coefficients of the liquid phase (Prausnitz et al. 1999). Downhill simplex optimization method (Vetterling et al. 1992) was used to determine the models’ parameters with the objective function (S) given in Eq. 2, where (N) is the number of experimental measurements and \(\left({\sigma }_{n}\right)\) is the temperature uncertainty, as proposed by Costa et al. 2007. The root mean square deviation (RMSD) was used to evaluate the deviation between the calculated temperature and the experimental ones.
Results and discussion
Pure tristearin thermal properties
The thermal properties of fatty acids and fatty alcohol compounds were previously presented (Pelaquim et al. 2019). Thus, Table 2 shows the transition temperatures of tristearin, which are represented by exothermic peaks or endothermic peaks, due to events such as residual melting temperature or polymorphs transformation; the melting temperature, which is the endothermic peak that occurs at higher temperature; the molar enthalpy of fusion; and the experimental standard deviations presented in parentheses, as well as the literature data of tristearin. These data were done in triplicate and standard deviation was calculated according to Eq. 3.
where \({s}_{x}\) is the standard deviation, n is the number of data points, \({x}_{i}\) is each of the values of the data, and \(x^{\prime}\) is the mean value of \({x}_{i}\).
Equation 4 was used to calculate the average relative deviation (ARD), which consists of the deviation between the melting temperature and molar enthalpies determined in this study (\({\Gamma }_{{\text{This}} \, {\text{study}}}\)) and in the literature (\({\Gamma }_{\text{Literature}}\)).
The ARD of melting temperature and the ARD of molar enthalpy were 0.17% and 2.15%, respectively. Both results show a good agreement between our experimental data and literature data.
TAGs are well known by their ability to crystallize under different crystal forms (Garti and Sato 1988). The most common ones are α, β′, and β forms, in which α is the less stable and β is the most stable form (Timms 1984; Persson 2008). The generation of tristearin β′-form is possible only under a special thermal conditioning treatment because of the speediness of the α to β-form transformation (Singh et al. 1999).
Figure 1 compares the DSC curve of tristearin determined in this study and that the one determined by Singh et al. 1999 using a heating rate equal to 2.5 K min−1. One can observe that the curve determined in this study is similar to the tristearin curve from the literature, showing four well-defined peaks (Timms 1984; Singh et al. 1999; Persson 2008) despite the different heating rate and thermal treatment used by the authors. According to the literature (Singh et al. 1999), the first peak observed in Fig. 1 is an endothermic peak that indicates a residual α-form melting at 328.61 K (peak number 1 indicated by the black arrow). This endothermic peak is followed by an exothermic one observed at 330.42 K, which is attributed to the transformation from α-form to β-form (peak number 2). Peak number 2 is overlapped by peak number 3, which is a residual β-form crystallization at 332.84 K; and peak number 4 is related to tristearin β-form melting temperature, at 345.73 K.
Experimental SLE data
Six SLE phase diagrams were constructed in this study, formed by tristearin + fatty acids (capric acid, lauric acid, and myristic acid) and tristearin + fatty alcohol compounds (1-decanol, 2-dodecanol, and 1-tetradecanol). The experimental data are shown in Tables 3, 4, 5, 6, 7 and 8, where x1 corresponds to tristearin molar fraction. The thermal curves are presented in the Supplementary Information (Figs. S1 to S6).
Phase diagrams and microscopy analysis of tristearin + fatty acids
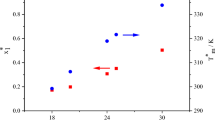
Figure 2 presents the phase diagram of tristearin + fatty acids. All of these systems present a monotectic behavior that was also observed in the literature for tristearin + rice bran oil system (Humphrey and Narine 2005; Costa et al. 2010). All the phase diagrams presented in Fig. 2 present a monotectic behavior; however, the exact composition of such point is not known due to its proximity to the pure fatty acid (Inoue et al. 2004a, b). Nevertheless, the liquidus line calculated using the ideal approach, three-suffix Margules, and NRTL models for the tristearin (1) + myristic acid (4) system indicated the presence of a eutectic point at 0.02 tristearin molar fraction. The model results suggest the occurrence of a eutectic reaction (Fig. 2C) and once more the proximity of pure myristic acid makes its composition determination by DSC a hard task.
SLE phase diagram of the A tristearin (1) + capric acid (2) system; B tristearin (1) + lauric acid (3) system; C tristearin (1) + myristic acid (4) system: (filled circle) melting temperature; (filled square) eutectic reaction temperature; (filled diamond) exothermic transition temperature; (filled diamond) melting process from optical microscopy; (*) pure component transition temperature; (grey line) ideal model; (red line) three-suffix Margules; (blue line) NRTL; (dashed line) guide to the eyes
The ideal model underestimated the liquidus line for all three phase diagrams but follow the same trend of the experimental data. Three-suffix Margules, and NRTL models presented a much better match with the liquidus line, in which the lines almost overlapped the experimental dots. The phase diagrams also show that the solid–liquid region decreases with the fatty acids chain length increase. Thus, tristearin (1) + capric acid (2) system presented the bigger solid–liquid region and tristearin (1) + myristic acid (4) system the smaller region. Concerning the phase diagrams regions, in Fig. 2C is possible to identify the region 1 above of liquidus line composed by a liquid phase, region 2 that is a solid–liquid equilibrium domain composed by solid tristearin with a liquid phase, region 3 below of the solidus line formed by a solid mixture of both components and region 4 formed by a solid solution of the mixture components.
To better understand and evaluate this behavior and the transitions, optical microscope images were captured at 0.2000 and 0.9000 tristearin molar fractions of tristearin (1) + lauric acid (3) system, presented in Figs. 3 and 4, and at 0.6000 and 0.7999 tristearin molar fractions of tristearin (1) + myristic acid (4) system, presented in Figs. 5 and 6.
Figure 2C, tristearin (1) + myristic acid (4) system, also shows exothermic events observed in DSC curves at tristearin molar fractions of 0.4001, 0.6001, and 0.7999, approximately. The exothermic peaks around 320 K probably indicate the α-form crystallization of tristearin (Kellens, Reynaers 1992). Besides, tristearin (1) + myristic acid (4) and tristearin (1) + lauric acid (3) systems present a solid solution starting at about 0.7999 molar fraction. Optical microscope images were captured to better explain this behavior (Figs. 5 and 6).
In Fig. 3A, the mixture of tristearin (1) + lauric acid (3) is in a solid phase at 315.15 K. With the increase of temperature, the mixture starts to melt at 317.15 K, as shown by the white arrows (Fig. 3B), indicating that solid lauric acid coexists with the liquid mixture. The mixture remains in both solid and liquid phases, almost without change until 320.45 K, where the melting process became noticeable again (Fig. 3C). This behavior indicates the occurrence of a monotectic behavior. After that, the temperature increase promotes the complete melting of the mixture at 345.65 K (Fig. 3D).
Initially, the tristearin (1) + lauric acid (3) system at 0.9000 tristearin molar fraction was in solid phase at 313.35 K, as indicated by Fig. 4A. Increasing the temperature up to 314.05 K, it is easy to note the brightness loss of the sample, as indicated by the white arrows in Fig. 4B. This phenomenon can be explained by a recrystallization probably due to a change in the crystal form of the mixture. This process continues until 317.35 K. The mixture remains in a solid phase (Fig. 4C) up to almost 330.95 K. This behavior can be related to tristearin polymorphism. When the temperature reaches 330.95 K, the mixture starts the melting process, which is perceptible by the increase in sample brightness in Fig. 4D, and the melting process only stops with the complete sample melting at 347.45 K. The fact that a liquid and a solid phase coexistence was not seen from 317 K in this mixture indicates the presence of a solid solution in the tristearin-rich region of the phase diagram, as shown in Fig. 2 (B) by the dotted lines (guide to the eyes).
Some images were also captured for tristearin (1) + myristic acid system and are presented in Fig. 5. The image taken at 318.15 K shows the sample in a solid state (Fig. 5A); the temperature increase at 318.45 K makes it possible to note that the crystals became darker, indicating a recrystallization process into a new solid phase through which it is more difficult to pass light (Fig. 5B). This transition was also observed in the differential thermal curves at 321.22 K (Fig. S3 in the Supplementary Information), and it was plotted in the phase diagram (Fig. 2C), represented by the dark diamond symbol. The melting process begins at 326.95 K, as shown in Fig. 5C, and, at this composition and temperature, liquid myristic acid coexists with a solid phase mixture. The melting process continues with the temperature increase until total sample melting at 346.35 K (Fig. 5D–F).
In Fig. 6B, one can observe the recrystallization discussed before. The temperature increase made the sample become darker, which is very clear in Fig. 6A–C, from 318.15 up to 344.45 K. However, at this sample composition, the melting process does not start at about 327 K, but only at 344.45 K, approximately 18 degrees above the thermal events observed by DSC, and the sample is totally melted at 346.35 K (Fig. 6D). Thus, the images confirm the formation of a solid solution in the extreme of the phase diagram that is rich in tristearin, since it was not observed a coexistence of a liquid + solid phase from 327 K, approximately, which is the assumed eutectic reaction temperature.
To understand the behavior of different TAGs on SLE phase diagram with fatty acids, the liquidus line determined in this study containing tristearin (1) + myristic acid (4) was plotted with three liquidus lines previously determined, containing tripalmitin + myristic acid (Pelaquim et al. 2019), trilaurin + myristic acid (Matos et al. 2016), and triolein + myristic acid (Matos et al. 2015), as shown in Fig. 7.
Considering the melting point of myristic acid, one can observe two different behaviors of the liquidus line. The first one is observed for tristearin or tripalmitin + myristic acid systems (Fig. 7A), in which the eutectic point moves from xmyristicacid \(\cong\) 0.90 to xmyristicacid \(\cong\) 1.00, a monotectic point. The second one is observed for trilaurin or triolein + myristic acid systems (Fig. 7B); in this case, the eutectic point moves from xmyristicacid \(\cong\) 0.50 to xmyristicacid \(\cong\) 0.00, a monotectic point. The eutectic point moves in the direction of the pure compound with the smaller melting temperature, in the case of saturated TAGs in the direction of the smaller carbon chain length TAG, as previously reported for systems formed by triolein and trilaurin + fatty alcohol compounds or fatty acids (Maximo et al. 2019).
Phase diagrams and microscopy analysis of tristearin + fatty alcohol compounds
DSC curves and phase diagram of tristearin (1) + 1-tetradecanol (7) system are presented in Figs. 8 and 9, respectively. Figure 8 shows that the DSC curves for the mixtures present at least two thermal events. The first thermal event, around 310 K, is attributed to the start of the phase change process, and the second, at highest temperature, represents the full sample melting. The TAG composition increase promotes a severe increase in the second endothermic peak and a significantly decrease in the first endothermic peak. As generally observed for fat systems (Carareto et al. 2014; Matos et al. 2015), here it is also note the occurrence of overlapped peaks at 0.1999, 0.4004, 0.5999, and 0.7997 tristearin molar fractions, at lower temperature (first peak), as indicated by the black arrows in Fig. 8. The overlapped peaks can be related to the melting of tristearin polymorphs, and the small spread exothermic peak at the 0.7997 tristearin molar fraction is probably due to tristearin α-form crystallization (Kellens and Reynaers 1992). Even though the peaks are overlapped, the most pronounced peak observed at 311 K has its intensity increased with the rise in tristearin molar fraction, as it happens for eutectic systems (Inoue et al. 2004a, b). According to the literature (Craven and Lencki 2011), in this case the phase diagram is apparently considered to present a monotectic behavior (Fig. 9). Thus, to better understand and evaluate this behavior, microscope optical images were taken at 0.1999 and 0.7997 tristearin molar fractions.
SLE phase diagram of the tristearin (1) + 1-tetradecanol (7) system: (filled circle) melting temperature; (filled square) solid transition temperature; (filled diamond) exothermic transition temperature; (triangle) solid transition temperature; (*) pure component transition temperature; (Θ) solid–liquid transition temperature determined by optical microscope (black line) ideal model; (red line) three-suffix Margules; (blue line) NRTL; (dashed line) guide to the eyes of solid solution formation according to microscope results
Initially, the binary mixture of tristearin (1) + 1-tetradecanol (7) at 0.1999 tristearin molar fraction is solid at 305.25 K (Fig. 10A). With the temperature increase, we can note the progress of the crystallization process at 310.55 K (Fig. 10B), as observed for the previous systems The crystallization process continues until 312.65 K and is perceptible due to brightness loss of the images from Fig. 10A to C. This process is confirmed by DSC curves in the region with the overlapped peaks around 311 K (Fig. 8). The peak temperatures of each overlapped peak were also plotted in the phase diagram using the Δ symbol (Fig. 9). Contrary to expectations, the solid phase remained up to 332.65 K (Fig. 10D), 30 degrees above the supposed solid–liquid transition temperature, as can be seen in the phase diagram presented in Fig. 9. A liquid phase in equilibrium with a solid phase can be clearly observed only at 332.65 K in Fig. 9E, represented by the Θ symbol in the phase diagram (Fig. 9). Finally, at 344.05 K, the mixture is completely melted (Fig. 10F). This behavior agrees with the peak obtained by the DSC experiments, the peak observed at higher temperature. Thus, these images indicate that the tristearin (1) + 1-tetradecanol (7) system does not obey the description of a simple eutectic system. The images confirm the formation of a solid solution in all composition range, and as was observed, this solid solution goes through some changes in its solid phase due to a crystal restructuring (polymorphic change), clearly perceptible because of the crystal darkness with the temperature increase, as can be better seen in the gif 1 at the Supplementary Information. A better understanding of such behavior requires the use of other analysis techniques, such as x-ray diffraction, and it is not the main goal of this study.
The same behavior observed for the 0.1999 tristearin molar fraction was observed for the 0.7997 tristearin molar fraction of the same system (Fig. 11), i.e., a recrystallization process takes place at 316.35 K (Fig. 11B) and the melting process started at 345.85 K (Fig. 11C), very close to the liquidus line temperature. This result corroborates the previously discussed behavior and can be better seen in the gif 2 at the Supplementary Information.
Figure 12 shows the phase diagrams of tristearin (1) + 1-decanol (5) and tristearin (1) + 1-dodecanol (6) systems. One can observe that both systems present, according to the experimental data and microscopy analysis, the same characteristics of the previously discussed system. Therefore, these systems might also present a solid solution region in all molar fraction composition range.
SLE phase diagram of the A tristearin (1) + 1-decanol (5) system; B tristearin (1) + 1-dodecanol (6) binary system: (filled circle) melting temperature; (filled square) solid transition temperature; (filled diamond) exothermic transition temperature; (*) pure component transition temperature; (black line) ideal model; (red line) three-suffix Margules; (blue line) NRTL; (dashed line) guide to the eyes of solid solution formation
In general, solid solution phase may be occur in binary solid mixtures, when the two components are miscible in all proportions in a liquid state. The solid solution changes the behavior of the melting profile of the systems, especially the beginning of the melting temperature which difficult its identification by using DSC data (Maximo et al. 2019). In addition, the increase of the triacylglycerol carbon chain and the increase of the difference between the melting temperatures of the pure components leads to a larger solid solution region (Maximo et al. 2014a, b).
As for the systems with fatty acids, the ideal behavior underestimates the liquidus line of mixtures composed by tristearin + fatty alcohol compounds and three-suffix Margules and NRTL presented a good match. In Fig. 12A it is possible to observe the 3 regions composed by this kind of phase diagram: region 1 is formed by a liquid phase, region 2 is a solid–liquid equilibrium area composed by solid tristearin with a liquid phase and region 3 is the solid solution of both components.
Thermodynamic modeling of SLE data
The adjusted parameters obtained by three-suffix Margules and NRTL are presented in Table 9. One can observe that the root mean square deviation (RMSD) values for all the systems studied are low, which means that all models describe the experimental data in a good way, including the ideal assumption, which needs a smaller computational effort. Besides, in this thermodynamic study, the solid phase was considered ideal, and, for all the systems, including those that presented a solid solution in all the composition range, the models described very well the liquidus line.
Conclusion
The DSC technique was used to determine the phase diagram of the studied systems and it is considered an efficient and reliable method to study the SLE behavior of fatty mixtures. In this study, the melting temperature of pure tristearin was determined, and this property agrees very well with the data found in the literature. The study of the solid–liquid equilibrium (SLE) of fatty mixtures is essential for the improvement and optimization of the processing of fatty based products. Due to the high complexity of the melting profile of fatty compounds and their mixtures, as tristearin + fatty acids and tristearin + fatty alcohol compounds, critical thermodynamic evaluation is important for process design and industrial quality requirements of products.
The phase diagrams of tristearin + fatty acids presented a monotectic behavior. Moreover, the tristearin (1) + lauric acid (3) and tristearin (1) + myristic acid (4) systems presented a solid solution close to the pure triacylglycerol molar fraction, according to optical microscope images. The systems formed by tristearin + fatty alcohol compounds seem to exhibit a solid solution formation in all the molar fraction composition range.
All the six studied systems were modeled using the ideal assumption, three-suffix Margules, and NRTL models. All models were able to describe the liquidus line. However, the three-suffix Margules and NRTL models presented the best results, since they are more complex than ideal assumption. Thus, both models, three-suffix Margules can be considered thermodynamic.
Availability of data and material
The authors declare that the data and material are available.
Code availability
Not applicable.
Abbreviations
- ARD:
-
Average relative deviation
- DSC:
-
Differential scanning calorimetry
- NRTL:
-
Non-random two-liquid model
- RMSD:
-
Root mean square deviation
- SLE:
-
Solid–liquid equilibrium
- SSS:
-
Tristearin
- TAG:
-
Triacylglycerols
References
Carareto NDD, Costa MC, Meirelles AJA, Pauly J (2015) High pressure solid-liquid equilibrium of fatty alcohols binary systems from 1-Dodecanol, 1-Tetradecanol, 1-Hexadecanol, and 1-Octadecanol. J Chem Eng Data 60:2966–2973
Carareto NDD, Dos Santos AO, Rolemberg MP, Cardoso LP, Costa MC, Meirelles AJA (2014) ‘On the solid-liquid phase diagrams of binary mixtures of even saturated fatty alcohols: Systems exhibiting peritectic reaction’, Thermochimica Acta. Elsevier B.V., 589, pp 137–147. https://doi.org/10.1016/j.tca.2014.05.022.
Costa MC, Rolemberg MP, Boros LAD, Krähenbühl MA, de Oliveira MG, Meirelles AJA (2007) Solid−liquid equilibrium of binary fatty acid mixtures. J Chem Eng Data 52:30–36. https://doi.org/10.1021/je060146z
Costa MC, Rolemberg MP, Dos Santos AO, Cardoso LP, Krähenbühl MA, Meirelles AJA (2010) Solid-liquid equilibrium of tristearin with refined rice bran and palm oils. J Chem Eng Data 55:5078–5082. https://doi.org/10.1021/je100647b
Costa MC, Boros LAD, Souza JA, Rolemberg MP, Krähenbühl MA, Meirelles AJA (2011) Solid–liquid equilibrium of binary mixtures containing fatty acids and triacylglycerols. J Chem Eng Data 56:3277–3284. https://doi.org/10.1021/je200033b
Craven RJ, Lencki RW (2011) Binary phase behavior of diacid 1,3-diacylglycerols. J Am Oil Chem Soc 88:1125–1134. https://doi.org/10.1007/s11746-011-1777-0
Da Silva RC, De Martini Soares FAS, Maruyama JM, Dagostinho NR, Silva YA, Ract JNR, Gioielli LA (2016) Microscopic approach of the crystallization of tripalmitin and tristearin by microscopy. Chem Phys Lipid 198:1–9. https://doi.org/10.1016/j.chemphyslip.2016.04.004
de Matos FC, da Costa MC, Meirelles AJ, Batista EAC (2015) Binary solid-liquid equilibrium systems containing fatty acids, fatty alcohols and triolein by differential scanning calorimetry. Fluid Phase Equilib 404:1–8. https://doi.org/10.1016/j.fluid.2015.06.015
de Matos FC, da Costa MC, Meirelles AJ, Batista EAC (2016) Binary solid-liquid equilibrium systems containing fatty acids, fatty alcohols and trilaurin by differential scanning calorimetry. Fluid Phase Equilib 423:74–83. https://doi.org/10.1016/j.fluid.2016.04.008
Dorighello Carareto ND, Costa MC, Meirelles AJA, Pauly J (2015) High pressure solid-liquid equilibrium of fatty alcohols binary systems from 1-DODECANOL, 1-Tetradecanol, 1-Hexadecanol, and 1-Octadecanol. J Chem Eng Data 60:2966–2973. https://doi.org/10.1021/acs.jced.5b00330
Eckert KA, Dasgupta S, Selge B, Ay P (2016) Solid liquid phase diagrams of binary fatty acid mixtures—palmitic/stearic with oleic/linoleic/linolenic acid mixture. Thermochim Acta 630:50–63. https://doi.org/10.1016/j.tca.2016.02.008
Fisk PR, Wildey RJ, Girling AE, Sanderson H, Belanger SE, Veenstra G, Nielsen A, Kasai Y, Willing A, Dyer SD, Stanton K (2009) Environmental properties of long chain alcohols. Part 1: physicochemical, environmental fate and acute aquatic toxicity properties. Ecotoxicol Environ Saf 72:980–995. https://doi.org/10.1016/j.ecoenv.2008.09.025
Garti N, Sato K (1988) Crystallization and polymorphism of fats and fatty acids. Marcel Dekker, Nova Iorque
Höhne GWH, Hemminger WF, Flammerseim HJ (2003) Differential Scanning Calorimetry, 2nd edn. Springer, New York
Humphrey KL, Narine SS (2005) Lipid phase behavior. In: Marangoni AG (ed) Fat crystal networks. Taylor & Francis, New York, p 854
Inoue T, Hisatsugu Y, Ishikawa R, Suzuki M (2004a) Solid-liquid phase behavior of binary fatty acid mixtures: 2. Mixtures of oleic acid with lauric acid, myristic acid, and palmitic acid. Chem Phys Lipid 127:161–173. https://doi.org/10.1016/j.chemphyslip.2003.10.013
Inoue T, Hisatsugu Y, Yamamoto R, Suzuki M (2004b) Solid-liquid phase behavior of binary fatty acid mixtures: 1. Oleic acid/stearic acid and oleic acid/behenic acid mixtures. Chem Phys Lipid 127:143–152. https://doi.org/10.1016/j.chemphyslip.2003.09.014
Johnson RW (1988) Fatty alcohols. Fatty acids in industry. Marcel Dekker, New York, pp 217–231
Kellens M, Reynaers H (1992) Study of the polymorphism of saturated monoacid triglycerides I: melting and crystallization behavior of tristearin. Fat Sci Technol 94:94–100. https://doi.org/10.1002/lipi.19920940304
Kellens M, Meeussen W, Gehrke R, Reynaers H (1991) Synchrotron radiation investigations of the polymorphic transitions of saturated monoacid triglycerides. Part 1: Tripalmitin and tristearin. Chem Phys Lipid 58:131–144. https://doi.org/10.1016/0009-3084(91)90119-V
Kogan A, Garti N (2006) Microemulsions as transdermal drug delivery vehicles. Adv Coll Interface Sci 123–126:369–385. https://doi.org/10.1016/j.cis.2006.05.014
Matovic M, Van Miltenburg JC, Los J, Gandolfo FG, Flöter E (2005) Thermal properties of tristearin by adiabatic and differential scanning calorimetry. J Chem Eng Data 50:1624–1630. https://doi.org/10.1021/je050092d
Maximo GJ, Costa MC, Meirelles AJA (2013) Solid-liquid equilibrium of triolein with fatty alcohols. Braz J Chem Eng 30:33–43. https://doi.org/10.1590/S0104-66322013000100005
Maximo GJ, Carareto NDD, Costa MC, dos Santos AO, Cardoso LP, Krähenbühl MA, Meirelles AJA (2014a) On the solid-liquid equilibrium of binary mixtures of fatty alcohols and fatty acids. Fluid Phase Equilib 366:88–98. https://doi.org/10.1016/j.fluid.2014.01.004
Maximo GJ, Costa MC, Meirelles AJA (2014b) The Crystal-T algorithm: a new approach to calculate the SLE of lipidic mixtures presenting solid solutions. Phys Chem Chem Phys 16:16740–16754. https://doi.org/10.1039/c4cp01529k
Maximo GJ, Carareto NDD, Costa MC (2019) Solid-Liquid Equilibrium in Food Processes. In: Thermodynamics of Phase Equilibria in Food Enhineering. 1st edn. Academic Press, Cambridge, p 684
Oh JH, McCurdy AR, Clark S, Swanson BG (2002) Characterization and thermal stability of polymorphic forms of synthesized tristearin. J Food Sci 67:911–2917. https://doi.org/10.1111/j.1365-2621.2002.tb08837.x
Pelaquim FP, Cardoso de Matos F, Cardoso LP, Caldas Batista EA, de Almeida J, Meirelles A, Conceição da Costa M (2019) Solid-liquid phase equilibrium diagrams of binary mixtures containing fatty acids, fatty alcohol compounds and tripalmitin using differential scanning calorimetry. Fluid Phase Equilib 497:19–32. https://doi.org/10.1016/j.fluid.2019.05.020
Pernetti M, van Malssen KF, Flöter E, Bot A (2007) Structuring of edible oils by alternatives to crystalline fat. Curr Opin Colloid Interface Sci 12:221–231. https://doi.org/10.1016/j.cocis.2007.07.002
Persson M (2008) Alpha stable fats. Lipid Technol 20:13–16. https://doi.org/10.1002/lite.200700099
Prausnitz JM, Lichtenthaler RN, de Azevedo EG (1999) Molecular thermodynamics of fluid-phase equilibria. 3rd edn, Nopardazan. Prentice Hall, Nova Jersey. https://doi.org/10.1016/0021-9614(70)90078-9
Singh SK, Jalali AF, Aldén M (1999) Modulated temperature differential scanning calorimetry for examination of tristearin polymorphism: 2. Isothermal Crystallization of Metastable Forms. J Am Oil Chem Soc 76:507–510. https://doi.org/10.1007/s11746-999-0031-5
Tadie M, Bahadur I, Reddy P, Ngema PT, Naidoo P, Deenadayalu N, Ramjugernath D (2013) Solid-liquid equilibria measurements for binary systems comprising (butyric acid + propionic or pentanoic acid) and (heptanoic acid + propionic or butyric or pentanoic or hexanoic acid). J Chem Thermodyn 57:485–492. https://doi.org/10.1016/j.jct.2012.09.019
Timms RE (1984) Phase behaviour of fats and their mixtures. Prog Lipid Res 23:1–38. https://doi.org/10.1016/0163-7827(84)90004-3
Vetterling WT, Teukolsky SA, Press WH, Flannery BP (1992) Numerical Recipes in F77. 2nd edn, Numerical Methods for Fortran 77: the art of scientific computing. 2nd edn. Press Syndicate of the University of Cambridge. http://www.papers3://publication/uuid/42C52282–20B5–4159–9E1B-2E89F2631AAA
Webster GR, Bisset NB, Cahill DM, Jones P, Killick A, Hawley A, Boyd BJ (2018) Tristearin as a model cuticle for high-throughput screening of agricultural adjuvant systems. ACS Omega 3:16672–16680. https://doi.org/10.1021/acsomega.8b02656
Wei D, Han S, Wang B (2014) Solid-liquid phase equilibrium study of binary mixtures of n-octadecane with capric, and lauric acid as phase change materials (PCMs). Fluid Phase Equilib 373:84–88. https://doi.org/10.1016/j.fluid.2014.04.020
Wesdorp LH (1990) Liquid-Multiple solid phase equilibria in fats. Universidade de Tecnologia de Delft
Wright AJ, Marangoni AG (2005) Physical Properties of Fats and Oils. Handbook of Functional Lipids. CRC Press, Florida, pp 135–162
Yui K, Itsukaichi Y, Kobayashi T, Tsuji T, Fukui K, Maeda K, Kuramochi H (2017) Solid-liquid equilibria in the binary systems of saturated fatty acids or triglycerides (C12 to C18) + hexadecane. J Chem Eng Data 62:35–43. https://doi.org/10.1021/acs.jced.6b00355
Zhang Z, Yuan Y, Zhang N, Cao X (2015) Thermophysical Properties of Some Fatty Acids/Surfactants as Phase Change Slurries for Thermal Energy Storage. J Chem Eng Data 60:2495–2501. https://doi.org/10.1021/acs.jced.5b00371
Zhao P, Yue Q, He H, Gao B, Wang Y, Li Q (2014) Study on phase diagram of fatty acids mixtures to determine eutectic temperatures and the corresponding mixing proportions. Appl Energy 115:483–490. https://doi.org/10.1016/j.apenergy.2013.10.048
Funding
The authors thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–Brazil (CAPES)–Finance Code 001, FAPESP (2014/21252-0 and 2018/19198-9), CNPq (305870/2014-9, 406963/2014-4, 306666/2020-0, 309780/2014-4, and 132149/2017-6), and FAEPEX/UNICAMP for the financial support. The authors also thank Espaço da Escrita–Pró-Reitoria de Pesquisa–UNICAMP–for the language services provided.
Author information
Authors and Affiliations
Contributions
PFP and MFC designed research; CMC, BEAC, MAJA conducted review, editing, and provided funding acquisition, project administration, and resources; PFP wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
43153_2022_227_MOESM1_ESM.docx
Experimental data and Ideal assumption, NRTL and three-suffix Margules UNIFAC model plots to the systems studied: SSS+C10; SSS+C12; SSS+C14; SSS+C10OH; SSS+C12OH; SSS+C14OH (DOCX 301 KB)

SI Figure 1.
Hemolymph (N = 6–12) and urinary (N = 4-12) essential amino acid composition of immersed (0-h) and emersed crabs over a 160-hour time-course exposure. Where data in not available for urinary concentrations no detectable amino acid presence was found. Values are represented as the mean ± S.E.M. (JPG 43 KB)

SI Figure 2.
Hemolymph (N = 6–12) non-essential amino acid composition of immersed (0-h) and emersed crabs over a 160-hour time-course exposure. Where data in not available for urinary concentrations no detectable amino acid presence was found. Values are represented as the mean ± S.E.M. (JPG 80 KB)
Rights and permissions
About this article
Cite this article
Pelaquim, F.P., de Matos, F.C., Batista, E.A.C. et al. Solid–liquid phase equilibrium diagrams of binary mixtures containing fatty acids, fatty alcohol compounds, and tristearin. Braz. J. Chem. Eng. 40, 269–286 (2023). https://doi.org/10.1007/s43153-022-00227-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43153-022-00227-5