Abstract
This work compares the biodegradability of polyesters produced by an esterification reaction between glycerol and oleic di-acid (D 18:1) issued from green chemical pathways, via either classical thermo-chemical methods, or an enzymatic method using the immobilized lipase of Candida antartica B (Novozym 435). An elastomeric polymer synthesized by enzymatic catalysis is more biodegradable than an elastomeric thermo-chemical polyester synthesized by a standard chemical procedure. This difference lies in percentage of the dendritic motifs, in values of the degree of substitution, and certainly in cross-links inducing an hyper-branched structure less accessible to the lipolytic enzymes in a waste treatment plant. However, when the elastomeric polymer synthesized by enzymatic catalysis is processed at high temperature as required for certain industrial applications, it presents an identical rate of biodegradation than the chemical polyester. The advantages of the thermo-chemical methods are greater speed and lower cost. Enzymatic synthesis appears be suited to producing polyesters, devoid of metallic catalysts, which must be used without processing at high temperature to keep a high biodegradability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Production of synthetic polymers from petroleum compounds dates back to the beginning of the 20th century and played a major role in the economic development of industrialized countries. Since 1970, however, it has been recognized that these polymers are resistant to degradation by microorganisms, once used, create problems of pollution and disposal both in the natural medium and in waste treatment plants. When designing new materials, therefore, efforts have been made to provide for not only their texture, their mechanical resistance or their moistness, but also for their biodegradability [1], while ensuring that this does not occur during use.
Taking polyethylene as an example, there is practically no diffusion of water and oxygen in the polymer. Only the surface, with a reduced number of free chains, is open to attack by extracellular enzymatic reactions. Addition of pro-oxidant derivatives (Manganese and Cobalt salts) leads to release free radicals which, exposed to light, allow the formation of hydroperoxides and then lead to the cleavage of the polyethylene [2–4]. These conditions [5] reduce the molecular weight, but only 20 % of fragments with Mw < 1000 g/mol can be mineralized by microorganisms. These fragments, which are hydrocarbons, follow various known metabolic pathways for alkanes with terminal, di-terminal or sub-terminal oxidation [6]. Thus, such polymers are not biodegradable according to norm NE 13432 for compostability. The long-term effects of accumulation of oligo- and poly-olefins in soils are not yet known [7].
Other biodegradable polymers can be synthesized using various natural resources and processes [8]. Examples include agro-polymers produced from vegetal biomasses [9], polymers produced from microbial metabolism such as poly-hydroxyalkanoates [10] and polymers synthesized from monomers produced by bacterial fermentations, i.e. poly(lactic acid) [11, 12] which is the principal polyester based on renewable raw material commercialized at industrial scale [13].
There are two ways of catalysing the poly-condensation of poly-acids and poly-ols to obtain polyesters: using either organo-metallic compounds [14] or enzymes [12]. The chemical method is efficacious and rapid, allowing the synthesis of many important polymers. Yet the various organo-metallic catalysers [15, 16] in these reactions cannot be fully eliminated after synthesis with the ensuing risk that they will confer toxicity to the polymers, and accumulation in soils. Chemical synthesis of polyesters requires high temperatures, sometimes exceeding 200 °C. Such temperatures can lead to secondary reactions liable to modify the stoechiometry of the polymerization through, for example, dehydration of diols or degradation of glycerol into acroleine. However, polymerization using chemical catalysis allows for the production polymers on a large scale and in a short reaction time, with limited purification steps afterwards.
The enzymatic synthesis of biodegradable polymers from renewable resources has attracted great interest. Thus, lipases which are enzymes used in many biotechnological fields [17, 18] can hydrolyse polyesters or catalyse the inverse reaction, the esterification. Balance between hydrolyse and esterification reactions is controlled by the quantity of water in the reacting medium. For the esterification reactions, the quantity of water allowing the optimal activity corresponds to the water molecules hardly bound to protein structure which are necessary to maintain their enzymatic conformation.
Whatever the mode of polymer synthesis, it is necessary to distinguish degradation from biodegradation. In the first case, the polymer undergoes an irreversible alteration of its chemical structure, leading to loss of its properties and functions. These alterations can be caused by abiotic phenomena such as mechanical hindrances, light, heat and hydrolysis or oxidation reactions [19]. Biodegradation is a degradation catalysed by microorganisms which can divide polymers into monomers. These monomers are then either mineralized into H2O and CO2 with energy production, or transformed into biomass and secondary metabolites [20]. Contrary to transformation by abiotic phenomena, catalysis by microorganisms allows organic matter recycling.
Here, we aimed to determine the effect of the mode of polyester synthesis, namely chemical or enzymatic or even a combination of the two methods, on the chemical structure of these polymers, and thus on their biodegradability. In particular, we focused on the effect of the temperature during the thermic phase, used in certain processing, on the biodegradability of polyesters obtained from glycerol and oleic di-acid (D18-1). These two compounds are issued from a green chemical pathway: glycerol is issued from the biodiesel production [21] and the oleic di-acid results of the enzymatic oxidation of the alkyl extremity of the oleic acid by Candida tropicalis [22]. Kulshrestha et al. [23], Yang et al. [24] and Zhang et al. [25] already reported the use of glycerol and oleic di-acid as polymer precursor but only under the chemical aspect of the synthesized products. Our study was conducted, either by chemical techniques or by the lipase [26] of Candida antarctica B (Novozym 435) immobilized on a polyacrylic matrix. To measure the biodegradability of polyesters, the lipase of Rhizopus arrhizus was often used [14, 27], but in this work we chose the most common laboratory test based on standardized respirometric techniques resulting in oxygen consumption and CO2 release [28] when microorganisms present, for example, in activated mud or compost or soil, are placed in contact with a polymer.
Experimental
Synthesis of Polyesters
Monomers used: cis 9 octadecen dioic acid (oleic di-acid: D 18:1) was supplied by Cognis (France). It was first purified by solubilisation in dichloromethane to remove insoluble saturated by-products and then recrystallized in petroleum ether to remove soluble monoacids. Its purity was evaluated by GC at 97 %. Glycerol was supplied by Acros Organics (purity 99 %).
Five polymers were synthesized from D 18:1 and glycerol in an equimolecular mixture: (1) a thermal polymer obtained by heating the monomer mixture at 65 °C, the temperature used in enzymatic methods; (2) monomers mixed by rotary stirring and heated at 160 °C for 3 h and at 180 °C for 1 h; (3) a polyester synthesized by first heating at 160 °C under nitrogen flux for 8 h in a 100 mL reactor with blade stirring and then dissolving the mixture in dichloromethane, precipitated in cold methanol (−40 °C < t° < −30 °C) and recovering it; (4) an enzymatic polymer obtained with Novozym 435 (0.1 %) at 65 °C in a glass reactor 100 mm high for 6 days; (5) a polyester synthesized by heating at 160 °C for 4 h of a pre-polymer obtained by enzymatic catalyse such as for the polymer 4 but stopped at a viscous liquid physical state.
Polymers 1, 4 and 5 were stirred in a glass reactor with an ARZR1 (Heidolph) motor equipped with a rod supporting a mixing blade (4.5 cm diameter). All the reactor were immersed in an oil-bath.
Elementary Analysis
Carbon, hydrogen and oxygen atom percentages in polymers were determined with a Thermo Finnigan EA 1112 Elementary Analyser.
Measure of the MW and Mn Polymers
This measure was realized by steric exclusion chromatography with a PLgel 5 μm MiniMIX-D column (250 × 4.6 mm). This column was protected by a PLgel 5 μm MiniMIX-D Guard pre-column (50 × 4.6 mm). Both columns were provided by Polymer Lab. They were supplied continuously with a gas-free tetrahydrofurane (THF) via a Waters 515 pump with a 0.3 mL min−1 delivery. Prior to analysis, solutions were filtered through 0.45 μm Millex-HV filters from Polymer Lab. 20 μL at 3 g L−1 were injected at room temperature. This chromatographic chain was equipped both with a Waters 410 refractometer (thermostated at 35 °C) and a UV Waters 2487 detector. Data acquisition was realized with OmniSEC by Viscotek.
Nuclear Magnetic Resonance
The insoluble samples were analyzed by 13C High Resolution Magic Angle Spinning (HRMAS) technique on a Bruker Avance 400 MHz spectrometer operating at a 13C resonance frequency of 106 MHz and using a HRMAS Bruker double-bearing probe. About 3–4 mg of sample were swollen with 50 µL of CDCl3 in a 4 mm zirconium dioxide rotor, equipped with Teflon spacers, and spun at 4 kHz. The soluble sample was analyzed by 13C Liquid State Bruker Avance 300 MHz spectrometer operating at a 13C resonance frequency of 76 MHz and using a commercial Bruker BBI probe. About 10 mg of sample were solubilized in a mixture of CDCl3-d1 (77.1 ppm) and DMSO-d6 (39.7 ppm) in a 5 mm NMR tube. Attributions of carbons of the glycerol units were deduced from Rabiller and Maze [29] and Mazur et al. [30]. All experiments were performed at room temperature and the 13C chemical shifts were referenced to tetramethylsilane (TMS).
Respirometric Method
Measures of respiratory activities were realized in an Oxytop System WTW apparatus composed of a manometer and a 1 l hermetic flask. Biomasses used were from sludge sampled in the waste treatment plant of Brignoles (Var, France). Into the Oxytop flask were introduced the polymer (0.25 g), mixed with the biomass (2.5 g) and a flask containing 50 mL of 0.2 M NaOH. Oxytop flasks were incubated at 20 °C. Quantities of oxygen consumed were calculated from the lowering of pressure measured by the Oxytop manometers according to the perfect gas law. They were expressed as mg of O2 consumed per g of biomass.
Quantities of CO2 released were measured by titration with barium hydroxide of Na2CO3 formed by reaction of CO2 and NaOH. They were expressed as mg of CO2 released per g of biomass. Measures of CO2 were realized every 7 days when the Oxytop flasks refilled with air and NaOH.
The biodegradability of polyesters 2, 3, 4 and 5 was compared to the biodegradability of commercial polymers single-use cups formed either with a vegetal pulp (polymer 6) or with a poly(lactic acid) commonly called PLA (polymer 7). The cups were pulverized and the powder thus obtained was dispersed into the biomass in the Oxytop Flasks in the proportions cited above for polyesters.
For each polymer tested, an assay was realized without polymer to measure the respiratory activity of the biomass itself. All the assays were realized in triplicate.
Results and Discussion
Effect of Synthesis Methods on Polyester Structure
Glyceridic motifs present in polyesters synthesized by esterification of glycerol by D18-1 di-acid are presented in Fig. 1 and the respective 13C Nuclear Magnetic Resonance (NMR) spectrum is shown in Fig. 2 corresponding to the polymer 2. Glyceridic motifs were identified, as further indicated in the experimental part, and their proportions were calculated using integrals of signals of methine carbons. The differences observed in the chemical shifts (1–1.5 ppm), compared to the attributions of Kulshrestha et al. [23] were principally due to the different mass concentration and the real sample temperature (about 10–15 °C) due to sample Magic Angle Spinning at 4 kHz.
Extended zone of the 13C NMR spectrum corresponding to the principal glyceridic motifs in a polyester obtained from esterification of glycerol by oleic di-acid. Attributions (cf. Fig. 1): 62.3 ppm L 1,2 G; 63.3 ppm De B; 63.6 ppm L 1,2 F; 64.5 ppm T1 I; 66.1 ppm L 1,3 D; 66.3 ppm T 1 H; 69.1 ppm L 1,3 C; 70.1 ppm De A; 71.3 ppm T 1 J; 73.3 ppm L 1,2 E
Whether using chemical, enzymatic or mixed techniques for polyester synthesis, we observed that proportions of the principal different glyceridic motifs (Fig. 1) depended on the experimental conditions during the successive sequences of polymer synthesis (Table 1). In particular, experiment 1, conducted at 65 °C, shows that when this temperature is used for enzymatic catalysis, weak, but not negligible polymerization, is induced.
Table 1 shows that, for all the polymers, primary hydroxyls are more esterified than secondary hydroxyls. This high percentage is partly due to monoglyceride formation in sn1 or sn3 positions, the presence of monoglycerides in sn2 position (T2 motif) not being detected.
Migration of acyl groups from the sn2 position towards sn1 and sn3 positions, and a higher probability of forming 1- or 3- monoglyceride, explain the absence of 2-monoglyceride [31]. When a second esterification is realized on a 1- or 3-monoglyceride, the other external position is preferentially esterified. The highest proportion of esters in sn2 position is present in dendritic motifs (De), indicating that the decrease in the proportion of available primary hydroxyl induces esterification in sn2 position.
Experiments 2, 3 and 4 show differences between chemical and enzymatic catalysis in percentages of both the T1 and dendritic motifs and values of the degree of substitution, defined as the mean number of ester bindings by glycerol unit. Degree of substitution increases with temperature in Experiments 2 and 3 (2.1). The two polyesters 2 and 3 thus obtained have 32 and 26 % of the dendritic motifs respectively, which means that their hydroxyl functions sn2 are likely to be involved, in similar proportions, in the increase of the degree of substitution. Nevertheless, the difference in their physical state and their Mn corresponds to different proportions of the other glyceridic motifs. Enzymatic catalysis with Novozym 435 (polymer 4) induces percentages of L1,3 esters (36 %) identical to those observed in polymer 2 obtained by thermal esterification (36 %), but great difference concern the percentages of dendritic, L1,2 and T1 motifs.
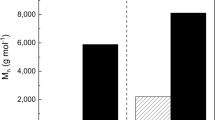
For polymer 5, which presents an elastomeric state, the pre-catalysis with Novozym 435, which leads to a viscous liquid (MW = 28,000 g/mol, Mn = 2830 g/mol), has an effect on the polymer structure. Its structure is closer to that of the polymer 4 than to that of the polymer 2. Thus, polymers 2 and 5 differ greatly, particularly in proportion of both T1 and dendritic motifs and in degree of substitution in spite they both undergo a thermal phase.
Biodegradability of the Polyesters
The degradation of these different polyesters by a biomass from urban sludge mud was measured at 20 °C by the oxygen consumed and the CO2 released, taking into account the endogen respiration of this biomass. It is important to note that all the polymers tested were treated according to the same experimental protocol, i.e. they were not shaken in Oxytop System. This test of biodegradability differs from the test using the lipase of R. arrhizus [14, 27]. An urban sludge contains a great number of microorganism species able to synthesise various types of lipases, thus increasing potentialities of ester-binding hydrolyses.
Two commercial polymers (polymers 6 and 7), considered as biodegradable under current legislation, were selected as references to test the methodology used here to study the biodegradability of the polyesters under consideration.
Consumption of O2 and release of CO2 by a biomass from sludge mud placed in contact with polymers 6 and 7 are given in Fig. 3; only polymer 6 is degraded in the Oxytop System condition, i.e. at 20 °C.
The high potential of microorganisms to synthesize cellulases and hemicellulases may explain this high biodegradation rate of the polymer 6 formed with a vegetal pulp. No degradation of polymer 7 formed with PLA was observed in the Oxytop System. To eliminate the hypothesis that the processing at high temperature [32] of the PLA cups could lead to a non-biodegradable structure, a racemic mixture of polylactic acid isomers (white powder) was subjected to the respiratory Oxytop System. Results are identical to those from experiments conducted with PLA. The crystallinity of the PLA is certainly [33] an important parameter which can be taken into account. Indeed, crystalline zones are less hydrolysable than amorphous zones. Moreover, enzymes degrade only the surface of the solid substrate, because they cannot penetrate the polymer systems [34]. As already demonstrated by Weir et al. [35, 36], the increasing of temperature above 50 °C, temperature reached in industrial compost plants [37, 38], modifies the crystalline zones turning them into an amorphous structure, more accessible and thus more biodegradable, so meeting the specifications of three international standards: ASTM D5338, ISO1855 and NF14352. However, in a real soil environment and home composting, the temperature usually does not exceed 30 °C. These results corroborate those of Rudnik and Briassoulis [39] who demonstrated by respirometric methods that PLA materials are not degraded at 30 °C. Consequently, the respiratory Oxytop System allows to differentiate the biodegradability, measured at 20 °C, of polymers studied in this work.
Figure 4 shows results of Experiments 2, 3, 4 and 5, indicating that polymers obtained from an equimolecular mixture of glycerol and D 18:1 are mineralized whatever the synthesis mode. Yet the polymers 3 and 4 present a biodegradability higher than the polymers 2 and 5. Thus, 52 mg and 45 mg of CO2 were respectively released for 35 days from polymers 3 and 4 by 1 g of biomass from sludge mud. In the same conditions, only 10 mg of CO2 were released from the polymers 2 and 5. For the polymer 4, it is important to eliminate the hypothesis that the residual Novozym may act in hydrolysis in the degradation system, thus releasing glycerol and D 18:1. There is need to point out, first, that no free glycerol was detected in polymer 4 at the end of its synthesis (results not shown). Second, when polymer 4 was mixed with urban sludge mud, Novozym 435 was at a maximum concentration of 0.01 versus 0.1 % for its synthesis phase, and the temperature was at 20 °C, whereas the maximum activity of this enzyme occurs between 65 and 80 °C [40].
These results seem indicate that the difficulties for microbial lipases to reach and thus to hydrolyse the ester bindings are not only dependent of the dendritic structure of the polymer. Probably, the thermic treatment subjected by the polymers 2 and 5 results in cross-links and thus in a higher branched structure than in the enzymatic polymer. The higher degree of branching between linear chains, as defined by Hölter et al. [41], the greater the likelihood that a hyper-branched polyester will have a typical globular dendrimer structure.
The thermic treatment subjected by the polymer 3, limited at a viscous liquid state, was insufficient to create such structures. Its weak Mw and its viscous state certainly allowed a better accessibility of the esters bindings to the microbial lipases as it was also observed by Umare et al. [42] with 1,3-propanediol based polyesters.
Polymers 4 and 5, despite having fairly similar structures and an elastomeric physical state, are not biodegraded at the same rate. It seems that the enzymatic pre-polymerization interferes with the thermal phase by reducing the proportion of dendritic motifs in polymer 5 as compared to polymer 4, the processing at 160 °C for 4 h then leading to cross-links which confer to the polymer 5 the same biodegradability than the polymer 2.
Based both on the percentage of carbon in each polymer structure and on the rate of CO2 production (Figs. 3, 4), it is possible to estimate the approximate time required for 1 g of biomass sampled in a waste treatment plant to transform 100 mg of polyester into CO2. In the Oxytop System, all the organic carbon can be considered to be transformed into CO2, organic carbon transformation into biomass probably being negligible. Indeed, the lack of no renewable elements such as nitrogen and phosphorus induces an energy decoupling. An extrapolation can be made from this result to a waste treatment plant, where there is permanent nitrogen and phosphorus supplies, taking into account that about 50 % of carbon (C) could be transformed into biomass [43, 44]. In this case, the time required to transform the organic carbon into CO2 and biomass in the Oxytop System, must be divided by approximately 2.
Table 2 shows the approximate time required for mineralization by 1 g of biomass of 100 mg of polymers 2, 3, 4, 5 and 6.
The findings of this study indicate that the mode of synthesis of a polymer should be chosen according both to its intended use and to the properties required. Enzymatic synthesis leads to polyesters which are more biodegradable than those obtained by chemical means, while providing an identical elastomeric state (polymers 2 and 4). A weak molecular mass and a viscous state facilitate biodegradation. A thermal phase induces a higher proportion of dendritic motifs, except after a pre-polymerization phase (polymer 5). The environmental conditions to which the polyesters are subjected after use (compost, urban sewage sludge, soil) also affect the action of microorganisms (polymer 7).
Conclusion
This work shows that the synthesis method is a parameter allowing or not the biodegradability of polyesters in an elastomeric state. Indeed, such polymers synthesized by enzymatic methods are thus more biodegradable than those synthesized by chemical methods. However, this difference disappears under processing at high temperature often required in industrial applications of a biodegradable polymer, which could drastically decrease its biodegradability.
The choice of the synthesis method depends on the utilisation envisaged for a polyester. Chemical synthesis is rapid and can be applied to a large mass of monomers, or when polymers need to have a slow biodegradation kinetic. Enzymatic synthesis is more expensive, suitable for specific products with high added value which do not require processing at high temperature. Enzymatic synthesis can also be used to obtain polymers intended to disappear via biodegradation after use.
References
Nair LS, Laurencin CT (2007) Prog Polym Sci 32:762–798
Weyland M, Daro A, David C (1995) Polym Degrad Stab 48:275–289
Jakubowicz I (2003) Polym Degrad Stab 80:39–43
Koutny M, Sancelme M, Dabin C, Pichon N, Delort A-M, Lemaire J (2006) Polym Degrad Stab 91:1496–1503
Kawai F, Watanabe M, Shibata M, Yokoyama S, Sudate Y, Hayashi S (2004) Polym Degrad Stab 86:105–114
Muller R (2003) Synthèse du projet européen SMT sur la biodégradabilité des matériaux
Bewa H (2005) Biodégradabilité et matériaux polymers biodegradables. Note de synthèse ADEME. http://www2.ademe.fr/servlet/getBin?name=8E9C123D5A10CF6479D7A6AABEEE91641140709716872.pdf
Avérous L (2004) J Macromol Sci 44:231–274
Gandini A (2011) Green Chem 13:1061–1083
Suriyamongkol P, Weselake R, Narine S, Moloney M, Shah S (2007) Biotechnol Adv 25:148–175
Lunt J (1998) Polym Degrad Stab 59:145–152
Edlund U, Albertson A-C (2003) Adv Drug Deliv Rev 55:585–609
Wolf O, Crank M, Patel M, Marscheider-Weidermann F, Schleich J, Husing B, Angerer G (2005) Techno-economic feasibility of large-scale production of bio-based polymers in Europe. Polylactic acid (PLA). European Science and Technology Observatory, EUR 22103 EN, pp 50-64
Roumanet P-J, Laflèche F, Jarroux N, Raoul Y, Claude S, Guégan Ph (2013) Eur Polym J 49:813–822
Fradet A, Maréchal E (1982) Adv Polym Sci 43:51–142
Fradet A, Tessier M (2003) Polyesters. In: Rogers ME, Long TE (eds) Synthetic methods in step-growth polymers. Wiley, Hoboken, pp 17–132
Jaeger KE, Eggert T (2002) Curr Opin Biotechnol 13:390–397
Hasan F, Shah AA, Hameed A (2006) Enzyme Microb Technol 39:235–251
Lucas N, Bienaime C, Belloy C (2008) Chemosphere 73:429–442
Shah AA, Hasan F, Hameed A, Ahmed S (2008) Biotechnol Adv 26:246–265
Da Silva G, Mack M, Contiero J (2009) Biotechnol Adv 27:30–39
Yang Y, Lu W, Zhang X, Xie W, Cai M, Gross R (2010) Biomacromolecules 11:259–268
Kulshrestha AS, Gao W, Gross R (2005) Macromolecules 38:3193–3204
Yang Y, Lu W, Cai J, Hou Y, Ouyang S, Xie W, Gross AA (2011) Macromolecules 44:1977–1985
Zhang Y-R, Spinella S, Xie W, Cai J, Yang Y, Wang Y-Z, Gross R (2013) Eur Polym J 49:793–803
Christensen MW, Andersen L, Husum TL, Kirk O (2003) Euro J Lipid Sci Technol 105:318–321
Montaudo G, Rizzarelli P (2000) Polym Degrad Stab 70:305–314
Massardier-Nageotte V, Pestre C, Cruard-Pradet T, Bayard R (2006) Polym Degrad Stab 91:620–662
Rabiller C, Maze F (1989) Magn Reson Chem 27:582–584
Mazur AW, Hiler GD, Lee SSC, Armstrong MP, Wendel JD (1991) Chem Phys Lipids 60:189–199
Spyros A, Phillipidis A, Photis P (2004) J Agric Food Chem 52:157–164
Lim L-T, Auras R, Rubino M (2008) Prog Polym Sci 33:820–852
Gleadall A, Pan J, Atkinson H (2012) Polym Degrad Stab 97:1616–1620
Mochizuki M, Hirami M (1997) Polym Adv Technol 8:203–209
Weir N, Buchanan F, Orr J, Dickson G (2004) Proc Inst Mech Eng H 218:307–319
Weir N, Buchanan F, Orr J, Farrar D, Dickson G (2004) Proc Inst Mech Eng H 218:321–330
Sawada H (1998) Polym Degrad Stab 59:365–370
Ikada Y, Tsuji H (2000) Macromol Rapid Commun 21:117–132
Rudnik E, Brassioulis D (2011) Ind Crops Prod 33:648–658
Widjaja A, Yeh T-H, Ju Y-H (2008) J Chin Inst Chem Eng 39:413–418
Hölter D, Burgath A, Frey H (1997) Acta Polym 48:30–35
Umare SS, Chandure AS, Pandey RA (2009) Polym Degrad Stab 92:464–479
Gottschalk G (1979) Bacterial metabolism. Springer, New York, pp 34–78
Stanier RY, Ingraham JL, Wheelis ML, Painter PR (1986) The microbial world, vol 07632, 5th edn. Prentice-Hall, Englewood Cliffs, pp 183–195
Acknowledgments
This work was supported by ONIDOL (France). We would like to thank Marjorie Sweetko for English language revision and Virgile Calvert for his technical help.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Goujard, L., Roumanet, PJ., Barea, B. et al. Evaluation of the Effect of Chemical or Enzymatic Synthesis Methods on Biodegradability of Polyesters. J Polym Environ 24, 64–71 (2016). https://doi.org/10.1007/s10924-015-0742-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-015-0742-7