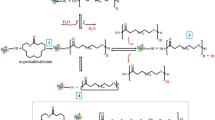
Abstract
Aliphatic polyesters are widely used in biomedical and environmental areas, and their use has grown due to environmental issues. The use of lipases as catalysts for the synthesis of polyesters is an environmentally benign alternative. In this work, the effect of monomer chain length on polyester synthesis was studied with seven diacids and six diols in a bulk system using immobilized Candida antarctica lipase B (Novozym®435). Firstly, the reaction temperature and the thermal stability of Novozym®435 (N435) were evaluated. The half-life of the commercial lipase was 24.8 h at 90 °C, and N435 maintained a residual activity of 38% after 96 h of incubation. The biocatalyst played an essential role in the reactions using monomers with longer alkylene chain length diacids (azelaic and sebacic acids) and diols (1,4-butanediol, 1,6-hexanediol, and 1,8-octanediol), giving a higher reactivity than reactions of shorter chain-length diacids (oxalic, malonic, succinic, glutaric and adipic acids) and diols (ethylene glycol and 1,3-propanediol). Polycondensation reactions carried out with 2,3-butanediol did not present a significative molecular weight. Otherwise, the reaction performed with 1,6-hexanediol resulted in polyesters with weight-average molecular weights (Mw) of 18,346 g mol−1 and 27,121 g mol−1 by reacting with azelaic and sebacic acids, respectively, at 90 °C using 5 wt.% of N435. The thermal properties of polyazelates and polysebactes were analyzed by DSC and TGA, which showed that aliphatic polyesters are practically stable at up to 380 °C, indicating their high thermal stability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aliphatic polyesters are a class of polymers widely used in biomedical, environmental, food, and automobile applications (Azim et al. 2006; Umare et al. 2007; Pellis et al. 2016). Regarding the extensive use of aliphatic polyesters and the increasing environmental concern about plastics, three problems should be addressed: the development of cleaner synthesis processes, the use of monomers produced from renewable resources, and the degradability of the polymer.
In general, the aliphatic polyesters are synthesized by polycondensation of dicarboxylic acids and diols (Pellis et al. 2016; Albertsson and Varma 2002) in the presence of a variety of metal catalysts at temperatures in the range of 180–280 °C. The use of high temperatures favors undesirable side reactions such as dehydration of diols and β-scission of polyesters (Gross et al. 2010). Besides, high reaction temperatures are not appropriate when the monomers have thermal- and chemically-unstable functional groups. Residues of metals present in the catalysts can be found in the final product, making it harmful to the environment and toxic for biomedical applications (Gross et al. 2010).
In order to circumvent the drawbacks of chemical catalysis, the use of enzymes in polymerization reactions has emerged. The substitution of a chemical catalyst by enzymes is a promising green alternative to produce polyesters under mild operational conditions, concerning temperature and toxicity. Besides, due to the high selectivity (enantio-, chemo- and regioselectivity) of enzymes, steps of protection-deprotection are minimized, resulting in products with improved quality (Douka et al. 2018; Miletic et al. 2011).
Many hydrolase enzymes, including especially lipases (EC 3.1.1.3) and cutinases (EC 3.1.1.74), have been used for polymerization of diacids and diols (Wallace and Morrow 1989; Okumura et al. 1984; Binns et al. 1993; Linko et al. 1995; Mahapatro et al. 2003; Feder and Gross 2010; Hunsen et al. 2007). Lipases have been widely used as a biocatalyst in polymers synthesis because they accept a wide range of substrates, with high activity, and are quite stable in non-aqueous media (Veld and Palmans 2011). The most widely used commercial immobilized lipase in polycondensation is Novozym®435 (N435), an immobilized preparation of Candida antarctica Lipase B (CALB), due to its high activity and thermal stability (Douka et al. 2018; Kobayashi 2010). CALB has a deep and narrow active site, and aliphatic alcohols, aliphatic esters, and acids are excellent substrates (Veld and Palmans 2011).
The synthetic routes to produce polyesters using lipase catalysis are ring-opening polymerizations (ROPs) of cyclic monomers, polycondensation reactions of AA-BB-type monomers such as diacids or diesters and diols, and polycondensation reactions of AB-type monomers such as ester or acid alcohol monomers. Condensations of the A-B type generate a leaving group that must be efficiently removed in order to obtain high molecular weight polyesters. The AA and BB-type monomers must be mixed in precisely equimolar quantities in order to obtain high molecular weight polymers (Koltzenburg et al. 2017; Miletic et al. 2011).
A range of polyesters has been synthesized by enzymatic polycondensation of simple diacids with diols (AA-BB-type monomers) (Varma et al. 2005). The water produced during the polycondensation of diacid and diol must be removed to shift the equilibrium towards polymerization (Veld and Palmans 2011; Varma et al. 2005). Lipases do not require the exclusion of water and air for polyester synthesis compared to traditional chemical initiators.
Biodegradable polymers such as polyesters have been synthesized using enzyme-catalyzed routes. Some aliphatic polyesters are biodegradable materials since their hydrolytic and enzymatic degradation products can be naturally metabolized into non-toxic substances (Douka et al. 2018; Pellis et al. 2016; Chen and Patel 2012). The properties and biodegradability of the aliphatic polyesters depend on the structure, that is, the combination of diacids and diols used (Bordes et al. 2009; Edlund and Albertsson 2003).
Polymers synthesized from biomass-derived monomers can often be biologically and/or hydrolytically degradable (Rowe et al. 2016). The development of biobased polymers meets environmental demand, reducing the use of fossil resources. Concerning the aliphatic polyesters, several biobased monomers can be used to produce polyesters such as the diacids succinic, malonic, glutaric, azelaic, and sebacic acids, and the diols 1,3-propanediol, 1,4-butanediol, and 2,3-butanediol (Pellis et al. 2016).
Succinic acid is a strategic platform chemical that can be produced industrially from renewable resources (Becker et al. 2015; Bechthold et al. 2008). Succinic acid is employed in the synthesis of polybutylene succinate (PBS) and polybutylene succinate-co-butylene adipate (PBSA). PBS is widely used for packaging, and it has the advantage of being enzymatic hydrolyzed to non-toxic substances, giving this polymer a leading position in biodegradable materials (Douka et al. 2018). Malonic acid and 1,3-propanediol are interesting derivatives of glycerol (Rowe et al. 2016), and they can be used for polypropylene malonate synthesis, which is a plasticizer and drug delivery medium (Doğan and Küsefoğlu 2008).
Glutaric and azelaic acid are monomers produced from renewable resources that contain an odd number of carbon atoms. Odd-chain dicarboxylic acids can influence the crystallinity, biodegradability, and thermo-mechanical properties of polyesters synthesized with them (Lu et al. 2017; Yu et al. 2017; Kong et al. 2014). Glutaric acid is an important building block for biopolymers since it presents the lowest melting point among all dicarboxylic acids (Mishra et al. 2013). The use of oligoesters based on azelaic and adipic acids constitutes a remarkable alternative for commercial plasticizers for polyethylene terephthalate (PET) (Langer et al. 2015). However, few studies reported the synthesis of polymers using azelaic acid and chemical catalysts (Yu et al. 2017; Ravi et al. 2011; Papageorgiou et al. 2010, 2011), and this diacid has not yet been completely addressed in polymer synthesis using biocatalysts (Feder and Gross 2010; Curia et al. 2015; Nguyen et al. 2016).
Another interesting diacid that can be produced in a biobased process is sebacic acid. Thus, like adipic and azelaic acids, sebacic acid is considered attractive because it can improve the cold fracture temperature of plasticized polyvinyl chloride (PVC) (Rahman and Brazel 2004), the fifth most produced polymer worldwide (Geyer et al. 2017). Besides, it is used as a valuable building block in the synthesis of polyamides, polyesters, and polyurethanes (Fuessl et al. 2012).
The use of biobased molecules will permit the development of new bioprocesses for polymer synthesis (Kobayashi 2010; Lu et al. 2017). The above references established the feasibility of enzymatic polymerization reactions between diacids and diols. Therefore, this work aimed to contribute to this area by focusing on monomer chain length effects. A large number of monomers (seven diacids and six diols) were tested in the absence of solvent, making enzymatic polycondensations more sustainable. The enzymatic reactivity of the primary and secondary hydroxyl groups was also evaluated in the reactions carried out for 96 h using butanediol and seven diacids. The temperature (80 and 90 °C) effect on the polysebacates synthesis was evaluated since it is a crucial parameter to perform the reaction in better conditions for the biocatalyst and synthesis. Considering the high temperature and time used in the polycondensation reactions, we used first mathematical approach to enzymatic thermal stability in this paper.
Experimental
Reagents
Oxalic (98%), malonic (99%), succinic (≥ 99%), glutaric (99%), azelaic (98%) and sebacic (99%) acids were purchased from Sigma-Aldrich as well as the diols ethylene glycol (≥ 99%), 1,3-propanediol (98%), 1,4-butanediol (99%), 2,3-butanediol (98%), 1,6-hexanediol (99%) and 1,8-octanediol (98%). Butanol (P.A.) was obtained from Vetec Química Fina Ltda (Rio de Janeiro, Brazil). Adipic acid (99.5%) was provided by Fluka Analytical. Novozym®435 (N435) was supplied by Novozymes Latin America Ltda (Brazil). N435 consists of Candida antarctica lipase B immobilized on a hydrophobic macroporous resin of poly(methyl methacrylate) crosslinked with divinylbenzene (Lewatit VPOC-1600), with 1.4% concentration of water. The particle size distribution ranges from 0.3 to 0.9 mm in diameter (Douka et al. 2018).
Enzyme activity
Esterification activity was determined by oleic acid consumption in the esterification reactions with butanol (oleic acid:butanol molar ratio of 1), at 45 °C, in a batch reactor magnetically stirred (400 rpm). One esterification unit (U) was defined as the enzyme amount that consumes 1 μmol of oleic acid per minute per g of enzymatic preparation (Corrêa et al. 2011).
Polyester synthesis
The enzymatic polycondensations between diacid and diol were carried out for 96 h in a closed 15 mL batch reactor magnetically stirred (400 rpm) and coupled to a condenser, and the temperature was kept at 90 °C. The reaction mixture consisted of 0.06 mol of diol (ethylene glycol, 1,3-propanediol, 1,4-butanediol, 2,3-butanediol, 1,6-hexanediol, and 1,8-octanediol) and 0.06 mol of diacid (oxalic, malonic, succinic, glutaric, adipic, azelaic and sebacic acid) using 5 wt.% of N435 relative to diacid. Control experiments without N435 were performed in the same reaction conditions in order to verify the influence of biocatalyst in the reaction (Blank test). At the end of the reaction (96 h), the product was extracted with chloroform, and the immobilized lipase was separated by vacuum filtration. These samples were analyzed by volumetric analysis and Gel Permeation Chromatography (GPC) analysis.
Thermal stability of Candida antarctica lipase B
The N435 thermal stability was investigated by incubating the enzyme preparation in butanol at 90 °C, for 96 h, in a 10 mL reactor vessel (EasyMax™ 102, Mettler Toledo) provided with magnetic stirring (400 rpm). The medium was cooled (45 °C) at appropriate time intervals, and oleic acid was added to determine enzyme activity, according to item 2.2. These assays were carried out in duplicate.
The residual activity data were fitted using a deactivation model that consists of a series-type mechanism and involves two first-order steps with one active intermediate (El) and a final enzyme state (E2) (Henley and Sadana 1985). Equation 1 describes the deactivation scheme, where E, E1, and E2 are specific activities, and k1 and k2 are first-order deactivation rate coefficients. Thus, the residual activity (a) is a weighted function of the specific activities ratio in the two enzyme states, α1 and α2, and is expressed in Eq. 2. The parameters were estimated numerically based on the Levenberg–Marquardt algorithm using Origin 8.1 software, whose convergence criterion was the chi-square minimization with the tolerance of 10–9, and the maximum number of iterations equal to 50.
Volumetric analysis
The consumption of carboxyl groups in the enzymatic polycondensations, after 96 h, was determined by acid–base volumetric analysis with 0.02 mol L−1 sodium hydroxide, using a Mettler Toledo T50 Titrator. The reaction medium samples were dissolved in a 35 mL acetone/ethanol mixture (1:1) before titration. The initial amount of carboxyl groups was calculated from the acid mass used in the reaction. The acid conversion was defined as the ratio between the number of moles of reacted carboxyl groups and the number of moles of initial carboxyl groups.
GPC analysis
The molecular weight of the products was determined by Gel Permeation Chromatography (Shimadzu chromatograph model UFLC). The chromatograph was equipped with a refractive index detector and a GPC-803 column (300 × 8.0 mm, Shimadzu). The analyzes were performed at 30 °C using tetrahydrofuran or chloroform as the eluent at a flow rate of 1.0 mL min−1. Sample concentrations of 0.4 wt.% and injection volumes of 20 μL were used. The number-average molecular weights (Mn), weight-average molecular weights (Mw), and dispersity index (ÐM, Mw/Mn) were determined by a universal calibration method using narrow dispersity polystyrene standards in the range 450–402,000 g mol−1. A typical GPC chromatogram is shown in the Supplementary Material.
Thermal analysis
Thermal transition properties of the products were determined by Differential Scanning Calorimetry (DSC) using a TA Q1000 calorimeter (TA Instrument). The samples were analyzed using a heat-cool-heat procedure from 10 to 90 °C under nitrogen flow. The heating and cooling rates were 10 °C min−1. The first heating step was carried out to erase the polymer thermal history. Then the cooling step and the second heating were accomplished in order to determine the crystallization temperature (Tc) and the melt transition temperature (Tm), respectively.
The thermal stability of the products was evaluated by thermogravimetric analysis (TGA) using a Q500 thermo analyzer (TA Instrument). Measurements were carried out under nitrogen flow at a heating rate of 25 °C min−1 from 30 to 700 °C. The temperatures at 5% mass loss (T5%) and a maximum weight loss (Tmax) were determined.
Results and discussion
Effect of temperature
Some studies observed that enzymatic polyesterification reactions of diacids and diols with longer carbon chains produced polyesters with higher molecular weight than systems with shorter ones (Mahapatro et al. 2003; Uyama et al. 2000; Feder and Gross 2010; Douka et al. 2018). The temperature increase favored the synthesis of high molecular weight polyesters in bulk reactions using N435 (Poojari et al. 2008; Frampton et al. 2013; Douka et al. 2018). Therefore, first, the effect of temperature (80 and 90 °C) was investigated in the polycondensation of 1,4-BDO and the highest carbon chain diacid evaluated in this work, sebacic acid. The molar ratio (sebacic acid: 1,4-BDO) was equal to 1, since this ratio is most suitable for AA + BB enzymatic polycondensation to obtain a high molar mass polyester (Liu et al. 2011; Linko et al. 1995; Koltzenburg et al. 2017; Miletic et al. 2011). As the polyesterification of these two monomers is low due to the solubility of sebacic acid in 1,4-BDO (Liu et al. 2011), the reaction was carried out for 96 h and the results are presented in Fig. 1.
The temperature increase enhanced (27.4%) the Mn of the polymer. A control experiment without using N435 as biocatalyst (blank test) was also performed under the same reaction conditions at 90 °C. Comparing the results of the blank test with those obtained in the presence of N435, it can be observed that the use of lipase significantly improved the reaction between sebacic acid and 1,4-BDO.
Uyama et al. (2000) studied the effect of temperature (50, 60, and 70 °C) on the enzymatic polymerization of 1,4-BDO and sebacic acid using immobilized Candida antarctica lipase. They observed that the polymerization at 50 °C led to the polymer with a lower weight-average molecular weight (MW), and lower yield compared to those obtained at 60 or 70 °C. This finding was attributed to the higher mobility of the substrates and the resulting polymer at a higher temperature.
An increase in temperature improves the solubility of substrates and reduces viscosity, and thus mass transfer limitations. However, high temperatures and long reaction times may cause a drop in catalytic activity due to the denaturation process. N435 is reported to be a thermally stable lipase and enables higher activities even at 90 °C (Kokkonen et al. 2019; Mei et al. 2002). Frampton et al. (2013) (Frampton and Zelisko 2013) studied the effect of temperature for an N435-catalyzed polymerization, and the authors observed that hydrophobic long-chain substrates are conducive to protecting this lipase at higher temperatures.
However, as the time required for enzymatic polycondensation is long, the thermal stability of N435 was evaluated to determine if the enzyme would be active during the whole time of reaction. The residual activity was determined by esterification of oleic acid and butanol at 45 °C, after different incubation times at 90 °C. The results are plotted in Fig. 2, and the estimated parameters are shown in Table 1.
The initial esterification activity obtained for lipase N435 was 2459 U. Two serial decay kinetics of first-order were observed; this is possible because of the two N435 forms, labile, and resistant (Pérez-Venegas et al. 2020). In the labile form, the residual activity rapidly decreased in the first hours and, after 48 h, it remained almost constant at 38%, which characterizes the resistant form. The half-life of the enzyme was calculated as 24.8 h with a high determination coefficient (R2 = 0.99), and this means that the model proposed by Henley and Sadana (1985) is adequate. However, the dependence of the intrinsic kinetic parameters was high, showing that the model becomes overparameterized, and it cannot be consistently verified from the data. This behavior may suggest a reversible mechanism in the first step (E0 \(\leftrightarrows\) E1) (Polakovič and Vrábel 1996). So, in order to solve the problem of the overparameterized equation, the parameter k1 was fixed, and this constraint allowed obtaining a valid solution. Indeed, a value of k1 of 1.7 h−1 provided an a2 parameter that describes the remained activity equal to 0.373 ± 0.015, compared to the observed value of 0.38. Besides, the deactivation rate coefficient k2 was close to zero, showing that the deactivation of the second state occurs slowly.
These results corroborate that N435 presents significant thermal stability (Mei et al. 2002). As the use of 90 °C yielded polyesters with higher Mn and allowed the maintenance of enzyme activity during the reaction time, further experiments in this work were carried out at 90 °C.
Polycondensation of dicarboxylic acids and diols
Usually, polymerizations are carried out in two stages: a first-stage of oligomerization, and a second-stage of polymerization (Liu et al. 2011; Linko et al. 1995). The first-stage reaction is performed under atmospheric pressure, converting the monomers to oligomers. After that, the by-product (water) removal is performed to shift the reaction equilibrium towards polymerization. The water removal by vacuum, the presence of molecular sieves, or dry nitrogen flow is essential in order to obtain a high average molar mass product (Douka et al. 2018). However, the application of vacuum during the early stages of the polymerization could promote the removal of volatile unreacted monomers, such as short-chain diols and diacids, and of low-molecular-weight oligomers, that would result in a change in the molar ratio of the two substrates (Douka et al. 2018). Besides, water removal by the use of a vacuum or desiccant at the beginning of the reaction can promote an excessive stripping of the essential water needed for enzyme activity (Aguieiras et al. 2011). In this work, only the first-stage of reaction of dicarboxylic acids and diols was studied in a bulk reaction system.
The reactivity of diols such as ethylene glycol (EG), 1,3-propanediol (1,3-PDO), 1,4-butanediol (1,4-BDO), 2,3-butanediol (2,3-BDO), 1,6-hexanediol (1,6-HDO) and 1,8-octanediol (1,8-ODO) and of diacids such as oxalic (C2), malonic (C3), succinic (C4), glutaric (C5), adipic (C6), azelaic (C9), and sebacic (C10) acids was evaluated taking into account the carbon chain length of these compounds.
The reaction medium of the enzymatic polycondensation of the diacids and diols is heterogeneous not only due to the presence of the immobilized enzyme, but also because of the physical state of the reactants. All of the diacids (oxalic, malonic, succinic, glutaric, adipic, azelaic, and sebacic acids) are solids with melting points in the range 97–190 °C. Regarding the diols, 1,6-HDO and 1,8-ODO are solids with melting points of 42 and 61 °C, respectively, and EG, 1,3-PDO, 1,4-BDO, and 2,3-BDO are viscous liquids. The presence of solvents can help to minimize this heterogeneity, but it makes this reaction system less sustainable since toxic organic solvents are becoming more restricted for many applications due to industrial, social, and environmental implications. Therefore, the enzymatic polycondensation reactions were carried out in the absence of solvent, making this procedure greener and more sustainable. As reported in the literature (Binns et al. 1998), the reaction medium becomes more homogeneous with time. For both temperatures tested (80 and 90 °C), it was observed that, during the reaction time, the low initial homogeneity of the reaction medium decreased, as indicated by the literature (Binns et al. 1998).
The results obtained in the reactions with and without N435 are presented in Table 2. The dispersity index (ÐM) for all polyesters was determined. Most oligomers or polymers showed narrow ÐM between 1.20 and 1.54. According to the results, the chain length of the diol and the diacid did not influence the dispersity. This lower dispersity (ÐM < 1.5) observed for polyesters formed by N435-catalyzed polycondensation suggests that chain growth occurs selectively. Thus, lipase produces oligomers/polymers with more uniform chain lengths (Mahapatro et al. 2004). On the other hand, the dispersity of polymers obtained by chemical step-growth processes presents values greater than or equal to 2 (Mahapatro et al. 2004).
It is possible to observe that there was no relation between product molecular weight and acid conversion after 96 h reaction. Thus, in order to evaluate the polymerization reaction, the weight-average molecular weight (Mw) was used, and these results are illustrated in Fig. 3.
Weight-average molecular weight (Mw) of polyesters obtained by polycondensation of oxalic (A), malonic (B), succinic (C), glutaric (D), adipic (E), azelaic (F), and sebacic (G) acids, and the diols EG, 1,3-PDO, 2,3-BDO, 1,4-BDO, 1,6-HXO, and 1,8-ODO in the absence (blank test, cross-hatch column) or presence (5 wt.%, black column) of Novozym 435 at 90 °C
As shown in Fig. 3A, products with low Mw were obtained in the reactions using oxalic acid. Uyama et al. (2000) studied reactions carried out for 8 h, using several diacids and diols and Candida antarctica lipase (Novo Nordisk), in a bulk system, at 60 °C. The authors observed that no product was formed when ethylene glycol was used in the reaction with oxalic acid, but the use of 1,4-BDO resulted in a poly(butylene oxalate) with Mw equal to 1700 g mol−1. An oligomer with a higher Mw (2317 g mol−1) was synthesized from these monomers in the present work.
Regarding the polycondensation of malonic acid and different diols (EG, 1,3-PDO, 1,4-BDO, and 2,3-BDO) in the presence of N435 and without the biocatalyst, a significant consumption of malonic acid was observed for all reactions (Table 2). However, the enzymatic polycondensation of malonic acid occurred only in the presence of 1,3-PDO and 1,4-BDO, producing oligomers with Mw and degree of polymerization of 1287 (ÐX = 7.9) and 1359 (ÐX = 7.7) g mol−1, respectively (Fig. 3B). It is important to highlight that an odor of acetic acid was detected during the experiments, indicating the decomposition of malonic acid, that was also confirmed by thermogravimetric analysis. Although the Mw of poly(propylene malonate) was low, this result was similar to the one reported using aluminum chloride as catalyst (1400 g mol−1) (Rowe et al. 2016). The synthesis of poly(ethylene malonate) was studied in the presence of immobilized Candida antarctica lipase B (Fermase CALB™ 10,000), in solvent-free conditions using ultrasound and poly(ethylene terephthalate) beads to adsorb the products, carried out with ethylene glycol diacetate and dibenzyl malonate (Tomke et al. 2017). The authors also observed a low degree of polymerization (ÐX = 3.9).
The experimental conditions used in this work for the polycondensation of succinic acid and different diols (Fig. 3C) gave a poly(propylene succinate) with Mw of 2210 g mol−1 and a degree of polymerization of 12.5 (Table 2). However, similar results were achieved in the blank test (without N.435), which could be explained by an autocatalytic reaction. Chang and Karalis (1993) reported that the diacid monomer acts as its own catalyst for the esterification reaction in the absence of an externally added strong acid. As the chain length of the diacid increases, and consequently the difference between its first and second dissociation constants decreases, the main reaction (polyesterification) gives way to the first step of reaction (monoesterification) (Vancsó-Szmercsányi et al. 1969).
The synthesis of poly(propylene succinate) has been studied (Debuissy et al. 2017a, b; Parcheta and Datta 2017, 2018; Parcheta et al. 2018), but only one report used an enzyme (N435) as a catalyst in the transesterification of 1,3-PDO and diethyl succinate in the presence of diphenyl ether (Debuissy et al. 2017a). A product of Mw 11,200 g mol−1 was obtained at 90 °C after 72 h with 10 wt.% of N435. The higher Mw achieved in this procedure can be related to the higher amount of enzyme and the use of solvent.
The polycondensation of glutaric acid with EG, 1,3-PDO and 1,4-BDO produced oligomers with Mw and degrees of polymerization of 1270 (ÐX = 7.2), 1558 (ÐX = 8.2) and 3654 (ÐX = 17.9) g mol−1, respectively, as seen in Fig. 3D and Table 2. Some studies reported the production of poly(ethylene glutarate) by the interesterification of ethylene glycol diacetate and diethyl glutarate in a solvent-free system using Candida antarctica lipase B (Fermase CALB™ 10,000) at 40 °C (Tomke et al. 2017; Zhao et al. 2016). A poly(ethylene glutarate) with a ÐX value of 31 was achieved using a higher enzyme amount (20% w/v) and a reaction procedure of vacuum/ultrasound/vacuum that allowed obtaining a polymer with a higher degree of polymerization. However, when these authors used 5% w/v of the enzyme, the ÐX was equal to 3.0 (Zhao et al. 2016). In this work, the poly(ethylene glutarate) produced without enzyme at 90 °C after 96 h presented ÐX of 7.2 (Mw = 1270 g mol−1).
As observed for the other short-chain length diacids (oxalic, malonic, succinic, and glutaric acids), N435 did not show significant reactivity in the polycondensation of adipic acid with the diols EG, 1,3-PDO, and 2,3-BDO (Table 2; Fig. 3E). Binns et al. (1998) investigated the polyesterification between adipic acid and 1,4-BDO in a bulk system using N435 and obtained an oligomer with Mw of 2227 g mol−1. Similar results were also observed in the present work. Mahapatro et al. (2003) studied the polycondensation under vacuum between adipic acid and different diols in a solvent-free system at 70 °C. The Mn of the polyadipates obtained from 1,4-BDO, 1,6-HDO, and 1,8-ODO after 72 h were 5340, 8720, and 20,000 g mol−1, respectively. The water removal by vacuum application shifted the equilibrium to the polymerization and enhanced the Mn values of the product.
The polycondensation of the diacids with higher carbon chain, i.e., azelaic and sebacic acids, resulted in products with a higher Mw. Therefore, the use of diols with a higher carbon chain (1,6-HDO and 1,8-ODO) was also investigated for these acids. The enzyme was effective in the polycondensation of azelaic and sebacic acids, increasing the molecular weight significantly (Fig. 3F and G).
The products of the polycondensation of azelaic acid with EG, 1,3-PDO, 1,4-BDO, 1,6-HDO, and 1,8-ODO presented Mw of 1680, 7546, 13718, 18346 and 8137 g mol−1, respectively (Fig. 3F). N435 provided a poly(1,6-hexanediol azelate) with a higher Mw (18,346 g mol−1) than that reported in the literature for Candida antarctica lipase in toluene medium at 60 °C (6860 g mol−1) (Uyama et al. 2003).
The products derived from sebacic acid showed higher Mw and ÐX than polyazelates. Thus, an increase of one methyl group increased the polymerization. The polycondensation of sebacic acid with EG, 1,3-PDO, 1,4-BDO, 1,6-HDO, and 1,8-ODO resulted in products with Mw of 1117, 6827, 16,504, 27,121 and 18,311 g mol−1, respectively (Fig. 3G). For azelaic and sebacic acids, the maximum value of Mw was found using 1,6-HDO, suggesting that 1,8-ODO is too large to access the active site (Yeniad et al. 2010). Feder and Gross (2010) also observed that oligomers obtained in the polyesterification of sebacic acid with 1,6-HDO had higher Mn than those obtained with 1,8-ODO, after 120 h.
The influence of the length of diacids and diols on the polycondensation in the presence of N435 was already reported in the literature (Pellis et al. 2016; Mahapatro et al. 2003; Feder and Gross 2010; Uyama et al. 1998, 2000; Debuissy et al. 2017c), indicating that the monomers with longer chain lengths are more reactive than shorter ones. The catalytic properties of enzymes are influenced by the compatibility and the access of reactants to the active site (Kokkonen et al. 2019). The active site of lipases has distinct binding sites for the alcohol and acid portion of esters (Bornscheuer and Kazlauska 2006). The scissile fatty acid binding site of CalB is relatively short (C13) and has a small hydrophobic area located at the wall of the funnel-like binding site (Pleiss et al. 1998). The number 13 indicates the length of the longest fatty acid that completely binds inside the binding pocket (Bornscheuer and Kazlauska 2006). The alcohol binding site is similar in all lipases. Therefore, in esterification experiments, the ratio of specificity constants depends on the fatty acid moiety rather than the chemical structure of the alcohol (Pleiss et al. 1998).
The acidity of the diacids can lead to irreversible inactivation of N435, decreasing the enzyme activity due to the protonation of catalytically or structurally important residues within CalB (Hollmann et al. 2009). However, Mahapatro et al. (2003) did not observe this effect in enzymatic polymerization. They determined the percent activity remaining after 48 h of bulk polymerization of 1,8-octanediol with succinic (80%), glutaric (78%), or sebacic acids (79%). The percent activity was also evaluated after 48 h of reaction of adipic acid with 1,4-BDO (78%), 1,6-HDO (79%), and 1,8-ODO (79%). As the values of percent activity were similar, they concluded that the large differences observed in reaction rate as a function of the diol and diacid chain length were not due to differences in lipase activity retained during the reaction. There was no substantial change in the percent retention of enzyme activity due to the monomer structures used.
The correlation of the hydrophobicity and the reactivity for polymerization of the reactants in the presence of N435 was evaluated to better understand the performance of the biocatalyst in the polymerization of diacids and diols. The octanol/water partition coefficient (Kow), used to characterize the hydrophobicity/polarity of compounds, is defined as the ratio of the concentrations of a substance partitioning between octanol and water (Sangster 1989). The values of log Kow or logP for many compounds are available in the literature or can be calculated using various computer programs. Figure 4 shows the logP values for the monomers used in this work.
According to the results shown in Figs. 3 and 4, the reactions using diacids with longer and more hydrophobic carbon chains such as azelaic acid (logP = 1.60) and sebacic acid (logP = 2.27) were enzymatically catalyzed. In contrast, for acids with 6 or fewer carbon atoms that are less hydrophobic, no significant catalytic effect was noted. As shown before (Fig. 3; Table 2), sebacic acid produced polymers with Mw and ÐX higher than those of azelaic acid, indicating that the higher hydrophobicity of sebacic acid contributed to favor the polycondensation. According to Li et al. (2008), diacids with a shorter chain length had higher hydrophilicity, hampering their diffusion into the hydrophobic active site of the enzyme. Concerning the diols, the polarity of the acids seems to be more important than that of the diols. The presence of EG, which is the least hydrophobic diol (logP = − 1.69), restricted the polyester production. As the diol carbon chain increased (from 1,3-PDO to 1,6-HDO), the Mw and the ÐX of the products also increased, probably because the hydrophobicity of the diols also increased. When the reaction was carried out in the presence of 1,8-ODO, the degree of polymerization decreased, despite its higher hydrophobicity, probably due to steric hindrance (Bornscheuer and Kazlauska 2006).
For all polymerizations of 2,3-BDO (Fig. 3; Table 2) and the dicarboxylic acids (oxalic, malonic, succinic, glutaric, adipic, azelaic, and sebacic acids), the polyesters produced did not present an Mw above 412 g mol−1, indicating that the access to vicinal hydroxyl groups is hampered by steric hindrance. Uyama et al. (2000) studied the synthesis of aliphatic polyesters by polycondensation in a solvent-free system using N435 at 60 °C for 8 h. The authors tested diols with secondary hydroxyl groups (1,2-propanediol, 1,3-butanediol, and 2,4-pentanediol), and only 1,3-butanediol was polymerized with sebacic acid, resulting in a product with a molar mass of 6400 g mol−1. It is also important to consider that the reactivity of secondary hydroxyl groups is low, and that N435 is more selective for primary hydroxyl groups (Yoon et al. 2012). Even though N435 can form ester bonds with secondary hydroxyl groups (Gustini et al. 2015; Yoon et al. 2012), products with low molecular weight are reported in the literature (Debuissy et al. 2017c; Yoon et al. 2012; Jiang et al. 2015).
Given that the highest Mw and DP values were found for polyazelates and polysebacates, only these polyesters were analyzed by TGA and DSC in order to compare their main characteristics.
Thermal properties of the products
The thermal stability and the melting process are essential characteristics of polyesters since these properties indicate the performance of the polymers during processing (Parcheta and Datta, 2017), applicability (Vouyiouka et al. 2013), and biodegradation (Laplaza et al. 2014).
Figure 5 shows the thermogravimetric profiles, and Table 3 summarizes the melting (Tm) and crystallization (Tc) temperatures of polyazelates and polysebacates. The Tm and the Tc are directly related to the lengths of the diol and diacid carbon chains, indicating an increase in crystallizability. This behavior was described in the literature concerning the carbon-chain of diacids (Lu et al. 2017; Kong et al. 2014; Jiang et al. 2015; Vouyiouka et al. 2013; Pellis et al. 2018; Kanelli et al. 2014) and diols (Pellis et al. 2018; Celli et al. 2007; Yu et al. 2018).
Different characteristics of the carbon-chain of diacids and diols influence the melting process of a polymer. Polymers with longer carbon-chain diacids and diols are expected to be more flexible and more promptly crystallized, resulting in a decrease of the melting temperature (Lu et al. 2017; Kong et al. 2014; Vouyiouka et al. 2013; Pellis et al. 2018; Kanelli et al. 2014). The conformation of the carbon-chain is also important. For example, long aliphatic-chain entanglements in the molten state increase the viscosity and decrease the chain mobility, increasing the melting temperature (Celli et al. 2007). An odd carbon-chain in the polymer can affect the crystal packing because of the distortion of the chain, lowering the melting temperature (Kong et al. 2014; Papageorgiou et al. 2011; Celli et al. 2007). It is called the even–odd effect on crystal packing. The density of ester groups is also mentioned as a factor that impacts the melting process (Vouyiouka et al. 2013; Kanelli et al. 2014; Celli et al. 2007). These groups act as defects along the polyester structure that difficult the folding and the crystal perfection. Consequently, when the distance between ester groups increases and the density of defects decreases, the melting temperature increases.
As can be seen in Fig. 6, poly(butylene azelate) showed two melting points. Multiple melting points for polyesters are reported in the literature (Kong et al. 2014; Wang et al. 2000; Marand et al. 2000; Sun and Woo 1999; Soccio et al. 2007; Yasuniwa and Satou 2002; Yoo and Im 1999; Li et al. 2008). The presence of multiple melting points can be related to two different processes: the melting of multimodal distributions of crystals with different stability (Marand et al. 2000; Hsiao et al. 1993) and melting-recrystallization-remelting (Blundell 1987; Rim and Runt 1984). The melt-crystallization mechanism differs from melt-recrystallization, and the melt crystallization rate depends somewhat on the melt-recrystallization rate since both are based on the mobility of the molecular chains (Yoo and Im 1999). Besides, the structure of a semicrystalline polymer can reorganize itself during the heating of the calorimetric is measurement, hampering the identification of the reorganization processes observed in multiple melting (Melnikov et al. 2018).
Poly(butylene azelate) was synthesized in a two-stage melt polycondensation method (esterification and polycondensation) using titanium tetrabutoxide as a catalyst (Mincheva et al. 2013; Vouyiouka et al. 2013; Arandia et al. 2015; Díaz et al. 2014). This polymer is reported in the literature as presenting only one melting point, different from the poly(butylene azelate) produced with N435, indicating that it has a different structure.
The thermal stability of polymers is important because it can limit their application. So, the thermal stability of polyazelates and polysebacates was evaluated using the temperatures where the 5% (T5%) and maximum (Tmax) weight loss occur. Most of the polymers studied presented T5% and Tmax around 380 and 409 °C, respectively, while poly(butylene azelate) showed slightly lower temperatures (T5% = 375 and Tmax = 404 °C). No clear relation between the thermal decomposition temperatures and the diacid or diol length was observed, as reported by Vouyiouka et al. (2013). These aliphatic polyesters present high weight-loss temperatures.
Conclusions
Novozym®435 efficiently catalyzed the polycondensation of dicarboxylic acids and diols in a solvent-free system, especially at 90 °C. The N435 thermal stability was investigated, and the excellent agreement between experimental data and the series-type enzyme deactivation model validated its use in the measurement of residual activity (R2 = 0.995). Besides, it was observed that 38% of the immobilized enzyme activity remained after 96 h at the reaction temperature. Concerning the monomer chain length, it was observed that this considerably affected the reaction. The final products of reactions conducted with diacids of 2, 3, 4, 5, and 6 carbon atoms had lower weight-average molecular weights (4000 g mol−1) than polyesters derived from acids with higher carbon chain length (azelaic, C9, and sebacic, C10, acids). The biocatalyst played an essential role in the reactions using these diacids (azelaic and sebacic acids) and diols (1,4-butanediol, 1,6-hexanediol, and 1,8-octanediol), remarkably increasing the Mw values, showing that enzymatic catalysis is favored in the presence of substrates with longer carbon chains. Polymers with higher Mw (27,121 g mol−1) were obtained using 5 wt.% of N435 after 96 h reaction using a simple, environmentally-friendly process. The thermal stabilities of the polyazelate and polysebacate products are quite comparable, all samples being practically stable up to 380 °C, which indicates high thermal stability and a high degree of polymerization.
References
Aguieiras ECG, Veloso CO, Bevilaqua JV, Rosas DO, Silva MAP, Langone MAP (2011) Estolides synthesis catalyzed by immobilized lipases. Enzyme Res 2011:1–7
Albertsson AC, Varma IK (2002) Degradable aliphatic polyesters. Springer, Berlin
Arandia I, Mugica A, Zubitur M, Arbe A, Liu G, Wang D, Mincheva R (2015) How composition determines the properties of isodimorphic poly(butylene succinate- ran -butylene azelate) random biobased copolymers: From single to double crystalline random copolymers. Macromolecules 48:43–57
Azim H, Dekhterman A, Jiang Z, Gross RA (2006) Candida antarctica lipase B-catalyzed synthesis of poly(butylene succinate): shorter chain building blocks also work. Biomacromol 7:3093–3097
Bechthold I, Bretz K, Kabasci S, Kopitzky R, Springer A (2008) Succinic acid: a new platform chemical for biobased polymers from renewable resources. Chem Eng Technol 31:647–654
Becker J, Lange A, Fabarius J, Wittmann C (2015) Top value platform chemicals: bio-based production of organic acids. Curr Opin Biotechnol 36:168–175
Binns F, Roberts SM, Taylor A, Williams CF (1993) Enzymic polymerization of an unactivated diol/diacid system. J Chem Soc Perkin Trans 1:899–904
Binns F, Harffey P, Roberts SM, Taylor A (1998) Studies of lipase-catalyzed polyesterification of an unactivated diacid/diol system. J Polym Sci Part A Polym Chem 36:2069–2080
Blundell DJ (1987) On the interpretation of multiple melting peaks in poly(ether ether ketone). Polymer (guildf) 28:2248–2251
Bordes P, Pollet E, Avérous L (2009) Nano-biocomposites: Biodegradable polyester/nanoclay systems. Prog Polym Sci 34:125–155
Bornscheuer UT, Kazlauska RJ (2006) Hydrolases in organic synthesis: regio- and stereoselective biotransformations, 2nd edn. Wiley VCH, Weinheim
Celli A, Barbiroli G, Berti C, Francesco DC, Lorenzetti C, Marchese P, Marianucci E (2007) Thermal properties of poly(alkylene dicarboxylate)s derived from 1,12-dodecanedioic acid and even aliphatic diols. J Polym Sci Part B Polym Phys 45:1053–1067
Chang WL, Karalis T (1993) Polyesterification reactions of adipic acid-based polyesters. J Polym Sci Part A Polym Chem 31:493–504
Chen GQ, Patel MK (2012) Plastics derived from biological sources: present and future: a technical and environmental review. Chem Rev 112:2082–2099
Corrêa INDS, Souza SL, Catran M, Bernardes OL, Portilho MF, Langone MAP (2011) Enzymatic biodiesel synthesis using a byproduct obtained from palm oil refining. Enzyme Res 2011:1–8
Curia S, Barclay AF, Torron S, Johansson M, Howdle SM (2015) Green process for green materials: viable low-temperature lipase-catalysed synthesis of renewable telechelics in supercritical CO2. Philos Trans R Soc A Math Phys Eng Sci 373:1–16
Debuissy T, Pollet E, Avérous L (2017a) Lipase-catalyzed synthesis of biobased and biodegradable aliphatic copolyesters from short building blocks. Effect of the monomer length. Eur Polym J 97:328–337
Debuissy T, Sangwan P, Pollet E, Avérous L (2017b) Study on the structure-properties relationship of biodegradable and biobased aliphatic copolyesters based on 1,3-propanediol, 1,4-butanediol, succinic and adipic acids. Polymer (guildf) 122:105–116
Debuissy T, Pollet E, Avérous L (2017c) Enzymatic synthesis of biobased poly(1,4-butylene succinate-ran-2,3-butylene succinate) copolyesters and characterization. Influence of 1,4- and 2,3-butanediol contents. Eur Polym J 93:103–115
Díaz A, Franco L, Puiggalí J (2014) Study on the crystallization of poly(butylene azelate-co-butylene succinate) copolymers. Thermochim Acta 575:45–54
Doğan E, Küsefoğlu S (2008) Synthesis and in situ foaming of biodegradable malonic acid ESO polymers. J Appl Polym Sci 110:1129–1135
Douka A, Vouyiouka S, Papaspyridi LM, Papaspyrides CD (2018) A review on enzymatic polymerization to produce polycondensation polymers: the case of aliphatic polyesters, polyamides and polyesteramides. Prog Polym Sci 79:1–25
Edlund U, Albertsson AC (2003) Polyesters based on diacid monomers. Adv Drug Deliv Rev 55:585–609
Feder D, Gross RA (2010) Exploring chain length selectivity in HiC-catalyzed polycondensation reactions. Biomacromol 11:690–697
Frampton MB, Zelisko PM (2013) Synthesis of lipase-catalyzed silicone-polyesters and silicone-polyamides at elevated temperatures. Chem Commun 49:9269–9271
Frampton MB, Séguin JP, Marquardt D, Harroun TA, Zelisko PM (2013) Synthesis of polyesters containing disiloxane subunits: Structural characterization, kinetics, and an examination of the thermal tolerance of Novozym-435. J Mol Catal B Enzym 85–86:149–155
Fuessl A, Yamamoto M, Schneller A (2012) Opportunities in bio-based building blocks for polycondensates and vinyl polymers. Polymer Sci A Compr Ref 10:49–70
Geyer R, Jambeck JR, Law KL (2017) Production, use, and fate of all plastics ever made. Sci Adv 3:25–29
Gross RA, Ganesh M, Lu W (2010) Enzyme-catalysis breathes new life into polyester condensation polymerizations. Trends Biotechnol 28:435–443
Gustini L, Noordover BAJ, Gehrels C, Dietz C, Koning CE (2015) Enzymatic synthesis and preliminary evaluation as coating of sorbitol-based, hydroxy-functional polyesters with controlled molecular weights. Eur Polym J 67:459–475
Henley JP, Sadana A (1985) Categorization of enzyme deactivations using a series-type mechanism. Enzyme Microb Technol 7:50–60
Hollmann F, Grzebyk P, Heinrichs V, Doderer K, Thum O (2009) On the inactivity of Candida antartica lipase B towards strong acids. J Mol Catal B Enzym 57:257–261
Hsiao BS, Gardner KH, Wu DQ, Chu B (1993) Time-resolved X-ray study of poly(aryl ether ketone) crystallization and melting behavior: 1. Crystallization. Polymer (guildf) 34:3986–3995
Hunsen M, Azim A, Mang H, Wallner SR, Ronkvist A, Wenchun X, Gross RA (2007) A cutinase with polyester synthesis activity. Macromolecules 40:148–150
Jiang Y, Woortman AJJ, Alberda Van Ekenstein GOR, Loos K (2015) A biocatalytic approach towards sustainable furanic-aliphatic polyesters. Polym Chem 6:5198–5211
Kanelli M, Douka A, Vouyiouka S, Papaspyrides CD, Topakas E, Papaspyridi LM, Christakopoulos P (2014) Production of biodegradable polyesters via enzymatic polymerization and solid state finishing. J Appl Polym Sci 131:2–9
Kobayashi S (2010) Lipase-catalyzed polyester synthesis—a green polymer chemistry. Proc Jpn Acad Ser B Phys Biol Sci 86:338–365
Kokkonen P, Bednar D, Pinto G, Prokop Z, Damborsky J (2019) Engineering enzyme access tunnels. Biotechnol Adv 37:107386
Koltzenburg S, Maskos M, Nuyken O (2017) Polymer chemistry. Springer-Verlag, Berlin Heidelberg
Kong X, Qi H, Curtis JM (2014) Synthesis and characterization of high-molecular weight aliphatic polyesters from monomers derived from renewable resources. J Appl Polym Sci 131:4–10
Langer E, Was̈kiewicz S, Lenartowicz-Klik M, Bortel K (2015) Application of waste poly(ethylene terephthalate) in the synthesis of new oligomeric plasticizers. Polym Degrad Stab 119:105–112
Laplaza J, Beardslee T, Eirich D, Picataggio S (2014) Verdezyne Inc. WO 2014/100461 A2, 26 Jun 2014
Li G, Yao D, Zong M (2008) Lipase-catalyzed synthesis of biodegradable copolymer containing malic acid units in solvent-free system. Eur Polym J 44:1123–1129
Linko YY, Wang ZL, Seppälä J (1995) Lipase-catalyzed synthesis of poly(1,4-butyl sebacate) from sebacic acid or its derivatives with 1,4-butanediol. J Biotechnol 40:133–138
Liu W, Chen B, Wang F, Tan T, Deng L (2011) Lipase-catalyzed of aliphatic polyesters and properties characterization. Process Bioch 46:1993–2000
Lu J, Wu L, Li BG (2017) High molecular weight polyesters derived from biobased 1,5-pentanediol and a variety of aliphatic diacids: synthesis, characterization, and thermo-mechanical properties. ACS Sustain Chem Eng 5:6159–6166
Mahapatro A, Kalra B, Kumar A, Gross RA (2003) Lipase-catalyzed polycondensations: Effect of substrates and solvent on chain formation, dispersity, and end-group structure. Biomacromol 4:544–551
Mahapatro A, Kumar A, Kalra B, Gross RA (2004) Solvent-free adipic acid/1,8-octanediol condensation polymerizations catalyzed by Candida antartica lipase B. Macromolecules 37:35–40
Marand H, Alizadeh A, Farmer R, Desai R, Velikov V (2000) Influence of structural and topological constraints on the crystallization and melting behavior of polymers. 2. Poly(arylene ether ether ketone). Macromolecules 33:3392–3403
Mei Y, Kumar A, Gross RA (2002) Probing water-temperature relationships for Lipase-catalyzed lactone ring-opening polymerizations. Macromolecules 35:5444–5448
Melnikov AP, Rosenthal M, Ivanov DA (2018) What thermal analysis can tell us about melting of semicrystalline polymers: exploring the general validity of the technique. ACS Macro Lett 7:1426–1431
Miletic N, Loos K, Gross RA (2011) Biocatalysis in polymer chemistry. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim
Mincheva R, Delangre A, Raquez JM, Narayan R, Dubois P (2013) Biobased polyesters with composition-dependent thermomechanical properties: synthesis and characterization of poly(butylene succinate-co-butylene azelate). Biomacromol 14:890–899
Mishra MK, Varughese S, Ramamurty U, Desiraju GR (2013) Odd-even effect in the elastic modulii of α, ω- alkanedicarboxylic acids. J Am Chem Soc 135:8121–8124
Nguyen HD, Löf D, Hvilsted S, Daugaard AE (2016) Highly branched bio-based unsaturated polyesters by enzymatic polymerization. Polymers (basel) 8:1–12
Okumura S, Iwai M, Tominaga Y (1984) Synthesis of ester oligomer by Aspergillus niger Lipase. Agric Biol Chem 48:2805–2808
Papageorgiou GZ, Bikiaris DN, Achilias DS, Karagiannidis N (2010) Synthesis, crystallization, and enzymatic degradation of the biodegradable polyester poly(ethylene azelate). Macromol Chem Phys 211:2585–2595
Papageorgiou GZ, Bikiaris DN, Achilias DS, Papastergiadis E, Docoslis A (2011) Crystallization and biodegradation of poly(butylene azelate): comparison with poly(ethylene azelate) and poly(propylene azelate). Thermochim Acta 515:13–23
Parcheta P, Datta J (2017) Structure analysis and thermal degradation characteristics of bio-based poly(propylene succinate)s obtained by using different catalyst amounts. J Therm Anal Calorim 130:197–206
Parcheta P, Datta J (2018) Kinetics study of the fully bio-based poly(propylene succinate) synthesis. Functional group approach. Polym Degrad Stab 155:238–249
Parcheta P, Koltsov I, Datta J (2018) Fully bio-based poly(propylene succinate) synthesis and investigation of thermal degradation kinetics with released gases analysis. Polym Degrad Stab 151:90–99
Pellis A, Herrero-Acero E, Gardossi L, Ferrario V, Guebitz GM (2016) Renewable building blocks for sustainable polyesters: new biotechnological routes for greener plastics. Polym Int 65:861–871
Pellis A, Comerford JW, Maneffa AJ, Sipponen MH, Clark JH, Farmer TJ (2018) Elucidating enzymatic polymerizations: chain-length selectivity of Candida antarctica lipase B towards various aliphatic diols and dicarboxylic acid diesters. Eur Polym J 106:79–84
Pérez-Venegas M, Tellez-Cruz MM, Solorza-Feria O, López-Munguía A, Castillo E, Juaristi E (2020) Thermal and mechanical stability of immobilized Candida antarctica Lipase B: an approximation to mechanochemical energetics in enzyme catalysis. Chem Cat Chem 12:803–811
Pleiss J, Fischer M, Schmid RD (1998) Anatomy of lipase binding sites: the scissile fatty acid binding site. Chem Phys Lipids 93:67–80
Polakovič M, Vrábel P (1996) Analysis of the mechanism and kinetics of thermal inactivation of enzymes: critical assessment of isothermal inactivation experiments. Process Biochem 31:787–800
Poojari Y, Palsule AS, Cai M, Clarson SJ, Gross RA (2008) Synthesis of organosiloxane copolymers using enzymatic polyesterification. Eur Polym J 44:4139–4145
Rahman M, Brazel CS (2004) The plasticizer market: an assessment of traditional plasticizers and research trends to meet new challenges. Prog Polym Sci 29:1223–1248
Ravi A, Nanthini R, Karunanighi M, Jaisankar V (2011) Synthesis and characterization of certain biodegradable random aliphatic copolyesters. Asian J Chem 23:556–560
Rim PB, Runt JP (1984) Melting point depression in crystalline/compatible polymer blends. Macromolecules 17:1520–1526
Rowe MD, Eyiler E, Walters KB (2016) Bio-based plasticizer and thermoset polyesters: a green polymer chemistry approach. J Appl Polym Sci 133:1–7
Sangster J (1989) Octanol water partition coefficients of simple organic compounds. J Phys Chem Ref Data 18:1111–1229
Soccio M, Lotti N, Finelli L, Gazzano M, Munari A (2007) Aliphatic poly(propylene dicarboxylate)s: effect of chain length on thermal properties and crystallization kinetics. Polymer (guildf) 48:3125–3136
Sun YS, Woo EM (1999) Relationships between polymorphic crystals and multiple melting peaks in crystalline syndiotactic polystyrene. Macromolecules 32:7836–7844
Tomke PD, Zhao X, Chiplunkar PP, Xu B, Wang H, Silva C, Rathod VK (2017) Lipase-ultrasound assisted synthesis of polyesters. Ultrason Sonochem 38:496–502
Umare SS, Chandure AS, Pandey RA (2007) Synthesis, characterization and biodegradable studies of 1,3-propanediol based polyesters. Polym Degrad Stab 92:464–479
Uyama H, Inada K, Kobayashi S (1998) Enzymatic polymerization of dicarboxylic acid and glycol to polyester in solvent-free system. Chem Lett 27:1285–1286
Uyama H, Inada K, Kobayashi S (2000) Lipase-catalyzed synthesis of aliphatic polyesters by polycondensation of dicarboxylic acids and glycols in solvent-free system. Polym J 32:440–443
Uyama H, Wada S, Fukui T, Kobayashi S (2003) Lipase-catalyzed synthesis of polyesters from anhydride derivatives involving dehydration. Biochem Eng J 16:145–152
Vancsó-Szmercsányi I, Maros-Gréger K, Makay-Bödi E (1969) Investigations on polyesterification reactions. Eur Polym J 5:155–161
Varma IK, Albertsson A-C, Rajkhowa R, Srivastava RK (2005) Enzyme catalyzed synthesis of polyesters. Prog Polym Sci 30:949–981
Veld MAJ, Palmans ARA (2011) Enzymatic Polymerization. Springer-Verlag Berlin Heidelberg, Berlin
Vouyiouka SN, Topakas E, Katsini A, Papaspyrides CD, Christakopoulos P (2013) A green route for the preparation of aliphatic polyesters via lipase-catalyzed prepolymerization and low-temperature postpolymerization. Macromol Mater Eng 298:679–689
Wallace JS, Morrow CJ (1989) Biocatalytic synthesis of polymers. II. Preparation of [AA–BB]x polyesters by porcine pancreatic lipase catalyzed transesterification in anhydrous, low polarity organic solvents. J Polym Sci Part A Polym Chem 27:3271–3284
Wang Z-G, Wang X-H, Hsiao BS, Marand PH, Alizadeh A, Farmer R, Desai R, Velikov V (2000) Structure and morphology development in syndiotactic polypropylene during isothermal crystallization and subsequent melting. J Polym Sci Part B Polym Phys 39:2982–2995
Yasuniwa M, Satou T (2002) Multiple melting behavior of poly(butylene succinate). I. Thermal analysis of melt-crystallized samples. J Polym Sci Part B Polym Phys 40:2411–2420
Yeniad B, Naik H, Heise A (2010) Lipases in polymer chemistry. Springer, Berlin
Yoo ES, Im SS (1999) Melting behavior of poly(butylene succinate) during heating scan by DSC. J Polym Sci Part B Polym Phys 37:1366
Yoon KR, Hong SP, Kong B, Choi IS (2012) Polycondensation of sebacic acid with primary and secondary hydroxyl groups containing diols catalyzed by candida antarctica lipase B. Synth Commun 42:3504–3512
Yu Y, Sang L, Wei Z, Leng X, Li Y (2017) Unique isodimorphism and isomorphism behaviors of even-odd poly(hexamethylene dicarboxylate) aliphatic copolyesters. Polymer (guildf) 115:106–117
Yu Y, Wei Z, Liu Y, Hua Z, Leng X, Li Y (2018) Effect of chain length of comonomeric diols on competition and miscibility of isodimorphism: a comparative study of poly(butylene glutarate-co-butylene azelate) and poly(octylene glutarate-co-octylene azelate). Eur Polym J 105:274–285
Zhao X, Bansode SR, Ribeiro A, Abreu AS, Oliveira C, Parpot P, Gogate PR (2016) Ultrasound enhances lipase-catalyzed synthesis of poly (ethylene glutarate). Ultrason Sonochem 31:506–511
Acknowledgements
The authors thank IFRJ, UERJ, Petrobras for funding, and LCPRB/IMA/UFRJ for GPC, TGA, and DSC analysis. The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
43153_2021_137_MOESM1_ESM.tif
Supplementary Figure 1: Gel permeation chromatogram of poly(butylene azelate) obtained at 30 °C using tetrahydrofuran as the eluent. Reaction conditions: molar ratio of 1,4-BDO/azelaic acid equal to 1, t = 96 h, T = 90 °C, 5 wt.% of Novozym 435 (TIF 126 KB)
Rights and permissions
About this article
Cite this article
Campisano, I.S.P., de Queiros Eugenio, E., de Oliveira Veloso, C. et al. Solvent-free lipase-catalyzed synthesis of linear and thermally stable polyesters obtained from diacids and diols. Braz. J. Chem. Eng. 38, 549–562 (2021). https://doi.org/10.1007/s43153-021-00137-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43153-021-00137-y