Abstract
Microenvironment plays a key role in controlling stem cell fate and thereby regulating tissue homeostasis and repair. It consists of acellular and cellular components that interact with stem cells and their progenitors and through signaling cascades influence balance between self-renewal, differentiation and dormancy. Under pathological conditions, disruptions in microenvironment can generate signals that stimulate untimely or aberrant stem cell differentiation or self-renewal, or activate de-differentiation of progenitor cells, leading to diseased states such as cancer. However, while unaltered microenvironment can restrain transformed cell behavior inhibiting malignant phenotypes, transformed cancer cells that exhibit resistance to conventional therapies and tumor initiating capacity are capable of inducing more permissive and immunotolerant microenvironment that promotes tumor growth and metastasis. Better understanding of their behavior and interactions with microenvironment opens up novel avenues for devising more efficacious cancer therapies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Epithelial-to-mesenchymal transition
- Hypoxia
- Immunosurveillance
- Microenvironment
- Niche-stem cell interactions
- Stem cells
- behavior
- Tumorigenesis
Introduction
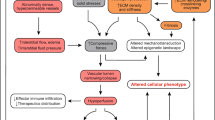
Stem cells represent populations of cells within organism that are capable of self-renewal and differentiation into one or more cell types that form a specific tissue (uni- or multi- potent stem cells, respectively) or the whole body (pluripotent or omnipotent stem cells). Orderly development and tissue repair require tight balance between arrested stem cell state (dormancy), stem cell self-renewal and differentiation, and in case of multipotent and pluripotent stem cells, between multiple differentiation pathways. This is achieved by stringent control of stem cell behavior exerted through signals from their microenvironment (niche). For instance, signals stemming from cell-cell and cell-matrix interactions, changes in oxygen concentration (i.e. hypoxia) and nutrient availability, all affect stem cell state and phenotype. Escape from and/or deregulation of the signaling cues present in microenvironment loosens control over stem cell function and results in disruption of tissue homeostasis leading to pathological states. Understanding the mechanisms involved in the cross talk between acellular and cellular elements of the microenvironment and their effects on normal and cancer stem cell behavior is crucial for the development of more efficacious treatments for pathological states associated with aberrant stem and/or progenitor cell phenotypes such as cancer.
Stem Cells’ Behavior Is Controlled by Their Niches: Examples of Epidermal Stem Cell Niche and of Hypoxia
Embryonic development and multiple physiologic processes depend on timely and regulated activation and differentiation of stem cells, processes controlled by signals from the niche. Typically, the stem cell niche is a defined anatomical compartment that includes cellular and acellular components that integrate both systemic and local cues to regulate the biology of stem cells. Cells, blood vessels, matrix glycoproteins, and the three-dimensional space that is formed from this architecture provide a highly specialized microenvironment for a stem cell. Stem cell niches facilitate the interaction between the stem cell and surrounding cells in a spatially and temporally defined manner that maintains tissue homeostasis. Niche includes extracellular components as well as diffusible factors to provide the proper regulation of the stem cell.
Epidermal stem cell niche. We will illustrate the role of the niche with an example of interfollicular epidermal stem cells in human skin. In humans large portions of the skin lack hair folicules and stem cells appear to be dispersed along the basal compartment of the interfollicular epidermis. While here, specific niche is difficult to define in morphological terms, patterns or gradients in structural elements and/or positive and negative signals generate niches that enable maintenance and functionality of the stem cell population. Even in the absence of morphological separation present in more commonly described epidermal stem cell hair buldge niche of the mouse, it still incorporates all the key interactions present in other stem cell niches. In both cases, epidermal stem cells reside in fine-tuned microenvironments that are controlled by constant cell–cell and cell–matrix interactions. They sit on and, through integrin and other receptors interact with, a specialized extracellular matrix layer called basement membrane that provides an interface between the epidermis and the underlying dermis. It is made from a complex network of extracellular matrix molecules, including several laminins, type IV collagen, nidogen and perlecan, all of which are necessary for its native structure and epidermal tissue formation. Cellular constituents of the niche include fibroblasts, endothelial cells and inflammatory cells and, presumably, also neighboring keratinocytes and melanocytes, Merkel cells and Langerhans cells. When three-dimensional in vitro model of human skin (dermal equivalent), was modified in a way that allowed for the formation of an authentic dermis-like matrix, it enabled long-term regeneration of the epidermis (Boehnke et al. 2007). These studies also confirmed the major impact of keratinocytes on ECM assembly and maturation in the dermis and clearly underlined the relevance of mutual epithelial–mesenchymal interaction for establishing a proper stem cell niche.
Epidermal stem cell maintenance is likely regulated by Notch ligand Delta1 whose expression is confined to the basal layer of human epidermis, with highest levels in regions presumably harboring stem cells. Furthermore, epidermal stem cell differentiation and maintenance of interfollicular epidermis are determined by a fine-tuned balance between intracellular levels of Notch and p63. Another crucial regulator of homeostasis in the interfollicular epidermis is the EGFR with its multiple mediators such as amphiregulin, epiregulin, heparin binding-epidermal growth factor and transforming growth factor (TGF)-α, all acting in an autocrine and paracrine manner. The role for several major EGFR-activated pathways is described including MAPK, PI3K/AKT, JAK/STAT and PKC cascades. Antagonizing interaction of EGFR and Notch family members in the differentiating epidermis resulted in their mutual downregulation strongly arguing for their essential functional interdependence.
Role of hypoxia. Stem cell fate is further regulated by conditions in the microenvironment. For example, low oxygen tension (hypoxia) maintains undifferentiated states of embryonic, hematopoietic, mesenchymal, and neural stem cell phenotypes and also influences their proliferation and cell-fate commitment. It has been hypothesized that the presence of low oxygen tension in stem cell niches offers a selective advantage that is well suited to their particular biological role. That is, essentially all cells that undergo aerobic metabolism are subject to some degree of oxidative stress through the generation of reactive oxygen species that can damage DNA. This effect is demonstrated by the fact that mouse embryonic fibroblasts accumulate more mutations and senesce faster when cultured under 20% oxygen than cells cultured under 3% oxygen (Parrinello et al. 2003). By residing in anatomical compartments that experience relatively low oxygen tensions (in the range of 1–9%), stem cells may escape this damage and maintain low proliferation rate. In addition, hypoxia has been shown in multiple stem cell systems to activate molecular pathways that control Oct4 and Notch signaling, two important regulators of stemness. Indeed, human ESC derivation from single embryonic cells (blastomeres) has been enhanced under mildly hypoxic conditions (8%) and eliminated the need for serum, essential ingredient for blastomere derivation under 20% oxygen. This gives further support to the notion that lower oxygen tension promotes better survival and self-renewal of pluripotent ESC (Ilic et al. 2009). Finally, oxygen tensions as low as 1% appear to decrease proliferation and maintain ESC pluripotency, while higher oxygen tensions (3–5%) appear to maintain pluripotency with no effect on proliferation. These results suggest that proliferation and perhaps even stem cell dormancy may be regulated by gradients of oxygen tension supplied by stem cells’ local niche.
Hypoxia appears to maintain an immature blast-like quality in mouse hematopoietic stem cells (HSC) with a primitive phenotype and enhanced engraftment capabilities (Eliasson and Jonsson 2010). Several investigators have demonstrated that slow-cycling HSC are more likely to localize in the low oxygen areas of the marrow, away from blood vessels, whereas fast cycling early hematopoietic progenitors with limited capacity for self-renewal reside in areas much closer to vasculature. Although hypoxic cultivation of bone marrow cells has been shown to increase their ability to repopulate and engraft, it is still unclear whether these effects are due to direct action on HSCs or other stromal elements, as many of these experiments were performed with whole marrow or partially purified cell populations (Eliasson and Jonsson 2010). HSCs present in the hypoxic niche express higher levels of Notch-1, telomerase, and the cell-cycle inhibitor p21 than cells closer to the vasculature (Jang and Sharkis 2007). Remarkably, extremely low oxygen tensions (0.1%) push CD34+ cells into an essentially quiescent state (Hermitte et al. 2006). HIF-1 has emerged as a likely candidate for this regulatory mechanism, as several groups have demonstrated that HIF can mediate cell-cycle arrest in multiple cell lines. In addition, mice with defective HIF signaling exhibit numerous hematopoietic pathologies with prominent defects in hematopoiesis that are embryonically lethal (Eliasson and Jonsson 2010). Collectively, this evidence suggests that hypoxia is a critical component of the HSC niche, and exposure of HSC to elevated oxygen tensions negatively affects their self-renewal while promoting cell-cycle entry and differentiation.
Hypoxia also likely plays a role in a neural stem cell (NSC) maintenance. In the human brain, partial oxygen pressure (pO2) varies from approximately 3% (23 mmHg) to 4% (33 mmHg), demonstrating a physiological oxygen gradient that is the highest in the alveolar space and the lowest in tissues where NSC likely reside. It is thus, likely that NSCs in the brain are located in a relatively hypoxic environment. Thus, in addition to the intercellular signals, soluble factors, blood vessels, and the extracellular matrix proteins found in neurogenic niches, oxygen tension may be an additional important component of the neural stem cell niche. In vitro, hypoxia is able to promote an undifferentiated state in neural crest stem cells and NSC. Observations regarding an enhancement in survival and proliferation of NSCs in mild hypoxic conditions have also been made (Pistollato et al. 2007). It has been shown that p53 phosphorylation increases in cultures maintained at 20% oxygen resulting in cell-cycle arrest, decreased proliferation, and differentiation of NSCs toward the glial lineage (Pistollato et al. 2007). This finding suggests that oxygen tensions in the environment influence both NSC stemness (or the maintenance of an undifferentiated state) by inhibiting their differentiation and their specific fate by modulating important intracellular pathways such as p53 and Notch signaling (Pistollato et al. 2007). Therefore, oxygen tension in the neural niche functions to maintain stem cell self-renewal and an undifferentiated state. Although direct measurements of oxygen tension of the subventricular zone (SVZ) have not been made, our current understanding of the cytoarchitecture and its relation to adjacent blood cells suggests that oxygen can be limiting near the ependymal surface where the neural stem cells reside. In conclusion, a hypoxic microenvironment facilitates stemness and prevents NSC from differentiating. Changes in redox status or other local cues mobilize the NSC population to proliferate, differentiate, or migrate.
Other factors such as nutrient availability also affect stem cell behavior. The mammalian target of rapamycin (mTOR) seems to play a key role within a cell in integrating a myriad of external and internal signals including niche oxygen levels and nutrient availability, cell energy status, presence of cellular stressors, and growth factors. The finely tuned response of mTOR to these stimuli results in alterations in stem cell metabolism, differentiation and cell growth, playing a major role in stem cell homeostasis and lifespan determination (reviewed in (Russell et al. 2011)).
Disruption of Niche-Stem Cell Interactions Induces Pathological States: Premature Aging and Tumorigenesis
Disruptions/failures in stem cell regulation lead to pathological states such as premature aging and cancer. For example, untimely and altered differentiation of mesenchimal stem cells (MSC) by upregulated downstream Notch signaling causes premature-aging disease Hutchinson–Gilford Progeria Syndrome (HGPS). This is due to a mutant version of lamin A protein called progerin that increases availability of the SKIP, nuclear matrix-associated co-activator of the Notch targets’ transcription. Significantly, activation of Notch pathway not only induced premature differentiation of MSC, but also enhanced osteogenesis while inhibiting differentiation of hMSCs into adipose tissue altering a balance between multiple differentiation pathways (Scaffidi and Misteli 2008). Alterations in stem cell regulation can also be caused by external signals and insults from the microenvironment such as ionizing radiation. For example, ionizing radiation-induced premature differentiation of melanocyte stem cells and resulting melanocyte stem cells depletion lead to irreversible hair graying (Inomata et al. 2009).
The altered niche environment can also induce aberrant activation of stem cell phenotype and self-renewal, the phenomenon that may lead to tumorigenesis. There is strong evidence to support the role of permissive microenvironment in promoting tumorigenesis both at premalignant and malignant stages. Multiple studies have shown that tumor-associated stroma can promote tumorigenesis by creating pro-inflammatory microenvironment. For example, tumor associated-fibroblasts isolated from the tumor stroma produce plethora of pro-inflammatory cytokines and growth factors and stimulate malignant transformation of multiple epithelial cell types including breast and prostate (Aboussekhra 2011). Pro-inflammatory secretome including IL-1, IL-6, IL-8 and GROα, has also been shown to contribute to pro-carcinogenic microenvironment associated with aging and stress-induced cell senescence (Coppe et al. 2010). Moreover, these cytokines have been shown to play key role in supporting cancer stem cell phenotypes and stem cell self-renewal (Krtolica et al. 2011; Korkaya et al. 2012). Indeed, recent evidence suggests that secretion of IL-1 by carcinoma cells attracts MSC to tumor-associated stroma and via prostaglandin E2 signaling induces MSC to generate pro-inflammatory cytokines that promote β catenin activation and cancer stem cell phenotype (Li et al. 2012).
However, microenvironment can also restrict cell behavior and, in some instances, restrain frankly malignant state through direct control of growth and invasiveness. For example, Weaver and colleagues have shown that malignant phenotype of breast tumor cells can be reversed in three-dimensional culture and in vivo by integrin β1 blocking antibodies which induced them to form polarized acini and cease growth (Weaver et al. 1997). Additionally, microenvironment can exert control through immunosurveillance – immune system-mediated tumor cell recognition and consequent destruction (see discussion below).
Tumor-Initiating and Cancer Stem Cell-Like State Is Affected by Niche/Microenvironment
There is currently growing evidence for the presence of cancer stem-like or tumor initiating cells in multiple tumor types – both hematological malignancies and solid cancers (Bonnet and Dick 1997; Reya et al. 2001). Cancer stem cells (CSC) or tumor initiating cells (TIC) are functionally defined by their potential to recapitulate tumor from which they were isolated at the single cell level. To this end, they are usually identified using serial transplantation experiments where limited number of cells isolated from the original tumor are transplanted into recipient animals and, once tumor is formed, this procedure is repeated multiple times with additional animals demonstrating CSC/TIC ability to initiate new tumors. New evidence demonstrates CSC/TIC clonal capacity even within natural tumor niches (Chen et al. 2012; Driessens et al. 2012; Schepers et al. 2012). These characteristics imply an unlimited proliferative capacity and also an ability to differentiate into all cell types present in the given tumor. What makes CSC/TIC-like populations within tumor even more therapeutically relevant is that their phenotype is often associated with high resistance to common therapeutic modalities of cancer treatment: chemotherapy and ionizing radiation. It is therefore, hypothesized, that they may be the major source of tumor re-growth and patient relapse after therapy. Indeed, there is growing clinical evidence that this may be the case (Li et al. 2008).
The ability of cancer cells to establish themselves in a foreign cellular environment is an essential characteristic of successful metastasis and a defining characteristic of CSC/TICs. Furthermore, there is some evidence that CSC/TIC phenotype may significantly overlap with phenotype of cells undergoing epithelial to mesenchymal transition (EMT), a phenomenon associated with increased tumor aggressiveness and metastasis. EMT is driven by transcription factors, including SNAIL1/2, ZEB1/2, or TWIST1/2, which increase the invasiveness of epithelial cells. In several studies, the induction of EMT has been shown to enhance self-renewal and the acquisition of CSC characteristics (Ansieau et al. 2008). In contrast, some studies demonstrate that tumor cells with an epithelial, not mesenchymal, phenotype survive in the circulation and form distant metastasis (Celia-Terrassa et al. 2012). For example, in prostate cancer cell lines, subpopulations with a strong epithelial gene program were enriched in highly metastatic CSC/TIC, whereas mesenchymal subpopulations showed reduced numbers of CSC/TIC. Collectively, these studies illustrate cancer stem cell plasticity and the fact that cell-type specific characteristics govern their self-renewal and mesenchymal gene interactions (Celia-Terrassa et al. 2012). Nevertheless, these data taken together with CSC/TIC chemo- and radiation-therapy resistance and capacity to form new tumors, suggest that it is quite likely that the cells with CSC/TIC characteristics may be the main sources of metastasis.
Importantly, every aspect of CSC/TIC behavior is under influence of microenvironment. For example, presence of drugs in tumor microenvironment and circulation may support survival and provide growth advantage to drug resistant tumor cells such as those expressing CSC/TIC phenotypes. Another example are hypoxic tumor microenvironments that often harbor quiescent (non-dividing) and tumor initiating cell populations. Hypoxia maintains the stem-like phenotype and prevents differentiation of CSC/TIC. It has been shown to promote self-renewal of glioblastoma and colorectal cancer stem cell-like CSC/TIC populations by inducing PI(3)K and ERK 1/2 pathways and regulating CDX1 and Notch1, respectively. While the degree to which quiescent and CSC/TIC populations overlap in hypoxic regions remains to be elucidated and may vary between different stages and types of tumors, it is clear that hypoxic tumor cells exhibit high resistance to common chemotherapeutic agents and are thus, likely responsible for tumor reoccurrence and, potentially, metastasis. Consequently, tumor hypoxia has been shown to correlate with poorer patient outcomes in multiple cancer types including colon, breast, prostate, and brain cancer.
Yet another effect of microenvironment is exerted through provisional extracellular matrix laid down by tumor-associated fibroblasts. It may promote migration and invasiveness thru integrin-fibronectin interactions and support survival of cells that have undergone EMT and are capable of metastasizing from the primary tumor. Tumor-associated fibroblasts and MSC present in tumor-activated stroma can also secrete a number of cytokines and growth factors creating a pro-inflammatory environment. This, in turn, can both directly affect CSC/TIC proliferation and motility (see discussion above) and also, modulate immune response (see next section).
Modulating the Effects of Microenvironment: Examples of Escaping Immunosurveillance and of Promoting Epithelial-to-Mesenchymal Transition
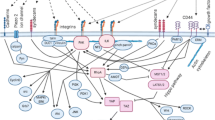
Tumor cell microenvironment contains, and is altered by, various components of both innate and adaptive immune system and their products. Depending on tumor immunogenicity, both initial tumor formation and progression of the disease can be significantly affected by host anti-tumor responses. By targeting premalignant and malignant cells in a process called immunosurveillance, immune responses can eliminate cancer cells prior to tumors becoming clinically apparent or can attenuate tumor growth and progression. However, mounting evidence shows that CSC/TIC effectively evade host immunosurveillance through multiple mechanisms including altered immunogenicity, production of immunomodulatory factors and direct interactions with tumor-infiltrating immune cells. We will discuss these different mechanisms in the following paragraphs.
MHC class I molecules are one of two primary classes of major histocompatibility complex (MHC) molecules and are found on every nucleated cell of the body. Their function is to present fragments of proteasome-degraded cellular and external proteins to cytotoxic T cells (CTL, CD8+). T cell receptors (TCR) and CD8 co-receptors on CTL plasma membrane interact with MHC I molecules that present the foreign protein fragments (antigens) on the surface of the affected cell. This interaction activates CTL to lyse the foreign antigen presenting cell.
MHC class I molecules play a key role in the immune recognition of transformed cells. It was recently reported that CSC/TIC may predominantly lose MHC class I molecules, and selectively silence the expression of tumor-associated antigens (TAAs), whose presence is associated with differentiated state, leading to resistance to immune rejection. Indeed, selective or general downregulation of MHC class I molecules may suppress the ability of class I MHC-restricted CTL to lyse CSC/TIC.
Consequently, flow cytometry analysis of glioblastoma multiforme (GBM) and astrocytoma CSC/TIC-enriched CD133+ cell fractions revealed that the majority of CD133+ cells did not express detectable MHC class I molecules or natural killer (NK) cell activating ligands on their cell surface (Wu et al. 2007). This may render them resistant to adaptive and innate immune surveillance. In addition, defects were found in the expression of antigen-processing machinery (APM) components in the cultured population of GBM-derived CSC/TIC (GSC; (Di Tomaso et al. 2010)). APM molecules included MHC class I molecules and their heavy chains (i.e., A-HC), β2-microglobulin, constitutive proteasome subunits (δ, MB1, and Z), immunoproteasome (LMP2, LMP7, and LMP10), transporter molecules (TAP), and chaperon molecules (tapasin, calnexin, calreticulin, and ERp57). While low levels of expression were also detected in corresponding fetal bovine serum-derived tumor cell lines in most cases expression was higher in tumor cell lines than in CSC/TIC (CSC/TIC are typically isolated under serum-free conditions, since serum constituents induce their differentiation into progenitor tumor cell types that have lower tumorigenic capacity). These results are in line with the previously reported decreased expression of MHC and APM molecules in a variety of human tumors and derived cell lines and suggest that in CSC/TIC isolated from these tumor types there is low efficiency in antigen processing and presentation. Therefore, CSC/TIC may display unique immunophenotypes, such as downregulation of MHC class I molecules, differentiation-associated TAAs and APM components, that enable them to effectively evade host immunosurveillance.
Another mechanism by which CSC/TIC may avoid the attack of immune system, is by inducing immunogenic tolerance through functional inactivation of antigen-reactive T lymphocytes or activation of regulatory T cells (Treg). Lymphocyte tolerance or anergy is likely induced by direct T cell inhibition via secretion of immunosuppressive cytokines, including IL-4, IL-10 and TGF-β. For example, researchers detected high levels of IL-4 and IL-4R in CD133+ stem-like cells in colon cancer. IL-4 has previously been reported to suppress apoptosis by enhancing the expression of anti-apoptotic proteins cFLIP, Bcl-xL, and PED in many tumor cell lines, including chronic lymphocytic leukemia B cells, as well as prostate, breast, and bladder tumor cell lines. Additionally, IL-4 has the capability of inhibiting the proliferation and immune responses of helper T (Th, CD4+) cells, and also exhibits immunoregulatory effect on B cells, mastocytes, and macrophages. Recent evidence shows that GSC can prevent CTL mediated specific immune cytotoxicity, and GSC can strongly inhibit the proliferation of Th cells. Effects of CSC/TIC IL-4 signaling may include autocrine inhibition of the apoptosis and induction of immune tolerance. In addition, TGF-β signaling pathway is specifically activated in the CSC/TIC fraction of breast cancers (Shipitsin et al. 2007), and secreted morphogens in the TGF-β super family as well as their receptors are preferentially expressed by CD133+ brain tumor CSC/TIC (Piccirillo et al. 2006) and by ABCB5+ malignant melanoma CSC/TIC (Schatton et al. 2008). It was shown that TGF-β negatively influences antitumor capabilities of host CTL. This activity is multi-directional and is based on the impairment of Fas-mediated apoptosis of tumor cells, downregulation of IFN-γ secretion and disturbed expression of perforin and granzymes by CTL. Indeed, T cell-specific blockade of TGF-β signaling was found to enhance the antitumor immune response in mice challenged with live tumor cells. Moreover, CSC/TIC may induce high levels of Treg cells to suppress the antitumor immune response and ultimately promote tumorigenic growth.
Immunogenic tolerance may also be achieved through clonal anergy of macrophages and dendritic cells (DC). Tumor associated macrophages (TAM) constitute one of the major immune cell populations responsible for both tumor rejection and promotion. The high expression of CD47 on leukemia stem cells (LSC) of AML patients can reduce the macrophage-induced phagocytosis of LSC and thus, decrease LSC clearance by innate immune system. CD47, also known as integrin-associated protein (IAP), can inhibit macrophage-mediated phagocytosis by binding signal-regulatory protein-α (SIRPα) on their surface (Barclay and Brown 2006). Disruption of the CD47–SIRPα interaction with a monoclonal antibody against CD47 preferentially enabled the phagocytosis of AML LSC by human macrophages. In addition, CD47–SIRPα interaction between CD47 expressed by LSC and SIRPα present on DC surface can also inhibit DC activation (Barclay and Brown 2006). Moreover, functional inactivation of DC, a major type of antigen-presenting cells, can affect the activation of initial T cells and inhibit the adaptive immune response.
Expression of another immunosuppressive protein, CD200, was significantly higher in the CSC/TIC relative to differentiated tumor cell fractions isolated from prostate, breast, colon and brain tumors. Additionally, CD200 was co-expressed with CSC/TIC markers. CD200 is a type Ia membrane protein which exerts suppressive effects through binding to its receptor, CD200R. CD200R is present on the surface of myeloid DCs, monocyte/macrophage lineage and on T lymphocytes. It was shown that the stimulation of CD200R on DCs triggered tumor supporting reactions mediated by Th2 cytokines and increased Treg activity, thought to hamper tumor-specific effector T cell immunity (Curiel et al. 2004). Conversely, blockade of CD200/CD200R interactions with monoclonal anti-CD200 antibodies resulted in a shift towards Th1 activity and attenuated immune tolerance (Rygiel et al. 2012).
While attenuation of the immune response may promote carcinogenesis, the activity of immune system itself can also promote tumor development. For example, chronic inflammatory responses mediated by activated B cells and associated antibodies have been directly shown to be critical in the initiation of skin cancer in K14-HPV16 mice (de Visser et al. 2005). Furthermore, tumor growth could be promoted by TAM. TAM can contribute to either a pro-tumorigenic or anti-tumorigenic environment depending on their capacity to present antigens, produce inflammatory cytokines, stimulate angiogenesis, and enable cytotoxic activity. While tumors evade macrophage phagocytosis through the expression of anti-phagocytic signals, including CD200 and CD47 as discussed above, cytokine production and antigen presentation by macrophages have also been shown to directly impact tumor growth.
Moreover, the evidence suggests that immune effectors can induce EMT following an acute or chronic inflammatory response. Likely, CTL cells trafficking into the tumor microenvironment can produce direct mediators of EMT, or alternatively, can produce other cytokines or chemokines (e.g., MCP-1), which can attract additional immune effectors (e.g., macrophages) that provide the stimuli. When epithelial tumors from neu-transgenic mice, that express the cell surface neu oncogene under control of the mammary epithelial cell-specific mouse mammary tumor virus (MMTV) promoter, were transplanted into nontransgenic syngeneic mice, a T-cell-dependent rejection occurred. However, the mice subsequently relapsed with tumors enriched in neu-negative variant tumor cells that had a mesenchymal phenotype. CTL cells were required for outgrowth of the neu-negative mesenchymal variants suggesting local induction of EMT (Santisteban et al. 2009). Furthermore, CTL cells isolated from mice primed with neu positive tumor cells were able to induce antigen loss when co-cultured with neu-positive tumor cells. Tumor cells isolated from relapsed mice showed that these tumors had characteristics of breast cancer stem cells (BCSC), as indicated by the cell surface CD24−/loCD44+ marker profile, enhanced mammosphere formation and tumorigenicity, elevated expression of drug transporters, DNA repair enzymes, and enhanced resistance to chemotherapy and radiation. In accordance with characteristics of true CSC/TIC, BCSC gave rise in vivo to tumors with a heterogeneous tumor population consisting of both CD24− and CD24+ tumor cells with predominant neu-positive epithelial phenotype, suggesting that the immune induced EMT was fully reversible. Thus, in contrast to their typically ascribed protective role, CTL cells were capable of inducing tumors to undergo EMT and to acquire BCSC properties and a more aggressive tumor phenotype.
Therapeutic Implications
In devising anti-cancer therapy, it is important to take into account the effects of microenvironment. Probably best known example and most used cancer treatment that relies on altering microenvironment, is inhibition of angiogenesis by anti-VEGF antibody (bevacizumab/avastin) and thus, deprivation of tumors of their oxygen and nutrition supply. Another example is inhibition of hedgehog pathway through inactivation of smoothened (Saridegib/IPI-926, GDC-0449/vismodegib, LDE-225/erismodegib) that may act to eliminate fibrous tissue that hinders drugs from reaching the cancer, while also directly affecting TIC/CSC growth. Tumor site allografts of healthy endothelial cells embedded in polymer matrix (PVS-30200) delivered at the time of surgical tumor removal to block tumor growth have also shown promise in preclinical studies.
There are multiple other novel approaches that may be tackled and are at different stages of preclinical/clinical development. For example, eliminating CSC/TIC through stimulating external signals that activate their differentiation may serve to sensitize tumor cells to standard therapy. Oxygenating hypoxic regions of tumors has the potential to promote cell cycle entry of quiescent tumor cells and to induce differentiation of hypoxic niche-dependent CSC/TIC, reducing resistance to antineoplastic therapies. Inhibiting promotion of EMT and lowering chronic inflammation while activating anti-tumor immune responses could provide additional approaches. Chronic inflammation is often associated with increased cancer risk in humans: patients with inflammatory bowel disease have an increased risk of colorectal cancer; Helicobacter pylori infection is associated with gastric adenocarcinomas and mucosa associated lymphomas; and chronic hepatitis is associated with hepatocellular carcinoma. For patients with chronic inflammatory conditions, therefore, suppressing the immune response can reduce subsequent cancer development. Considering the complexity of the disease, most likely the best ways for treating cancer patients are going to be individualized combination therapies based on well stratified patient populations and may include one or multiple of aforementioned modalities in conjunction with more traditional therapies.
A series of therapeutic strategies targeting CSC/TIC have been developed, such as inhibiting proliferation, promoting differentiation, inducing apoptosis, and enhancing the sensitivity of chemoradiation. Preliminary experimental results indicate that these strategies can target CSC/TIC and inhibit their functions albeit so far with limited success. Therefore, there is an urgent need for further in-depth investigations of the mechanisms that may lead to rational basis for treatment development.
Although identification of therapies that selectively target CSC/TIC is an important goal, a parallel and perhaps equally efficacious approach would be to target the mechanisms of plasticity that generate and maintain the CSC/TIC population in tumors, namely elements of their microenvironment. These include both extracellular factors controlled on systemic and local levels such as hypoxia, cytokines, growth factors and extracellular matrix, as well as different cellular components, including niche and stromal cells and various constituents of the immune system that contribute to tumor milieu. For example, it has been suggested by Reiman and colleagues (Reiman et al. 2010) that because immunity is able to induce BCSCs, one approach would be to define and to target the specific immune effectors of this process. Although activated CTL cells are the critical effectors of EMT in mouse mammary tumor cells, it is not possible to generally target CTL cells given their important role in protection against infection. Skewing of the macrophage response within the tumor microenvironment from a M2 (tumor-promoting) to M1 (tumor-eradicating) phenotype may have the potential to reduce tumor invasion and metastases. Having a Th2 or Treg response may promote breast cancer metastases, suggesting that agents (e.g., vaccines) that shift from Th2/Treg to an antitumor Th1 response may be useful.
Another approach to inhibit EMT-associated tumor progression is to target the pathways involved in the induction of EMT that specifically lead to the acquisition of CSC/TIC characteristics, as recently shown for inhibitors of EMT mediated by TGFβ or Hedgehog pathways. The immune system has long been viewed as a co-conspirator with developing tumors; more recent data have shown that it can also selectively target tumor cells at early stages of cancer progression. An important goal now is to identify how to reduce the tumor promotion abilities of the immune system while preserving or increasing its ability to eliminate tumor cells.
In conclusion, while tumor cell populations undergoing malignant transformation may not in some cases represent CSC or TIC (and in many cases may not arise from normal stem cells), they inevitably harbor within their cells that share with CSC/TIC some of their key properties: the ability to self-renew and to give rise to tumors and, often, also the capacity to differentiate into multiple tumor-associated cell types. It is these characteristics that may be selected for and/or supported by permissive microenvironment and are thus, important to be studied in that context. The underlying mechanisms promise to open up novel approaches to developing drug targets and therapies that may lead to increased disease-free survival and reduction in metastatic disease.
References
Aboussekhra A (2011) Role of cancer-associated fibroblasts in breast cancer development and prognosis. Int J Dev Biol 55:841–849
Ansieau S, Bastid J, Doreau A, Morel AP, Bouchet BP, Thomas C, Fauvet F, Puisieux I, Doglioni C, Piccinin S, Maestro R, Voeltzel T, Selmi A, Valsesia-Wittmann S, Caron de Fromentel C, Puisieux A (2008) Induction of EMT by twist proteins as a collateral effect of tumor-promoting inactivation of premature senescence. Cancer Cell 14:79–89
Barclay AN, Brown MH (2006) The SIRP family of receptors and immune regulation. Nat Rev Immunol 6:457–464
Boehnke K, Mirancea N, Pavesio A, Fusenig NE, Boukamp P, Stark HJ (2007) Effects of fibroblasts and microenvironment on epidermal regeneration and tissue function in long-term skin equivalents. Eur J Cell Biol 86:731–746
Bonnet D, Dick JE (1997) Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med 3:730–737
Celia-Terrassa T, Meca-Cortes O, Mateo F, de Paz AM, Rubio N, Arnal-Estape A, Ell BJ, Bermudo R, Diaz A, Guerra-Rebollo M, Lozano JJ, Estaras C, Ulloa C, Alvarez-Simon D, Mila J, Vilella R, Paciucci R, Martinez-Balbas M, de Herreros AG, Gomis RR, Kang Y, Blanco J, Fernandez PL, Thomson TM (2012) Epithelial-mesenchymal transition can suppress major attributes of human epithelial tumor-initiating cells. J Clin Invest 122:1849–1868
Chen J, Li Y, Yu TS, McKay RM, Burns DK, Kernie SG, Parada LF (2012) A restricted cell population propagates glioblastoma growth after chemotherapy. Nature 488:522–526
Coppe JP, Desprez PY, Krtolica A, Campisi J (2010) The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol 5:99–118
Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W (2004) Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 10:942–949
de Visser KE, Korets LV, Coussens LM (2005) De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell 7:411–423
Di Tomaso T, Mazzoleni S, Wang E, Sovena G, Clavenna D, Franzin A, Mortini P, Ferrone S, Doglioni C, Marincola FM, Galli R, Parmiani G, Maccalli C (2010) Immunobiological characterization of cancer stem cells isolated from glioblastoma patients. Clin Cancer Res 16:800–813
Driessens G, Beck B, Caauwe A, Simons BD, Blanpain C (2012) Defining the mode of tumour growth by clonal analysis. Nature 488:527–530
Eliasson P, Jonsson JI (2010) The hematopoietic stem cell niche: low in oxygen but a nice place to be. J Cell Physiol 222:17–22
Hermitte F, Brunet de la Grange P, Belloc F, Praloran V, Ivanovic Z (2006) Very low O2 concentration (0.1%) favors G0 return of dividing CD34+ cells. Stem Cells 24:65–73
Ilic D, Giritharan G, Zdravkovic T, Caceres E, Genbacev O, Fisher SJ, Krtolica A (2009) Derivation of human embryonic stem cell lines from biopsied blastomeres on human feeders with minimal exposure to xenomaterials. Stem Cells Dev 18:1343–1350
Inomata K, Aoto T, Binh NT, Okamoto N, Tanimura S, Wakayama T, Iseki S, Hara E, Masunaga T, Shimizu H, Nishimura EK (2009) Genotoxic stress abrogates renewal of melanocyte stem cells by triggering their differentiation. Cell 137:1088–1099
Jang YY, Sharkis SJ (2007) A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood 110:3056–3063
Korkaya H, Kim GI, Davis A, Malik F, Henry NL, Ithimakin S, Quraishi AA, Tawakkol N, D’Angelo R, Paulson AK, Chung S, Luther T, Paholak HJ, Liu S, Hassan KA, Zen Q, Clouthier SG, Wicha MS (2012) Activation of an IL6 inflammatory loop mediates trastuzumab resistance in HER2+ breast cancer by expanding the cancer stem cell population. Mol Cell 47:570–584
Krtolica A, Larocque N, Genbacev O, Ilic D, Coppe JP, Patil CK, Zdravkovic T, McMaster M, Campisi J, Fisher SJ (2011) GROalpha regulates human embryonic stem cell self-renewal or adoption of a neuronal fate. Differentiation 81:222–232
Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, Wu MF, Hilsenbeck SG, Pavlick A, Zhang X, Chamness GC, Wong H, Rosen J, Chang JC (2008) Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst 100:672–679
Li HJ, Reinhardt F, Herschman HR, Weinberg RA (2012) Cancer-stimulated mesenchymal stem cells create a carcinoma stem cell niche via prostaglandin E2 signaling. Cancer Discov 2:840–855
Parrinello S, Samper E, Krtolica A, Goldstein J, Melov S, Campisi J (2003) Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nat Cell Biol 5:741–747
Piccirillo SG, Reynolds BA, Zanetti N, Lamorte G, Binda E, Broggi G, Brem H, Olivi A, Dimeco F, Vescovi AL (2006) Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature 444:761–765
Pistollato F, Chen HL, Schwartz PH, Basso G, Panchision DM (2007) Oxygen tension controls the expansion of human CNS precursors and the generation of astrocytes and oligodendrocytes. Mol Cell Neurosci 35:424–435
Reiman JM, Knutson KL, Radisky DC (2010) Immune promotion of epithelial-mesenchymal transition and generation of breast cancer stem cells. Cancer Res 70:3005–3008
Reya T, Morrison SJ, Clarke MF, Weissman IL (2001) Stem cells, cancer, and cancer stem cells. Nature 414:105–111
Russell RC, Fang C, Guan KL (2011) An emerging role for TOR signaling in mammalian tissue and stem cell physiology. Development 138:3343–3356
Rygiel TP, Karnam G, Goverse G, van der Marel AP, Greuter MJ, van Schaarenburg RA, Visser WF, Brenkman AB, Molenaar R, Hoek RM, Mebius RE, Meyaard L (2012) CD200-CD200R signaling suppresses anti-tumor responses independently of CD200 expression on the tumor. Oncogene 31:2979–2988
Santisteban M, Reiman JM, Asiedu MK, Behrens MD, Nassar A, Kalli KR, Haluska P, Ingle JN, Hartmann LC, Manjili MH, Radisky DC, Ferrone S, Knutson KL (2009) Immune-induced epithelial to mesenchymal transition in vivo generates breast cancer stem cells. Cancer Res 69:2887–2895
Scaffidi P, Misteli T (2008) Lamin A-dependent misregulation of adult stem cells associated with accelerated ageing. Nat Cell Biol 10:452–459
Schatton T, Murphy GF, Frank NY, Yamaura K, Waaga-Gasser AM, Gasser M, Zhan Q, Jordan S, Duncan LM, Weishaupt C, Fuhlbrigge RC, Kupper TS, Sayegh MH, Frank MH (2008) Identification of cells initiating human melanomas. Nature 451:345–349
Schepers AG, Snippert HJ, Stange DE, van den Born M, van Es JH, van de Wetering M, Clevers H (2012) Lineage tracing reveals Lgr5+ stem cell activity in mouse intestinal adenomas. Science 337:730–735
Shipitsin M, Campbell LL, Argani P, Weremowicz S, Bloushtain-Qimron N, Yao J, Nikolskaya T, Serebryiskaya T, Beroukhim R, Hu M, Halushka MK, Sukumar S, Parker LM, Anderson KS, Harris LN, Garber JE, Richardson AL, Schnitt SJ, Nikolsky Y, Gelman RS, Polyak K (2007) Molecular definition of breast tumor heterogeneity. Cancer Cell 11:259–273
Weaver VM, Petersen OW, Wang F, Larabell CA, Briand P, Damsky C, Bissell MJ (1997) Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol 137:231–245
Wu A, Wiesner S, Xiao J, Ericson K, Chen W, Hall WA, Low WC, Ohlfest JR (2007) Expression of MHC I and NK ligands on human CD133+ glioma cells: possible targets of immunotherapy. J Neurooncol 83:121–131
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Krtolica, A. (2014). Role of Microenvironment in Regulating Stem Cell and Tumor Initiating Cancer Cell Behavior and Its Potential Therapeutic Implications. In: Hayat, M. (eds) Stem Cells and Cancer Stem Cells, Volume 11. Stem Cells and Cancer Stem Cells, vol 11. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-7329-5_14
Download citation
DOI: https://doi.org/10.1007/978-94-007-7329-5_14
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-7328-8
Online ISBN: 978-94-007-7329-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)