Abstract
A structural or functional cervix problem prevents a woman from carrying a full-term pregnancy, which leads to the disease known as cervical insufficiency. Cervical insufficiency is partially inherited, and in certain situations, variations in genes related to connective tissue metabolism may be involved. The main objective of this investigation was to describe the collagen type I alpha 1 chain (COL1A1) gene rs1800012 polymorphism and the transforming growth factor beta 1 (TGFB1) gene rs1800471 polymorphism in a cohort of patients suffering from cervical insufficiency. Polymerase chain reaction (PCR) and restriction fragment length polymorphism (RFLP) assays have been used to analyze the DNAs of 93 patients with cervical insufficiency and 103 healthy controls. The chi-square test was used for statistical analysis. There were significant differences in the genotype frequencies of the COL1A1 gene rs1800012 (G > T) and TGFB1 gene rs1800471 (G > C) polymorphisms between the patient and the control groups (p = 0.049 and p = 0.049, respectively). Also, the C allele of the TGFB1 rs1800471 polymorphism was significantly higher in the patient group than the control group (p = 0.016). Following clinical assessment, the COL1A1 rs1800012 polymorphism was found to be connected to the history of cerclage (p = 0.010). Additionally, the frequency of the TT/GG composite genotype of COL1A1 rs1800012/TGFB1 rs1800471 polymorphisms was significantly lower in the patient group than the control group (p = 0.049). The TT genotype of COL1A1 rs1800012 polymorphism was found to be protective against cervical insufficiency, while the C allele of TGFB1 rs1800471 polymorphism was found to predispose to the disease. It appears that the TT/GG composite genotype of COL1A1 rs1800012/TGFB1 rs1800471 polymorphisms protects against cervical insufficiency.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cervical insufficiency is a second-trimester pregnancy loss caused by the cervix's inability to support pregnancy without uterine contractions [31]. The prevalence of cervical insufficiency is estimated to be 20% of second-trimester pregnancy losses that happen before 24 weeks, 8% of recurrent mid-trimester losses in women, and 1% of all pregnancies [2, 10].
Cervical insufficiency's pathogenesis is not well understood. These women's cervical insufficiency could be explained by inherited biomechanical weakness. The structural defects [22, 23, 37], congenital Müllerian anomalies [26], birth trauma [36], induced abortion [30], or conization [21, 35] could be the cause of this weakness. Another hypothesis is that cervical insufficiency could result from early maturation [29] or be associated with an inappropriate inflammatory response [15, 28].
Since collagen (an extracellular matrix protein) makes up 85% of the dry weight of the cervix, congenital uterine abnormalities, connective tissue disorders, or impairment of normal collagen development and function abnormalities that compromise its integrity are among the risk factors for cervical insufficiency [23]. Type I and Type III collagens are the main collagens of the cervix. The Type I collagen molecule is composed of one alpha 2 and two alpha 1 chains that are encoded by the collagen type I alpha 2 chain (COL1A2) and collagen type I alpha 1 chain (COL1A1) genes, respectively. The Type III collagen molecule is composed of three alpha-1 chains. It was reported that women with a history of cervical insufficiency have less cervical collagen [34]. Polymorphisms in the human COL1A1 gene, located on chromosome 17q21.33, have been identified that may modify COL1A1 expression, hence affecting collagen type I characteristics and causing a predisposition to injuries. The most researched genetic polymorphism is the one that affects a binding site for the transcription factor Sp1 and makes a G to T alter within the gene's first intron (rs1800012). This variant was first identified in 1996 [9]. Studies have reported that the Sp1 transcription factor shows enhanced binding affinity to the Sp1 binding site of the COL1A1 gene in the presence of the T allele and enhances the synthesis of the collagen alpha 1 chain [17].
Transforming growth factor beta 1 (TGFB1) as a versatile peptide regulates several cell types' processes, including differentiation, proliferation, and other processes. TGFB1 is synthesized by a large number of cells and promotes the synthesis and deposition of extracellular matrix [5, 19]. TGFB1 belongs to the pro-fibrotic cytokine family, and it is the main regulator of collagen synthesis in human skin [32]. It was indicated that TGFB1 plasma concentration was mostly controlled by genetic variations [8]. For this reason, variations that cause elevated or lowered levels of TGFB1 may impact the communication between cells and the extracellular matrix. The human TGFB1 gene localizes on chromosome 19q13.2 and has seven exons [4]. The rs1800471 polymorphism, also known as + 915 G > C, c. + 74 G > C, and Arg25Pro, is located in the signal sequence of the TGFB1 gene and is linked to inter-individual differences in TGFB1 production levels [13]. This polymorphism modifies the transit of protein across the endoplasmic reticulum membrane, hence influencing its concentration.
Given the close functional relationship between TGFB1 and collagen in the extracellular matrix, we aimed to assess the potential involvement of COL1A1 rs1800012 and TGFB1 rs1800471 gene polymorphisms as susceptibility markers for cervical insufficiency.
Materials and Methods
Supply of Biological Materials and Profiles of Patients
Overall, 196 women were incorporated into this case–control study. The patients with cervical insufficiency were diagnosed in the gynecology and obstetrics outpatient clinics of Tokat Gaziosmanpasa University (32 patients) and Ondokuz Mayis University (61 patients) (mean age: 28.78 ± 4.506 years). The control group consisted of 103 women who gave birth at term (mean age: 27.62 ± 4.073 years). In this case–control study, details about the patients were obtained from hospital records. As a criterion for inclusion in the study, patients who underwent cerclage due to a history of miscarriage or second trimester cervical insufficiency, who have cervical shortness or cervical dilatation, or who were detected to have cervical insufficiency during follow-up. The control group included in the study consisted of 103 women who applied to Tokat Gaziosmanpasa University Hospital at the same time as the patient group and gave birth at term without any known comorbidities. As a criterion for exclusion from the study, individuals with systemic disorders (chronic hypertension, vascular diseases, and diabetes mellitus) identified either before or after pregnancy, repeated pregnancies, and fetal abnormalities, patients diagnosed with eclampsia and hemolysis elevated liver enzymes low platelet (HELLP) syndrome. It was decided that the research was ethically applicable by the Tokat Gaziosmanpasa University Clinical Research Ethics Committee (Approval No: 21-KAEK-186). We acquired informed consent from all the subjects.
Applied Methods
A commercially available DNA isolation kit (Invitrogen LifeTechnologies, Carlsbad, CA) was used to obtain DNA from whole blood collected in EDTA tubes. Polymerase chain reaction (PCR) and restriction fragment length polymorphism (RFLP) methods were employed to evaluate the rs1800012 polymorphism in the first intron of the COL1A1 gene (Gene ID: 1277) and the rs1800471 polymorphism in the first exon of the TGFB1 gene (Gene ID: 7040). The primer sequences used in the analysis of each polymorphism, the hybridization temperature of the primers, the length of the resulting PCR products, the restriction enzymes, and the sizes of the fragments obtained as a result of RFLP according to genotypes are shown in Table 1 (Figs. 1 and 2).
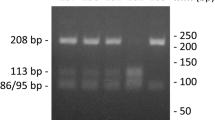
The genotypes of COL1A1 gene rs1800012 (G > T) polymorphism, which is defined by the PflMI restriction endonuclease. Lines 1 and 7: heterozygous GT genotypes; lines 2, 3,4 and 6: homozygous GG genotypes; line 5 homozygous TT genotype. Line 8: 50 bp DNA Ladder RTU (GeneDirex). The DNA Ladder contains the following 17 fragments (in base pairs): 1500, 1200, 1000, 900, 800, 700, 600, 500, 450, 400, 350, 300, 250, 200, 150, 100, 50
The genotypes of TGFB1 gene rs1800471 (G > C) polymorphism, which is defined by the BgII restriction endonuclease. Lines 2, 3, 4, 5, 6, 7, 8, 9 and 10: homozygous GG genotypes; line 11: heterozygous GC genotype. Line 1: 50 bp DNA Ladder RTU (GeneDirex). The DNA Ladder contains the following 17 fragments (in base pairs): 1500, 1200, 1000, 900, 800, 700, 600, 500, 450, 400, 350, 300, 250, 200, 150, 100, 50
Statistical Evaluation
Statistical packages for the social sciences (IBM SPSS Statistics, version 22) and OpenEpi Info software package version 3.01 (www.openepi.com) were applied for statistical analyses. To determine whether there was a deviation from Hardy–Weinberg equilibrium (HWE) for COL1A1 and TGFB1 gene polymorphisms, a chi-square test was applied. Chi-square and Fisher's exact tests were used to compare the allele and genotype frequencies of cervical insufficiency patients and healthy controls. The correlation between clinical and demographic characteristics of the patients and COL1A1 and TGFB1 gene polymorphisms was evaluated using Fisher's exact, analysis of variance (ANOVA), and chi-square tests. 95% confidence interval (CI) and odds ratio (OR) were calculated for risk factor detection. P values were considered significant if < 0.05 and 2-tailed p values were taken into account.
According to the results of the power analysis performed with the help of the G*Power 3.1.9.4 program with the two sample arrangements in independent groups, a total of 198 samples were determined for the study, taking into account the power of 80%, the margin of error of 5%, and the effect size of 0.07.
Results
Allele and genotype distributions of TGFB1 and COL1A1 gene polymorphisms were demonstrated in Table 2. A statistically notable difference was detected between cervical insufficiency patients and healthy controls in regards to genotype distributions of the COL1A1 rs1800012 (G > T) polymorphism (p = 0.049). The TT genotype was approximately 7 times more common in controls than in patients, suggesting protection against cervical insufficiency (OR = 0.15, 95% Cl = 0.006–0.997). A statistically notable difference was also detected between cervical insufficiency patients and healthy controls in regards to genotype and allele distributions of the TGFB1 rs1800471 (G > C) polymorphism (p = 0.049 and p = 0.016, respectively). The C allele was approximately 3 times more common in patients than in controls, suggesting a predisposition to cervical insufficiency (OR = 2.6, 95% Cl = 1.18–6.22). Although the genotype distributions of COL1A1 rs1800012 (G > T) polymorphism were compatible with HWE in both cervical insufficiency patients and healthy controls, the genotype distributions of TGFB1 rs1800471 (G > C) polymorphism were not compatible with HWE in cervical insufficiency patients, but they were compatible with HWE in controls.
As a result of the composite genotype analysis of both polymorphisms, it was determined that the TT/GG (COL1A1 rs1800012 G > T/TGFB1 rs1800471 G > C) composite genotype differed significantly between patients and controls (p = 0.049) (Table 3). The coexistence of the homozygous mutant genotype of COL1A1 rs1800012 polymorphism and the homozygous wild genotype of TGFB1 rs1800471 polymorphism was more common in controls, suggesting protection against cervical insufficiency.
When the clinical and demographic data of cervical insufficiency patients were compared with COL1A1 rs1800012 (G > T) and TGFB1 rs1800471 (G > C) polymorphisms, a statistically significant relationship was detected between history of cerclage and COL1A1 rs1800012 (G > T) polymorphism (p = 0.010) (Table 4). The frequency of GT + TT genotypes was lower in those with a history of cerclage than in those without a history of cerclage (15.5% vs. 40.0%).
Discussion
According to our results, there were significant differences in the genotype frequencies of COL1A1 gene rs1800012 (G > T) and TGFB1 gene rs1800471 (G > C) polymorphisms between cervical insufficiency patients and healthy controls (p = 0.049 and p = 0.049, respectively). Also, the C allele of the TGFB1 rs1800471 polymorphism was significantly higher in the patient group than the control group (p = 0.016). Following clinical assessment, the COL1A1 rs1800012 polymorphism was found to be connected to the history of cerclage (p = 0.010). Additionally, the frequency of the TT/GG composite genotype of COL1A1 rs1800012/TGFB1 rs1800471 polymorphisms was significantly lower in patients than in healthy controls (p = 0.049).
The results of this investigation support the theory that extracellular matrix-encoding genes may play a role in the complex condition known as cervical insufficiency. In this case–control association study, we verified the association of the COL1A1 rs1800012 (G > T) and TGFB1 rs1800471 (G > C) polymorphisms with cervical insufficiency in a Turkish population. Data evaluation revealed that the TT genotype of the COL1A1 rs1800012 (G > T) polymorphism was approximately 7 times more common in controls than in patients. Moreover, the frequency of the GT + TT genotype was lower among patients who have previously undergone cerclage as opposed to those who have not, suggesting that the TT genotype may provide protection against cervical insufficiency and the severity of the disease. When the previous literature was reviewed, two studies investigating the relationship between cervical insufficiency and COL1A1 rs1800012 (G > T) polymorphism were found, one in American and one in Danish populations [37, 33]. In the Danish population study [33], no correlation was observed between COL1A1 rs1800012 (G > T) polymorphism and cervical insufficiency, whereas in the American population study, a statistically significant association was found [37]. However, in contrast to our results, TT genotype was found more frequently in the patient group (10.8% vs. 3.1%, p = 0.04) [37]. In gel shift tests, the rare rs1800012 T allele has demonstrated a strong binding affinity for the Sp1 transcription factor and a threefold increase in primary COL1A1 messenger RNA (mRNA) in comparison to the G allele. [11, 17]. Furthermore, the T allele of rs1800012 was correlated with a high alpha 1/alpha 2 protein ratio, reflecting an increased COL1A1/COL1A2 mRNA ratio (2.3:1), which plays a part in the production of the type 1 collagen molecule [17]. The pro-alpha1 chains of type I collagen are encoded by the COL1A1 gene. Type I collagen, which forms fibrils, is prevalent in bones, the cornea, the dermis, and tendons. This information is consistent with our results, where we found the TT genotype more frequently in controls. In other words, the TT genotype increases pro-alpha1 chains of type I collagen production and provides protection against the disease. Additionally, mutations in COL1A1 are known to be linked to a number of well-known connective tissue illnesses, including idiopathic osteoporosis, Caffey disease, Ehlers-Danlos syndrome classical type, Ehlers-Danlos syndrome type VIIA, and osteogenesis imperfecta type I–IV. The association between the COL1A1 gene and cervical insufficiency was also examined by studying different polymorphisms of this gene. The COL1A1 gene intronic rs2586490 polymorphism was examined in patients with cervical insufficiency in a recent study conducted in a Brazilian population. The patient group's homozygote mutant genotype was found to be greater than the control group's (p = 0.023) [1].
TGFB1, a powerful immune regulator cytokine, is involved in both the attachment of trophoblast cells to the extracellular matrix and the implantation phase of the embryo [16]. Additionally, it promotes the synthesis of proteolytic enzymes, which let the trophoblast enter the prepared endometrium more easily [24]. This cytokine is crucial for preserving pregnancy as it is involved in the immunological divergence from Th1 to Th2 [25]. Many reproductive problems, such as pre-eclampsia, endometriosis, recurrent spontaneous miscarriage, unexplained infertility, penile fibrosis, prostate cancer, and breast cancer, appear to have decreased TGFB1 expression [12]. A correlation between pregnancy failure and poor expression of TGFB1 was also reported [7]. Numerous single nucleotide polymorphisms (SNPs) in exons 1 and 2 of the human TGFB1 gene have been studied in light of the knowledge that TGFB1 plays an important role in successful pregnancy [3]. In our study, we found that the TGFB1 rs1800471 (G > C) polymorphism was associated with cervical insufficiency on both an allele and a genotype basis. The frequencies of the C allele and the C allele-carrying genotypes (GC + CC) were significantly elevated in patients in contrast to controls, suggesting that the C allele and C allele-carrying genotypes create a predisposition to cervical insufficiency. Similarly, Warren et al. [37], in their study of the American population, found an association between cervical insufficiency and TGFB1 rs1800471 (G > C) polymorphism. The C allele-carrying genotypes (GC + CC) were also found to be increased in patients compared to controls in their study (p < 0.001). In silico analysis showed that the TGFB1 rs1800471 (G > C, Arg25Pro) polymorphism significantly altered the secondary structure of mRNA, which can lower TGFB1 levels by reducing mRNA half-life [18]. Additionally, this polymorphism made the TGFB1 signal peptide more hydrophobic, which hindered the protein's passage through the endoplasmic reticulum. The association between the TGFB1 gene and cervical insufficiency was also examined by studying different polymorphisms of this gene. In a recent study carried out in a Brazilian population, the TGFB1 gene 3’untranslated region polymorphism (rs1800468) was studied in cervical insufficiency patients, and no association was found [1].
The cervix is composed of up to 85% collagen, is firm, serves as a barrier to stop the spread of infection, and provides mechanical strength [2]. During pregnancy, the cervix remodels with the degradation of collagen and increased softness and elasticity of the cervix [10, 20]. Any disruption to this process could result in cervical dystocia and cervical insufficiency, which could lead to preterm birth. Second-trimester biopsy samples from women with cervical insufficiency have been shown to include a significant percentage of newly generated collagen with low biomechanical strength [27]. Cervical remodeling is influenced by numerous factors, such as hormones (progesterone, estrogen), cross-linking enzymes of collagen biosynthesis (lysyl oxidase, LOX), macrophages, metalloproteinases, collagenases and proteoglycans [14]. Also, anti-inflammatory cytokines are necessary for pregnancy maintenance [6]. Anti-inflammatory cytokines block pro-inflammatory cytokines, and a reduction in anti-inflammatory cytokine production may lead to preterm birth by increasing an individual's susceptibility to infections.
Therefore, studying collagen biosynthesis genes such as COL1A1, COL1A2, LOX, TGFB1, and cytokine genes such as interleukin 6 (IL6), IL10, and tumor necrosis factor (TNF) may be potential candidates in women who have cervical insufficiency.
Conclusion
To the best of our knowledge, this is the first study that identifies the function of COL1A1 rs1800012 (G > T) and TGFB1 rs1800471 (G > C) polymorphisms in cervical insufficiency in the Turkish population. A lower frequency of the TT genotype of COL1A1 rs1800012 (G > T) polymorphism and a higher frequency of the C allele and C allele-carrying genotypes of TGFB1 rs1800471 (G > C) polymorphism were found in cervical insufficiency patients. While the TT genotype of COL1A1 rs1800012 (G > T) polymorphism appears to be protective against cervical insufficiency and disease severity, the C allele and C allele-carrying genotypes of TGFB1 rs1800471 (G > C) polymorphism appear to show susceptibility to cervical insufficiency. Our study's findings indicate that functional analysis is required to assess the impact of TGFB1 and COL1A1 gene polymorphisms on mRNA and protein expression because polymorphisms in the genes, impair protein synthesis and function.
Data Availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
Alves APVD, Freitas AB, Levi JE, et al. COL1A1, COL4A3, TIMP2 and TGFB1 polymorphisms in cervical insufficiency. J Perinat Med. 2021;49(5):553–8. https://doi.org/10.1515/jpm-2020-0320.
Brown R, Gagnon R, Delisle MF, MATERNAL FETAL MEDICINE COMMITTEE. Cervical insufficiency and cervical cerclage. J ObstetGynaecol Can. 2013;35(12): 1115-1127. https://doi.org/10.1016/S1701-2163(15)30764-7. Erratum in: J Obstet Gynaecol Can. 2014 36(1):13.
Cebinelli GCM, Trugilo KP, Garcia SB, de Oliveira KB. TGF-β1 functional polymorphisms: a review. Eur Cytokine Netw. 2016;27(4):81–9. https://doi.org/10.1684/ecn.2016.0382.
Derynck R, Rhee L, Chen EY, Van Tilburg A. Intron-exon structure of the human transforming growth factor-beta precursor gene. Nucleic Acids Res. 1987;15(7):3188–9. https://doi.org/10.1093/nar/15.7.3188.
Derynck R, Akhurst RJ, Balmain A. TGF-beta signaling in tumor suppression and cancer progression. Nat Genet. 2001; 29(2): 117-129. https://doi.org/10.1038/ng1001-117. Erratum in: Nat Genet 2001 29(3):351.
Engel SA, Olshan AF, Savitz DA, Thorp J, Erichsen HC, Chanock SJ. Risk of small-for-gestational age is associated with common anti-inflammatory cytokine polymorphisms. Epidemiology. 2005;16(4):478–86. https://doi.org/10.1097/01.ede.0000164535.36412.6b.
Gorivodsky M, Torchinsky A, Zemliak I, Savion S, Fein A, Toder V. TGF beta 2 mRNA expression and pregnancy failure in mice. Am J Reprod Immunol. 1999;42(2):124–33.
Grainger DJ, Heathcote K, Chiano M, et al. Genetic control of the circulating concentration of transforming growth factor type beta1. Hum Mol Genet. 1999;8(1):93–7. https://doi.org/10.1093/hmg/8.1.93.
Grant SF, Reid DM, Blake G, Herd R, Fogelman I, Ralston SH. Reduced bone density and osteoporosis associated with a polymorphic Sp1 binding site in the collagen type I alpha 1 gene. Nat Genet. 1996;14(2):203–5. https://doi.org/10.1038/ng1096-203.
Iams JD, Cebrik D, Lynch C, Behrendt N, Das A. The rate of cervical change and the phenotype of spontaneous preterm birth. Am J Obstet Gynecol. 2011;205(2):130.e1-6. https://doi.org/10.1016/j.ajog.2011.05.021.
Jin H, van't Hof RJ, Albagha OM, Ralston SH. Promoter and intron 1 polymorphisms of COL1A1 interact to regulate transcription and susceptibility to osteoporosis. Hum Mol Genet. 2009; 18(15): 2729-2738. https://doi.org/10.1093/hmg/ddp205.
Kajdaniuk D, Marek B, Borgiel-Marek H, Kos-Kudła B. Transforming growth factor β1 (TGFβ1) in physiology and pathology. Endokrynol Pol. 2013;64(5):384–96. https://doi.org/10.5603/EP.2013.0022.
Khani M, Amani D, Taheripanah R, Sanadgol N, Feizollahzadeh S, Rahmani Z. Transforming growth factor beta-1 (TGF-β1) gene single nucleotide polymorphisms (SNPs) and susceptibility to pre-eclampsia in Iranian women: A case-control study. Pregnancy Hypertens. 2015;5(4):267–72. https://doi.org/10.1016/j.preghy.2015.01.002.
Kurt I, Kulhan M, AlAshqar A, Borahay MA. Uterine collagen cross-linking: biology, role in disorders, and therapeutic implications. Reprod Sci. 2024;31(3):645–60. https://doi.org/10.1007/s43032-023-01386-7.
Lee SE, Romero R, Park CW, Jun JK, Yoon BH. The frequency and significance of intraamniotic inflammation in patients with cervical insufficiency. Am J Obstet Gynecol. 2008;198(6):633.e1-8. https://doi.org/10.1016/j.ajog.2007.11.047.
Li Q. Transforming growth factor β signaling in uterine development and function. J Anim Sci Biotechnol. 2014;5(1):52. https://doi.org/10.1186/2049-1891-5-52.
Mann V, Hobson EE, Li B, et al. A COL1A1 Sp1 binding site polymorphism predisposes to osteoporotic fracture by affecting bone density and quality. J Clin Invest. 2001;107(7):899–907. https://doi.org/10.1172/JCI10347.
Marhemati F, Rezaei R, Mohseni Meybodi A, Taheripanah R, Mostafaei S, Amani D. Transforming growth factor beta 1 (TGFβ1) polymorphisms and unexplained infertility: a genetic association study. Syst Biol Reprod Med. 2020;66(4):267–80. https://doi.org/10.1080/19396368.2020.1773575.
Mezzano SA, Ruiz-Ortega M, Egido J. Angiotensin II and renal fibrosis. Hypertension. 2001;38(3 Pt 2):635–8. https://doi.org/10.1161/hy09t1.094234.
Norman JE. Preterm labour. Cervical function and prematurity. Best practice & research. Clin Obstet Gynaecol. 2007;21(5):791–806. https://doi.org/10.1016/j.bpobgyn.2007.03.002.
Ortoft G, Henriksen T, Hansen E, Petersen L. After conisation of the cervix, the perinatal mortality as a result of preterm delivery increases in subsequent pregnancy. BJOG. 2010;117(3):258–67. https://doi.org/10.1111/j.1471-0528.2009.02438.x.
Oxlund BS, Ørtoft G, Brüel A, Danielsen CC, Oxlund H, Uldbjerg N. Cervical collagen and biomechanical strength in non-pregnant women with a history of cervical insufficiency. Reprod Biol Endocrinol. 2010;8:92. https://doi.org/10.1186/1477-7827-8-92.
Petersen LK, Uldbjerg N. Cervical collagen in non-pregnant women with previous cervical incompetence. Eur J Obstet Gynecol Reprod Biol. 1996;67(1):41–5. https://doi.org/10.1016/0301-2115(96)02440-2.
Poniatowski ŁA, Wojdasiewicz P, Gasik R, Szukiewicz D. Transforming growth factor Beta family: insight into the role of growth factors in regulation of fracture healing biology and potential clinical applications. Mediators Inflamm. 2015;2015:137823. https://doi.org/10.1155/2015/137823.
Qiu T, Teng Y, Wang Y, Xu L. Adoptive transfer of transforming growth factor-β1-induced CD4+ CD25+ regulatory T cells prevents immune response-mediated spon-taneous abortion. Reprod Fertil Dev. 2016;28(11):1788–97. https://doi.org/10.1071/RD14503.
Rackow BW, Arici A. Reproductive performance of women with müllerian anomalies. Curr Opin Obstet Gynecol. 2007;19(3):229–37. https://doi.org/10.1097/GCO.0b013e32814b0649.
Rechberger T, Uldbjerg N, Oxlund H. Connective tissue changes in the cervix during normal pregnancy and pregnancy complicated by cervical incompetence. Obstet Gynecol. 1988;71(4):563–7.
Romero R, Gonzalez R, Sepulveda W, et al. Infection and labor. VIII. Microbial invasion of the amniotic cavity in patients with suspected cervical incompetence: prevalence and clinical significance. Am J Obstet Gynecol. 1992;167(4 Pt 1):1086–91. https://doi.org/10.1016/s0002-9378(12)80043-3.
Schlembach D, Mackay L, Shi L, Maner WL, Garfield RE, Maul H. Cervical ripening and insufficiency: from biochemical and molecular studies to in vivo clinical examination. Eur J Obstet Gynecol Reprod Biol. 2009;144(Suppl 1):S70–6. https://doi.org/10.1016/j.ejogrb.2009.02.036.
Shah PS, Zao J, Knowledge Synthesis Group of Determinants of preterm/LBW births. Induced termination of pregnancy and low birthweight and preterm birth: a systematic review and meta-analyses. BJOG. 2009;116(11):1425-42. https://doi.org/10.1111/j.1471-0528.2009.02278.x.
Shennan A, Jones B. The cervix and prematurity: aetiology, prediction and prevention. Semin Fetal Neonatal Med. 2004;9(6):471–9. https://doi.org/10.1016/j.siny.2004.09.001.
Shi X, Luo J, Weigel KJ, et al. Cancer-associated fibroblasts facilitate squamous cell carcinoma lung metastasis in mice by providing TGFβ-mediated cancer stem cell niche. Front Cell Dev Biol. 2021;9:668164. https://doi.org/10.3389/fcell.2021.668164.
Sundtoft I, Uldbjerg N, Steffensen R, Sommer S, Christiansen OB. Polymorphisms in genes coding for cytokines, mannose-binding lectin, collagen metabolism and thrombophilia in women with cervical insufficiency. Gynecol Obstet Invest. 2016;81(1):15–22. https://doi.org/10.1159/000381620.
Sundtoft I, Langhoff-Roos J, Sandager P, Sommer S, Uldbjerg N. Cervical collagen is reduced in non-pregnant women with a history of cervical insufficiency and a short cervix. Acta Obstet Gynecol Scand. 2017;96(8):984–90. https://doi.org/10.1111/aogs.13143.
Voigt M, Henrich W, Zygmunt M, Friese K, Straube S, Briese V. Is induced abortion a risk factor in subsequent pregnancy? J Perinat Med. 2009;37(2):144–9. https://doi.org/10.1515/JPM.2009.001.
Vyas NA, Vink JS, Ghidini A, et al. Risk factors for cervical insufficiency after term delivery. Am J Obstet Gynecol. 2006;195(3):787–91. https://doi.org/10.1016/j.ajog.2006.06.069.
Warren JE, Silver RM, Dalton J, Nelson LT, Branch DW, Porter TF. Collagen 1Alpha1 and transforming growth factor-beta polymorphisms in women with cervical insufficiency. Obstet Gynecol. 2007;110(3):619–24. https://doi.org/10.1097/01.AOG.0000277261.92756.1a.
Acknowledgements
This research was supported by Tokat Gaziosmanpasa University Research Fund (Project No: 2021/83).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
The authors report there are no competing interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gulucu, S., Onal, M. & Karakus, N. Genetic Polymorphisms of COL1A1 Promoter Region (rs1800012) and TGFB1 Signal Peptide (rs1800471): Role in Cervical Insufficiency Susceptibility?. Reprod. Sci. (2024). https://doi.org/10.1007/s43032-024-01684-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43032-024-01684-8