Abstract
Cells of Aspergillus oryzae IPT-301 rich in fructosyltransferase (FTase) were successfully immobilized by crosslinking with glutaraldehyde and used for the transfructosylation reaction of sucrose. The glutaraldehyde concentration and pH used in the immobilization process were optimized for maximizing the transfructosylation activity (AT) and minimizing the hydrolytic activity (AH). Also, the operational stability and the influence of temperature, pH and sucrose concentration on the enzymatic activities of the free and crosslinked cells were evaluated. Both the maximum AT and minimum AH were obtained for cells immobilized with glutaraldehyde concentration of 2.1% (v/v) and pH 7.9. Crosslinked cells showed considerably higher AT/AH ratio than free cells at several temperatures, pH and sucrose concentrations in the reaction media. Kinetics data suggested that crosslinked cells present higher substrate-enzyme affinity and transfructosylation rate than free cells. Furthermore, after 12 batch reaction cycles the FTase present in the immobilized cell kept 88.9% of its initial AT, demonstrating a considerably higher operational stability than the FTase present in the free cell, which showed 50.3% of its initial AT. These results suggest the potential use of crosslinked cells of Aspergillus oryzae IPT-301 for the large-scale production of fructooligosaccharides (FOS).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fructosyltransferases (FTase, E.C. 2.4.1.9) are enzymes which catalyze transfructosylation reactions in sucrose molecules for the production of fructooligosaccharides (FOS) (Antosová and Pokovic 2001; Antosová et al. 2008; Muniz-Márques et al. 2016). They are industrially produced by fermentation from fungi belonging to the genera Aspergillus, Penicillum, Aureobasidium, Fusarium and Rhodotorula, being excreted to the culture medium (extracellular FTase) and/or remaining adhered to the microbial cells (mycelial FTase) (Wang 2015; L’Hocine et al. 2000; Ghazi et al. 2005; Aguiar-Oliveira and Maugeri 2010). FOS are fructose oligomers, whose fructosyl (F) units are bound to the terminal sucrose molecule (GF) by β-(2 → 1) glycosidic bonds, in which their main constituents are the sugars 1-kestose (GF2), nystose (GF3) and 1-β-fructofuranosyl nystose (GF4) (Yun 1996). The consumption of FOS, with low degree of polymerization, presents benefits to the human health for being low-calorie prebiotic sugars, non-cariogenic, for increasing the absorption of calcium and magnesium by the human organism, for reducing the levels of total cholesterol in the blood and for promoting the selectivity of bifidobacteria in the gut microbiota, helping in the elimination of pathogenic microorganisms and in the prevention of colon cancer (Vanková et al. 2008; Nobre et al. 2018).
One of the main parameters of evaluation associated to the enzymatic production of FOS refers to the ratio between the transfructosylation and hydrolytic activities (AT/AH) on the sucrose molecules (Hidaka et al. 1988). Route of FOS production consists in immobilizing extracellular FTase, present in the fermented broth or extracted from the microbial cells, in supports such as polymethacrylate (Ghazi et al. 2005), Amberlite IRA 900® (Platková et al. 2006) and a mixture of gelatin, sodium alginate and calcium chloride (Kamimura et al. 2009). Enzyme immobilization allows its separation from the reaction medium and the increase in its operational stability. The transfructosylation reaction can also be performed using microbial cells (Nobre et al. 2018; Sheu et al. 2013). Studies performed by Cuervo-Fernandez et al. (2007) showed that the mycelial FTase present in the microbial cells of Aspergillus oryzae IPT-301 presented a higher transfructosylation activity and, therefore, a higher potential for FOS production, among seventeen filamentous fungal strains investigated, with a ratio between activities (AT/AH) equal to 10.86. Ottoni et al. (2012) reported an FTase production with maximum transfructosylation activity of 950 U g−1, respectively, employing the same microorganism, cultivated under optimized concentrations of sucrose, urea and yeast extract. Perna et al. (2018), cultivating Aspergillus oryzae IPT-301 by submerged fermentation, obtained a maximum ratio between activities (AT/AH) equal to 17, operating a 10 L bioreactor with a synthetic culture medium at 50 °C and pH 5.0. The immobilization of microbial cells is an alternative which enables the increase in operational stability (López-Gallego et al. 2005) and, furthermore, can be more advantageous than the extracellular enzymes immobilization, since it is not necessary to extract the enzyme from the microorganism and the natural structure of the biomass itself, in which the enzyme is adhered, can be used as support (Canilha et al. 2006). Chien et al. (2001) immobilized mycelial FTase from Aspergillus japonicus by encapsulation in gluten, obtaining a stable biocatalyst during 24 h of reaction. Ganaie et al. (2014) immobilized the mycelial FTase from Aspergillus flavus NFCCI 2364 by encapsulation in chitosan and alginate. The immobilization with alginate produced the highest transfructosylation activity (45 U mL−1), and allowed the FTase reuse for 15 consecutive cycles, however, with a drop of 40% in FOS yield between the first and the last cycle. Similarly, the immobilization of Aspergillus aculeatus, in alginate, showed a FOS yield of 65.47% w/w, and it was reused during 15 cycles without significant drop in the enzymatic activity (Huang et al. 2016). Mussatto et al. (2009) and Castro et al. (2017) reported the immobilization of cells with mycelial FTase from Aspergillus japonicus ATCC 20,236 and Aureobasidium pullulans, in polyurethane foam, plant fibers, molecular sieves and glass foams. The highest FOS yields were reported for the cells immobilized in high porosity reticulated polyurethane foam.
Despite the increase in operational stability resulting from microbial cells immobilization, the use of external supports might impose diffusional restrictions to the reagents and products, limiting the enzymatic activity, or causing its total deactivation (Krasnan et al. 2016; Shuler and Kargi 2002). One of the techniques proposed to circumvent this problem is cells immobilization by reticulation, in which the cell structure itself is used as support (without the addition of an external support) by the addition of a chemical agent, which promotes cell crosslinking (Migneault et al. 2004a). The reticulation with glutaraldehyde of Aspergillus flavus with lipase adhered to the mycelium enabled an increase in operational stability, allowing biomass reuse for 13 consecutive cycles (Long et al. 1996). Sun et al. (2010) used whole Rhizopus oryzae cells reticulated with glutaraldehyde in the methanolysis reaction for biodiesel production from renewable oils. The reticulation promoted an increase in the operational stability of the cells from 10 to 15 cycles without significant drop in activity, which was attributed to the reduction in lipase desorption.
An increase in FOS production from mycelial FTase depends on an elevated transfructosylation activity, a high AT/AH and enough operational stability to enable its reuse in several cycles and, also, its application in continuous processes. Hence, cells rich in mycelial FTase must be immobilized so that the diffusion limitations and the loss of enzymatic activity are minimized. Few are the works in the literature on cell reticulation to obtain bioproducts and there are no studies on the immobilization by reticulation of cells of Aspergillus oryzae IPT-301 for FOS production. Therefore, the purpose of this work was to study the influence of the immobilization variables (glutaraldehyde concentration and pH) for glutaraldehyde-crosslinked cells from Aspergillus oryzae IPT-301, aiming at maximizing the transfructosylation activity, and minimizing the hydrolytic activity. Also, the operational stability and the influence of the process parameters (temperature, pH and concentration of sucrose of the reaction medium) on enzymatic activities of free and crosslinked cells were evaluated.
Materials and methods
Materials
All chemical reagents used were of analytical grade. Yeast extract, sucrose, KH2PO4, MnCl2.4H2O and FeSO4·7H2O were acquired from Labsynth® (Diadema, Brazil). Glycerin and phenol were obtained from Isofar® (Duque de Caxias, Brazil). Glucose, NaBH4, NaNO3, MgSO4·7H2O, NaOH, Na2S2O5, C7H4N2O7 and KNaC4H4O6.4H2O were purchased from Dinamica® (Diadema, Brazil). Potato dextrose agar was obtained from Kasvi® (São José dos Pinhais, Brazil) and the solution of glutaraldehyde Grade I (25% in water) was acquired from Sigma-Aldrich® (São Paulo, Brazil). The enzymatic colorimetric kit GOD-PAP for glucose determination was purchased from Laborlab® (Campinas, Brazil).
Microorganism and culture conditions
The fungus Aspergillus oryzae IPT-301was provided by the Institute for Technological Research (IPT/SP). The strain was cultivated in dishes with solid medium composed of (in %, m/v): potato dextrose agar 2.0, glycerin 2.5, yeast extract 0.5 and glucose 2.5 at 30 °C for 7 days. The inoculum was prepared by the suspension of spores in a 0.95% NaCl solution (m/v) and 0.1% Tween-80 (v/v) and dilution in glycerin solution at 20% (m/v) to obtain the concentration of 1 × 107 spores.mL−1.
The experiments were performed in Erlenmeyer flasks containing 50 mL of culture medium with the following composition (in % m/v): sucrose 15.0, yeast extract 0.5, NaNO3 0.5, KH2PO4 0.2, MgSO4·7H2O 0.05, MnCl2·4H2O 0.03 and FeSO4 7H2O 0.001. The pH of the medium was adjusted to 5.5 before sterilization. The flasks were inoculated with 0.5 mL of the spore suspension and incubated in an orbital shaker at 30 °C and 200 rpm for 64 h. At the end of the experiment, the samples were collected and filtered in filter paper (Whatman nº1) and the microbial cells (mycelium) was stored at 4 °C for further immobilization assays.
Assays of microbial cells crosslinking
Experimental design and statistical analysis
The experimental design of the type rotational central composite design 22 was chosen for the study of two factors: glutaraldehyde concentration (GLU) and pH of the reaction medium in microbial cells crosslinking assays, each one in five levels (Table 1). The values for the factors were chosen after a series of preliminary assays. The factors effects on the transfructosylation activity (AT), on the hydrolytic activity (AH) and on the ratio between the activities (AT/AH) were investigated for the crosslinked cells. The experimental matrix and the statistical analysis (Analysis of Variance—ANOVA) were obtained employing the software Statistica® version 7.0 (StatSoft. Inc. 2007, USA).
The response surface model was adjusted for two response variables, Y, named transfructosylation activity (U g−1) and hydrolytic activity (U g−1) of the crosslinked cells containing mycelial FTase. The enzymatic activity assays were performed as described in Sect. 2.4.1. The second-order response functions adjusted for the two factors were given by Eq. (1) and the differences were considered significant for p values ≤ 0.05.
in which A and B represent the levels of the factors GLU (%, v/v) and pH of the reaction medium, respectively, while β0, β1, β2, β12, β11 and β22 represent the estimated coefficients.
Assays of cells immobilization by reticulation
The microbial cells (mycelium) immobilization assays were performed according to the adaptation of the methodology reported by Sun et al. (2010) and Vescovi et al. (2016). Immobilization was done in Erlenmeyer flasks containing 0.1 g of cells in 10 mL of reaction medium composed of a 25% glutaraldehyde solution (v/v) and a tris–acetate buffer solution at 0.2 mol L−1. The pH of the buffer solution and the glutaraldehyde concentration were defined by the experimental design (Sect. 2.3.1). The immobilization reaction was performed in an orbital shaker at 25 °C and 200 rpm for 1 h and terminated, for further 30 min, by the addition of 0.1 mL of NaBH4 100 g L−1 previously dissolved in a NaOH solution at 1 × 10–3 mol L−1. At the end of the reaction, the samples were collected and filtered in filter paper (Whatman no. 1). The immobilized mycelium (crosslinked cells) was abundantly washed with distilled water and stored at 4 °C for further characterization studies of the process parameters (temperature, pH and concentration of sucrose from the reaction medium) and operational stability assays.
Analytical methods
Enzymatic activity assays
The free and crosslinked cells transfructosylation (AT) and hydrolytic (AH) activities were determined as follows: 0.05 g of mycelium (free or crosslinked cells) were added to 3.7 mL of sucrose at 480.2 g L−1 and 1.2 mL of a tris–acetate buffer at 0.2 mol L−1 pH 5.5. The reaction was performed in a Dubnoff bath at 50 °C and 190 rpm for 1 h and interrupted by boiling (boiling water) for 10 min and ice bath for 5 min (Cuervo-Fernandez et al. 2007; Ottoni et al. 2012; Cunha et al. 2019). The reaction medium was filtered in filter paper (Whatman no. 1) and the concentrations of reducing sugars and glucose were quantified by DNS and GOD-PAP® methods (Sect. 2.4.2), respectively. One unit of transfructosylation and hydrolytic activity was defined as the amount of enzyme which transfers 1 µmol of fructose (transfructosylated fructose) or releases 1 µmol of fructose, respectively, per minute at the experimental conditions assayed (Cuervo-Fernandez et al. 2007; Ottoni et al. 2012; Ganaie et al. 2014; Cunha et al. 2019).
Analysis of carbohydrates
The glucose [G] and reducing sugars [RS] concentrations were obtained by the enzymatic colorimetric GOD-PAP® (glucose oxidase peroxidase 4-aminoantipyrine) method (Vega and Zúniga-Hansen 2011; Ganaie et al. 2014; Cunha et al. 2019) and DNS (3,5-dinitrosalicylic acid) (Miller 1959), respectively. The fructose [F] and transfructosylated fructose [FT] concentrations in the reaction medium were determined by Eqs. (2) and (3) (Chen and Liu 1996; Cunha et al. 2019).
Temperature-activity and pH-activity profiles
Enzymatic activities of free and crosslinked cells were measured in the temperature range of 30 °C–65 °C (pH 5.5) and at pH 3.5–7.5 (50 °C), using sucrose solution (480.2 g L−1) as substrate. Transfructosylation (AT) and hydrolytic (AH) activities were determined under conditions as described in Sect. 2.4.1. The experiments were conducted in triplicate.
Effects of sucrose concentration in the reaction media and determination of kinetic parameters
Enzymatic activities of free and crosslinked cells were determined using 3.7 mL of sucrose p.a. solution at different concentration (75.5, 151.0, 264.3, 377.5, 480.2 and 604.1 g L−1) plus 1.2 mL tris acetate buffer 0.2 mol L−1 at pH 5.5. Transfructosylation (AT) and hydrolytic (AH) activities were determined under conditions as described in Sect. 2.4.1. The experiments were conducted in triplicate. For enzymatic kinetics evaluation, the sets of experimental data obtained for the AT activities were fitted to the Michaelis–Menten model and the Hill model. Kinetic parameters such as maximum reaction rate (Vmax) and Michaelis constant (Km), and Hill constant (n) were obtained using a nonlinear regression analysis.
Operational stability assays of the free and crosslinked cells
Assays of operational stability of the free and crosslinked cells at the optimal conditions were performed. For this, 0.05 g of cells, free or cross-linked, containing mycelial FTase, was added to the reaction medium containing 3.7 mL of sucrose 480.2 g L−1 and 1.2 mL of tris–acetate buffer at 0.2 mol L−1 pH 5.5. The enzymatic reaction assays were conducted according to the method described in Sect. 2.4.1. At the end of each cycle, corresponding to 1 h of reaction, the free or crosslinked cells was removed from the reaction medium by vacuum filtration, washed with 100 mL of distilled water for the reagents molecules removal and/or products of its microenvironment. After washing, the microbial cell was introduced again in a new and similar reaction medium. The transfructosylation activity was evaluated considering the number of reaction cycles. The experiments were performed in triplicate.
Results and discussion
Optimization of the variables for crosslinked cells by experimental planning
In Table 2, the transfructosylation (AT) and hydrolytic (AH) activities values of crosslinked cells are presented in relation to the immobilization variables (glutaraldehyde concentration and pH), according to the experimental design described in Sect. 2.3.1. The values of AT varied between 357.38 and 844.08 U g−1, whereas the values of AH varied between 113.26 and 282.38 U g−1. On the other hand, the values of AT and AH, obtained in the central point, presented low variation, which indicates a good reproducibility of the reticulation process.
As described in Table 3, for the transfructosylation activity (AT) the linear and quadratic terms of the glutaraldehyde concentration effects and the quadratic effect of the pH were significant, for a level of significance of 5% (p < 0.05). These variables were used to describe the quadratic model (Eq. 4). The pH linear and the interaction effects between the two variables were not significant.
in which AT, [GLU] and pH are the crosslinked cells transfructosylation activity, glutaraldehyde concentration and the pH employed in the immobilization, respectively.
Table 4 presents the analysis of variance (ANOVA) for the transfructosylation activity (AT) quadratic model of the crosslinked cells (Eq. 4). The coefficient of error determination (R2) obtained indicates that 95.84% of the variability of the responses observed can be explained by this model. By the F Test, it was verified that the model explains a significant amount of variation of the experimental data, since, for the level of significance of 5%, the calculated F value was higher than the tabulated F value of a reference frequency distribution (F; degrees of freedom of the model; degrees of freedom of the deviation; level of significance) (Rodrigues and Iemma 2009). The high R2 and the F test value indicate that the amount of variation due to the model is higher than the unexplained variation and, therefore, the model can be considered valid.
According to the response surface (Fig. 1a) and the contour curve (Fig. 1b), obtained from the AT model (Eq. 4), there is an optimum region of values of glutaraldehyde concentration, between 1.65 and 2.3% (v/v), and pH, from 7.5 to 8.3, which enable the AT maximization of the crosslinked cells.
For the AH of the crosslinked cells, only the linear and quadratic effects terms of the glutaraldehyde concentration were significant, for a level of significance of 5% (p < 0.05) (Table 5) and, therefore, they were used in the interaction model (Eq. 5). The effects of the immobilization pH and the interaction between the two variables were not significant:
in which AH and [GLU] are the crosslinked cells hydrolytic activity and the glutaraldehyde concentration used in mycelium immobilization, respectively.
Table 6 presents the analysis of variance (ANOVA) for the quadratic model with interaction applied to the hydrolytic activity of the crosslinked cells (Eq. 5). The coefficient of error determination (R2) indicates that 88.14% of the variability of the responses observed can be explained by this model. The value of the calculated F (33.44) for the model was higher than the value of the tabulated F (4.26) at a level of significance of 5%. The high value of the R2 and the F test indicate that the model is valid to represent the experimental data of the hydrolytic activity of crosslinked cells.
The response surface (Fig. 2a) and the contour curve (Fig. 2b), obtained from the model described by Eq. 5, indicate that there is an optimal region of glutaraldehyde concentration, between 2.0 and 2.5% (v/v), for which the cells hydrolytic activity reaches its lowest values, regardless of the immobilization pH. It is worth highlighting that, for this same region of glutaraldehyde concentration, the transfructosylation activity reached its highest values (Fig. 1), which represents a simultaneous gain in the ratio AT/AH.
Correlating the transfructosylation (AT) and hydrolytic (AH) activities values of the crosslinked cells in relation to the immobilization variables (Table 2), it was observed that the highest values of the ratio AT/AH were obtained for the glutaraldehyde concentration of 2.1% (v/v) and pH 7.9, conditions in which the transfructosylation and hydrolytic activities were maximized (Fig. 1) and minimized (Fig. 2), respectively. The high values of the ratio AT/AH obtained suggest a rise in the affinity between the mycelial FTase (enzyme adhered to the microbial cells) and the sucrose molecules (acceptor substrate), caused by possible alterations of the enzyme structure after crosslinked cells with glutaraldehyde. As reported by Antosová and Polakovic (2001), Perna et al. (2018), Cunha et al. (2019) and Huang et al. (2016), FTase presents, concomitantly, transfructosylation and hydrolytic activities. The low affinity of FTase by the water molecules present in the reaction medium, which reduces the hydrolytic activity (Antosová and Polakovic 2001), and the transfructosylation activity predominance for a sucrose concentration higher than 200 g L−1 favor high AT/AH ratios (Kim et al. 2000). The results obtained suggest that the reticulation of the microbial cell wall with glutaraldehyde can affect its hydrophilicity, limiting its interaction with water and, therefore, reducing the hydrolytic activity because of the lack of water molecules in the cellular microenvironment. On the other hand, the gain in the transfructosylation activity might be associated to the rise in the sucrose concentration molecules on the fungal cell wall, favored by the lower disaccharide polarity when compared to the water molecule. Therefore, an increase in the ratio AT/AH is observed and, consequently, a favorable situation for FOS production with higher yields (Aguiar-Oliveira and Maugeri 2010; Hidaka et al. 1988; Maresma et al. 2010).
It must be emphasized that it was not possible to directly analyze the behavior of the ratio (AT/AH) by experimental design, since, when analyzing the values obtained for the response ratio (AT/AH), by means of the Shapiro–Wilk adherence test regarding population normality (Rodrigues and Iemma 2009), it is observed that the results did not constitute a normal distribution (Gauss distribution). Since the experimental design is based on parametric statistics and, therefore, is strongly dependent on the normality of the data analyzed, results from an analysis performed by a rotational central composite design would not be trustworthy for the response ratio AT/AH.
Effect of reaction temperature on the enzymatic activities of the free and crosslinked cells
Figure 3a, b show the influence of reaction temperature on the enzymatic activities of the free and crosslinked cells. The free and immobilized cells showed the highest AH at 65 °C and showed a rise of the AT and AH at higher temperatures, which can be attributed to the rise of collisions between substrate molecules and active sites (Fields 2001; Shuler and Kargi 2002). Also, these results are in agree with the optimum temperature ranges reported for the transfructosylation reaction of sucrose (Almeida et al. 2005; Ganaie et al. 2014; Schuurmann et al. 2014). Free cells from Aspergillus niger ATCC 20,611, Aspergillus sp. and Xanthophyllomyces dendrorhous also showed the highest AT at 60 °C (Hirayama et al. 1989; Cuervo-Fernandez et al. 2004; Ning et al. 2010). In the present work, the free and crosslinked cells showed the highest AT at 65 °C and 60 °C, respectively (Fig. 3a, b). Also, the AT obtained from crosslinked cells at 60 °C was higher than the AT showed by free cells at the same temperature. The opposite effect of reaction temperature on the AT was reported by Ganaie et al. (2014) for cells from Aspergillus flavus NFCCI 2364 encapsulated in sodium alginate. In their work the free cells showed the highest AT at 50 °C while immobilized cells showed the highest AT at 60 °C. In the present work the highest AT/AH ratio of the free cell (9.44 ± 0.82) was obtained at 50 °C (Fig. 3a), in accordance with Cuervo-Fernandez et al. (2007). The crosslinked cells of Aspergillus oryzae IPT-301 showed a considerable increase in the highest AT/AH ratio (14.38 ± 1.19) at the same temperature (50 °C), suggesting that the cell immobilization by crosslinking with glutaraldehyde is favorable for FOS production.
Effect of reaction pH on the enzymatic activities of the free and crosslinked cells
Figure 4a, b show the influence of the reaction pH on the enzymatic activities of the free and crosslinked cells. The free and crosslinked cells showed the highest AT (803 ± 34 U g−1 and 1492 ± 111 U g−1, respectively) at pH 5.5. However, the crosslinked cells showed higher AT than the free cells at all the reaction pHs, suggesting that the crosslinking process made the enzyme more resistant to variations of ionic forces in the reaction medium (Long et al. 1996; Shuler and Kargi 2002). Similarly, Ganaie et al. (2014) reported changes in the effect of the reaction pH after immobilization of cells from Aspergillus flavus NFCCI 2364 by encapsulation in sodium alginate. In that study the free cells showed the highest AT at pHs between 5.0 and 7.0, and maximum AT (40 U mL−1) at pH 6.0, while the immobilized cells showed the highest AT at pHs between 5.0 and 8.0 with maximum AT (45 U mL−1) at pH 7.0. Ning et al. (2010) also reported the highest AT (15 U g−1) at pH 7.0 for enzyme present in cells from Xanthophyllomyce dendrorhous. In the present work the free and crosslinked cells showed the highest AH (707 ± 102 U g−1 and 1174 ± 257 U g−1, respectively) at pH 3.5 (Fig. 4a, b), in agree with the optimum pH reported for acid hydrolysis of sucrose (Aguiar-Oliveira and Maugeri 2010). The free cells showed the highest AT/AH ratios at pHs between 5.5 and 7.5, and the highest AT/AH ratio (7.49 ± 0.36) at pH 5.5. The crosslinked cells showed the highest AT/AH ratios at pHs between 5.5 and 7.0, and its highest value (19.97 ± 3.74) at pH 6.5. Also, at pH 5.5 the AT/AH ratio of the crosslinked cells was considerable higher (19.33 ± 2.68) than that showed by the free cells (Fig. 4a), suggesting that the immobilization of cells of Aspergillus oryzae IPT-301 by crosslinking changed the influence of the reaction pH on the AT and AH, leading to the increase of the AT/AH, which is favorable for FOS production.
Kinetic parameters of the transfructosylation reaction of sucrose on free and crosslinked cells
Figure 5a, b show the effect of the sucrose concentration on the enzymatic activities of the free and crosslinked cells, respectively. The highest AT of the free and crosslinked cells were obtained at sucrose concentration of 377.5 g L−1. However, the crosslinked cells showed higher AT than the free cells at all the sucrose concentrations. The crosslinked cells showed a reduction of the AH as the sucrose concentration increased, while the free cells showed the opposite behavior. Similarly, Zeng Kang et al. (2016) reported a reduction of the AH as the sucrose concentration increased. The crosslinked cells showed the highest AT/AH ratio (15.1 ± 3.3) at 480.2 g L−1.
The kinetics of the transfructosylation reaction of sucrose on free and crosslinked cells of Aspergillus oryzae IPT-301were fit to the Hill and Michaelis–Menten models (Fig. 6a, b). The kinetic of the free cells showed an error determination coefficient (R2) of 0.940 and 0.910 for the Hill and Michaelis–Menten models, respectively. The kinetic of the crosslinked cells showed a R2 of 0.957 and 0.954 for the Hill and Michaelis–Menten models, respectively. Hence, the Hill model showed the best fit for free and immobilized cells. Ghazi et al. (2005) reported that the Hill model is applicable for enzymes that transfer fructose groups. Also, this model is suitable for enzymes that show more than one catalytic site and multiple subunits such as FTases, which are found in dimeric form (Weiss 1997; Aguiar-Oliveira and Maugeri 2010; Luscher et al. 1996).
The kinetic parameters obtained for the Hill and Michaelis–Menten are listed in Table 7. The Hill coefficients (n) obtained for the free and crosslinked cells suggest that there is a positive cooperative behavior, in which the first sucrose molecule that reacts on the enzyme causes an increase in affinity between the rest of active sites and substrate molecules. Also, according to this coefficient the crosslinked cells showed less cooperativity than the free cells. Furthermore, the crosslinked cells showed higher K0,5 than the free cells, while the free cells showed higher Km than the crosslinked cells, suggesting an increase in the substrate-enzyme affinity after immobilization (Shuler and Kargi 2002). Besides that the crosslinked cells showed higher Vmax than the free cells. These results are in agreement with the increase of AT/AH ratios obtained after immobilization, suggesting that the crosslinking process improved the transfer of fructose groups to the sucrose molecule and decreased the hydrolytic activity.
Operational stability of the free and crosslinked cells
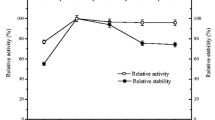
Figure 7 presents the results for the operational stability assays for the free and crosslinked cells, characterized by the relative transfructosylation activity along sequential reaction cycles, determined according to Sect. 2.7.
It is observed that after 12 reaction cycles, the free cells suffered a dramatic fall in activity, with final relative activity of 50.3 ± 3.9%, whereas the crosslinked cells presented a relative activity of 88.9 ± 2.2%. This result indicates that there was a significant extension in the mycelial FTase operational stability after the immobilization by reticulation with glutaraldehyde. Currently, there are no studies reported on crosslinked cells, containing mycelial FTase, for FOS production. The main applications reported on the reticulation of enzymes adhered to the mycelium aimed at the immobilization of whole lipase cells for biodiesel production. Nevertheless, it is important to highlight that, similarly to the results obtained in this work, Sun et al. (2010) reported an increase in the operational stability of Rhizopus oryzae whole cell, containing mycelial lipase, after reticulation with glutaraldehyde. The free and crosslinked cells presented 10% and 90% of their initial activities after ten reaction cycles in batch, respectively. Likewise, Long et al. (1996) reported an increase in thermal and operational stability of Aspergillus flavus mycelial FTase after reticulation with glutaraldehyde, maintaining the enzymatic activity for 13 sequential batch cycles. Additionally, Szczesna-Antczak et al. (2004) presented that the process of reticulation with glutaraldehyde was more efficient than the technique of calcium alginate and polyvinyl alcohol encapsulation for the immobilization of pellets of Mucor circinelloides mycelial lipase used in the conversion of caprylic acid for five reaction cycles.
The whole cells with mycelial FTase have been mainly immobilized by encapsulation or absorption during fermentation, but few studies have reported the effect of these immobilizations on the operational stability of the whole cell. Ganaie et al. (2014) reported the operational stability of the mycelial FTase from Aspergillus japonicus ATCC 20,236, immobilized by encapsulation with alginate and chitosan. After 12 reaction cycles, FOS yield decreased from 65 to 50% using alginate, and from 42 to 2% using chitosan. These results indicate that encapsulation provided an inferior operational stability to that obtained by reticulation in this work, since FOS yield is directly associated to the transfructosylation activity.
The rise in the operational stability of the microbial cells, after reticulation with glutaraldehyde, can be related to a higher resistance to the drag of mycelial enzymes by water during the washing after each cycle, provided by the bonds imposed by the reticulation between the aldehyde and the amino groups of the protein and microbial cell, which strengthen the interaction between enzyme and mycelium (Long et al. 1996; Sun et al. 2010; Barbosa et al. 2012; Monsan 1978). Crosslinked cells with glutaraldehyde occurs by bonds between the amino groups of the cells and the aldehyde groups by the formation of Schiff bases (imine groups) (Monsan 1978; Migneault et al. 2004b). These bonds can still extend to the amino groups of lysine residues present in several proteins, so that the reticulation of the enzyme and cells ensemble can increase enzymatic stability, providing an increase in enzyme resistance to the extraction by water during the process of mycelium washing (Long et al. 1996).
The rise in operational stability of the microbial cells containing mycelial FTase can allow its reuse as biocatalyst in sequential batches for FOS production (Ganaie et al. 2014). This is a crucial factor for cost reduction in the industrial FOS production, since it reduces the production of catalytic cells. The high operational stability, together with the high value of the AT/AH ratio, obtained after reticulation, indicate great operational advantages of Aspergillus oryzae IPT-301 crosslinked cells with glutaraldehyde, increasing its potential of application in FOS production.
Conclusion
The variables of immobilization by reticulation of Aspergillus oryzae IPT-301 cells (pH and glutaraldehyde concentration) were optimized, enabling the maximization of the transfructosylation activity and minimization of the hydrolytic activity and, therefore, the maximization of the ratio between the activities AT/AH, which represents a favorable scenario for FOS production. Crosslinked cells provided higher AT/AH ratios at several temperatures, pHs and sucrose concentrations in the reaction media, as well as an expressive gain in the operational stability in comparison with the free cells. These results demonstrate the potential use of the cross-linked cells with glutaraldehyde in FOS production during sequential reaction cycles, also enabling its application in continuous processes.
References
Aguiar-Oliveira E, Maugeri F (2010) Characterization of the immobilized Fructosyltranferase from Rhodotorula sp. Int J Food Eng. https://doi.org/10.2202/1556-3758.1894
Almeida ACS, Araújo LC, Costa AM, Abreu CAM, Lima MAGA, Palha MAPF (2005) Sucrose hydrolysis catalyzed by auto-immobilized invertase into intact cells of Cladosporium cladosporioides. Electron J Biotechnol 8(1):54–62
Antosová M, Polakovic M (2001) Fructosyltransferase: The enzymes catalyzing production of fructooligosaccharides. Chem Pap Chem Zvesti 55:350–358
Antosová M, Illeová V, Vandáková M, Druzkovská A, Polakovic M (2008) Chromatographic separation and kinetic properties of fructosyltransferase from Aureobasidium pullulans. J Biotechnol 135:58–63
Barbosa O, Torres R, Ortiz C, Fernandez-Lafuente R (2012) Versatility of glutaraldehyde to immobilize lipases: Effect of the immobilization protocol on the properties of lipase B from Candida Antarctica. Process Biochem. https://doi.org/10.1016/j.procbio.2012.04.019
Canilha L, Carvalho W, Silva JBA (2006) Biocatalisadores imobilizados: uso de células e enzimas imobilizadas em processos biotecnológicos. Biotecnol Cienc Desenvolv 36:48–57
Castro CC, Nobre C, Duprez ME, Weireld G, Hantson AL (2017) Screening and selection of potential carriers to immobilize Aureobasidium pullulans cells for fructo-oligosaccharides production. Biochem Eng J 118:82–90
Chen WC, Liu CH (1996) Production of β-fructofuranosidase by Aspergillus japonicus. Enzyme Microb Technol. https://doi.org/10.1016/0141-0229(95)00099-2
Chien CS, Lee WC, Lin TJ (2001) Immobilization of Aspergillus japonicus by entrapping cells in gluten for production of fructooligosaccharides. Enzyme Microb Technol 29(4-5):252-257. https://doi.org/10.1016/S0141-0229(01)00384-2
Cuervo-Fernandez R, Maresma BG, Juárez A, Martínez J (2004) Production of fructoligosaccharides by β-fructofuranosidase from Aspergillus sp. 27H. J Chem Technol Biotechnol. https://doi.org/10.1002/jctb.967
Cuervo-Fernandez R, Ottoni CA, Silva ES, Matsubara RMS, Carter JM, Magossi LR, Wada MA, Andrade MF, Maresma BG, Maiorano AE (2007) Screening of β-fructofuranosidase-producing microorganisms and effect of pH and temperature on enzymatic rate. Appl Microbiol Biotechnol 74(1):87–93
Cunha JS, Ottoni CA, Morales SAV, Silva ES, Maiorano AE, Perna RF (2019) Synthesis and characterization of fructosyltransferase from Aspergillus oryzae IPT-301 for high fructooligosaccharides production. Braz J Chem Eng. 36(2):657–668. https://doi.org/10.1590/0104-6632.20190362s20180572
Fields PA (2001) Review: Protein function at thermal extremes: balancing stability and flexibility. Comp Biochem Physiol Part A Mol Integr Physiol. https://doi.org/10.1016/S1095-6433(00)00359-7
Ganaie MA, Gupta US (2014) Recycling of cell culture and efficient release of intracelular fructosyltransferase by ultrasonication for the production of fructooligosaccharides. Carbohydr Polym 110:253–258
Ganaie MA, Lateef A, Gupta US (2014) Enzymatic trends of fructooligosaccharides production by microorganisms. Appl Biochem Biotechnol 172:2143–2159
Ghazi I, Fernandez-Arrojo L, Garcia-Arellano H, Ferrer M, Ballesteros A, Plou FJ (2005) Purification and kinetic characterization of a fructosyltransferase from Aspergillus aculeatus. J Biotechnol 128(1):204–211
Hidaka H, Hirayama M, Sumi N (1988) A fructooligosaccharide producing enzyme from Aspergillus niger ATCC 20611. Agric Biol Chem. https://doi.org/10.1080/00021369.1988.10868810
Hirayama M, Sumi N, Hidaka H (1989) Purification and properties of a fructooligosaccharideproducing β-fructofuranosidase from Aspergillus niger ATCC 20611. Agric Biol Chem. https://doi.org/10.1271/bbb1961.53.667
Huang MP, Wu M, Xu QS, Mo DJ, Feng JX (2016) Highly efficient synthesis of fructooligosaccharides by extracellular fructooligosaccharide-producing enzymes and immobilized cells of Aspergillus aculeatus M105 and purification and biochemical characterization of a fructosyltransferase from the fungus. J Agric Food Chem. https://doi.org/10.1021/acs.jafc.6b02115
Kamimura ES, Silva GS, Covre LM, Carvalho RA, Santos AMP (2009) Immobilization of fructosyltransferase from Pichia pastoris in blend of alginate and gelatin. New Biotechnol. https://doi.org/10.1016/j.nbt.2009.06.485
Kim BW, Kwon HJ, Park HY, Nam SW, Park JP, Yun JW (2000) Production of a novel transfructosylating enzyme from Bacillus macerans EG-6. Bioprocess Eng. https://doi.org/10.1007/s004499900078
Krasnan V, Stloukal R, Rosenberg M (2016) Rebros M (2016) Immobilization of cells and enzymes to LentiKats®. Appl Microbiol Biotechnol 100(6):2535–2553
L’Hocine L, Wang Z, Jiang B, Xu S (2000) Purification and partial characterization of fructosyltransferase and invertase from Aspergillus niger AS0023. J Biotechnol 81(1):73–84
Long K, Ghazali HM, Ariff A, Ampon K, Bucke C (1996) In situ crosslinking of Aspergillus flavus lipase: improvement of activity, stability and properties. Biotechnol Lett. https://doi.org/10.1007/BF00128587
López-Gallego F, Montes T, Fuentes M, Alonso N, Grazu V, Betancor L, Guisán JM, Fernández-Lafuente R (2005) Improved stabilization of chemically aminated enzymes via multipoint covalent attachment on glyoxyl supports. J Biotechnol. https://doi.org/10.1016/j.jbiotec.2004.09.015
Lüscher M, Erdin C, Sprenger N, Hochstrasser U, Boller T, Wiemken A (1996) Inulin synthesis by a combination of purified fructosyltransferases from tubers of Helianthus tuberosus. FEBS Lett. https://doi.org/10.1016/0014-5793(96)00343-2
Maresma BG, Castillo BG, Fernández RC, Silva ES, Maiorano AE, Rodrigues MFA (2010) Mutagenesis of Aspergillus oryzae IPT-301 to improve the production of β-fructofuranosidase. Braz J Microbiol. https://doi.org/10.1590/S1517-83822010000100027
Migneault I, Dartiguenave C, Bertrand MJ, Waldron KC (2004a) Glutaraldehyde: Behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. Biotechniques. https://doi.org/10.2144/04375RV01
Migneault I, Dartiguenave C, Vinh J, Bertrand MJ, Waldron KC (2004b) Comparison of two glutaraldehyde immobilization techniques for solid-phase tryptic peptide mapping of human hemoglobin by capillary zone electrophoresis and mass spectrometry. Electrophoresis. https://doi.org/10.1002/elps.200305861
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. https://doi.org/10.1021/ac60147a030
Monsan P (1978) Optimization of glutaraldehyde activation of a support for enzyme immobilization. J Mol Catal. https://doi.org/10.1016/0304-5102(78)80026-1
Muñiz-Márquez DB, Contreras JC, Rodríguez R, Mussatto SI, Teixeira JA, Aguilar CN (2016) Enhancement of fructosyltransferase and fructooligosaccharides production by A. oryzae DIA-MF in solid-state fermentation using aguamiel as culture medium. Bioresour Technol. https://doi.org/10.1016/j.biortech.2016.03.022
Mussatto SI, Aguilar CN, Rodrigues LR, Teixeira JA (2009) Colonization of Aspergillus japonicus on synthetic materials and application to the production of fructooligosaccharides. Carbohydr Res. https://doi.org/10.1016/j.carres.2009.01.025
Ning Y, Wang J, Chen J, Yang N, Jin Z, Xu X (2010) Production of neo-fructooligosaccharides using free-whole-cell biotransformation by Xanthophyllomyces dendrorhous. Bioresour Technol. https://doi.org/10.1016/j.biortech.2010.04.026
Nobre C, Filho EGA, Fernandes FAN, Brito ES, Rodrigues S, Teixeira JA, Rodrigues LR (2018) Production of fructo-oligosaccharides by Aspergillus ibericus and their chemical characterization. Food Sci Technol. https://doi.org/10.1016/j.lwt.2017.10.015
Ottoni CA, Cuervo-Fernandez R, Piccoli RM, Moreira R, Guilarte-Maresma B, Silva ES, Rodrigues MFA, Maiorano A (2012) Media optimization for β-Fructofuranosidase production by Aspergillus oryzae. Braz J Chem Eng 29(1):49–59. https://doi.org/10.1590/S0104-66322012000100006
Perna RF, Cunha JS, Gonçalves MCP, Basso RC, Silva ES, Maiorano AE (2018) Microbial fructosyltransferase: production by submerged fermentation and evaluation of pH and temperature effects on transfructosylation and hydrolytic enzymatic activities. Int J Eng Res Sci 4(3):43–50
Platková Z, Polakovič M, Stefuca V, Vandáková M, Antošová M (2006) Selection of carrier for immobilization of fructosyltransferase from Aureobasidium pullulans. Chem Pap 60(6):469–472
Rodrigues MI, Iemma AF (2009) Planejamento de experimentos & otimização de processos. Casa do Espírito Amigo Fraternidade Fé e Amor,Campinas
Schuurmann J, Quehl P, Festel G, Jose J (2014) Bacterial whole-cell biocatalysts by surface display of enzymes: toward industrial application. Appl Microbiol Biotechnol 98(19):8031–8046. https://doi.org/10.1007/s00253-014-5897-y
Sheu D-C, Chang J-Y, Chen Y-J, Lee C-W (2013) Production of high-purity neofructooligosaccharides by culture of Xanthophyllomyces dendrorhous. Bioresour Technol. https://doi.org/10.1016/j.biortech.2013.01.061
Shuler ML, Kargi F (2002) Bioprocess engineering: basic concepts. Prentice-Hall Inc, Upper Saddle River
Sun T, Du W, Liu D, Dai L (2010) Improved catalytic performance of GA crosslinking treated Rhizopus oryzae IFO 4697 whole cell for biodiesel production. Process Biochem 45(7):1192–1195
Szczesna-Antczak M, Antczak T, Rzyska M, Modrzejewska Z, Patura J, Kalinowska H, Bielecki S (2004) Stabilization of an intracellular Mucor circinelloides lipase for application in non-aqueous media. J Mol Catal B Enzym 29(1–6):163–171
Vaňková K, Onderková Z, Antošová M, Polakovič M (2008) Design and economics of industrial production of fructooligosaccharides. Chem Pap 62(4):375–381
Vega R, Zúniga-Hansen ME (2011) Enzymatic synthesis of fructooligosaccharides with high 1-kestose concentrations using response surface methodology. Bioresour Technol 102:10180–10186
Vescovi V, Kopp W, Guisán JM, Giordano RLC, Mendes AA, Tardioli PW (2016) Improved catalytic properties of Candida antarctica lipase B multi- attached on tailor-made hydrophobic silica containing octyl and multifunctional amino- glutaraldehyde spacer arms. Process Biochem. https://doi.org/10.1016/j.procbio.2016.09.016
Wang T-H (2015) Synthesis of Neofructooligosaccharides. Org Chem Insights 51-6. https://doi.org/10.4137/OCI.S13222
Weiss JN (1997) The Hill equation revisited: uses and misuses. FASEB J. https://doi.org/10.1096/fasebj.11.11.9285481
Yun JW (1996) Fructooligosaccharides—Occurrence preparation and application. Enzyme Microb Technol 19(2):107–117. https://doi.org/10.1016/0141-0229(95)00188-3
Zeng Kang XA, Dong-mei Z, Liu C Brennan S, Brennan M, Jin-song Z, Shu-jua Yu (2016) Preparation of fructooligosaccharides using Aspergillus niger 6640 whole-cell as catalyst for bio-transformation. LWT - Food Sci Technol 651072–1079. https://doi.org/10.1016/j.lwt.2015.09.031
Acknowledgements
The authors gratefully acknowledge the financial support from the National Council for Scientific and Technological Development—CNPq (Proc. 421540/2018-4), Foundation for Research of the State of Minas Gerais (FAPEMIG, Process APQ-02131-14) and Coordination for the Improvement of Higher Education Personnel (CAPES).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Garcia, R.L., Dias, G.S., Morales, S.A.V. et al. Glutaraldehyde-crosslinked cells from Aspergillus oryzae IPT-301 for high transfructosylation activity: optimization of the immobilization variables, characterization and operational stability. Braz. J. Chem. Eng. 38, 273–285 (2021). https://doi.org/10.1007/s43153-021-00110-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43153-021-00110-9