Abstract
Purpose of Review
The tumor microenvironment (TME) is an amalgam of multiple dysregulated biophysical cues that can alter cellular behavior through mechanotransductive signaling and epigenetic modifications. Through this review, we seek to characterize the extent of biophysical and epigenetic regulation of cancer stemness and tumor-associated immune cells in order to identify ideal targets for cancer therapy.
Recent Findings
Recent studies have identified cancer stemness and immune action as significant contributors to neoplastic disease, due to their susceptibility to microenvironmental influences. Matrix stiffening, altered vasculature, and resultant hypoxia within the TME can influence cancer stem cell (CSC) and immune cell behavior, as well as alter the epigenetic landscapes involved in cancer development.
Summary
This review highlights the importance of aberrant biophysical cues in driving cancer progression through altered behavior of CSCs and immune cells, which in turn sustains further biophysical dysregulation. We examine current and potential therapeutic approaches that break this self-sustaining cycle of disease progression by targeting the presented biophysical and epigenetic signatures of cancer. We also summarize strategies including the normalization of the TME, targeted drug delivery, and inhibition of cancer-enabling epigenetic players.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer research has consistently conveyed that tumor initiation and progression are achieved through acquisition and accumulation of genetic mutations that drive clonal expansion from a single cancer cell [1,2,3]. These driver mutations contribute largely to tumor cell heterogeneity, as they can offer different growth advantages that can be positively selected for during the development of cancer, allowing one sub-clonal population to drive tumor progression over another. Because the acquisition of self-renewal capabilities by subclones can lead to the formation of cancer stem cells (CSCs), cancer cells that have acquired stem-like phenotypes can therefore further drive tumor progression [4]. Although the exact origin of CSCs remains uncertain, the shaping of tumor heterogeneity by both epigenetic mechanisms and the tumor microenvironment (TME) suggests that the formation and maintenance of CSCs likely involve the contribution of changing epigenetic signatures driven by the co-evolution of cancer cells and the TME.
Tumors represent an ecosystem comprised of malignant cells surrounded by the hypoxic, chronically inflamed, and biomechanically aberrant TME, which is also occupied by resident and tumor-infiltrating immune cells. Tumor immune cells play an instrumental role in eliminating neoplasms and can exhibit both pro- and anti-oncogenic phenotypes, which can be used to determine the clinical outcome of malignancies [5]. Tumor biophysical signals guide immune cell behavior directly by mechanotransduction and indirectly by stimulating the production of abnormal cytokines, chemoattractants, and growth factors that impose survival constraints to eliminate effector types while simultaneously fostering niche-specific regulatory forms. Effectively targeting cancer would therefore likely require an understanding of the influences of the TME on its immune population.
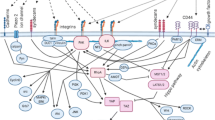
In this review, we discuss the driving forces of cancer initiation and progression through a closer look at how the TME introduces both biophysical cues and consequent epigenetic alterations that manipulate tumor cellular plasticity, invasiveness, and immune action (Fig. 1). We also discuss how the resulting acquisition of stem-like properties and aberrant immune cell phenotypes contributes to tumorigenesis and long-term tumor maintenance. Finally, we consider current and potential therapeutics designed to target the anomalous biophysical and epigenetic signatures of the TME to halt the self-sustaining cycle of tumor progression, kickstart homeostatic reforms, and promote healing.
Changing biophysical cues alter cellular phenotypes in the tumor microenvironment through mechanotransductive signaling and epigenetic changes. Schematic depicting the regulatory circuits involved in the biophysical modulation of several cancer processes including the emergence of stemness and regulatory immune behavior. Changes in the microvasculature and ECM composition resulting from dysregulated cell signaling and metabolic pathways trigger cascades of accumulating biophysical effects that also contribute to the hypoxic and acidic conditions of the TME. These changes ultimately regulate cell behavior within the TME through modulating immune cell activity and promoting stem-like properties. We highlight the self-sustaining feed-forward loop that drives tumor progression. Abnormal biophysical cues established by altered cell behavior further modulate cell behavior. This reciprocal relationship can be targeted by therapeutics that promotes TME normalization, causing a disruption of the mechanisms by which biophysical cues and cell behavior regulate each other.
Epigenetic and Biophysical Regulation of Cancer Stemness and Associated Pathways
Cancer stemness refers to the ability of a select subpopulation of tumoral cells to exhibit stem-like properties (namely, the ability to both differentiate and self-renew) [4]. Cancer stem cells (CSCs) usually account for less than 5% of all cancer cells, as has been observed in multiple myeloma and breast tumors [6,7,8]; however, their contribution to tumor heterogeneity impedes the development of successful cancer treatments. The CSC hypothesis proposes that a rare (or elite) population of CSCs contributes to long-term tumor maintenance (or relapse) and cancer progression [9, 10] by seeding new tumors, proliferating extensively, and giving rise to non-CSCs that promote tumor heterogeneity [11, 12]. While it was believed that self-renewing normal and neoplastic stem cells lie at the top of the cellular hierarchy of tumor tissues and their differentiated progeny are not self-renewing, recent research has observed spontaneous dedifferentiation by human mammary epithelial cells in the absence of genetic manipulation, leading to their reversion into a stem-like state. Oncogenic transformation further promotes this spontaneous conversion so that non-CSCs give rise to CSCs. Acquired plasticity of non-CSCs alludes to a possible resolution to the current inconsistencies presented by the CSC model [11]; however, the drivers of this transition toward stem-like states are still emerging.
Altered Epigenetic Signatures Can Induce Stem-Like Phenotypes in Tumor Cells
The acquisition of stem-like properties can result from alterations to a cell’s epigenetic profile [13, 14]. Epigenetic modifications involve changes to the chromatin components that influence gene expression without disrupting the nucleotide sequence. These alterations include DNA methylation, histone modifications, structural remodeling of chromatin, and dysregulation of miRNAs, all of which can result in alterations in gene regulation through changes in DNA accessibility, protein-DNA interactions, or direct RNA silencing/post-transcriptional regulation. In cancer, epigenetic abnormalities contribute significantly to tumor initiation and the acquisition of stem-like properties. For example, the levels of linker histone H1.0—important in restricting cancer cell proliferation potential—are heterogeneous within cancer cell populations, with low levels being associated with cells exhibiting CSC properties and high levels being associated with differentiated cell states [15]. In the case of glioblastoma, which is an aggressive brain cancer, mutations in the H3.3 Histone A (H3F3A) gene have been shown to facilitate a genome-wide decrease of the repressive histone mark H3K27me3 which leads to an increase in oncogenic self-renewal potential. Interestingly, glioblastoma is also known to show higher frequencies of CSCs [14, 16]. It has also been observed that approximately 25% of acute myeloid leukemia (AML) patients harbor activity-reducing mutations in DNA methyltransferase 3A (DNMT3A), which is thought to drive atypical expression of stem cell markers and a reemergence of stem cell properties that contribute to leukemia-initiating stem cell formation and expansion, although the mechanisms have yet to be fully elucidated [13, 17]. Table 1 addresses other observed epigenetic signatures that contribute to the acquisition and maintenance of stem-like properties in cancer cells.
Epithelial-to-Mesenchymal Transition and Cancer Stemness Enable Optimization of Tumor Metastatic and Proliferative Potential
The epithelial-to-mesenchymal transition (EMT) describes a cellular transformation that allows epithelial cells to acquire mesenchymal phenotypes, including improved migratory capacity and increased expression of extracellular matrix (ECM) components. While EMT allows for body plan establishment and tissue regeneration in normal developmental programs, it can also be reactivated in tumors to enable penetration of blood and lymphatic vessels, thereby facilitating tumor invasion and metastasis [18]. Spontaneous dedifferentiation of cells in the TME can be triggered by biophysical cues that also drive EMT. Recent studies have established several molecular commonalities between the acquisition of cancer stemness and EMT, including shared antigen signatures, regulatory mechanisms, and signaling pathways [18]. While CSCs are believed to contribute largely to tumor invasion and metastasis, the relationship between CSCs and EMT is reflected by the ability of cells undergoing EMT to acquire stem-like phenotypes. For example, induction of EMT in immortalized human mammary epithelial cells (HMLEs) resulted in the acquisition of fibroblast-like morphology and a CD44high/CD24low expression pattern—a unique surface antigen signature attributed to neoplastic mammary stem cells [19]. It has also been shown that immune cell-mediated induction of EMT in breast cancer tumors produced a CD24-/lowCD44+ surface antigen signature, representative of breast cancer stem cells [20]. In prostate cancer cells, it was revealed that cancer-associated fibroblast (CAF)-mediated induction of EMT, as verified by changes in cell morphology, upregulation of EMT regulators SNAIL and TWIST, and downregulation of E-cadherin, leads to downregulated expression of CD24 and upregulated expression of CD44 [21]. Correlations between EMT and the acquisition of stemness in cancer suggest that they may interact in a reciprocal fashion in the context of tumor progression. It has been speculated that CSCs undergo EMT to maximize their metastatic potential as they obtain migratory properties allowing for travel to distant sites before reversion to an epithelial state that is more ideal for proliferation and establishment of a metastatic tumor via a mesenchymal-to-epithelial transition (MET) [22]. This hypothesis has been supported by flow cytometry and transcriptomic analyses indicating that CSCs in squamous cell carcinoma can switch between migratory and proliferative phenotypes [23]. Observations of acquired stemness in human mammary epithelial cells, as exhibited by elevated expression levels of stem cell marker CD44 and acquired mammosphere formation ability, also support the notion that such transitions yield changes in mechanical behavior [11].
The Shared Signaling Pathways Between EMT and Cancer Stemness Are Epigenetically and Biophysically Influenced
The similarities between EMT and cancer stemness in tumor malignancy can also be observed through their shared signaling pathways. The Wnt signaling pathway is involved in regulating cell development, differentiation, and proliferation through its modulation of gene activation via transcription factor β-catenin and contributes to both EMT and cancer stemness [24, 25]. Wnt signaling has been observed to activate EMT through mediation of miR-300 activity [24] as well as through stimulation of Survivin expression and activation of the PI3K/Akt pathway [26]. In the context of cancer stemness, interactions between β-catenin and Lef1 transactivate the miR-371-373 cluster that mediates CSC self-renewal [27]. In colorectal cancer, poor prognosis has been associated with the methylation of Wnt target genes involved in cancer stemness, which results in an increase in the number of CSCs. This observation suggests that Wnt activation is involved in the differentiation of CSCs and that the activation mechanism is epigenetically regulated [28]. The role of Wnt signaling components in mechanisms pertaining to both EMT and cancer stemness is also supported by the observation of EMT upregulation following Wnt activation by a CSC marker G-protein coupled receptor 5 (LGR5) [29]. In addition, Notch signaling is highly involved in promoting tumorigenesis, and its activation via increased expression of Notch-1 and transcription factor Hes1 upon exposure of lung cancer cells to fine particulate matter has been reported to drive both EMT and stem-like properties [30]. Retroviral transduction of Notch-1 into colon cancer cells has also been shown to increase expression of EMT- and stemness-associated proteins CD44, Slug, Smad-3, and Jagged-1 [31]. Furthermore, the transforming growth factor beta (TGF-β) signaling pathway is involved in cell growth and proliferation and can promote EMT and stemness in carcinomas; Katsuno et al. showed that prolonged exposure of breast carcinoma HMLER cells to TGF-β led to mesenchymal morphology and an increased amount of CD24low/CD44high cells [32]. Yet another notable shared signaling pathway between EMT and cancer stemness is Sonic Hedgehog (SHH), which can drive both properties in cholangiocarcinoma cells [33•]. While hypoxia induces SHH signaling in cholangiocarcinoma [33•], SHH-driven medulloblastoma stem cells are susceptible to epigenetic regulation via miR-466f-3p, the low expression of which sustains EMT [34]. The susceptibility of these shared signaling pathways to both epigenetic regulation and the influence of the hypoxic TME (which is associated with extensive matrix remodeling) alludes to the critical role that epigenetic and biophysical cues play in regulating EMT and cancer stemness.
Matrix Remodeling, Compressive Stress, and Hypoxia Promote EMT and Cancer Stemness
Biophysical signaling (i.e., through substrate or matrix rigidity, cell morphology, surface topography) and mechanical force have been shown to play critical roles in the control and maintenance of stem cell properties (i.e., proliferation, differentiation) [35,36,37] and regulation of EMT [38] in the context of tumor initiation and invasion. The microenvironment of solid tumors exhibits hallmark mechanical changes including increases in shear, compressive, and tensile stress as well as heightened matrix stiffness and density [39, 40].
Changes in matrix stiffness and composition influence adhesome dynamics and migratory potential, which can result in the promotion of stem-like properties. A study by Tang et al. demonstrates that while HCT-8 human ileocecal colorectal adenocarcinoma cells attach to substrates and form colonies on 21–47 kPa gels, they begin to dissociate after 7 days, as downregulation of cell-cell adhesion molecule E-cadherin and increased motility by dissociated cells are observed [41]. Interestingly, such a phenotype does not exist on both soft (1 kPa) and extremely stiff (3.6 GPa) substrates [41]. This finding suggests that matrix stiffness can play a key role in regulating EMT-associated characteristics such as adhesion and migratory potential. Collagens, which comprise the main structural element of the ECM, are overexpressed in CSCs and particular collagen subtypes can contribute to EMT induction and tumor initiation [42]. For example, collagen I has been shown to inhibit differentiation and promote stemness in human colorectal carcinoma cells through its interactions with α2β1 integrin [43]. Additionally, CSCs are dependent on integrin signaling activated by ECM proteins and several integrin subunits such as β3, α6, and β1 contribute to the self-renewal and maintenance of CSCs and serve as CSC biomarkers [44]. The fascin-mediated upregulation of integrin subunit β1, a key adhesion molecule, was also associated with CSC enrichment and worse prognosis in breast cancer patients, which further suggests that biophysical cues largely affect cancer stemness [45]. Also, in breast cancer, matrix stiffening has been shown to activate integrin-linked kinase (ILK), which is responsible for transmitting extracellular signals from the ECM to regulate anchorage-dependent growth, differentiation, and tumor angiogenesis. Activated ILK then signals through the PI3K/Akt pathway to regulate cancer stemness by inducing expression of CD44, β1 integrin, and Nanog [46]. The kinase Akt, which contributes to key cellular processes like cell proliferation, transcription, and cell migration, is speculated to be a master regulator of ECM-driven induction of EMT and CSC phenotypes [42]. The PI3K/Akt pathway is also activated by the binding of hyaluronan, an important polysaccharide for structural and compositional maintenance of the ECM, to stem cell marker CD44. This hyaluronan-CD44 interaction has been shown to promote stemness in breast and ovarian CSCs [42]. CSCs are also thought to remodel their ECM through differential expression of matrix metalloproteinases to maximize their survival. The upregulated expression of metalloproteinases by CSCs in glioblastoma and ovarian cancer has been observed to result in increased invasive and migratory potential [47, 48].
Enhanced migratory capacity by CSCs can be triggered by other biophysical cues as well. For example, applied compressive stress increases motility in breast cancer cells by stimulating the formation of “leader cells” with filopodial protrusions, thereby promoting a more invasive phenotype [49]. This finding suggests that the increased migratory potential of CSCs can result from compressive stress that is introduced by CSC proliferation. Additionally, it is suspected that the conversion of non-CSCs to CSCs may be driven by biophysical cues in the TME that drive EMT, as is observed by a decrease in epithelial properties and increase in mesenchymal properties [18, 50]. For example, hypoxic conditions and constitutive expression of hypoxia-inducible factors (HIFs) have been shown to induce EMT, as reported by a shift of epithelial to mesenchymal marker expression and increase in migratory capacity, via direct activation of EMT transcription factor TWIST [51].
While spontaneous conversion of non-stem cells to stem-like cells has been observed before, it is likely that biophysical factors play a significant role in the regulation of stemness acquisition [11]. Nuclear reprogramming has been observed in cells encountering lateral confinement without the presence of biochemical inducers and, similarly, this confinement also triggered the activation of cancer stemness-related genes (i.e., OCT4, CD44, and SNAI1) within MCF7 breast cancer cells, suggesting that stemness programs can be activated in response to specific biophysical cues [52••]. The observed biophysically mediated activation of stem and migrative properties in cancer cells demonstrates the importance of the mechanical components of the TME in promoting both tumor initiation and invasion.
Additionally, biophysical cues from the TME can also promote EMT and cancer stemness indirectly through alteration of epigenetic signatures. Histone modifiers, which are largely involved in shaping the epigenetic profile of tumor cells, have been reported to be responsive to hypoxic conditions [53]. Furthermore, while the culturing of tumor repopulating melanoma cells on rigid plastic substrates has been shown to inhibit self-renewal, matrix softness has been observed to regulate the plasticity of tumor-repopulating cells by inducing H3K9 demethylation and Sox2 expression [54]. A study by Tan et al. demonstrates that disrupting actin filaments or microtubules in melanoma cells with Latrunculin A or colchicine, respectively, and inhibiting myosin light chain kinase with ML7 leads to significantly decreased H3K9 methylation levels [54]. Additionally, Tan et al. show that the silencing of methyltransferases G9a and SUV39h1 via siRNA knockdown results in greatly decreased H3K9me2 and me3 levels in the Sox2 promoter region, as assessed by chromatin immunoprecipitation (ChIP), thereby increasing Sox2 expression significantly. This finding that biophysically induced H3K9 demethylation stimulates self-renewal in differentiated melanoma cells by promoting Sox2 expression highlights how both biophysical and epigenetic cues can interact to regulate tumor growth and proliferation [54]. Figure 2 outlines the TME biophysical cues that participate in the regulation of cancer stemness through modulation of the EMT phenotype and epigenetic alteration.
Biophysical and epigenetic factors within the tumor microenvironment drive cancer stemness, invasiveness, and immune evasion. The behaviors of cancer cells that occupy the tumor are influenced by alterations in biophysical cues. Classic aberrant extracellular cues in the TME include increased matrix stiffness, solid and fluid stresses, interstitial flow, and low perfusion leading to hypoxia and acidity. Cancer stemness results from the epigenetic mechanisms that originate in such an abnormal microenvironment. Tumor biophysical cues may also be involved in CSC development and serve to promote EMT. In addition, the TME biophysical cues and consequent epigenetic changes worsen cancer outcomes by evading, eliminating, or reorienting effector immune cells while also recruiting regulatory cells to the tumor
YAP/TAZ and MRTF in the Biophysical Regulation of Cancer Stemness
There exist other regulators relevant in tumor initiation and progression that are susceptible to biophysical cues. YAP/TAZ are the primary downstream effectors of the vertebrate Hippo signaling pathway, which is responsible for regulation of organ size, tissue homeostasis, as well as various cancers [55]. YAP/TAZ have also been shown to promote cancer stemness through their role in activating genes involved in proliferation [56]. Studies have revealed that expression of YAP/TAZ in non-stem breast cancer cells can induce reprogramming into cells with CSC characteristics [57]. YAP/TAZ-induced transdifferentiation of hepatocytes to biliary progenitors prior to tumorigenesis has also been observed in liver cancer [58]. Nuclear localization of YAP/TAZ is regulated by intracellular tension resulting from cells “sensing” stiffer substrates, extracellular shear from fluid flow, or by experiencing increased cell spreading or mechanical stress/strain [59]. For example, stabilization of the F-actin cytoskeleton and mechanical strain applied to E-cadherin cell-cell junctions have been proven to induce YAP/TAZ activity by nuclear translocation [60, 61].
Additionally, mechanosensitive myocardin-related transcription factors (MRTFs), which provide a link between cytoskeletal dynamics and cytoskeletal gene expression, are also critical mediators of EMT [62]. While high levels of G-actin retain MRTFs in the cytoplasm, their nuclear localization is triggered by Rho-induced incorporation of G-actin into F-actin [63]. Actin polymerization thus allows MRTFs to interact with their co-activator, transcription factor serum response factor (SRF), leading to the subsequent activation of cytoskeletal target genes [63]. Nuclear accumulation of MRTF-A has been shown to be responsive to disruptions in cell-cell junctions [64], restriction of cell spreading [65], and changes in matrix stiffness [38]. Recent studies of MRTFs demonstrate a correlation between MRTF-A RNA expression and breast and lung cancer metastasis [66, 67]. Increased expression of both MRTF-A and -B also stimulates the initiation of pancreatic cancer by promoting sphere formation by stem cell-like cells and the generation of cancer-initiating cells (CICs), as marked by upregulation of CIC markers CD44, Tspan8, and CD151 [68].
Biophysical Regulation of the Epigenetic Signatures Contributing to Cancer Stemness and Tumor Initiation
The shaping of epigenetic patterns by biophysical cues is a familiar concept that has been highlighted in many biological studies. Recent research has demonstrated that heterochromatin dynamics and telomere structure can be influenced by reduced matrix constraints [69]. It has also been observed that histone modifications are responsive to biophysical changes associated with a 3D environment [70••]. Cell geometric constraints have been shown to induce the mechanical regulation of histone deacetylase 3 (HDAC3) cytoplasmic-to-nuclear redistribution in an actomyosin-dependent manner [71]. Additionally, the activity of WD repeat domain 5, WDR5—a subunit of H3 methyltransferase—can be regulated by mechanomodulation, as upregulation of H3K4 methylation by WDR5 can be triggered by cellular confinement [72]. Extracellular cues associated with biophysical alterations within the TME also play an important role in the regulation of epigenetic patterns contributing to cancer initiation and progression; in particular, many enzymes involved in DNA and histone methylation are responsive to the hypoxic conditions of the TME. Interestingly, the interaction of HIF-1α-induced HDAC3 with WDR5 has been identified to be critical to hypoxia-induced EMT and metastasis in hypopharyngeal carcinoma cells [73]. In breast cancer, the activity of ten-eleven translocation enzymes TET1 and TET3 (which facilitate DNA demethylation) is deregulated by hypoxic conditions, which ultimately promotes tumor metastasis [74]. Hypoxia has also been shown to trigger global DNA demethylation through the upregulation of methionine adenosyltransferase 2A (MAT2A) in hepatoma cells [75]. These observations are implicative of the coaction of both biophysical and epigenetic factors in tumor initiation and progression.
As the proliferative and metastatic potential of CSCs is largely dependent on EMT and adhesome dynamics, normalization of the tumor vasculature and stromal matrix would result in amelioration of the hypoxic conditions and matrix stiffness that trigger EMT and aberrant adhesome gene expression, respectively. This review elaborates on therapeutic challenges and approaches in the “Therapeutic Normalization of the TME Can Improve Cancer Outcomes” section.
Biophysical Regulation and Epigenetics of Immune Cells in Cancer
It is often believed that normal tissue homeostasis and architecture can avert the emergence of malignancies and that anomalous biophysical cues shift this balance to precancerous tendencies [76]. This is no less applicable to the immune cells of the TME, as immunosurveillance and clearance of neoplastic cells are the cornerstones of restraining and eliminating cancer in healthy states. In fact, Hanahan and Weinberg’s updated list of cancer hallmarks suggests that immunoevasion and chronic inflammation are enabling characteristics of cancer, a distinction from their earlier description of cancer hallmarks that did not mention immune cells [77, 78]. While active adaptive immunity can lead to favorable clinical outcomes based on effective malignancy clearance, chronic inflammatory responses of innate immune cells in the vicinity of precancerous tissue may lead to tumorigenesis. Likewise, immune insufficiency can also result in increased cancer susceptibility, as seen in cases of primary immunodeficiency diseases [79], organ transplant-associated drug-induced immunosuppression [80], absence of immune cells including natural killer (NK), natural killer T cells (NKT), gamma delta (γδ) T cells, and other lymphocytes, or deficiency of immune products such as interferon-gamma (IFN-γ), perforins, and GM-CSF [81,82,83,84]. In this section, we discuss how cancer-associated biophysical cues influence immune cell phenotype through altered transcriptional and epigenetic programs (outlined in Fig. 2).
Both Pro- and Anti-oncogenic Immune Cell Types Exist in the Tumor and Dictate Cancer Outcomes
Cancer outcomes are often guided by the composition, location, and behavior of the immune cells that reside in or are recruited to the tumor. It has been recently suggested that immunological classification of tumors into “hot,” “cold,” and “immune-excluded” types, by analyzing spatial distributions of immune infiltrates at the tumor periphery and core may be used as a stratifying biomarker in immunotherapy [85]. It is speculated that such spatial patterns, frequently guided by chemoattractant gradients, can dictate tumor outcomes depending on the phenotype of the immune cells involved [85, 86]. Prominent tumor presence of effector CD8+ cytotoxic T lymphocytes (CTL) and Th1 cells is associated with favorable prognosis [87, 88], whereas pro-tumor types including Th17 cells [88], myeloid-derived suppressor cells (MDSC) [89], and tumor-associated macrophages (TAM) [90, 91] correlate with poor prognosis in several cancers. It is now widely understood that immune cells play multifarious functions in the TME, including those which exert pro- and anti-oncogenic influences [5]. Pro-tumor regulatory and secretory behaviors in immune cells are co-opted by malignancies to supplement the aberrant TME. The tumor immune response may also be marked by the exclusion of certain immune cell types. Reduced accumulation and migration of CTL in “cold” tumors can happen when the dense stromal ECM sequesters CTLs, physically blocking their access to the tumor core in a chemokine and ECM remodeling enzyme-dependent manner [86, 92•].
The burgeoning tumor mass results in the emergence of several deregulated biophysical cues as malignancy develops and proliferates. These include increased local tissue stiffness and fibrosis; increased intra- and extra-tumoral solid stresses and deforming forces; altered vasculature, perfusion, and permeability; and increased interstitial fluid pressure. In addition, such cues indirectly affect cellular phenotype and survival by reshaping the TME to be acidic, hypoxic, and nutrient deficient. All these direct and indirect cues may shape the behavior of both resident and recruited immune cells of the TME. In the upcoming subsections, we review the biophysical regulation of immune cell behaviors in the context of cancer progression and provide insights on how changes in the epigenetic landscape may contribute to these behaviors.
Tumor-Associated Matrix Cues Contribute to Immune Cell Activation Phenotypes
The Tumor ECM Modulates Immune Cells by Altered Mechanotransduction
Desmoplasia, or excessive ECM deposition that is largely attributable to cancer-associated fibroblast (CAF) activity, is only one of many biophysical deviations in the TME [78]. ECM composition acts as a powerful determinant of cellular behavior including growth, proliferation, and death, and is also believed to guide cell trafficking into and out of a tumor. Solid tumor development is concomitant with greater ECM remodeling, creating a stiffer, crosslinked, and less compliant tissue [54]. T cells, dendritic cells (DC), and monocyte-macrophages are among the tumor-infiltrating immune cells known to respond to stiffness stimuli. T lymphocyte activation requires the formation of an immunological synapse to the antigen-presenting cell (APC) that it engages with. Recent studies show that the compliance of a substrate exhibiting co-stimulatory ligands can influence the activation of CD4+ and CD8+ T cells through cytoskeletal dynamics and increased mechanical forces at the T cell receptor (TCR) complex [93, 94]. Stiffer polyacrylamide substrates conjugated with anti-CD3/CD28 allowed for better attachment and activation of naïve T cells, causing elevated IL-2 secretion [93]. Interestingly, although tumors are stiffer tissues, cancer cells of various etiologies have been described to be stiffness-insensitive, consequently staying soft even in relatively stiff microenvironments, and displayed lower traction forces than their non-cancerous counterparts [95]. It remains to be seen if T cell cytotoxic action is curtailed by the relative softening of cancer cells, conceivably through defective direct antigen presentation. Another study demonstrated that 3D cultures of T cells in high-density collagen impaired T cell proliferation in response to PMA and ionomycin resulting in lowered CD8+ CTL to CD4+ ratios when compared to low density collagen, a phenomenon also captured in vivo in breast cancer [92•]. It was also revealed that dense 3D collagen caused reduced cytotoxic effectiveness and elevated regulatory behavior of T cells, in comparison to low-density 3D collagen and regular 2D controls. This was evidenced by the downregulation of GZMB and IFNG and the upregulation of IL10, TGFB1, and FOXP3 genes in dense 3D cultures [92•]. These studies demonstrate the potential of extracellular stiffness cues in guiding T cell activation and proliferation.
Dendritic cells are the predominant APCs with which naïve T cells interact and are also influenced by substrate stiffness. Culturing DCs on soft (2 kPa) and stiff (12 kPa) polyacrylamide gels coated with fibronectin to mimic fibrotic stiffening has shown changes in gene expression of two distinct DC cell states. In immature cells, stiffer substrates reduce β2 integrin expression and podosome formation, whereas, in mature DCs, the expression of maturity markers CD83 (co-stimulatory molecule) and CCR7 is reduced [96]. DC immaturity in the tumor results in poor antigen presentation and leads to reduced T cell activation and proliferation. TCR stimulation by DCs lacking costimulatory ligands may also contribute to T cell anergy.
Similarly, macrophages have been extensively reported to be sensitive to ECM composition, stiffness, and topographical and adhesive cues [97]. On diverse 2D ECM-coated surfaces, macrophages adopt varied morphologies, while also demonstrating divergent potential for polarization [98]. Specifically, collagen type I coated substrates evoked the greatest TNFα secretion from inflammatory macrophages, and least IL-10 secretion from pro-healing polarized cells, compared to the other ECM coatings in the study [98]. ECM adhesive cues that force cellular elongation result in enhanced pro-healing M2 activation in macrophages [99]. Likewise, in 3D hydrogels containing adhesive ECM proteins, macrophage inflammatory programs are greatly ameliorated in an integrin binding-dependent manner, compared to non-ECM controls [100, 101]. On softer 3D fibrin hydrogels, macrophages display reduced tendencies for inflammatory polarization by soluble cues [102], a protective effect that diminished in magnitude as the hydrogels became stiffer with crosslinking [103]. Crosslinked fibrin also caused macrophages to display increased cell spread and motility [103]. Similarly, when macrophages were cultured on polyacrylamide gels of different stiffness, stiffer gels were pro-inflammatory in a TLR4-dependent manner, irrespective of the ECM protein coated [104]. While these evidence point to macrophage sensitivity to biophysical cues that might also exist in a tumor, the consequences of biophysical regulation of macrophages in the context of the TME (both anti-inflammatory like CSF-1, TGF-β, and IL-10, and pro-inflammatory like TNF-α) remain to be investigated.
Tumor ECM Composition Modulates Immune Responses
Studies show that the unique tumor ECM composition can reorient immune cell behaviors. For instance, decellularized human colorectal cancer matrices polarize macrophages toward an M2-like anti-inflammatory phenotype that elevates TGF-β and IL-10, and are capable of promoting cancer cell invasion through CCL18 upregulation [105•]. Such pro-oncogenic macrophages can recruit regulatory FOXP3+ T cells (Treg) by producing TGF-β and CCL22 [106] while also impeding CTL action by stromal sequestration [107], and expression of inhibitory immune checkpoint molecules [108]. Such macrophages also produce vascular endothelial growth factor (VEGF), which acts alongside CCL18 to promote angiogenesis in tumors [109]. Collectively, these studies suggest that the tumor ECM helps transform infiltrating macrophages toward an anti-inflammatory, pro-metastatic, and pro-angiogenic state resembling regulatory M2-like TAMs.
The collagen-rich ECM of the tumor plays an immunosuppressive role by acting as high-affinity ligands for the leukocyte-associated Ig-like receptor-1 (LAIR-1) [110]. LAIR-1 is prominently expressed in a variety of peripheral blood immune cells, including CD4+ and CD8+ T cells, B cells, NK cells, and monocytes, and serves as a self-recognition inhibitory signal. It engages with both extracellular and transmembrane collagens—the overexpression of either in cancer cells is associated with cancer progression, immune inhibition, and poor outcomes [110, 111]. Remarkably, while antibody-mediated disruption of the LAIR-1 engagement with matrix collagens rescued the activation of anti-cancerous Th1 cells, it resulted in inhibition of Th17 cells, which incidentally also expressed greater surface LAIR-1 than Th1 cells [112]. Activated Th17 cells are generally believed to play a pro-oncogenic influence on the TME by secreting IL-17, a pro-angiogenic and pro-inflammatory cytokine [88]. In addition to stromal T cell sequestration [92•], LAIR-1-mediated immunomodulation demonstrates the possible selective inhibition of certain immune cell types by the tumor matrix.
The Tumor ECM Shapes Immune Cell Behavior by Altered Epigenetic Machinery
Emerging evidence sheds light on the influences of the ECM substrate not only on transcriptional activity but also on the epigenetic landscape of cells [72, 113]. As migratory cells, immune cells often must squeeze through interstitial confines to extravasate to their targets. It had been recently shown that actomyosin contractility in T cells causes nuclear softening, enabling migration through confined ECM spaces [70••]. As cells elongate to migrate, cytoskeletal reorganization induces an upregulation of the histone methyltransferase WDR5, resulting in increased histone H3K4 trimethylation. This triggers chromatin decondensation and lowered nuclear stiffness, permitting cells to navigate restricted confines without damage to the nucleus. It was also shown that WDR5 silencing results in reduced migratory potential of T cells, stemming from a failure to produce stable elongated trailing tails [70••]. Indeed, the effects of cellular shape restrictions on epigenetic and transcriptional machinery have been described earlier in fibroblasts, where it was shown that cellular elongation by micropatterning causes an increase in WDR5, H3K4 methylation, and H3 acetylation, and a decrease in HDAC2 [72].
The mechanotransducer YAP, recently implicated in cancer transcriptional programs via its association with the histone hyperacetylation reader BRD4 [114], has been shown to be upregulated in Tregs compared to CD4+ T cells [115], as well as necessary for the expression of FoxP3 transcription factor and the immunosuppressive potential of Tregs [115]. YAP has also been demonstrated to be a negative regulator of T cell infiltration and activation in tumors [116]. Likewise, the stiffness- and confinement-responsive transcriptional coactivator MRTF-A is known to enhance inflammatory programs in macrophages by interacting with the histone H3K4 methyltransferase complex COMPASS, recruiting the methyltransferases ASH2 and WDR5, and opening NF-κB target gene promoters for transcription [117, 118]. Disruption of epigenetic pathways involving histone acetylation using the HDAC inhibitor Trichostatin A induced phenotypical changes including an elongated morphology, and heightened expression of both pro-inflammatory and pro-healing markers even in the absence of a polarizing stimulus [119]. Additionally, confinement of macrophages was shown to decrease histone deacetylase 3 (HDAC3) and MRTF-A in the nucleus, which led to reduced inflammatory activation in response to LPS [118]. Substrate stiffness can also regulate macrophage response, conceivably through epigenetic means—however, the influences of such biophysical cues in tandem with major TAM polarizing signals on the macrophage state remain largely unknown. As both the effector activation and regulatory phenotypes of immune cells involve epigenetic reorganization, the prospect of such biophysically derived and epigenetically driven mechanisms in tumor immune cells is worth exploring for the development of anti-cancer therapies.
Tumor-Associated Vasculature Abnormalities Contribute to Tumor Immunomodulation
Vascular Abnormalities of the TME
An abnormal tumor vasculature limits the trafficking of CTLs into the tumor, as a result of angiogenesis-induced endothelial cell anergy and irregular blood flow [120]. Additionally, the TME experiences elevated solid stresses and interstitial fluid pressure, owing to the rapidly expanding mass jostling with neighboring tissue for space. Consequently, cells of the TME experience compressive, tensile, and shear forces, which might all contribute towards shaping cellular behavior. In vitro studies have demonstrated the ability of adherent immune cells to respond to mechanical forces. Notably, extracellular pressure was observed to increase DC maturity [121]. By subjecting immature DCs to an elevated pressure of 40 mmHg, it was observed that DCs express inflammatory cytokines and maturity markers [121]. However, such pressure-matured DCs displayed no rise in the expression of MHC-I or CD40, molecules that are essential for cross-presentation and co-stimulation of T cells respectively [121]. Given that cytokines present in tumors, including IL-10 and TGF-β, have negative influences on DC maturation in tumors [122], it is quite possible that the antigen recognition, uptake, and presentation capabilities of such pressure-matured DCs in tumors may not be on par with those of typical cytokine-matured DCs. Using engineered APCs that possess mutated TCR ligands, which bind to the TCR complex without spontaneous activation, it has been shown that mechanical perturbing force applied across the immunological synapse using a micropipette was sufficient to trigger calcium mobilization and T cell activation [123]. Similarly, macrophages are also sensitive to interstitial flow (IF) arising from fluid pressure in the tumor. Using a 3D culture modeling IF in tumors, it was shown that IF induces macrophages polarization toward an M2-like phenotype through integrin/Src-mediated mechanotransduction pathways and STAT3/6 [124]. Under IF, macrophages secrete TGF-β, which enhances their ability to promote cancer cell migration [124]. Macrophage motility also increases under against the direction of flow, suggesting a flow-mediated mechanism for recruitment of macrophages to tumors [124].
Additionally, the tumor solid and fluid stresses influence immune cells by promoting vascular narrowing and hypoperfusion, leading to tumor hypoxia, acidity, and nutrient deprivation. Hypoxia in the TME fuels the expression of HIF-1α and the overproduction of angiogenic factors (primarily belonging to VEGF family), prompting abnormal capillaries that are numerous, tortuous, and heterogeneous. These dense capillary and lymphatic networks are hyperpermeable to plasma proteins, and cause elevated interstitial flow to the stroma, resulting in higher compressive forces on the vasculature and consequent impaired vascular perfusion. This results in a positive feedback loop that aggravates hypoxia and acidity in the tumor [125].
Vascular Abnormalities Influence Immune Response Through Hypoxia
The deficient microcirculation in tumors drives differential immune cell infiltration, differentiation, survival/proliferation, and activation through hypoxia [126]. Macrophages in the tumor are polarized toward an M2-like pro-tumor phenotype in response to hypoxic signaling, resulting in additional TGF-β and IL-10 production sustaining the M2-like TAM polarization [127]. Hypoxia also causes the differentiation of Treg and Th17 cells from CD4+ T cells—moreover, the expression of the Treg master regulator FOXP3 is mediated by HIF-1α [128••]. Hypoxia-induced TAMs and tumor cells may upregulate the expression of inhibitory immune checkpoint molecules that act to stem the activation or induce apoptosis in effector CTLs and NK cells [108]. Myeloid-specific deletion of HIF-1α reduces tumor growth, stemming from a reduced macrophage regulatory behavior and a resultant release of T cell suppression [129]. Hypoxia also results in altered patterns of chemoattractants that recruit suppressor TAMs, MDSCs, and Tregs, while attenuating CTLs and NK cells [108, 130, 131]. For instance, hypoxia-induced EMT in hepatocellular carcinoma cells leads to increased CCL20 secretion, increasing macrophage expression of the immune checkpoint molecule indoleamine 2,3-dioxygenase (IDO), and consequently promoting Treg activity, in addition to evoking anergy, reduced proliferation, and impaired IFN-γ production in CTLs [132]. In addition, hypoxia suppresses the maturity of DCs, compromising antigen presentation, and polarizing them to a pro-inflammatory phenotype [133]. Hypoxia may also indirectly affect immune cells in the TME by promoting active ECM crosslinking and remodeling through upregulated production of LOX family enzymes in cancer cells, CAFs, and endothelial cells, and matrix metalloproteases in cancer cells, CAFs, and TAMs [128••, 134]. Hypoxia also downregulates tissue inhibitors of metalloproteinases (TIMPs) in tumors [128••]. Vascular normalization therapies aimed at the chronic hypoxia and inflammation are therefore proposed to restore normal perfusion and alleviate some of the direct and indirect immune suppressive effects of the TME [126].
Hypoxia is a known determinant of cellular behavior by enacting epigenetic reorganization of the DNA and histone landscape. For instance, hypoxia drives cell fate changes by inhibition of histone demethylases acting on both activating (open chromatin—H3K4 and H3K36 trimethylation) [135] and inhibitory (heterochromatin—H3K27 trimethylation) [136] histone marks. Furthermore, hypoxia causes global DNA hypermethylation in fibrotic tissue via elevated expression of DNA methyltransferases DNMT1 and DNMT3B [137]. DNA and histone modifications are known to have significant roles in the activation of every immune cell. For instance, pro-inflammatory stimulation using lipopolysaccharides (LPS), and IFN-γ, and M2 pro-healing stimulation using IL-4 and IL-13 polarized macrophages have both been shown to orchestrate specific genetic programs through epigenetic means [138]. One such epigenetic signature is the LPS treatment-associated induction of KDM6B, an enzyme described to erase repressive H3K27me3 marks, which permits the upregulation of a subset of inflammatory genes [139]. Similar epigenetic changes also enable the activation of T cells. For instance, CD8+ T cell activation is concomitant with increased H3 acetylation at the promoter and enhancer regions of the IFNG gene, a change that is maintained in memory T cells, enabling faster cytotoxic response to a second stimulus [140]. While the effects of hypoxia on immune cells have been characterized extensively, the contribution of epigenetic changes to differential immune activation under hypoxic conditions remains largely undescribed. It is highly probable that hypoxia can either hamper tumor clearance or promote regulatory behavior of immune cells through epigenetic alterations. Taken together with its influences on EMT-driven cancer stemness and invasiveness, hypoxia presents as a promising target for tumor tissue normalization.
The TME biophysical cues prime the tumor to eliminate, evade, or reorient immune effector types and recruit pro-oncogenic immune cells that promote immune suppression. This happens directly through mechanosensation and downstream epigenetic and transcriptional regulation, or indirectly through altered soluble cues and signaling. Targeting the TME to remove biophysical abnormalities holds the promise of improved therapeutic outcomes.
Therapeutic Normalization of the TME Can Improve Cancer Outcomes
The emergence of abnormal biophysical cues within the TME sets in motion a series of changes in resident cell behavior that further reinforce these aberrant conditions. While this reciprocal relationship is a vicious self-sustaining loop that is critically important for tumor progression, most chemotherapeutic and immunomodulatory therapies are aimed at targeting intracellular molecular abnormalities. In the following sections, we consider the utility of therapies aimed at targeting the TME as adjuvants to conventional interventions used in cancer treatment (outlined in Fig. 3).
Therapeutic normalization of the aberrant TME cues encourages positive cancer outcomes. An overview outlining the normalization of dysregulated matrix composition, vasculature, and oxygen tension within the TME and facilitation of positive cancer responses. Restoring normal matrix composition, easing compressed vasculature, and reintroducing normoxic conditions can relieve conditions that promote cancer stemness and invasiveness. TME normalization can also be aimed at reducing inflammation and CTL apoptosis, while promoting an effective immune response that aids tumor clearance. A normalized TME may be achieved by targeting the factors that contribute to matrix stiffness, deviant vasculature, and hypoxic and acidic conditions. Targeting epigenetic modifications can also serve as a potent adjuvant therapy to enhance patient outcomes
Epigenetic Interventions and the TME
CSC targeting therapies include inhibition of relevant signaling pathways such as Wnt/β-catenin and Hedgehog [42]. However, therapeutic efforts to target CSCs are rife with challenges involving the diagnostic potential of CSC biomarkers as well as the spontaneous dedifferentiation of non-CSCs. To begin with, the target potential of CSC markers has been unclear, as a specific marker may only be enriched in certain cancer subtypes or disease stages and may not be generally applicable. This has been the case for several cancers including melanoma [141], ovarian cancer [142], and leukemia [143], in which tumorigenic cells were shown to exhibit heterogeneity in surface marker expression within the tumor sample and across different patients. The proposal of CSC-targeted therapy is further complicated by the ability of non-CSCs to spontaneously dedifferentiate into stem-like states, likely giving rise to new pools of CSCs [11]. Given that biophysical cues within the TME have been shown to drive cancer stemness through epigenetic changes in the cell, epigenetic drugs could be a powerful tool in targeting cancer stemness. Indeed, DNA methyltransferase inhibitors (DNMTi), histone deacetylase inhibitors (HDACi), and lysine-specific demethylase 1 inhibitors (LSD1i) have been shown to induce differentiation of CSCs, thereby reducing tumorigenesis, and improve clinical outcomes in various cancer contexts [144,145,146].
Epigenetic drugs have also shown promise in treating specific TME aberrancies. It has been well established that hypoxic conditions promote tumor growth through enhanced angiogenesis and are coupled with HDAC1, HDAC2, and HDAC3 overexpression [147]. This upregulation has been correlated with suppression of two tumor suppressor genes, p53 and pVHL, and is coupled with the upregulation of HIF-1α and VEGF. HDAC1 inhibitor Trichostatin A recovered p53 and pVHL expression while subsequently downregulating HIF-1α and VEGF, reducing angiogenesis in a mouse model [147]. A VHL-deficient human renal carcinoma cell line was treated with a different HDACi, dacinostat, and inhibited HIF-1α transcription via a VHL-independent means, indicating HIF-1α acetylation levels likely play an important role in gene expression and tumor angiogenesis [148]. Furthermore, in both in vitro and in vivo contexts, dacinostat induced apoptosis through cell cycle arrest in myeloid leukemia, extending survival of mice and showing promise as a combination therapy with an ABL inhibitor imatinib [149].
Epigenetic drugs also serve a promising role in cancer therapy as immune cell modulators. In chemo-resistant non-small cell lung cancer (NSCLC), an HDAC6 inhibitor, ricolinostat, provided an immunostimulatory effect by T cell activation and enhanced MHCI presentation in solid tumor cells [150]. Although specific combinations of epigenetic drugs can cause toxicity in humans due to their global effects, a combination of ricolinostat and bromodomain and extraterminal domain inhibitor (iBET) JQ1 reduced T-reg cell suppression and lead to attenuation of tumor growth and extended survival in mice with NSCLC [150]. Additionally, there has been a resurgence in the study of immunotherapies involving immune checkpoint blockers (ICB) [151]. ICBs were thought of as a failed therapy due to rapid development of resistance by cancer cells, as well as an overall lack of clinical benefit. However, recent preclinical research points to a combination of epigenetic drugs and ICBs as a means to reduce drug resistance to ICB, providing a lengthened window of opportunity for treatments to take effect [151].
Shared epigenetic characteristics between the deregulated components of the aberrant TME point to the use of epigenetic drugs as a complement with other tumor therapies as the current step in the frontier in fighting the multifaceted aberrancies persisting in solid tumor growth. It is promising that the global effects of epigenetic drugs could be tailored and targeted to effect cancer phenotypes at the TME, CSC, and immune cell levels.
TME Normalization as a Strategy to Improve Patient Outcomes
The Tumor Microenvironment Presents Significant Challenges to Existing Treatments
Malignancies represent a state of destabilized tissue homeostasis, embodied by various tumor-permissive physical and functional signals. In addition to the cancer cells, there are profound changes to the various stromal components, namely fibroblasts, vasculature, immune cells, and ECM. Because the TME is a complex niche where cells of numerous identities interact, it becomes imperative to appreciate the role non-cancer cells play in determining cancer cell fate. Stromal fibrosis and ECM deposits are phases of tumorigenesis and are proposed to represent lesions that herald cancers, even in otherwise non-malignant tissue [152]. Recent studies highlight that the stroma can pose significant challenges in treating malignancies [153••]. Therapies involving even small molecule pharmaceutics can fail owing to impaired pharmacological distribution. This happens due to diffusion limitations imposed by a desmoplastic ECM or abnormal vasculature and consequent hypoperfusion. Adoptive immunotherapy and other cellular therapies must overcome additional barriers such as stromal matricellular products and immune checkpoint molecules. Tumor hypoperfusion also introduces hypoxic and acidic challenges that blunt the activity of tumor-infiltrating cells, in addition to acting as a survival pressure that selects for more resilient pro-tumor cells. Solid stresses introduced by the expanding tumor causes a “mass effect,” which can also affect normal tissue surrounding it [154••]. Increased intratumoral fluid pressure, actuated by hyperpermeable vessels lacking pericyte coverage, can contribute to the compressive resistance of the ECM [125]. Such abnormal biophysical cues are instrumental for cancer stemness, migration, EMT, and metastatic escape to a distal site of invasion. Furthermore, extracellular mechanical signals can get transduced to intracellular tension and nuclear reorganization, resulting in differential gene expression [155••]. Targeting these cancer-enabling factors would help reestablish homeostasis and subsequent healing. More importantly, reengineering the TME to normalcy by restoring normal ECM and vascular properties can allow for the delivery and penetration of conventional chemotherapeutics.
Normalization of Tumor Stromal Matrix for Restorative Healing
The aberrant tumor ECM acts as an important conduit for the unusual extracellular instructions received by resident cells. Because the dense and stiff ECM acts as a premetastatic niche that fosters cancer cell colonization, the matrix and its components have also been direct targets of interest. TGF-β secreted by CAFs and TAMs serves as a prominent upstream determinant of tumor ECM and vascular properties. Although necessary for cell cycle arrest and apoptosis in normal cells, elevated TGF-β expression has been associated with poor cancer prognosis [156]. Activation of TGF-β pathway causes elevated collagen production, inhibition of vascular pericytes, and polarization of macrophages to regulatory TAMs (reviewed in [157]). It also enables CAFs to induce EMT and support tumor-initiating cells [157]. This highlights TGF-β as a key driver of chemoresistance and invasiveness of some cancers [157]. Antibody-based TGF-β blockade has been demonstrated to halt cancer progression through improved vascular maturation, TAM inhibition, reduced collagen deposition, lower interstitial fluid pressure (IFP), and improved drug penetration [158, 159]. The anti-hypertensive drug Losartan acts as an angiotensin-II receptor antagonist. Losartan has been demonstrated to exhibit anti-fibrotic activities, stemming from the suppression of TGF-β activators such as thrombospondin-1. It was shown to reduce collagen I levels in several mice tumor models, while also improving the distribution and therapeutic efficacy of pegylated liposomal doxorubicin [160]. Used in combination with FOLFIRINOX (leucovorin, 5-fluorouracil, irinotecan, and oxaliplatin) in a phase II clinical trial, Losartan use was associated with downstaging of advanced pancreatic cancer and an R0 resection (indicating complete remission) of 61% when paired with radiographical ablation [161]. Hypoxia-induced lysyl oxidases (LOX family) are responsible for matrix collagen crosslinking, causing an increase in stiffness. LOX is also implicated in a breast to bone cancer metastasis, proposed to happen in 85% of advanced-stage disease [162, 163]. LOX inhibition is therefore an ideal target for cancer treatment. LOX inhibition by small molecule drug β-aminopropionitrile attenuates the metastatic potential of breast cancer cells [164]. However, it was associated with toxicity in non-cancerous tissue [162]. Antibody-mediated LOX inhibition in a mouse breast cancer model shows reduced metastatic potential, subdued osteolytic lesions, and lower NFATc1-driven inflammatory osteoclastogenesis [163].
Hyaluronan-rich tumors have been a target of interest due to their association with poor prognosis [165]. The hyaluronan synthase inhibitor 4-methylumbelliferone (4-MU) has been demonstrated to reduce cancer proliferation and improve chemodrug efficacy [166]. Alternatively, enzymatic treatments using hyaluronidase have been touted, with the pegylated form PEGPH20 being shown to reduce stromal swelling, regulate IFP, re-expand vasculature, improve the efficacy of chemodrug gemcitabine, and double survival rates compared to gemcitabine-only controls [167]. It was also shown to enhance CD8+ T cell accumulation and the efficacy of anti-PD-L1 treatment in a mouse breast cancer model [168]. While this was one of the most clinically advanced ECM normalization therapy, it failed phase III trials recently and did not improve survival rates in pancreatic ductal adenocarcinoma patients compared to nab-paclitaxel/gemcitabine-only treatment controls [169]. Similar enzymes that degrade the ECM have been tested with limited success—bacterial collagenase caused increased risks of toxicity from products of degradation and bovine hyaluronidase caused a significant risk of immune reactions, while relaxin treatment increased the risk of cancer dissemination [160, 170]. ECM-remodeling enzymes mediate the metastatic escape of cancer cells and represent yet another attractive therapeutic target. MMP14 blockade through the monoclonal antibody DX-2400 has been more successful, selective, and non-toxic [171]. DX-2400 treatment decreased TGF-β, polarized macrophages to anti-oncogenic effector phenotype, and increased iNOS secretions that enhanced perfusion and response to radiotherapy [171]. Similarly, heparanase inhibition can result in lower stromal remodeling, invasiveness, and angiogenesis [172]. PG545 is one such heparan sulfate mimic that was well tolerated and improved T cell tumor infiltration in a phase I clinical study [173].
Normalization of Tumor Vasculature to Ameliorate Hypoxia
Tumor microvascular normalization is aimed at restoring normal function, perfusion, normoxia, pH, and effector immune infiltration in order to reverse adverse TME conditions. Curtailing the formation of numerous malforming vessels through anti-angiogenic treatments to combat hypoxia-induced VEGF overexpression has been explored to promote regularization of the vascular network. This may be achieved by using monoclonal antibodies against VEGF (bevacizumab) or its receptor (ramucirumab), small molecule tyrosine receptor kinase inhibitors that block VEGF receptors (sunitinib, sorafenib, axitinib, and others), or VEGF traps (Aflibercept). Several of these have FDA approvals and are in use in combination with chemodrugs or immune checkpoint inhibitors, in addition to several current trials for further indications [174]. In addition, these are subjects of several current trials for further indications [174]. Since HIF-1 is directly implicated in anti-angiogenic treatment resistance, it has served as a direct target of interest. HIF blockade takes the form of inhibition of upstream regulators of HIF (mTORC1/2 pathway inhibitors, PP242), inhibition of translation (antisense oligonucleotides, EZN-2968), inhibition of stabilizing proteins (HSP90 inhibitors, 17-AAG and 17-DMAG), or inhibition of HIF-1 dimerization (via acriflavine or PT2385) [175••, 176].
Restoration of normoperfusion in the tumor requires a reduction in the compressive stresses emanating from the dense stromal ECM that cause restricted vascular flow. Anti-fibrotic and anti-inflammatory medications have been proposed to restore normoxia by reducing compressive stresses on the tumor vasculature (reviewed in [177]). Anti-inflammatory medications (e.g., COX inhibitors) trigger inflammation resolution mediators that aid in reducing the permeability of tumor microvessels and thus can regulate IFP [177, 178]. Anti-fibrotic drugs (e.g., tranilast, pirfenidone) work by blocking proliferation and TGF-β production of CAFs, while also reducing the inflammatory mediator expression by immune cells [179, 180].
The extracellular environment in solid tumors has a pH of 6.2–6.9, significantly lower than normal tissue which is maintained at 7.3–7.4 [181]. The acidic TME has been the target of several treatment regimens aimed at blocking proton ATPases, sodium/hydrogen exchangers (specifically NHE1), and carboxylate-proton cotransporters (MCT1 and MCT4) that are involved in proton efflux and overexpressed/activated in tumor cells [182•]. The transmembrane hypoxia-induced enzyme carbonic anhydrase (CAIX) is yet another target aimed at de-acidifying the TME [182•]. Such interventions may remove extracellular acidity while also decreasing intracellular pH, resulting in reduced proliferation and induction of apoptosis in cancer cells [183]. Similar interventions that aim to restore normalcy in matrix and vascular cues presented to the cells of the TME hold the promise of driving restorative healing, potentially enhancing patient response to traditional treatment regimens.
Capitalizing on the Cues Within the TME for Targeted Cancer Drug Delivery
Conventional therapies target cancer in a highly non-localized fashion. New studies indicate that the abnormal conditions of the TME can be exploited for localized and selective delivery of therapeutics. Such strategies entail, for example, the use of non-toxic prodrugs that become activated by certain conditions that exist only in the TME. Desmoplasia, hypoxia, and acidity represent some TME conditions that could allow for the selective release of therapeutic payload.
Desmoplasia is one of the common features of solid cancers. A dense, crosslinked, and aligned matrix causes the tumor mass to become several folds stiffer than local non-tumorous tissue. Stiffer tissues trigger mechanotransduction, transmitting signals to the nucleus. This often occurs through the nuclear translocation of transcriptional factors such as YAP/TAZ, MRTF-A, and TWIST1. Interestingly, all of these have been implicated as pro-oncogenic signals and are overexpressed in certain tumors. For instance, driven to the nucleus by stiffer substrates, TWIST1 is involved in activation of the genes responsible for EMT, invasion, and metastasis, in addition to those of collagens, MMPs, and LOX [184].
A recent study has demonstrated the use of engineered mechanoresponsive cellular systems enacting stiffness-selective gene expression as a novel way to deliver active drugs [185•]. This was achieved by placing the exogenous production of cytosine deaminase (CD) under the control of a YAP/TAZ-responsive element, in mesenchymal stem cells (MSCs). Upon sensing the stiffer microenvironment of a tumor, these mechanoresponsive stem cells (MRSCs) produce CD allowing for the conversion of the prodrug 5-fluorocytosine to the active drug form 5-fluorouracil. This action is local and therefore proposed to happen near the targeted cells. Since various mechanoresponsive transcriptional factors are of dissimilar molecular weights, and therefore allow for different thresholds of the substrate stiffnesses for nuclear translocation [186], it might be possible to engineer such systems to various grade of tumor stiffness. Such stiffness responsive therapies had been proposed earlier as well. Cao et al. described phage peptide-based molecular probes that could selectively bind to strained fibronectin fibers [187]. In principle, such a system could be used for fastidious molecular targeting of ECM at altered states of pathological stress.
Tumor hypoxia is yet another widespread tumor property that can be utilized for cancer targeting. This would take advantage of the reduced tumor partial oxygen pressure, which was measured to be between 2- and 22-fold lower than corresponding normoxic tissue pO2 depending on the anatomical location [188]. The most prominent of cellular adaptations to lower oxygen availability come from the activity of HIFs, the function of which relies on the redox stabilization under hypoxic conditions [128••]. HIF family transcriptional factors are responsible for several pro-oncogenic genes and ECM components, and interact with the chromatin via hypoxia-responsive elements (HRE). We propose that cell-based therapeutics that target hypoxia can be designed in a manner similar to that of the stiffness-sensing MRSCs described above. The proposed system could exploit a prodrug-converting enzyme in the control of a HRE, allowing activation upon HIF expression in hypoxic tumors [185•].
The reduced oxygen tension in tumors can be also used for targeted administration of hypoxia-sensitive prodrugs (reviewed in [189]). Such drugs are often cytotoxic agents that are either bioreductive prodrugs that become activated only in the reducing tumor environment or are already active forms that become compromised in normoxic redox conditions. These drugs function by the interference of DNA replication, intercalation, or damage. Examples of hypoxia-activated prodrugs include AQ4N (banoxantrone) that gets reduced to a potent topoisomerase II inhibitor [190], TPZ (tirapazamine) gets reduced to a DNA-damaging radical [191], and PR-104 and TH-302 which both transform into DNA crosslinkers under hypoxic stress [190, 192]. Another study has also described 33 nm nanoparticle clusters targeted toward hypoxic centers by conjugating said particles with a CCL28 ligand [193]. These particles are MMP-reactive and hence break into smaller 5 nm particles to achieve deep tumor penetration, and act as radiosensitizers, consequently producing radicals upon radiation treatment [193]. Such chemokine-based targeting opens the possibility of similar targeted nanoparticle-based drug delivery systems.
The acidic TME can also be exploited using pH-responsive drug release systems to deliver chemodrugs. Mesoporous organosilica nanoparticles loaded with doxorubicin has been demonstrated to target the acidic TME in breast cancer mouse models and achieve selective drug distribution [194]. This was achieved by modifying the particles with a pH (low) insertion peptide (pHLIP). This polypeptide is derived from the bacteriorhodopsin C helix and is capable of localization to the acidic milieu and insertion into the cell membrane, making it a potent tool for the delivery of nanoparticles-drug complexes and antisense oligonucleotides [194]. Other strategies that prepare nanoparticles for targeted delivery of drugs include the use of pH-responsive linkers, corona coatings/shells, or acid-programmable dissociation (reviewed in [195]).
The TME thus provides several abnormal properties that can be targeted for normalization. This could consequently allow for homeostatic and healing reforms in the TME while eliminating the advantages that cancer and stromal cells have over non-cancerous counterparts. TME normalization therapies may also be employed as adjuvant therapies for improved chemodrug distribution, efficacy, and cancer outcomes.
Conclusions
In this review, we highlighted (i) the acquisition of stem-like characteristics and (ii) the ability to circumvent immune clearance as important driving forces of cancer development and progression. We examined the epigenetic mechanisms involved in promoting tumor initiation and progression through modulation of CSC and immune cell activities within the TME. Such epigenetic mechanisms include histone modifications, aberrant DNA methylation patterns, chromatin remodeling, and dysregulated miRNA activity that lead to activation of significant biological processes contributing to the upregulation of stem-like properties and modulation of immune activation. We also described the tumor as a complex ecosystem composed of malignant cells under the influence of aberrant biophysical cues presented by a hypoxic and chronically inflamed TME. Specifically, matrix rigidity and altered vasculature regulate stemness by promoting the activation of relevant pathways including EMT, Wnt signaling, and Notch signaling. Additionally, we explored how said biophysical cues guide the behavior of immune cells present in the TME, including that of T cells, macrophages, and dendritic cells.
More interestingly, we recognized the coaction of biophysical and epigenetic factors in driving cancer development through exploration of the biophysical regulation of epigenetic mechanisms. The shaping of epigenetic signatures by biophysical cues has been observed in numerous studies that portray the alteration of heterochromatin dynamics in response to changing biophysical cues. In the context of cancer, methyltransferases, acetyltransferases, and other enzymes involved in epigenetic regulation are responsive to the hypoxic conditions attributed to the biomechanically aberrant TME. These observations are highly implicative of the interplay between biophysical cues and epigenetics in regulating cancer stemness and immune action thereby driving tumor initiation and progression. Future studies aimed at elucidating the regulatory and signaling mechanisms that constitute this interplay will not only advance current understanding of how tumor heterogeneity contributes to the complexity of cancer but also identify more promising therapeutic targets that will enhance scientific efforts to improve cancer patient outcomes.
This review also assessed the challenges associated with investigating cancer stemness as a potential therapeutic target. In particular, we addressed the reliability of stem cell markers in cancer therapy. We also discussed the therapeutic challenges caused by the aberrant TME, as drug delivery can be limited by the cancer-enabling activity of various checkpoint molecules as well as by the diffusional constraints presented by an abnormal vasculature and a dense stromal ECM. Additionally, we reviewed several promising therapeutic strategies that aim to normalize the aberrant properties of the TME through anti-remodeling, anti-fibrotic, anti-angiogenic, and vasodilating interventions. We explored the approach of capitalizing on biophysical cues within the TME through therapies that exploit aberrant tumor properties, such as matrix stiffness and hypoxia, to achieve efficient and highly localized drug delivery. In addition to reviewing potential biophysical targets, we also looked at the use of epigenetic drugs or inhibitors of epigenetic readers, writers, and erasers in tumor initiation and progression as a combinatorial treatment for cancer.
Overall, we recognize the development and progression of cancer as a result of acquired hallmark traits that are subject to the influence of the biophysical cues, and epigenetic mechanisms presented by the TME. The coordination of gene regulatory mechanisms across multiple cell types is critical during tumor initiation and progression, as is reflected by the upregulation of stem-like properties in the tumor and modulation of immune cell action during cancer development. Dysregulated cellular activities can potentially be corrected by therapeutic approaches that target abnormal biophysical cues and epigenetic modifications. Still, challenges remain in further understanding the biomechanically aberrant TME, and targeting cancer stemness and regulatory immune cells. However, future studies to elucidate the biophysical and epigenetic regulatory mechanisms involved alongside therapeutic efforts to normalize the TME and undo the acquisition of stem-like properties and behavioral changes in immune cells will be of great importance for the advancement of therapies to improve cancer patient outcomes.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Nowell P. The clonal evolution of tumor cell populations. Science (New York, NY). 1976;194(4260):23–8. https://doi.org/10.1126/science.959840.
Yang M, Topaloglu U, Petty WJ, Pagni M, Foley KL, Grant SC, et al. Circulating mutational portrait of cancer: manifestation of aggressive clonal events in both early and late stages. J Hematol Oncol. 2017;10(1):100. https://doi.org/10.1186/s13045-017-0468-1.
Duesberg P, McCormack A. Immortality of cancers: a consequence of inherent karyotypic variations and selections for autonomy. Cell Cycle. 2013;12(5):783–802. https://doi.org/10.4161/cc.23720.
Prasad S, Ramachandran S, Gupta N, Kaushik I, Srivastava SK. Cancer cells stemness: a doorstep to targeted therapy. Biochim Biophys Acta (BBA) - Mol Basis Dis. 1866;2020(4):165424. https://doi.org/10.1016/j.bbadis.2019.02.019.
de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6(1):24–37. https://doi.org/10.1038/nrc1782.
Ponnusamy MP, Batra SK. Ovarian cancer: emerging concept on cancer stem cells. J Ovarian Res. 2008;1(1):4. https://doi.org/10.1186/1757-2215-1-4.
Matsui W, Huff CA, Wang Q, Malehorn MT, Barber J, Tanhehco Y, et al. Characterization of clonogenic multiple myeloma cells. Blood. 2004;103(6):2332–6. https://doi.org/10.1182/blood-2003-09-3064.
Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci. 2003;100(7):3983. https://doi.org/10.1073/pnas.0530291100.
Kreso A, Dick JE. Evolution of the cancer stem cell model. Cell Stem Cell. 2014;14(3):275–91. https://doi.org/10.1016/j.stem.2014.02.006.
Batlle E, Clevers H. Cancer stem cells revisited. Nat Med. 2017;23(10):1124–34. https://doi.org/10.1038/nm.4409.
Chaffer CL, Brueckmann I, Scheel C, Kaestli AJ, Wiggins PA, Rodrigues LO, et al. Normal and neoplastic nonstem cells can spontaneously convert to a stem-like state. Proc Natl Acad Sci. 2011;108(19):7950–5. https://doi.org/10.1073/pnas.1102454108.
Wainwright EN, Scaffidi P. Epigenetics and cancer stem cells: unleashing, hijacking, and restricting cellular plasticity. Trends Cancer. 2017;3(5):372–86. https://doi.org/10.1016/j.trecan.2017.04.004.
The Cancer Genome Atlas Research N. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368(22):2059–74. https://doi.org/10.1056/NEJMoa1301689.
Lewis PW, Muller MM, Koletsky MS, Cordero F, Lin S, Banaszynski LA, et al. Inhibition of PRC2 activity by a gain-of-function H3 mutation found in pediatric glioblastoma. Science (New York, NY). 2013;340(6134):857–61. https://doi.org/10.1126/science.1232245.
Torres CM, Biran A, Burney MJ, Patel H, Henser-Brownhill T, Cohen AHS et al. The linker histone H1.0 generates epigenetic and functional intratumor heterogeneity. Science (New York, NY). 2016;353(6307):aaf1644-aaf. doi:https://doi.org/10.1126/science.aaf1644.
Funato K, Major T, Lewis PW, Allis CD, Tabar V. Use of human embryonic stem cells to model pediatric gliomas with H3.3K27M histone mutation. Science (New York, NY). 2014;346(6216):1529–33. https://doi.org/10.1126/science.1253799.
Lu R, Wang P, Parton T, Zhou Y, Chrysovergis K, Rockowitz S, et al. Epigenetic perturbations by Arg882-mutated DNMT3A potentiate aberrant stem cell gene-expression program and acute leukemia development. Cancer Cell. 2016;30(1):92–107. https://doi.org/10.1016/j.ccell.2016.05.008.
Thiery JP, Sleeman JP. Complex networks orchestrate epithelial–mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7(2):131–42. https://doi.org/10.1038/nrm1835.
Mani SA, Guo W, Liao M-J, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133(4):704–15. https://doi.org/10.1016/j.cell.2008.03.027.
Santisteban M, Reiman JM, Asiedu MK, Behrens MD, Nassar A, Kalli KR, et al. Immune-induced epithelial to mesenchymal transition in vivo generates breast cancer stem cells. Cancer Res. 2009;69(7):2887–95. https://doi.org/10.1158/0008-5472.CAN-08-3343.
Giannoni E, Bianchini F, Masieri L, Serni S, Torre E, Calorini L, et al. Reciprocal activation of prostate cancer cells and cancer-associated fibroblasts stimulates epithelial-mesenchymal transition and cancer stemness. Cancer Res. 2010;70(17):6945–56. https://doi.org/10.1158/0008-5472.CAN-10-0785.
Brabletz T, Hlubek F, Spaderna S, Schmalhofer O, Hiendlmeyer E, Jung A, et al. Invasion and metastasis in colorectal cancer: epithelial-mesenchymal transition, mesenchymal-epithelial transition, stem cells and β-catenin. Cells Tissues Organs. 2005;179(1-2):56–65. https://doi.org/10.1159/000084509.
Biddle A, Liang X, Gammon L, Fazil B, Harper LJ, Emich H, et al. Cancer stem cells in squamous cell carcinoma switch between two distinct phenotypes that are preferentially migratory or proliferative. Cancer Res. 2011;71(15):5317–26. https://doi.org/10.1158/0008-5472.CAN-11-1059.
Zhang J-Q, Chen S, Gu J-N, Zhu Y, Zhan Q, Cheng D-F, et al. MicroRNA-300 promotes apoptosis and inhibits proliferation, migration, invasion and epithelial-mesenchymal transition via the Wnt/β-catenin signaling pathway by targeting CUL4B in pancreatic cancer cells. J Cell Biochem. 2018;119(1):1027–40. https://doi.org/10.1002/jcb.26270.
Fodde R, Brabletz T. Wnt/β-catenin signaling in cancer stemness and malignant behavior. Curr Opin Cell Biol. 2007;19(2):150–8. https://doi.org/10.1016/j.ceb.2007.02.007.
Lee SC, Kim O-H, Lee SK, Kim S-J. IWR-1 inhibits epithelial-mesenchymal transition of colorectal cancer cells through suppressing Wnt/β-catenin signaling as well as survivin expression. Oncotarget. 2015;6(29). https://doi.org/10.18632/oncotarget.4354.
Zhou AD, Diao LT, Xu H, Xiao ZD, Li JH, Zhou H, et al. β-Catenin/LEF1 transactivates the microRNA-371-373 cluster that modulates the Wnt/β-catenin-signaling pathway. Oncogene. 2012;31(24):2968–78. https://doi.org/10.1038/onc.2011.461.
de Sousa E Melo F, Colak S, Buikhuisen J, Koster J, Cameron K, de Jong Joan H et al. Methylation of cancer-stem-cell-associated wnt target genes predicts poor prognosis in colorectal cancer patients. Cell Stem Cell 2011;9(5):476-485. doi:https://doi.org/10.1016/j.stem.2011.10.008.
Zhang J, Cai H, Sun L, Zhan P, Chen M, Zhang F, et al. LGR5, a novel functional glioma stem cell marker, promotes EMT by activating the Wnt/β-catenin pathway and predicts poor survival of glioma patients. J Exp Clin Cancer Res. 2018;37(1):225. https://doi.org/10.1186/s13046-018-0864-6.
Wang Y, Zhong Y, Hou T, Liao J, Zhang C, Sun C, et al. PM2.5 induces EMT and promotes CSC properties by activating Notch pathway in vivo and vitro. Ecotoxicol Environ Saf. 2019;178:159–67. https://doi.org/10.1016/j.ecoenv.2019.03.086.
Fender AW, Nutter JM, Fitzgerald TL, Bertrand FE, Sigounas G. Notch-1 promotes stemness and epithelial to mesenchymal transition in colorectal cancer: notch signaling plays a key role in the phenotype of colon cancer cells. J Cell Biochem. 2015;116(11):2517–27. https://doi.org/10.1002/jcb.25196.
Katsuno Y, Meyer DS, Zhang Z, Shokat KM, Akhurst RJ, Miyazono K, et al. Chronic TGF-β exposure drives stabilized EMT, tumor stemness, and cancer drug resistance with vulnerability to bitopic mTOR inhibition. Sci Signal. 2019;12(570):eaau8544. https://doi.org/10.1126/scisignal.aau8544.
• Bhuria V, Xing J, Scholta T, Bui KC, Nguyen MLT, Malek NP et al. Hypoxia induced Sonic Hedgehog signaling regulates cancer stemness, epithelial-to-mesenchymal transition and invasion in cholangiocarcinoma. Exp Cell Res. 2019;385(2):111671. doi:https://doi.org/10.1016/j.yexcr.2019.111671. Highlights the biophysical influence of hypoxia in upregulating expression of cancer stem cell-associated transcription factors (Nanog, Oct4, Sox2) and epithelial-to-mesenchymal transition markers (N-cadherin, Vimentin) via activation of Sonic Hedgehog signaling.
Besharat ZM, Sabato C, Po A, Gianno F, Abballe L, Napolitano M, et al. Low Expression of miR-466f-3p Sustains Epithelial to Mesenchymal Transition in Sonic Hedgehog Medulloblastoma Stem Cells Through Vegfa-Nrp2 Signaling Pathway. Front Pharmacol. 2018;9:1281. https://doi.org/10.3389/fphar.2018.01281.
Kilian KA, Bugarija B, Lahn BT, Mrksich M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc Natl Acad Sci. 2010;107(11):4872–7. https://doi.org/10.1073/pnas.0903269107.
Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–89. https://doi.org/10.1016/j.cell.2006.06.044.
Kshitiz, Park J, Kim P, Helen W, Engler AJ, Levchenko A et al. Control of stem cell fate and function by engineering physical microenvironments. Integr Biol 2012;4(9):1008-1018. doi:https://doi.org/10.1039/c2ib20080e.
O'Connor JW, Riley PN, Nalluri SM, Ashar PK, Gomez EW. Matrix rigidity mediates TGFβ1-induced epithelial-myofibroblast transition by controlling cytoskeletal organization and MRTF-A localization: matrix rigidity mediates EMT via MRTF-A. J Cell Physiol. 2015;230(8):1829–39. https://doi.org/10.1002/jcp.24895.
Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nat Rev Cancer. 2009;9(2):108–22. https://doi.org/10.1038/nrc2544.
Jain RK, Martin JD, Stylianopoulos T. The Role of Mechanical Forces in Tumor Growth and Therapy. Annu Rev Biomed Eng. 2014;16(1):321–46. https://doi.org/10.1146/annurev-bioeng-071813-105259.
Tang X, Kuhlenschmidt TB, Zhou J, Bell P, Wang F, Kuhlenschmidt MS, et al. Mechanical force affects expression of an in vitro metastasis-like phenotype in HCT-8 cells. Biophys J. 2010;99(8):2460–9. https://doi.org/10.1016/j.bpj.2010.08.034.
Nallanthighal S, Heiserman JP, Cheon DJ. The role of the extracellular matrix in cancer stemness. Front Cell Dev Biol. 2019;7:86. https://doi.org/10.3389/fcell.2019.00086.
Kirkland SC. Type I collagen inhibits differentiation and promotes a stem cell-like phenotype in human colorectal carcinoma cells. Br J Cancer. 2009;101(2):320–6. https://doi.org/10.1038/sj.bjc.6605143.
Seguin L, Desgrosellier JS, Weis SM, Cheresh DA. Integrins and cancer: regulators of cancer stemness, metastasis, and drug resistance. Trends Cell Biol. 2015;25(4):234–40. https://doi.org/10.1016/j.tcb.2014.12.006.
Barnawi R, Al-Khaldi S, Colak D, Tulbah A, Al-Tweigeri T, Fallatah M, et al. β1 Integrin is essential for fascin-mediated breast cancer stem cell function and disease progression. Int J Cancer. 2019;145(3):830–41. https://doi.org/10.1002/ijc.32183.
Pang M-F, Siedlik MJ, Han S, Stallings-Mann M, Radisky DC, Nelson CM. Tissue stiffness and hypoxia modulate the integrin-linked kinase ILK to control breast cancer stem-like cells. Cancer Res. 2016;76(18):5277–87. https://doi.org/10.1158/0008-5472.CAN-16-0579.
Inoue A, Takahashi H, Harada H, Kohno S, Ohue S, Kobayashi K, et al. Cancer stem-like cells of glioblastoma characteristically express MMP-13 and display highly invasive activity. Int J Oncol. 2010;37(5):1121–31. https://doi.org/10.3892/ijo_00000764.
Long H, Xie R, Xiang T, Zhao Z, Lin S, Liang Z, et al. Autocrine CCL5 signaling promotes invasion and migration of CD133+ ovarian cancer stem-like cells via NF-κB-mediated MMP-9 upregulation. Stem Cells (Dayton, Ohio). 2012;30(10):2309–19. https://doi.org/10.1002/stem.1194.
Tse JM, Cheng G, Tyrrell JA, Wilcox-Adelman SA, Boucher Y, Jain RK, et al. Mechanical compression drives cancer cells toward invasive phenotype. Proc Natl Acad Sci. 2012;109(3):911–6. https://doi.org/10.1073/pnas.1118910109.
Brabletz T, Jung A, Reu S, Porzner M, Hlubek F, Kunz-Schughart LA, et al. Variable β-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc Natl Acad Sci. 2001;98(18):10356–61. https://doi.org/10.1073/pnas.171610498.
Yang M-H, Wu K-J. TWIST activation by hypoxia inducible factor-1 (HIF-1): Implications in metastasis and development. Cell Cycle. 2008;7(14):2090–6. https://doi.org/10.4161/cc.7.14.6324.
•• Roy B, Venkatachalapathy S, Ratna P, Wang Y, Jokhun DS, Nagarajan M et al. Laterally confined growth of cells induces nuclear reprogramming in the absence of exogenous biochemical factors. Proceedings of the National Academy of Sciences. 2018;115(21):E4741. doi:https://doi.org/10.1073/pnas.1714770115. This study demonstrates that the sustained laterally confined growth of mouse fibroblasts on micropatterned substrates can activate stem cell-like properties, thereby emphasizing the importance of biophysical cues in regulating stemness. Laterally confined growth was also shown to induce cancer stemness in the breast cancer cell line MCF7.
Hancock RL, Dunne K, Walport LJ, Flashman E, Kawamura A. Epigenetic regulation by histone demethylases in hypoxia. Epigenomics. 2015;7(5):791–811. https://doi.org/10.2217/epi.15.24.
Tan Y, Tajik A, Chen J, Jia Q, Chowdhury F, Wang L, et al. Matrix softness regulates plasticity of tumour-repopulating cells via H3K9 demethylation and Sox2 expression. Nat Commun. 2014;5:4619. https://doi.org/10.1038/ncomms5619.
Varelas X. The Hippo pathway effectors TAZ and YAP in development, homeostasis and disease. Development. 2014;141(8):1614–26. https://doi.org/10.1242/dev.102376.
Park JH, Shin JE, Park HW. The role of hippo pathway in cancer stem cell biology. Mol Cell. 2018;41(2):83–92. https://doi.org/10.14348/MOLCELLS.2018.2242.
Cordenonsi M, Zanconato F, Azzolin L, Forcato M, Rosato A, Frasson C, et al. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell. 2011;147(4):759–72. https://doi.org/10.1016/j.cell.2011.09.048.
Yimlamai D, Christodoulou C, Galli GG, Yanger K, Pepe-Mooney B, Gurung B, et al. Hippo pathway activity influences liver cell fate. Cell. 2014;157(6):1324–38. https://doi.org/10.1016/j.cell.2014.03.060.
Fischer M, Rikeit P, Knaus P, Coirault C. YAP-Mediated mechanotransduction in skeletal muscle. Front Physiol. 2016;7:41. https://doi.org/10.3389/fphys.2016.00041.
Aragona M, Panciera T, Manfrin A, Giulitti S, Michielin F, Elvassore N, et al. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell. 2013;154(5):1047–59. https://doi.org/10.1016/j.cell.2013.07.042.
Benham-Pyle BW, Pruitt BL, Nelson WJ. Cell adhesion. Mechanical strain induces E-cadherin-dependent Yap1 and β-catenin activation to drive cell cycle entry. Science (New York, NY). 2015;348(6238):1024–7. https://doi.org/10.1126/science.aaa4559.
Morita T, Mayanagi T, Sobue K. Dual roles of myocardin-related transcription factors in epithelial–mesenchymal transition via slug induction and actin remodeling. J Cell Biol. 2007;179(5):1027–42. https://doi.org/10.1083/jcb.200708174.
Miralles F, Posern G, Zaromytidou A-I, Treisman R. Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell. 2003;113(3):329–42. https://doi.org/10.1016/S0092-8674(03)00278-2.
Fan L, Sebe A, Péterfi Z, Masszi A, Thirone AC, Rotstein OD, et al. Cell contact-dependent regulation of epithelial-myofibroblast transition via the rho-rho kinase-phospho-myosin pathway. Mol Biol Cell. 2007;18(3):1083–97. https://doi.org/10.1091/mbc.e06-07-0602.
O’Connor JW, Gomez EW. Cell adhesion and shape regulate TGF-Beta1-induced epithelial-myofibroblast transition via MRTF-A signaling. PLoS One. 2013;8(12):e83188. https://doi.org/10.1371/journal.pone.0083188.
Cheng X, Yang Y, Fan Z, Yu L, Bai H, Zhou B, et al. MKL1 potentiates lung cancer cell migration and invasion by epigenetically activating MMP9 transcription. Oncogene. 2015;34(44):5570–81. https://doi.org/10.1038/onc.2015.14.
Liao XH, Wang N, Liu LY, Zheng L, Xing WJ, Zhao DW, et al. MRTF-A and STAT3 synergistically promote breast cancer cell migration. Cell Signal. 2014;26(11):2370–80. https://doi.org/10.1016/j.cellsig.2014.07.023.
Song Z, Liu Z, Sun J, Sun FL, Li CZ, Sun JZ, et al. The MRTF-A/B function as oncogenes in pancreatic cancer. Oncol Rep. 2016;35(1):127–38. https://doi.org/10.3892/or.2015.4329.
Makhija E, Jokhun DS, Shivashankar GV. Nuclear deformability and telomere dynamics are regulated by cell geometric constraints. Proc Natl Acad Sci. 2016;113(1):E32. https://doi.org/10.1073/pnas.1513189113.
•• Wang P, Dreger M, Madrazo E, Williams CJ, Samaniego R, Hodson NW et al. WDR5 modulates cell motility and morphology and controls nuclear changes induced by a 3D environment. Proc Natl Acad Sci U S A. 2018;115(34):8581-8586. https://doi.org/10.1073/pnas.1719405115. WD repeat domain 5 (WDR5) is an epigenetic modulator of H3K4 methylation that is essential to the regulation of nuclear deformability, cell polarity, and cell migration in vitro. This study reveals that the interactions between cells and a surrounding 3D environment leads to upregulation of WDR5-mediated H3K4 methylation, thus highlighting the recurring theme of biophysical and epigenetic regulation of cellular behavior.
Jain N, Iyer KV, Kumar A, Shivashankar GV. Cell geometric constraints induce modular gene-expression patterns via redistribution of HDAC3 regulated by actomyosin contractility. Proc Natl Acad Sci. 2013;110(28):11349. https://doi.org/10.1073/pnas.1300801110.
Downing TL, Soto J, Morez C, Houssin T, Fritz A, Yuan F, et al. Biophysical regulation of epigenetic state and cell reprogramming. Nat Mater. 2013;12(12):1154–62. https://doi.org/10.1038/nmat3777.
Wu M-Z, Tsai Y-P, Yang M-H, Huang C-H, Chang S-Y, Chang C-C, et al. Interplay between HDAC3 and WDR5 is essential for hypoxia-induced epithelial-mesenchymal transition. Mol Cell. 2011;43(5):811–22. https://doi.org/10.1016/j.molcel.2011.07.012.
Wu MZ, Chen SF, Nieh S, Benner C, Ger LP, Jan CI, et al. Hypoxia drives breast tumor malignancy through a TET-TNFα-p38-MAPK signaling axis. Cancer Res. 2015;75(18):3912–24. https://doi.org/10.1158/0008-5472.Can-14-3208.
Liu Q, Liu L, Zhao Y, Zhang J, Wang D, Chen J, et al. Hypoxia induces genomic DNA demethylation through the activation of HIF-1alpha and transcriptional upregulation of MAT2A in hepatoma cells. Mol Cancer Ther. 2011;10(6):1113–23. https://doi.org/10.1158/1535-7163.MCT-10-1010.
Bissell MJ, Hines WC. Why don't we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med. 2011;17(3):320–9. https://doi.org/10.1038/nm.2328.
Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. https://doi.org/10.1016/s0092-8674(00)81683-9.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. https://doi.org/10.1016/j.cell.2011.02.013.
Haas OA. Primary immunodeficiency and cancer predisposition revisited: embedding two closely related concepts into an integrative conceptual framework. Front Immunol. 2018;9:3136. https://doi.org/10.3389/fimmu.2018.03136.
Dantal J, Soulillou JP. Immunosuppressive drugs and the risk of cancer after organ transplantation. N Engl J Med. 2005;352(13):1371–3. https://doi.org/10.1056/NEJMe058018.
Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, et al. IFNγ and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410(6832):1107–11. https://doi.org/10.1038/35074122.
Girardi M, Oppenheim DE, Steele CR, Lewis JM, Glusac E, Filler R, et al. Regulation of cutaneous malignancy by gammadelta T cells. Science (New York, NY). 2001;294(5542):605–9. https://doi.org/10.1126/science.1063916.
Street SE, Cretney E, Smyth MJ. Perforin and interferon-gamma activities independently control tumor initiation, growth, and metastasis. Blood. 2001;97(1):192–7. https://doi.org/10.1182/blood.v97.1.192.
Enzler T, Gillessen S, Manis JP, Ferguson D, Fleming J, Alt FW, et al. Deficiencies of GM-CSF and interferon gamma link inflammation and cancer. J Exp Med. 2003;197(9):1213–9. https://doi.org/10.1084/jem.20021258.
Kather JN, Suarez-Carmona M, Charoentong P, Weis CA, Hirsch D, Bankhead P, et al. Topography of cancer-associated immune cells in human solid tumors. Elife. 2018;7. https://doi.org/10.7554/eLife.36967.
Salmon H, Franciszkiewicz K, Damotte D, Dieu-Nosjean MC, Validire P, Trautmann A, et al. Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J Clin Invest. 2012;122(3):899–910. https://doi.org/10.1172/JCI45817.
Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science (New York, NY). 2006;313(5795):1960. https://doi.org/10.1126/science.1129139.
Tosolini M, Kirilovsky A, Mlecnik B, Fredriksen T, Mauger S, Bindea G, et al. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res. 2011;71(4):1263–71. https://doi.org/10.1158/0008-5472.CAN-10-2907.
Solito S, Marigo I, Pinton L, Damuzzo V, Mandruzzato S, Bronte V. Myeloid-derived suppressor cell heterogeneity in human cancers. Ann N Y Acad Sci. 2014;1319:47–65. https://doi.org/10.1111/nyas.12469.
Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–44. https://doi.org/10.1038/nature07205.
Q-w Z, Liu L, Gong C-y, Shi H-s, Zeng Y-h, Wang X-z, et al. Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PLoS One. 2012;7(12):e50946. https://doi.org/10.1371/journal.pone.0050946.
• Kuczek DE, Larsen AMH, Thorseth ML, Carretta M, Kalvisa A, Siersbaek MS, et al. Collagen density regulates the activity of tumor-infiltrating T cells. J Immunother Cancer. 2019;7(1):68. https://doi.org/10.1186/s40425-019-0556-6This study connects 3D ECM density with the proliferation and functional activation of T cells using transcriptome analysis.
Judokusumo E, Tabdanov E, Kumari S, Dustin ML, Kam LC. Mechanosensing in T lymphocyte activation. Biophys J. 2012;102(2):L5–7. https://doi.org/10.1016/j.bpj.2011.12.011.
O'Connor RS, Hao X, Shen K, Bashour K, Akimova T, Hancock WW, et al. Substrate rigidity regulates human T cell activation and proliferation. J Immunol. 2012;189(3):1330–9. https://doi.org/10.4049/jimmunol.1102757.
Lin HH, Lin HK, Lin IH, Chiou YW, Chen HW, Liu CY, et al. Mechanical phenotype of cancer cells: cell softening and loss of stiffness sensing. Oncotarget. 2015;6(25):20946–58. https://doi.org/10.18632/oncotarget.4173.
Mennens SFB, Bolomini-Vittori M, Weiden J, Joosten B, Cambi A, van den Dries K. Substrate stiffness influences phenotype and function of human antigen-presenting dendritic cells. Sci Rep. 2017;7(1):17511. https://doi.org/10.1038/s41598-017-17787-z.
Meli VS, Veerasubramanian PK, Atcha H, Reitz Z, Downing TL, Liu WF. Biophysical regulation of macrophages in health and disease. J Leukoc Biol. 2019;106(2):283–99. https://doi.org/10.1002/jlb.Mr0318-126r.
Luu TU, Liu WF. Regulation of macrophages by extracellular matrix composition and adhesion geometry. Regen Eng Transl Med. 2018;4(4):238–46. https://doi.org/10.1007/s40883-018-0065-z.
McWhorter FY, Wang T, Nguyen P, Chung T, Liu WF. Modulation of macrophage phenotype by cell shape. Proc Natl Acad Sci U S A. 2013;110(43):17253–8. https://doi.org/10.1073/pnas.1308887110.
Armstrong JW, Chapes SK. Effects of extracellular matrix proteins on macrophage differentiation, growth, and function: comparison of liquid and agar culture systems. J Exp Zool. 1994;269(3):178–87. https://doi.org/10.1002/jez.1402690303.
Cha BH, Shin SR, Leijten J, Li YC, Singh S, Liu JC, et al. Integrin-mediated interactions control macrophage polarization in 3D hydrogels. Adv Healthc Mater. 2017, 21;6. https://doi.org/10.1002/adhm.201700289.
Hsieh JY, Smith TD, Meli VS, Tran TN, Botvinick EL, Liu WF. Differential regulation of macrophage inflammatory activation by fibrin and fibrinogen. Acta Biomater. 2017;47:14–24. https://doi.org/10.1016/j.actbio.2016.09.024.
Hsieh JY, Keating MT, Smith TD, Meli VS, Botvinick EL, Liu WF. Matrix crosslinking enhances macrophage adhesion, migration, and inflammatory activation. APL Bioeng. 2019;3(1):016103. https://doi.org/10.1063/1.5067301.
Previtera ML, Sengupta A. Substrate stiffness regulates proinflammatory mediator production through TLR4 activity in macrophages. PLoS One. 2016;10(12):e0145813. https://doi.org/10.1371/journal.pone.0145813.
• Pinto ML, Rios E, Silva AC, Neves SC, Caires HR, Pinto AT, et al. Decellularized human colorectal cancer matrices polarize macrophages towards an anti-inflammatory phenotype promoting cancer cell invasion via CCL18. Biomaterials. 2017;124:211–24. https://doi.org/10.1016/j.biomaterials.2017.02.004This study demonstrates the potential of the tumor matrix in educating macrophages towards the anti-inflammatory M2 phenotype that stimulated cancer cell invasion.
Savage ND, de Boer T, Walburg KV, Joosten SA, van Meijgaarden K, Geluk A, et al. Human anti-inflammatory macrophages induce Foxp3+ GITR+ CD25+ regulatory T cells, which suppress via membrane-bound TGFbeta-1. J Immunol. 2008;181(3):2220–6. https://doi.org/10.4049/jimmunol.181.3.2220.
Peranzoni E, Lemoine J, Vimeux L, Feuillet V, Barrin S, Kantari-Mimoun C, et al. Macrophages impede CD8 T cells from reaching tumor cells and limit the efficacy of anti-PD-1 treatment. Proc Natl Acad Sci U S A. 2018;115(17):E4041–E50. https://doi.org/10.1073/pnas.1720948115.
Noman MZ, Hasmim M, Messai Y, Terry S, Kieda C, Janji B, et al. Hypoxia: a key player in antitumor immune response. A review in the theme: cellular responses to hypoxia. Am J Phys Cell Physiol. 2015;309(9):C569–79. https://doi.org/10.1152/ajpcell.00207.2015.
Lin L, Chen YS, Yao YD, Chen JQ, Chen JN, Huang SY, et al. CCL18 from tumor-associated macrophages promotes angiogenesis in breast cancer. Oncotarget. 2015;6(33):34758–73. https://doi.org/10.18632/oncotarget.5325.
Lebbink RJ, de Ruiter T, Adelmeijer J, Brenkman AB, van Helvoort JM, Koch M, et al. Collagens are functional, high affinity ligands for the inhibitory immune receptor LAIR-1. J Exp Med. 2006;203(6):1419–25. https://doi.org/10.1084/jem.20052554.
Rygiel TP, Stolte EH, de Ruiter T, van de Weijer ML, Meyaard L. Tumor-expressed collagens can modulate immune cell function through the inhibitory collagen receptor LAIR-1. Mol Immunol. 2011;49(1-2):402–6. https://doi.org/10.1016/j.molimm.2011.09.006.
Agashe VV, Jankowska-Gan E, Keller M, Sullivan JA, Haynes LD, Kernien JF et al. Leukocyte-associated Ig-like receptor 1 inhibits T<sub>h</sub>1 responses but is required for natural and induced monocyte-dependent T<sub>h</sub>17 responses. J Immunol. 2018:ji1701753. doi:https://doi.org/10.4049/jimmunol.1701753.
Li Y, Chu JS, Kurpinski K, Li X, Bautista DM, Yang L, et al. Biophysical regulation of histone acetylation in mesenchymal stem cells. Biophys J. 2011;100(8):1902–9. https://doi.org/10.1016/j.bpj.2011.03.008.
Zanconato F, Battilana G, Forcato M, Filippi L, Azzolin L, Manfrin A, et al. Transcriptional addiction in cancer cells is mediated by YAP/TAZ through BRD4. Nat Med. 2018;24(10):1599–610. https://doi.org/10.1038/s41591-018-0158-8.
Ni X, Tao J, Barbi J, Chen Q, Park BV, Li Z, et al. YAP is essential for Treg-mediated suppression of antitumor immunity. Cancer Discov. 2018;8(8):1026–43. https://doi.org/10.1158/2159-8290.CD-17-1124.
Stampouloglou E, Cheng N, Federico A, Slaby E, Monti S, Szeto GL, et al. Yap suppresses T-cell function and infiltration in the tumor microenvironment. PLoS Biol. 2020;18(1):e3000591. https://doi.org/10.1371/journal.pbio.3000591.
Yu L, Weng X, Liang P, Dai X, Wu X, Xu H, et al. MRTF-A mediates LPS-induced pro-inflammatory transcription by interacting with the COMPASS complex. J Cell Sci. 2014;127(Pt 21):4645–57. https://doi.org/10.1242/jcs.152314.
Jain N, Vogel V. Spatial confinement downsizes the inflammatory response of macrophages. Nat Mater. 2018;17(12):1134–44. https://doi.org/10.1038/s41563-018-0190-6.
Cabanel M, Brand C, Oliveira-Nunes MC, Cabral-Piccin MP, Lopes MF, Brito JM, et al. Epigenetic control of macrophage shape transition towards an atypical elongated phenotype by histone deacetylase activity. PLoS One. 2015;10(7):e0132984. https://doi.org/10.1371/journal.pone.0132984.
Bellone M, Calcinotto A. Ways to enhance lymphocyte trafficking into tumors and fitness of tumor infiltrating lymphocytes. Front Oncol. 2013;3:231. https://doi.org/10.3389/fonc.2013.00231.
Craig DH, Shiratsuchi H, Basson MD. Increased extracellular pressure provides a novel adjuvant stimulus for enhancement of conventional dendritic cell maturation strategies. Biochem Biophys Res Commun. 2009;387(1):174–9. https://doi.org/10.1016/j.bbrc.2009.07.010.
Thepmalee C, Panya A, Junking M, Chieochansin T, Yenchitsomanus P-T. Inhibition of IL-10 and TGF-β receptors on dendritic cells enhances activation of effector T-cells to kill cholangiocarcinoma cells. Hum Vaccin Immunother. 2018;14(6):1423–31. https://doi.org/10.1080/21645515.2018.1431598.
Li YC, Chen BM, Wu PC, Cheng TL, Kao LS, Tao MH, et al. Cutting edge: mechanical forces acting on T cells immobilized via the TCR complex can trigger TCR signaling. J Immunol. 2010;184(11):5959–63. https://doi.org/10.4049/jimmunol.0900775.
Li R, Serrano JC, Xing H, Lee TA, Azizgolshani H, Zaman M, et al. Interstitial flow promotes macrophage polarization toward an M2 phenotype. Mol Biol Cell. 2018;29(16):1927–40.
Carmeliet P, Jain RK. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov. 2011;10(6):417–27. https://doi.org/10.1038/nrd3455.
Huang Y, Goel S, Duda DG, Fukumura D, Jain RK. Vascular normalization as an emerging strategy to enhance cancer immunotherapy. Cancer Res. 2013;73(10):2943–8. https://doi.org/10.1158/0008-5472.Can-12-4354.
Murdoch C, Lewis CE. Macrophage migration and gene expression in response to tumor hypoxia. Int J Cancer. 2005;117(5):701–8. https://doi.org/10.1002/ijc.21422.
•• Petrova V, Annicchiarico-Petruzzelli M, Melino G, Amelio I. The hypoxic tumour microenvironment. Oncogenesis. 2018;7(1):10. https://doi.org/10.1038/s41389-017-0011-9This review summarizes the contributions of hypoxia and the HIFs on the various components of the TME.
Doedens AL, Stockmann C, Rubinstein MP, Liao D, Zhang N, DeNardo DG, et al. Macrophage expression of hypoxia-inducible factor-1 alpha suppresses T-cell function and promotes tumor progression. Cancer Res. 2010;70(19):7465–75. https://doi.org/10.1158/0008-5472.CAN-10-1439.
Facciabene A, Peng X, Hagemann IS, Balint K, Barchetti A, Wang LP, et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature. 2011;475(7355):226–30. https://doi.org/10.1038/nature10169.
Multhoff G, Vaupel P. Hypoxia compromises anti-cancer immune responses. Adv Exp Med Biol. 2020;1232:131–43. https://doi.org/10.1007/978-3-030-34461-0_18.
Ye LY, Chen W, Bai XL, Xu XY, Zhang Q, Xia XF, et al. Hypoxia-induced epithelial-to-mesenchymal transition in hepatocellular carcinoma induces an immunosuppressive tumor microenvironment to promote metastasis. Cancer Res. 2016;76(4):818–30. https://doi.org/10.1158/0008-5472.CAN-15-0977.
Pierobon D, Bosco MC, Blengio F, Raggi F, Eva A, Filippi M, et al. Chronic hypoxia reprograms human immature dendritic cells by inducing a proinflammatory phenotype and TREM-1 expression. Eur J Immunol. 2013;43(4):949–66. https://doi.org/10.1002/eji.201242709.
Henze A-T, Mazzone M. The impact of hypoxia on tumor-associated macrophages. J Clin Invest. 2016;126(10):3672–9. https://doi.org/10.1172/JCI84427.
Batie M, Frost J, Frost M, Wilson JW, Schofield P, Rocha S. Hypoxia induces rapid changes to histone methylation and reprograms chromatin. Science (New York, NY). 2019;363(6432):1222. https://doi.org/10.1126/science.aau5870.
Chakraborty AA, Laukka T, Myllykoski M, Ringel AE, Booker MA, Tolstorukov MY, et al. Histone demethylase KDM6A directly senses oxygen to control chromatin and cell fate. Science (New York, NY). 2019;363(6432):1217. https://doi.org/10.1126/science.aaw1026.
Watson CJ, Collier P, Tea I, Neary R, Watson JA, Robinson C, et al. Hypoxia-induced epigenetic modifications are associated with cardiac tissue fibrosis and the development of a myofibroblast-like phenotype. Hum Mol Genet. 2014;23(8):2176–88. https://doi.org/10.1093/hmg/ddt614.
Kittan NA, Allen RM, Dhaliwal A, Cavassani KA, Schaller M, Gallagher KA, et al. Cytokine induced phenotypic and epigenetic signatures are key to establishing specific macrophage phenotypes. PLoS One. 2013;8(10):e78045. https://doi.org/10.1371/journal.pone.0078045.
De Santa F, Narang V, Yap ZH, Tusi BK, Burgold T, Austenaa L, et al. Jmjd3 contributes to the control of gene expression in LPS-activated macrophages. EMBO J. 2009;28(21):3341–52. https://doi.org/10.1038/emboj.2009.271.
Northrop JK, Thomas RM, Wells AD, Shen H. Epigenetic remodeling of the IL-2 and IFN-γ loci in memory CD8 T cells is influenced by CD4 T cells. J Immunol. 2006;177(2):1062. https://doi.org/10.4049/jimmunol.177.2.1062.
Quintana E, Shackleton M, Foster HR, Fullen DR, Sabel MS, Johnson TM, et al. Phenotypic heterogeneity among tumorigenic melanoma cells from patients that is reversible and not hierarchically organized. Cancer Cell. 2010;18(5):510–23. https://doi.org/10.1016/j.ccr.2010.10.012.
Stewart JM, Shaw PA, Gedye C, Bernardini MQ, Neel BG, Ailles LE. Phenotypic heterogeneity and instability of human ovarian tumor-initiating cells. Proc Natl Acad Sci. 2011;108(16):6468–73. https://doi.org/10.1073/pnas.1005529108.
Eppert K, Takenaka K, Lechman ER, Waldron L, Nilsson B, van Galen P, et al. Stem cell gene expression programs influence clinical outcome in human leukemia. Nat Med. 2011;17(9):1086–93. https://doi.org/10.1038/nm.2415.
Zagorac S, Alcala S, Fernandez Bayon G, Bou Kheir T, Schoenhals M, Gonzalez-Neira A, et al. DNMT1 inhibition reprograms pancreatic cancer stem cells via upregulation of the miR-17-92 cluster. Cancer Res. 2016;76(15):4546–58. https://doi.org/10.1158/0008-5472.CAN-15-3268.
Pathania R, Ramachandran S, Mariappan G, Thakur P, Shi H, Choi JH, et al. Combined Inhibition of DNMT and HDAC blocks the tumorigenicity of cancer stem-like cells and attenuates mammary tumor growth. Cancer Res. 2016;76(11):3224–35. https://doi.org/10.1158/0008-5472.CAN-15-2249.
Anastas JN, Zee BM, Kalin JH, Kim M, Guo R, Alexandrescu S, et al. Re-programing chromatin with a bifunctional LSD1/HDAC inhibitor induces therapeutic differentiation in DIPG. Cancer Cell. 2019;36(5):528–44 e10. https://doi.org/10.1016/j.ccell.2019.09.005.
Kim MS, Kwon HJ, Lee YM, Baek JH, Jang JE, Lee SW, et al. Histone deacetylases induce angiogenesis by negative regulation of tumor suppressor genes. Nat Med. 2001;7(4):437–43. https://doi.org/10.1038/86507.
Qian DZ, Kachhap SK, Collis SJ, Verheul HM, Carducci MA, Atadja P, et al. Class II histone deacetylases are associated with VHL-independent regulation of hypoxia-inducible factor 1 alpha. Cancer Res. 2006;66(17):8814–21. https://doi.org/10.1158/0008-5472.CAN-05-4598.
Weisberg E, Catley L, Kujawa J, Atadja P, Remiszewski S, Fuerst P, et al. Histone deacetylase inhibitor NVP-LAQ824 has significant activity against myeloid leukemia cells in vitro and in vivo. Leukemia. 2004;18(12):1951–63. https://doi.org/10.1038/sj.leu.2403519.
Adeegbe DO, Liu Y, Lizotte PH, Kamihara Y, Aref AR, Almonte C, et al. Synergistic immunostimulatory effects and therapeutic benefit of combined histone deacetylase and bromodomain inhibition in non-small cell lung cancer. Cancer Discov. 2017;7(8):852–67. https://doi.org/10.1158/2159-8290.CD-16-1020.
Topper MJ, Vaz M, Marrone KA, Brahmer JR, Baylin SB. The emerging role of epigenetic therapeutics in immuno-oncology. Nat Rev Clin Oncol. 2020;17(2):75–90. https://doi.org/10.1038/s41571-019-0266-5.
Brucher BL, Jamall IS. Epistemology of the origin of cancer: a new paradigm. BMC Cancer. 2014;14:331. https://doi.org/10.1186/1471-2407-14-331.
•• Valkenburg KC, de Groot AE, Pienta KJ. Targeting the tumour stroma to improve cancer therapy. Nat Rev Clin Oncol. 2018;15(6):366–81. https://doi.org/10.1038/s41571-018-0007-1. A comprehensive review that explores the evidence available on stromal normalization in cancer therapy.
•• Seano G, Nia HT, Emblem KE, Datta M, Ren J, Krishnan S, et al. Solid stress in brain tumours causes neuronal loss and neurological dysfunction and can be reversed by lithium. Nat Biomed Eng. 2019;3(3):230–45. https://doi.org/10.1038/s41551-018-0334-7. An excellent study that shows therapeutic intervention and microenvironment normalization in action in brain tumors. It also serves as an example of how tissue normalization can guide better clinical outcomes.
•• Uhler C, Shivashankar GV. Nuclear mechanopathology and cancer diagnosis. Trends Cancer. 2018;4(4):320–31. https://doi.org/10.1016/j.trecan.2018.02.009Explores the regulation of nuclear morphology by components of the cellular microenvironment, including cytoskeletal architecture and the extracellular matrix. This review also addresses the innovative use of machine learning to classify cancer cells via analysis of nuclear morphology.
Guinney J, Dienstmann R, Wang X, de Reynies A, Schlicker A, Soneson C, et al. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21(11):1350–6. https://doi.org/10.1038/nm.3967.
Colak S, Ten Dijke P. Targeting TGF-beta signaling in cancer. Trends Cancer. 2017;3(1):56–71. https://doi.org/10.1016/j.trecan.2016.11.008.
Liu J, Liao S, Diop-Frimpong B, Chen W, Goel S, Naxerova K, et al. TGF-beta blockade improves the distribution and efficacy of therapeutics in breast carcinoma by normalizing the tumor stroma. Proc Natl Acad Sci U S A. 2012;109(41):16618–23. https://doi.org/10.1073/pnas.1117610109.
Calon A, Lonardo E, Berenguer-Llergo A, Espinet E, Hernando-Momblona X, Iglesias M, et al. Stromal gene expression defines poor-prognosis subtypes in colorectal cancer. Nat Genet. 2015;47(4):320–9. https://doi.org/10.1038/ng.3225.
Diop-Frimpong B, Chauhan VP, Krane S, Boucher Y, Jain RK. Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proc Natl Acad Sci U S A. 2011;108(7):2909–14. https://doi.org/10.1073/pnas.1018892108.
Murphy JE, Wo JY, Ryan DP, Clark JW, Jiang W, Yeap BY, et al. Total neoadjuvant therapy with FOLFIRINOX in combination with losartan followed by chemoradiotherapy for locally advanced pancreatic cancer: a phase 2 clinical trial. JAMA Oncol. 2019;5(7):1020–7. https://doi.org/10.1001/jamaoncol.2019.0892.
Cox TR, Gartland A, Erler JT. Lysyl oxidase, a targetable secreted molecule involved in cancer metastasis. Cancer Res. 2016;76(2):188–92. https://doi.org/10.1158/0008-5472.CAN-15-2306.
Cox TR, Rumney RM, Schoof EM, Perryman L, Høye AM, Agrawal A, et al. The hypoxic cancer secretome induces pre-metastatic bone lesions through lysyl oxidase. Nature. 2015;522(7554):106–10.
Bondareva A, Downey CM, Ayres F, Liu W, Boyd SK, Hallgrimsson B, et al. The lysyl oxidase inhibitor, β-aminopropionitrile, diminishes the metastatic colonization potential of circulating breast cancer cells. PLoS One. 2009;4(5).
Karousou E, D'Angelo ML, Kouvidi K, Vigetti D, Viola M, Nikitovic D, et al. Collagen VI and hyaluronan: the common role in breast cancer. Biomed Res Int. 2014;2014:606458. https://doi.org/10.1155/2014/606458.
Nakazawa H, Yoshihara S, Kudo D, Morohashi H, Kakizaki I, Kon A, et al. 4-methylumbelliferone, a hyaluronan synthase suppressor, enhances the anticancer activity of gemcitabine in human pancreatic cancer cells. Cancer Chemother Pharmacol. 2006;57(2):165–70. https://doi.org/10.1007/s00280-005-0016-5.
Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21(3):418–29. https://doi.org/10.1016/j.ccr.2012.01.007.
Clift R, Lee J, Thompson CB, Huang Y. PEGylated recombinant hyaluronidase PH20 (PEGPH20) enhances tumor infiltrating CD8+ T cell accumulation and improves checkpoint inhibitor efficacy in murine syngeneic breast cancer models. AACR; 2017.
Tempero MA, Van Cutsem E, Sigal D, Oh D-Y, Fazio N, Macarulla T, et al. HALO 109-301: A randomized, double-blind, placebo-controlled, phase 3 study of pegvorhyaluronidase alfa (PEGPH20)+ nab-paclitaxel/gemcitabine (AG) in patients (pts) with previously untreated hyaluronan (HA)-high metastatic pancreatic ductal adenocarcinoma (mPDA). In: American Society of Clinical Oncology; 2020.
Dolor A, Szoka FC Jr. Digesting a path forward: the utility of collagenase tumor treatment for improved drug delivery. Mol Pharm. 2018;15(6):2069–83. https://doi.org/10.1021/acs.molpharmaceut.8b00319.
Ager EI, Kozin SV, Kirkpatrick ND, Seano G, Kodack DP, Askoxylakis V, et al. Blockade of MMP14 activity in murine breast carcinomas: implications for macrophages, vessels, and radiotherapy. J Natl Cancer Inst. 2015;107(4). https://doi.org/10.1093/jnci/djv017.
Dredge K, Hammond E, Handley P, Gonda TJ, Smith MT, Vincent C, et al. PG545, a dual heparanase and angiogenesis inhibitor, induces potent anti-tumour and anti-metastatic efficacy in preclinical models. Br J Cancer. 2011;104(4):635–42. https://doi.org/10.1038/bjc.2011.11.
Hammond E, Haynes NM, Cullinane C, Brennan TV, Bampton D, Handley P, et al. Immunomodulatory activities of pixatimod: emerging nonclinical and clinical data, and its potential utility in combination with PD-1 inhibitors. J Immunother Cancer. 2018;6(1):54. https://doi.org/10.1186/s40425-018-0363-5.
Hegde PS, Wallin JJ, Mancao C. Predictive markers of anti-VEGF and emerging role of angiogenesis inhibitors as immunotherapeutics. Semin Cancer Biol. 2018;52:117–24. https://doi.org/10.1016/j.semcancer.2017.12.002.
•• Rey S, Schito L, Wouters BG, Eliasof S, Kerbel RS. Targeting hypoxia-inducible factors for antiangiogenic cancer therapy. Trends Cancer. 2017;3(7):529–41. https://doi.org/10.1016/j.trecan.2017.05.002. The hypoxic tumor microenvironment triggers angiogenesis and can aid in tumor resistance to antiangiogenic cancer therapy. This review discusses the target potential of hypoxia-inducible factors in improving antiangiogenic cancer therapy.
Wigerup C, Pahlman S, Bexell D. Therapeutic targeting of hypoxia and hypoxia-inducible factors in cancer. Pharmacol Ther. 2016;164:152–69. https://doi.org/10.1016/j.pharmthera.2016.04.009.
Gkretsi V, Zacharia LC, Stylianopoulos T. Targeting inflammation to improve tumor drug delivery. Trends Cancer. 2017;3(9):621–30. https://doi.org/10.1016/j.trecan.2017.07.006.
Pakneshan P, Birsner AE, Adini I, Becker CM, D'Amato RJ. Differential suppression of vascular permeability and corneal angiogenesis by nonsteroidal anti-inflammatory drugs. Invest Ophthalmol Vis Sci. 2008;49(9):3909–13. https://doi.org/10.1167/iovs.07-1527.
King TE Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2083–92. https://doi.org/10.1056/NEJMoa1402582.
Ohshio Y, Hanaoka J, Kontani K, Teramoto K. Tranilast inhibits the function of cancer-associated fibroblasts responsible for the induction of immune suppressor cell types. Scand J Immunol. 2014;80(6):408–16. https://doi.org/10.1111/sji.12242.
Shirmanova MV, Druzhkova IN, Lukina MM, Matlashov ME, Belousov VV, Snopova LB, et al. Intracellular pH imaging in cancer cells in vitro and tumors in vivo using the new genetically encoded sensor SypHer2. Biochim Biophys Acta. 2015;1850(9):1905–11. https://doi.org/10.1016/j.bbagen.2015.05.001.
• Zhong S, Jeong JH, Chen Z, Chen Z, Luo JL. Targeting tumor microenvironment by small-molecule inhibitors. Transl Oncol. 2020;13(1):57–69. https://doi.org/10.1016/j.tranon.2019.10.001. Highlights the promise of targeting the more therapeutically accessible tumor microenvironment via small molecule inhibitors. This review summarizes recent advances in small-molecule inhibition of various tumor microenvironment components, including hypoxia, acidity, and immune signaling.
McCarty MF, Whitaker J. Manipulating tumor acidification as a cancer treatment strategy. Altern Med Rev. 2010;15(3):264–72.
Wei SC, Fattet L, Tsai JH, Guo Y, Pai VH, Majeski HE, et al. Matrix stiffness drives epithelial-mesenchymal transition and tumour metastasis through a TWIST1-G3BP2 mechanotransduction pathway. Nat Cell Biol. 2015;17(5):678–88. https://doi.org/10.1038/ncb3157.
• Liu L, Zhang SX, Liao W, Farhoodi HP, Wong CW, Chen CC, et al. Mechanoresponsive stem cells to target cancer metastases through biophysical cues. Sci Transl Med. 2017;9(400). https://doi.org/10.1126/scitranslmed.aan2966. This study uses the increased local stiffness of tumors to target them using novel mechanosensitive cell therapeutics.
Elosegui-Artola A, Andreu I, Beedle AEM, Lezamiz A, Uroz M, Kosmalska AJ et al. Force triggers YAP nuclear entry by regulating transport across nuclear pores. Cell 2017;171(6):1397-1410.e14. doi:https://doi.org/10.1016/j.cell.2017.10.008.
Cao L, Zeller MK, Fiore VF, Strane P, Bermudez H, Barker TH. Phage-based molecular probes that discriminate force-induced structural states of fibronectin in vivo. Proc Natl Acad Sci U S A. 2012;109(19):7251–6. https://doi.org/10.1073/pnas.1118088109.
McKeown SR. Defining normoxia, physoxia and hypoxia in tumours-implications for treatment response. Br J Radiol. 2014;87(1035):20130676. https://doi.org/10.1259/bjr.20130676.
Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer. 2011;11(6):393–410. https://doi.org/10.1038/nrc3064.
Papadopoulos KP, Goel S, Beeram M, Wong A, Desai K, Haigentz M, et al. A phase 1 open-label, accelerated dose-escalation study of the hypoxia-activated prodrug AQ4N in patients with advanced malignancies. Clin Cancer Res. 2008;14(21):7110–5. https://doi.org/10.1158/1078-0432.CCR-08-0483.
Cowen RL, Williams KJ, Chinje EC, Jaffar M, Sheppard FC, Telfer BA, et al. Hypoxia targeted gene therapy to increase the efficacy of tirapazamine as an adjuvant to radiotherapy: reversing tumor radioresistance and effecting cure. Cancer Res. 2004;64(4):1396–402. https://doi.org/10.1158/0008-5472.can-03-2698.
Meng F, Evans JW, Bhupathi D, Banica M, Lan L, Lorente G, et al. Molecular and cellular pharmacology of the hypoxia-activated prodrug TH-302. Mol Cancer Ther. 2012;11(3):740–51. https://doi.org/10.1158/1535-7163.MCT-11-0634.
Huo D, Liu S, Zhang C, He J, Zhou Z, Zhang H, et al. Hypoxia-targeting, tumor microenvironment responsive nanocluster bomb for radical-enhanced radiotherapy. ACS Nano. 2017;11(10):10159–74. https://doi.org/10.1021/acsnano.7b04737.
Zhang Y, Dang M, Tian Y, Zhu Y, Liu W, Tian W, et al. Tumor acidic microenvironment targeted drug delivery based on pHLIP-modified mesoporous organosilica nanoparticles. ACS Appl Mater Interfaces. 2017;9(36):30543–52. https://doi.org/10.1021/acsami.7b10840.
Feng L, Dong Z, Tao D, Zhang Y, Liu Z. The acidic tumor microenvironment: a target for smart cancer nano-theranostics. Natl Sci Rev. 2017;5(2):269–86. https://doi.org/10.1093/nsr/nwx062.
Broske AM, Vockentanz L, Kharazi S, Huska MR, Mancini E, Scheller M, et al. DNA methylation protects hematopoietic stem cell multipotency from myeloerythroid restriction. Nat Genet. 2009;41(11):1207–15. https://doi.org/10.1038/ng.463.
Lu R, Wang P, Parton T, Zhou Y, Chrysovergis K, Rockowitz S, et al. Epigenetic perturbations by Arg882-mutated DNMT3A potentiate aberrant stem cell gene-expression program and acute leukemia development. Cancer Cell. 2016;30(1):92–107.
Liu CC, Lin JH, Hsu TW, Su K, Li AFY, Hsu HS, et al. IL-6 enriched lung cancer stem-like cell population by inhibition of cell cycle regulators via DNMT1 upregulation. Int J Cancer. 2015;136(3):547–59.
Klarmann GJ, Decker A, Farrar WL. Epigenetic gene silencing in the Wnt pathway in breast cancer. Epigenetics. 2008;3(2):59–63.
Helou RE, Wicinski J, Guille A, Adelaide J, Finetti P, Bertucci F et al. A distinct DNA methylation signature defines breast cancer stem cells and predict cancer outcome. AACR; 2014.
Suzuki H, Watkins DN, Jair K-W, Schuebel KE, Markowitz SD, Dong Chen W, et al. Epigenetic inactivation of SFRP genes allows constitutive WNT signaling in colorectal cancer. Nat Genet. 2004;36(4):417–22. https://doi.org/10.1038/ng1330.
Yoda Y, Takeshima H, Niwa T, Kim JG, Ando T, Kushima R, et al. Integrated analysis of cancer-related pathways affected by genetic and epigenetic alterations in gastric cancer. Gastric Cancer. 2015;18(1):65–76.
Versteege I, Sévenet N, Lange J, Rousseau-Merck M-F, Ambros P, Handgretinger R, et al. Truncating mutations of hSNF5/INI1 in aggressive paediatric cancer. Nature. 1998;394(6689):203–6.
Wang Y, He L, Du Y, Zhu P, Huang G, Luo J, et al. The long noncoding RNA lncTCF7 promotes self-renewal of human liver cancer stem cells through activation of Wnt signaling. Cell Stem Cell. 2015;16(4):413–25.
Shi J, Whyte WA, Zepeda-Mendoza CJ, Milazzo JP, Shen C, Roe J-S, et al. Role of SWI/SNF in acute leukemia maintenance and enhancer-mediated Myc regulation. Genes Dev. 2013;27(24):2648–62.
Varela I, Tarpey P, Raine K, Huang D, Ong CK, Stephens P, et al. Exome sequencing identifies frequent mutation of the SWI/SNF complex gene PBRM1 in renal carcinoma. Nature. 2011;469(7331):539–42.
Yu A, Rual J-F, Tamai K, Harada Y, Vidal M, He X, et al. Association of dishevelled with the clathrin AP-2 adaptor is required for frizzled endocytosis and planar cell polarity signaling. Dev Cell. 2007;12(1):129–41.
Rheinbay E, Suvà ML, Gillespie SM, Wakimoto H, Patel AP, Shahid M, et al. An aberrant transcription factor network essential for Wnt signaling and stem cell maintenance in glioblastoma. Cell Rep. 2013;3(5):1567–79.
Chan KM, Han J, Fang D, Gan H, Zhang Z. A lesson learned from the H3. 3K27M mutation found in pediatric glioma: a new approach to the study of the function of histone modifications in vivo? Cell Cycle. 2013;12(16):2546–52.
Ghoshal P, Nganga AJ, Moran-Giuati J, Szafranek A, Johnson TR, Bigelow AJ, et al. Loss of the SMRT/NCoR2 corepressor correlates with JAG2 overexpression in multiple myeloma. Cancer Res. 2009;69(10):4380–7.
Liu C, Liu L, Shan J, Shen J, Xu Y, Zhang Q, et al. Histone deacetylase 3 participates in self-renewal of liver cancer stem cells through histone modification. Cancer Lett. 2013;339(1):60–9.
Chen X, Sun K, Jiao S, Cai N, Zhao X, Zou H, et al. High levels of SIRT1 expression enhance tumorigenesis and associate with a poor prognosis of colorectal carcinoma patients. Sci Rep. 2014;4(1):1–12.
Yu F, Jiao Y, Zhu Y, Wang Y, Zhu J, Cui X, et al. MicroRNA 34c gene down-regulation via DNA methylation promotes self-renewal and epithelial-mesenchymal transition in breast tumor-initiating cells. J Biol Chem. 2012;287(1):465–73.
Liu C, Kelnar K, Liu B, Chen X, Calhoun-Davis T, Li H, et al. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat Med. 2011;17(2):211.
Khorrami S, Hosseini AZ, Mowla SJ, Ghanbari R. Differential microRNA expression profile of colon cancer stem cells derived from primary tumor and HT-29 cell line. Int J Cancer Manag. 2017;10(8).
Acknowledgments
This work was supported by the National Institutes of Health (NIH) National Institute of Allergy and Infectious Disease (NIAID) Grant R21AI128519-01 to W.F.L, NIH National Institute of Biomedical Imaging and Bioengineering (NIBIB) Grant R21EB027840-01 to T.L.D and W.F.L, and NIH New Innovator Award (DP2) Grant DP2CA250382-01, a National Science Foundation (NSF) grant (DMS1763272) and a grant from the Simons Foundation (594598 QN) to T.L.D. N.A. was supported by a T32 training grant from the NIH (T32HL116270).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Cell Behavior Manipulation
Rights and permissions
About this article
Cite this article
Veerasubramanian, P.K., Trinh, A., Akhtar, N. et al. Biophysical and Epigenetic Regulation of Cancer Stemness, Invasiveness, and Immune Action. Curr. Tissue Microenviron. Rep. 1, 277–300 (2020). https://doi.org/10.1007/s43152-020-00021-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43152-020-00021-w